Abstract
Objective
To evaluate reproductive hormone patterns in women exposed to alkylating agent chemotherapy
Design
Prospective cohort
Setting
University Hospital
Patients
Normally menstruating, mid-reproductive age women (20-35 years old) who had previously been exposed to alkylating agent chemotherapy for cancer treatment were compared to two healthy control populations: similarly-aged women and late reproductive age women (43-50 years old).
Interventions
Subjects collected daily urine samples for one cycle.
Main Outcome Measures
Integrated urinary pregnanediol glucuronide (PDG) and estrone conjugates (E1c) and urinary excretion of gonadotropins (FSH and LH)
Results
38 women (13 survivors, 11 same-age controls, 14 late reproductive age controls) provided 1082 urine samples. Cycle length, luteal phase length, and evidence of luteal activity were similar between groups. As expected, ovarian reserve was impaired in cancer survivors compared to same-age controls but similar between survivors and late reproductive age controls. In contrast, survivors had total and peak PDG levels that were similar to same-age controls and higher than those observed in late reproductive age controls. Survivors had higher E1c levels than both same-age controls and late reproductive age controls. There was no difference in urinary gonadotropins among groups.
Conclusions
Women exposed to alkylating agents have a unique reproductive hormone milieu that is not solely explained by age or ovarian reserve. The urinary hormone profile observed in survivors appears more similar to same-age controls than late reproductive age women with similar ovarian reserve, which may suggest that age plays a more important role than ovarian reserve in the follicular dynamics of survivors.
Keywords: progesterone excretion, urinary hormones, PDG, E1c, cancer survivors
Introduction
Each year in the United States, over 100,000 women and girls under the age of 45 are diagnosed with cancer.(1) Of these, approximately 12,400 are younger than 20 years old at the time of diagnosis.(2) Cancer therapies have improved substantially in recent years, leading to dramatic increases in survival. Currently, there are estimated to be over 10 million adult cancer survivors in the United States. Among U.S. adults in their thirties, one in a thousand is a childhood cancer survivor. (3,4) As survival improves, there is increasing emphasis on optimizing health and quality of life among survivors.
Many cancer therapies are gonadotoxic, particularly alkylating agent chemotherapy and pelvic radiation.(5,6) These treatments result in acute destruction of ovarian follicles, which results in an overall depletion of the ovarian follicular pool. Indeed, multiple studies have shown that cancer survivors have decreased measures of ovarian reserve compared to unexposed women of similar age.(7) Depletion of the follicular pool may manifest clinically as menstrual cycle irregularity, subfertility and premature menopause. Even among those who resume regular menses after treatment, the risk of infertility and early menopause is increased. (6,8)
Accelerated reproductive aging in mid reproductive aged cancer survivors parallel observations during natural reproductive aging in late reproductive age women. (9) In the late reproductive years, follicular secretion of Anti-Mullerian Hormone (AMH) and Inhibin B declines, resulting in decreased pituitary suppression and a subsequent rise in Follicle Stimulating Hormone (FSH). (10-16) Ovarian antral follicle count also declines.(17,18) Furthermore, urinary hormone assessment reveals that compared to mid-reproductive age women, women in the late reproductive years are more likely to demonstrate luteal phase dysfunction and have elevated estradiol metabolites and gonadotropins levels. (19-21) Thus, in addition to impairment in oocyte number, studies suggest that among late reproductive age women, the quality of the ovarian follicles declines over time, even among women with regular menses.
Similar to natural reproductive aging, we know that chemotherapy causes decreases in oocyte number. In contrast, we do not know if the quality of the ovarian follicle is impaired. Indeed, little is known about how cancer therapy influences folliculogenesis and if ovulatory quality is compromised. The aim of this study is to evaluate ovulatory function in cancer survivors in comparison to naturally aging women in their mid and late reproductive years.
Methods
The Institutional Review Board at the University of Pennsylvania approved this prospective cohort study. We enrolled three groups of women: cancer survivors, similarly aged controls, and late reproductive age controls. Survivors were eligible for enrollment if they were between 20 and 35 years of age, previously exposed to alkylating agent chemotherapy, currently healthy and at least one year from completion of cancer treatment. Healthy women between 20 and 35 years old with no history of infertility and no exposure to chemotherapy were eligible for enrollment as same age controls. Healthy women between 43 and 50 years old with no history of infertility and no exposure to chemotherapy were recruited as late reproductive age controls. Subjects in all groups were required to have regular menstrual cycles every 21- 35 days and have a uterus and both ovaries.
Exclusion criteria included pregnancy or lactation within the previous three months, use of hormonal contraception or replacement within the previous three months, Body Mass Index (BMI) greater than 30 kg/m2, and excessive exercise (defined as greater than 1 hour per day). In addition, subjects were excluded if they had any medical condition other than cancer associated with premature ovarian failure (i.e. Turners Syndrome or Fragile X syndrome) or ovulatory dysfunction (i.e. thyroid disease, congenital adrenal hyperplasia, Cushing’s syndrome, hyperprolactinemia, and polycystic ovary syndrome). Subjects were identified from existing prospective cohort studies at the University of Pennsylvania, the Cancer Survivorship Program at the Children’s Hospital of Philadelphia, Abramson Cancer Center, and community referrals.
Study visits were scheduled in the early follicular phase and included a questionnaire to assess demographic information, transvaginal ultrasound for measurement of antral follicle count, and blood sample collection for measurement of FSH, E2 and AMH. The subjects were then asked to collect a urine sample daily starting on the first day of the menstrual cycle for one menstrual cycle. The sample was collected from the first morning void, poured into pre-labeled, glycerol containing tubes and stored in the freezer in a pre-labeled box. Samples were transported to the clinic in coolers. Samples were then thawed quickly using hot water, centrifuged, aliquoted into 1-1.25 mL samples, and refrozen at -80°C. Frozen samples were then shipped to the University of Michigan CLASS Laboratory for urinary hormone analysis. The subjects were asked to keep a diary, in which they recorded the collection time, spotting or bleeding, missing collection days and any problems with the collection.
The following analytes were measured in the urine for each day of the cycle: follicle stimulating hormone (FSH), luteinizing hormone (LH), pregnanediol glucuronide (PDG), which is a metabolite of circulating progesterone, estrone conjugate (E1c), which is a metabolite of circulating estrogens, and creatinine. Urinary FSH and LH were measured via two-site chemiluminescent immunoassay using two monoclonal antibodies. The sensitivity for the FSH assay was 1.05 mIU/mL, range was 1.05-244 mIU/mL, and inter and intra-assay variability was 10.9% and 3.9%, respectively. For the LH assay, the sensitivity was 1.0 mIU/mL, the range was 1.0-587 mIU/mL, and the inter and intra-assay variability was 10.7% and 4.8%, respectively. Creatinine was measured using a spectrophotometric assay with a sensitivity of 0.05 mg/mL, range of 0.05-1.4 mg/mL, inter-assay variability of 11.4% and intra-assay variability of 4.3%. PDG and E1c were measured via competitive immunoassay with direct chemoiluminometric technology. For the PDG assay, sensitivity was 25 ng/mL, range was 0.005-25 mcg/mL, inter-assay variability was 12.3%, and intra-assay variability was 7.7%. The E1c assay had a sensitivity of 5 ng/mL, range of 5.1-408 ng/mL, inter-assay variability of 11.0%, and intra-assay variability of 7.8%. Urinary FSH, LH, PDG and E1c were normalized to creatinine and integrated over one cycle to generate total cycle values. Peak values were also examined. Follicular and luteal integrated urinary hormone levels were assessed based on day of luteal transition, which was determined based on the LH peak. Early follicular phase serum samples were also obtained for the measurement of FSH, Estradiol (E2) and AMH (Gen II assay) by the University of Pennsylvania Clinical Translational Research Center. Serum samples were analyzed using ELISA kits for AMH (Diagnostic Systems, Gen II assay) and IRMA Coat-A-Count kits for FSH (Siemens) and E2 (Diagnostic Products Corporation). The range of the AMH assay was 0.050-10.0 ng/mL, with a sensitivity 0.025 ng/mL, inter-assay variability less than 8%, and intra-assay variability of 5%. The range of the FSH assay was 1.0 - 100 mIU/ml, with a sensitivity of 0.25 mIU/ml, inter-assay variability less than 8%, and intra-assay variability less than 4 %. The range of the E2 assay was 20-3,600 pg/mL, with a sensitivity of 7 pg/ml and inter- and intra-assay variability of less than 8% and less than 6%, respectively. For analysis, all hormones were natural log transformed to reduce the impact of large values. Results are back transformed and presented as geometric mean levels along with 95% confidence intervals. Linear regression models were controlled for cycle length and missing observations. Two previously validated methods, an absolute threshold and a relative threshold, were used to assess for evidence of luteal activity. Using the absolute threshold method, a cycle was considered ovulatory if creatinine-adjusted PDG levels rose to 3 ng/mg Cr or above for three consecutive days.(19) The relative threshold, or Kassam method, determined that there was evidence of luteal activity if adjusted PDG rose three times above a cycle-specific baseline.(19,22)
The primary outcomes were integrated urinary PDG, Integrated urinary E1c, and luteal phase length. Integrated urinary FSH and LH were also examined.
Results
Thirty-eight women (13 survivors, 11 same age controls, and 14 late reproductive age controls) provided 1082 urine samples for analysis. Mean age of cancer diagnosis among survivors was 13.1 years (standard deviation 7.3 years, range 2-26 years), and mean time since completion of cancer treatment was 14.4 years (standard deviation 7.1 years, range 5-29 years). Eight survivors had hematologic malignancies, four had sarcomas, and one had breast cancer. All survivors were treated with alkylating agent chemotherapy; among those, seven received high dose therapy (defined as cumulative dose of cyclophosphamide of 15g/m2 or higher, cumulative dose of ifosfamide of 40g/m2 or higher, or having had alkylating chemotherapy prior to a bone marrow transplant.) Six survivors were treated with radiation and one underwent bone marrow transplant.
The mean age of cancer survivors was 28.2 compared to 29.1 in same age controls. As expected, the women in these groups were significantly younger than those in the late reproductive age group (Table 1). There was no difference in BMI, race, smoking status or percentage with higher education. Survivors were more likely to be nulligravid compared to late reproductive age controls (69% vs. 21%, p<0.05). There was no significant difference in parity between survivors and same age controls. When comparing menstrual cycle characteristics, there was no difference in cycle length, evidence of luteal activity, or luteal phase length. A higher percentage of survivors had a luteal phase that was less than 12 days (39%) compared to healthy controls (20% ) and late reproductive age controls (14%), but this difference did not reach statistical significance. Twenty-seven percent of same age controls missed at least one day of urine collection compared to 15% and 14% of survivors and late reproductive age controls, respectively.
Table 1.
Demographic Data Among Cancer Survivors, Same Age Controls and Late Reproductive Age Controls
| Cancer survivors (n=13) | Same age controls (n=11) | Late reproductive age controls (n=14) | p value Survivors vs. Same age | p value Survivors vs. Late Reproductive age | p value Same age vs. Late Reproductive age | |
|---|---|---|---|---|---|---|
| Age, mean (sd) | 28.2 (4.6) | 29.1 (4.1) | 45.7 (2.6) | 0.47 | <0.01 | <0.01 |
| BMI, mean (sd) | 23.5 (3.3) | 23.4 (1.8) | 24.7 (3.6) | 0.88 | 0.36 | 0.49 |
| Caucasian race (%) | 92.3% | 90.9% | 92.9% | 1.00 | 1.00 | 1.00 |
| Current smoker (%) | 7.7% | 9.1% | 14.3% | 1.00 | 1.00 | 1.00 |
| Higher education (%) | 76.9% | 80.0% | 64.3% | 1.00 | 0.68 | 0.65 |
| Nulligravid (%) | 69.2% | 54.6% | 21.4% | 0.68 | 0.02 | 0.12 |
| Cycle length, median (range) | 29 (23-36) | 28 (26-34) | 29 (25-30) | 0.64 | 0.75 | 0.89 |
| Follicular phase length, median (range) | 15 (11-23) | 14 (12-20) | 14 (10-20) | 1.00 | 0.28 | 0.32 |
| Luteal phase length, median (range) | 12 (6-21) | 14 (6-15) | 14.5 (9-22) | 0.86 | 0.13 | 0.18 |
| Luteal phase <12 days | 39% | 20% | 14% | 0.39 | 0.08 | 0.57 |
| No missing days | 85% | 73% | 86% | 0.63 | 1.00 | 0.31 |
| Evidence of luteal activity (absolute threshold) | 100% | 72.3% | 92.9% | 0.08 | 1.00 | 0.29 |
| Evidence of luteal activity (relative threshold) | 69.2% | 63.6% | 64.3% | 1.00 | 1.00 | 1.00 |
As expected, when compared to same age controls, cancer survivors had impaired measures of ovarian reserve (Table 2). Geometric mean AMH was 0.53 ng/ml (95% CI 0.18-1.53) among survivors compared to 3.60 ng/ml (95% CI 2.16-6.00) among controls (p=0.01). Survivors had higher serum early follicular phase FSH levels (12.3 vs. 8.3 mIU/mL, p=0.02) and fewer antral follicles (11.3 vs. 38.1, p<0.01). There was no difference in early follicular phase serum estradiol levels 25.5 vs. 31.1 mIU/mL, p=0.37). Cancer survivors had similar serum AMH, serum FSH, serum estradiol, and antral follicles to late reproductive age women (Table 2).
Table 2.
Comparison of Ovarian Reserve Among Cancer Survivors, Same Age Controls and Late Reproductive Age Controls
| Cancer survivors | Same age controls | Late reproductive age controls | p value Survivors vs. Same age | p value Survivors vs. Late Reproductive age | p value Same age vs. Late Reproductive age | |
|---|---|---|---|---|---|---|
| AMH (ng/mL) | 0.53 (0.18-1.53) | 3.60 (2.16-6.00) | 0.28 (0.15-0.55) | 0.01 | 0.40 | <0.01 |
| FSH (mIU/mL) | 12.3 (8.9-17.1) | 8.3 (7.2-9.4) | 16.2 (11.6-22.6) | 0.02 | 0.31 | <0.01 |
| Estradiol (mIU/mL) | 25.5 (20.7-31.4) | 31.3 (22.5-43.4) | 20.3 (13.0-37.5) | 0.37 | 0.62 | 0.14 |
| Antral Follicle Count | 11.3 (7.1-17.9) | 38.1 (24.0-60.5) | 8.5 (4.6-15.8) | <0.01 | 0.38 | <0.01 |
Units: PDG mcg/mgCr, E1c ng/mgCr, FSH and LH mU/mgCr
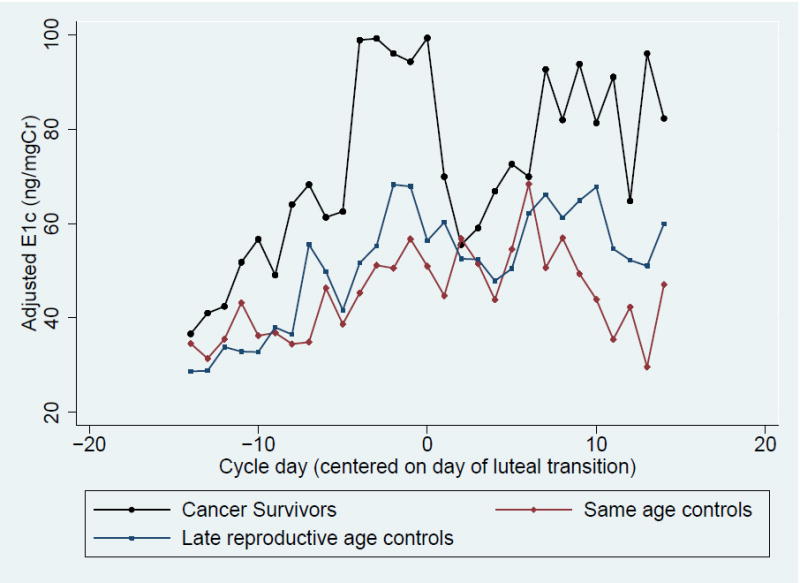
When urinary PDG levels were compared between groups, cancer survivors had total cycle, peak, luteal phase, and follicular phase PDG levels that were similar to their same age peers (Table 3, Figure 1A). When compared to late reproductive age controls, survivors demonstrated significantly higher total cycle and follicular PDG levels, and there was a trend toward high peak and luteal PDG levels. A similar pattern was observed when same age controls were compared to late reproductive age controls: total, peak, and follicular PDG levels were significantly higher in same age controls compared to late reproductive age women, and there was a trend toward higher luteal PDG (Table 3, Figure 1A). When comparing E1c between groups, cancer survivors had significantly higher total cycle, peak, and follicular E1c levels than both same age controls and late reproductive age controls (Table 3, Figure 1B).
Table 3.
Comparison of Urinary Pregnanediol Glucuronide (PDG), Estrone Conjugates (E1c), Follicle Stimulating Hormone (FSH), and Luteinizing Hormone (LH) Among Cancer Survivors, Same Age Controls and Late Reproductive Age Controls
| Cancer survivors (n=13) | Same age controls (n=11) | Late reproductive age controls (n=14) | p value Survivors vs. Same age | p value Survivors vs. Late Reproductive age | p value Same age vs. Late Reproductive age | |
|---|---|---|---|---|---|---|
| PDG Total | 40.0 (31.5-50.9) | 47.1 (36.1-61.5) | 28.2 (22.3-35.5) | 0.37 | 0.04 | <0.01 |
| PDG Peak | 3.78 (2.82-5.07) | 4.47 (3.23-6.18) | 2.57 (1.93-3.40) | 0.45 | 0.06 | 0.01 |
| PDG Luteal | 24.6 (18.4-33.1) | 28.3 (20.0-39.9) | 19.7 (14.9-26.1) | 0.55 | 0.27 | 0.11 |
| PDG Follicular | 12.9 (9.3-17.9) | 16.2 (11.3-23.3) | 6.8 (5.0-9.3) | 0.35 | <0.01 | <0.01 |
| E1c Total | 1863 (1482-2344) | 1109 (860-1430) | 1314 (1054-1638) | 0.01 | 0.03 | 0.32 |
| E1c Peak | 169 (128-222) | 76 (56-104) | 91 (69.8-118.4) | <0.01 | <0.01 | 0.39 |
| E1c Luteal | 848 (610-1178) | 570 (388-838) | 682 (497-936) | 0.13 | 0.34 | 0.47 |
| E1c Follicular | 908 (638-1291) | 537 (363-793) | 443 (316-621) | 0.05 | <0.01 | 0.46 |
| FSH Total | 839 (642-1096) | 804 (598-1081) | 841 (651-1087) | 0.83 | 0.99 | 0.82 |
| FSH Peak | 93 (64-134) | 64 (42-96) | 79 (56-113) | 0.18 | 0.54 | 0.42 |
| FSH Follicular | 475 (317-710) | 428 (274-669) | 357 (242-523) | 0.73 | 0.31 | 0.54 |
| FSH Luteal | 286 (210-386) | 305 (213-435) | 387 (289-519) | 0.78 | 0.15 | 0.30 |
| LH Total | 134 (99-181) | 126 (90-175) | 138 (104-184) | 0.78 | 0.89 | 0.66 |
| LH Peak | 26 (17-39) | 19 (12-31) | 23 (15-35) | 0.37 | 0.70 | 0.57 |
| LH Follicular | 67 (48-93) | 63 (43-92) | 56 (41-78) | 0.83 | 0.47 | 0.64 |
| LH Luteal | 44 (29-67) | 47 (29-77) | 56 (38-83) | 0.83 | 0.40 | 0.59 |
Data presented as geometric mean and 95% CI adjusted for cycle length and days missing
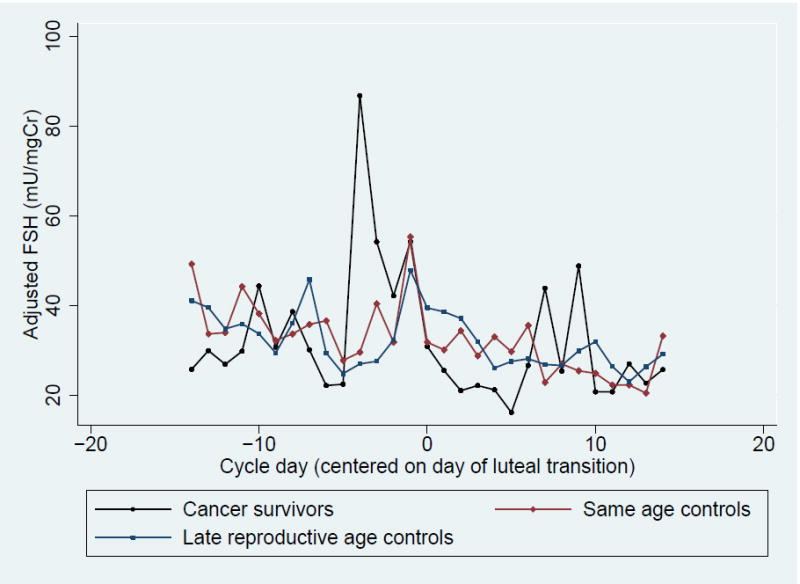
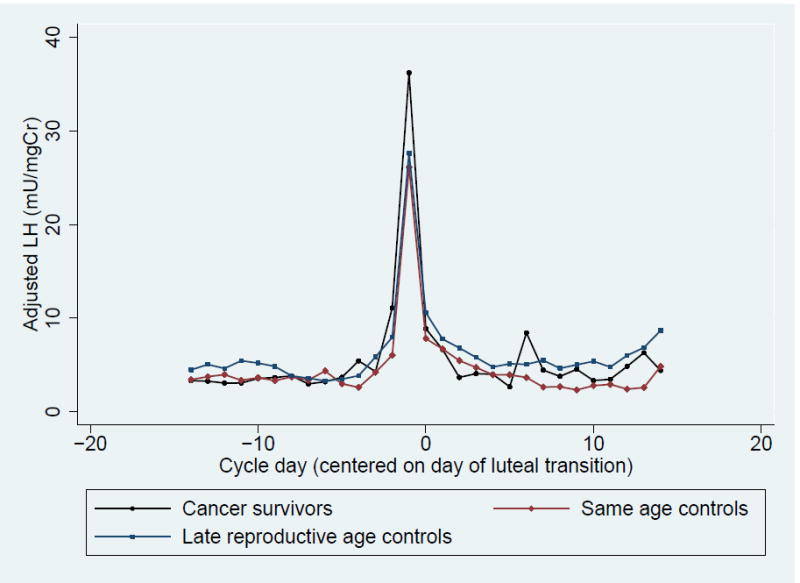
Figure 1. Daily Adjusted Urinary Metabolites Levels Among Cancer Survivors, Same Age Controls and Late Reproductive Age Controls.
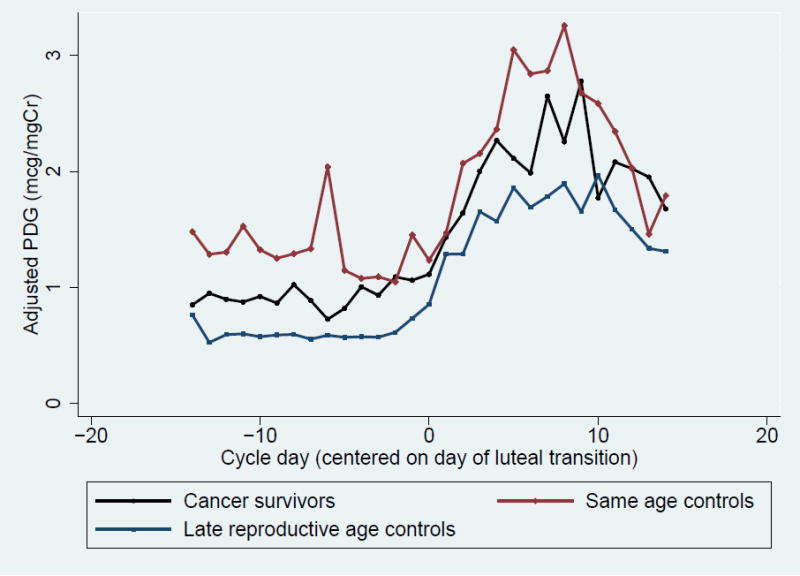
- Urinary Pregnanediol Glucuronide (PDG)
- Urinary Estrone Conjugates (E1c)
- Urinary Follicle Stimulating Hormone (FSH)
- Urinary Luteinizing Hormone (LH)
In contrast, there was no significant difference in total, peak, follicular phase and luteal phase urinary FSH and LH between groups (Table 3, Figure 1C and 1D). However, when examining integrated FSH levels graphically by cycle day, cancer survivors tended to have higher peak and follicular FSH levels (Table 3, Figure 1 C). This trend was more pronounced in survivors who received high dose therapy but was still present among survivors with low dose chemotherapy (Supplementary Figure 1). When integrated urinary FSH levels were compared between groups in the four days prior to ovulation, the trend toward higher FSH levels among survivors was more pronounced and corresponded to a significantly higher integrated E1c level over the same time period (Supplementary Table 1).
In summary, cancer survivors demonstrated lower ovarian reserve, similar progesterone excretion and higher estrogen excretion than their same age peers. In contrast, survivors have similar ovarian reserve to late reproductive age controls but had higher progesterone and estrogen excretion. There was no significant difference in FSH and LH between groups, but there was a trend toward higher late follicular FSH levels among cancer survivors.
Discussion
Despite having impaired ovarian reserve, women exposed to alkylating agent chemotherapy have progesterone excretion that is similar to their same age peers and higher than late reproductive age women. In addition, survivors demonstrated higher estrogen excretion than either control group despite similar urinary gonadotropin levels, indicating a unique reproductive hormone milieu that cannot be explained solely based on age or ovarian reserve.
Daily urinary hormone assessments have been previously measured in late reproductive age women in an effort to define characteristics associated with the menopausal transition. Santoro et al. analyzed daily urinary PDG levels in 848 late reproductive age women across one menstrual cycle yearly for three years. PDG was lower with advancing age and declined by 5.6% each year.(23) In our study, mid-reproductive age controls had the highest PDG levels and late reproductive age women had the lowest levels. Thus, our findings are in agreement with previous studies that suggest that folliculogenesis is impaired with advancing age. Additionally, Pal et al. examined daily urinary PDG for one menstrual cycle among women with infertility (mean age 34.1 years) secondary to diminished ovarian reserve (DOR) and in healthy, similarly aged controls. The authors observed that luteal phase urinary PDG excretion was significantly lower among women with DOR compared to controls.(24) In our analysis, there was no significant difference in total, peak, follicular, or luteal PDG levels between cancer survivors and same age controls. However, there was a trend toward lower PDG levels among cancer survivors compared to their peers. In addition, survivors were almost twice as likely as their peers to have a luteal phase length less then 12 days (39% for survivors vs. 20% for healthy controls, p=0.08). Taken together, these observations may indicate that alkylating agents cause some impairment in luteal function similar to that seen among infertile women with DOR but that our study was underpowered to detect a significant difference in luteal function between survivors and their healthy peers.
Interestingly, estrogen excretion throughout the cycle was higher in cancer survivors compared to same age women and late reproductive age women. This finding was unexpected as it does not reflect the pattern of estrogen secretion seen in healthy, infertile women. For example, Pal et al. found that luteal phase urinary E1c secretion was significantly lower among mid-reproductive age infertile women with DOR compared to healthy controls. (24) While both cancer survivors and infertile women with DOR have similar measures of ovarian reserve, the discrepancy in urinary E1c excretion compared to controls suggests unique follicular environments. Additionally, when urinary estrone conjugates were measured in the Daily Hormone Study of the SWAN study, late reproductive age women with the highest and lowest levels were more likely to have anovulatory cycles compared to women with intermediate levels. (23) In our analysis, the percentage of subjects with evidence of luteal activity/ovulation was similar among groups despite the difference in estrogen metabolism. Thus, unlike late reproductive age women, women exposed to alkylating agent chemotherapy seem to have elevated estrone metabolites while also having ovulatory cycles.
We observed a trend toward higher FSH levels in the late follicular phase among cancer survivors compared to same age controls and late reproductive age women. This pattern of FSH elevation was unexpected. Indeed, Pal and colleagues observed elevated urinary FSH levels among healthy, infertile women with DOR but only in the early follicular phase. (24) In addition, when examining integrated FSH levels graphically by cycle day, survivors exposed to high dose alkylating agent chemotherapy tended to have a more pronounced FSH rise, suggesting a dose response relationship. Furthermore, the rise in FSH in the late follicular phase corresponded to a significantly higher E1c among survivors over the same time frame, suggesting that altered pituitary gonadotropin production may help to explain the hormone profile observed among survivors. We hypothesize that alkylating agent chemotherapy is associated with impaired estrogen negative feedback, resulting in elevated FSH and increased estradiol secretion. It is possible that exposure to alkylating agent chemotherapy alters follicular dynamics, thereby increasing secretion of estrogens. Further studies are needed to confirm these findings and elucidate the underlying mechanism.
Our study has several strengths. This study is the first to explore the follicular dynamics of cancer survivors by measuring daily hormone excretion. The prospective design enabled us to perform rigorous data collection. In addition, by collecting daily urine samples, we were able to examine hormone excretion patterns across an entire menstrual cycle, allowing for a more comprehensive assessment than would be available with isolated measurements. Furthermore, we were able to compare urinary hormone levels with ovarian reserve measures. Finally, we included two control groups, one with similar age and the other with similar ovarian reserve to cancer survivors, which enabled us to compare follicular dynamics with healthy women at different points in the reproductive life. Indeed, comparison with two control groups showed that survivors have a hormone secretion pattern that is unique and not solely explained by age or ovarian reserve. Our findings provide novel insights into the long-term impact of alkylating agent chemotherapy on the ovarian follicle.
Limitations to this study include a small sample size. As a result, we had limited power to examine differences in luteal phase length between the groups. While survivors were twice as likely to have a short luteal phase, a significant difference was not observed due to small number of women in each group. Similarly, we observed trends toward higher urinary gonadotropin levels among cancer survivors, but we were underpowered to detect statistically significant differences in these outcomes. Additionally, by design, we only included survivors with regular menses. While these findings provide valuable information regarding follicular dynamics in regularly cycling reproductive age survivors, they may not be generalizable to survivors with menstrual irregularities.
In conclusion, women exposed to alkylating agents have a unique reproductive hormone milieu that is not solely explained by their age or ovarian reserve. Survivors appear to have a urinary hormone profile that is more similar to their same age peers than late reproductive age women with similar ovarian reserve, which may suggest that age plays a more important role than ovarian reserve in the follicular dynamics of survivors. If confirmed, this hypothesis would help to explain the better-than-expected pregnancy rates that our group has observed among survivors despite decreased ovarian reserve.(25) Further studies are needed to validate these findings and determine the impact of this hormone profile on fertility and timing of menopause. Ultimately, understanding the unique hormonal dynamics in cancer survivors may help to identify therapeutic targets to improve fertility in this population.
Supplementary Material
Acknowledgments
Funding support: Supported by the NIH Research Project Grant (1R01HD062797-- CG, MS), the NIH Mentored Research Scientist Career Development Grant (K01 L:1- CA-133839-03--CG), and the NIH T32 Reproductive Epidemiology Training Grant (HD 007440) (LJ)
Footnotes
Financial disclosures
The authors have nothing to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wingo PA, Ries LA, Rosenberg HM, Miller DS, Edwards BK. Cancer incidence and mortality 1973-1995 a report card for the U.S. Cancer. 1998;82(6):1197–207. doi: 10.1002/(sici)1097-0142(19980315)82:6<1197::aid-cncr26>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, et al. Cancer statistics 2008 CA. Cancer J Clin. 2008;58(2):71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 3.Jemal A, Clegg LX, Ward E, Ries LAG, Wu X, Jamison PM, et al. Annual report to the nation on the status of cancer 1975-2001, with a special feature regarding survival. Cancer. 2004;101(1):3–27. doi: 10.1002/cncr.20288. [DOI] [PubMed] [Google Scholar]
- 4.Bath LE, Wallace WHB, Critchley HOD. Late effects of the treatment of childhood cancer on the female reproductive system and the potential for fertility preservation. BJOG. 2002;109(2):107–14. doi: 10.1111/j.1471-0528.2002.t01-1-01007.x. [DOI] [PubMed] [Google Scholar]
- 5.Byrne J, Mulvihill JJ, Myers MH, Connelly RR, Naughton MD, Krauss MR, et al. Effects of treatment on fertility in long-term survivors of childhood or adolescent cancer. N Engl J Med. 1987;317(21):1315–21. doi: 10.1056/NEJM198711193172104. [DOI] [PubMed] [Google Scholar]
- 6.Chiarelli AM, Marrett LD, Darlington G. Early menopause and infertility in females after treatment for childhood cancer diagnosed in 1964-1988 in Ontario, Canada. Am J Epidemiol. 1999;150(3):245–54. doi: 10.1093/oxfordjournals.aje.a009995. [DOI] [PubMed] [Google Scholar]
- 7.Dillon KE, Sammel MD, Prewitt M, Ginsberg JP, Walker D, Mersereau JE, et al. Pretreatment antimullerian hormone levels determine rate of posttherapy ovarian reserve recovery: acute changes in ovarian reserve during and after chemotherapy. Fertil Steril. 2013;99(2):477–83. doi: 10.1016/j.fertnstert.2012.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sklar CA, Mertens AC, Mitby P, Whitton J, Stovall M, Kasper C, et al. Premature menopause in survivors of childhood cancer: a report from the childhood cancer survivor study. J Natl Cancer Inst. 2006;98(13):890–6. doi: 10.1093/jnci/djj243. [DOI] [PubMed] [Google Scholar]
- 9.Gracia CR, Sammel MD, Freeman E, Prewitt M, Carlson C, Ray A, et al. Impact of cancer therapies on ovarian reserve. Fertil Steril. 2012;97(1):134–40.e1. doi: 10.1016/j.fertnstert.2011.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Freeman EW, Sammel MD, Gracia CR, Kapoor S, Lin H, Liu L, et al. Follicular phase hormone levels and menstrual bleeding status in the approach to menopause. Fertil Steril. 2005;83(2):383–92. doi: 10.1016/j.fertnstert.2004.06.066. [DOI] [PubMed] [Google Scholar]
- 11.Welt CK, McNicholl DJ, Taylor AE, Hall JE. Female reproductive aging is marked by decreased secretion of dimeric inhibin. Journal of Clinical Endocrinology & Metabolism. 1999;84(1):105–11. doi: 10.1210/jcem.84.1.5381. [DOI] [PubMed] [Google Scholar]
- 12.Burger HG, Dudley EC, Hopper JL, Groome N, Guthrie JR, Green A, et al. Prospectively measured levels of serum follicle-stimulating hormone, estradiol, and the dimeric inhibins during the menopausal transition in a population-based cohort of women. Journal of Clinical Endocrinology & Metabolism. 1999;84(11):4025–30. doi: 10.1210/jcem.84.11.6158. [DOI] [PubMed] [Google Scholar]
- 13.Santoro N, Adel T, Skurnick JH. Decreased inhibin tone and increased activin A secretion characterize reproductive aging in women. Fertil Steril. 1999;71(4):658–62. doi: 10.1016/s0015-0282(98)00529-9. [DOI] [PubMed] [Google Scholar]
- 14.Hale GE, Zhao X, Hughes CL, Burger HG, Robertson DM, Fraser IS. Endocrine features of menstrual cycles in middle and late reproductive age and the menopausal transition classified according to the Staging of Reproductive Aging Workshop (STRAW) staging system. Journal of Clinical Endocrinology & Metabolism. 2007;92(8):3060–7. doi: 10.1210/jc.2007-0066. [DOI] [PubMed] [Google Scholar]
- 15.Landgren B-M, Collins A, Csemiczky G, Burger HG, Baksheev L, Robertson DM. Menopause transition: Annual changes in serum hormonal patterns over the menstrual cycle in women during a nine-year period prior to menopause. Journal of Clinical Endocrinology & Metabolism. 2004;89(6):2763–9. doi: 10.1210/jc.2003-030824. [DOI] [PubMed] [Google Scholar]
- 16.van Rooij IAJ, Broekmans FJM, Scheffer GJ, Looman CWN, Habbema JDF, de Jong FH, et al. Serum antimullerian hormone levels best reflect the reproductive decline with age in normal women with proven fertility: a longitudinal study. Fertil Steril. 2005;83(4):979–87. doi: 10.1016/j.fertnstert.2004.11.029. [DOI] [PubMed] [Google Scholar]
- 17.Ruess ML, Kline J, Santos R, Levin B, Timor-Tritsch I. Age and the ovarian follicle pool assessed with transvaginal ultrasonography. Am J Obstet Gynecol. 1996;174(2):624–7. doi: 10.1016/s0002-9378(96)70439-8. [DOI] [PubMed] [Google Scholar]
- 18.Sharara FI, McClamrock HD. The effect of aging on ovarian volume measurements in infertile women. Obstetrics & Gynecology. 1999;94(1):57–60. doi: 10.1016/s0029-7844(99)00242-2. [DOI] [PubMed] [Google Scholar]
- 19.Santoro N, Crawford SL, Allsworth JE, Gold EB, Greendale GA, Korenman S, et al. Assessing menstrual cycles with urinary hormone assays. Am J Physiol Endocrinol Metab. 2003;284(3):E521–30. doi: 10.1152/ajpendo.00381.2002. [DOI] [PubMed] [Google Scholar]
- 20.Santoro N, Lasley B, McConnell D, Allsworth J, Crawford S, Gold EB, et al. Body size and ethnicity are associated with menstrual cycle alterations in women in the early menopausal transition: The Study of Women’s Health across the Nation (SWAN) Daily Hormone Study. Journal of Clinical Endocrinology & Metabolism. 2004;89(6):2622–31. doi: 10.1210/jc.2003-031578. [DOI] [PubMed] [Google Scholar]
- 21.Santoro N, Brown JR, Adel T, Skurnick JH. Characterization of reproductive hormonal dynamics in the perimenopause. Journal of Clinical Endocrinology & Metabolism. 1996;81(4):1495–501. doi: 10.1210/jcem.81.4.8636357. [DOI] [PubMed] [Google Scholar]
- 22.Kassam A, Overstreet JW, Snow-Harter C, De Souza MJ, Gold EB, Lasley BL. Identification of anovulation and transient luteal function using a urinary pregnanediol-3-glucuronide ratio algorithm. Environ Health Perspect. 2006;104(4):408–13. doi: 10.1289/ehp.96104408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Santoro N, Crawford SL, Lasley WL, Luborsky JL, Matthews KA, McConnell D, et al. Factors related to declining luteal function in women during the menopausal transition. Journal of Clinical Endocrinology & Metabolism. 2008;93(5):1711–21. doi: 10.1210/jc.2007-2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pal L, Zhang K, Zeitlian G, Santoro N. Characterizing the reproductive hormone milieu in infertile women with diminished ovarian reserve. Fertil Steril. 2010;93(4):1074–9. doi: 10.1016/j.fertnstert.2008.10.069. [DOI] [PubMed] [Google Scholar]
- 25.Dillon KE, Sammel MD, Ginsberg JP, Lechtenberg L, Prewitt M, Gracia CR. Pregnancy after cancer: Results from a prospective cohort study of cancer survivors. Pediatr Blood Cancer. 2013;60:2001–6. doi: 10.1002/pbc.24701. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


