Abstract
Acute hepatitis C virus (HCV3) infection culminates in viral persistence in the majority of cases. Antibodies that recognize the envelope glycoproteins E1 and E2 are generated during the late stages of acute infection, yet their contribution to spontaneous viral clearance remains controversial. Investigation of the humoral responses during acute HCV infection have been limited by the inability to directly identify and characterize HCV-specific B cells. Here we describe the development of a novel tetramer of the E2 glycoprotein ectodomain (J6, genotype 2a strain), which allowed us to visualize E2-specific B cells longitudinally in the peripheral blood of HCV-infected individuals. HCV-specific class-switched memory B cells were detected in 3/7 participants during late acute infection, with a mean frequency of 0.63% for positive samples (range: 0.16 to 0.67) and in 7/7 participants with chronic infection with a mean frequency of 0.47% (range: 0.20% to 0.78%). In a cross-sectional study, E2 tetramer positive population was detected in 28/31 chronically infected individuals. Deep sequencing of the B cell receptor (BCR) from E2-specific class-switched memory B cells sorted from two independent participants revealed a focused repertoire suggestive of clonal selection. Tetramer-specific B cells exhibited skewed CDR3 length distribution and increased mutation frequency compared to naive B cells. This BCR profile is indicative of clonal expansion and affinity maturation. E2 tetramer allows for specific and sensitive ex vivo characterization of rare HCV-specific B cells in infected individuals, and will enable researchers to gain a better understanding of humoral immunity in HCV infection.
Introduction
Hepatitis C virus (HCV) infection remains a global public health problem. In the United States, infection rates have increased steadily over the past decade primarily due to injection drug use among adolescents and young adults (1). Although direct acting antivirals (DAA4) are generally safe and most persistent infections are cured within 2–3 months of therapy (2), their high cost, limited availability and the asymptomatic nature of most infections remain important challenges. Furthermore, successful treatment of chronic HCV infection with DAAs does not prevent reinfection, which is a recurrent problem for the high risk population (3). The development of a prophylactic vaccine to inhibit HCV transmission is still a major goal. An interesting candidate, currently in phase 2 clinical trials is based on the prime-boost strategy with the first immunization using a chimpanzee adenovirus together with a boost using a modified vaccinia Ankara (MVA) vector both expressing the non-structural region of HCV genome. In phase 1 clinical study, this vaccine was well tolerated and induced a strong T cell response (4). However, an optimal vaccine would combine both T and B cell responses (5). It was shown that immunization with recombinant HCV E1E2 glycoproteins elicited a cross-reactive neutralizing antibody response in humans (6). In order to better evaluate the potential of a vaccine eliciting an effective humoral response, better insight into the development of a protective B cell response during acute HCV infection is required.
Seroconversion to HCV envelope glycoproteins 1 (E1) and 2 (E2) usually occurs several weeks after infection, regardless of whether the virus is cleared or persists (7, 8). The neutralizing effect of anti-HCV antibodies was demonstrated in both the chimpanzees (9, 10) and humanized mouse models of HCV infection (11, 12). In these studies, incubation of an HCV inoculum with anti-HCV antibodies prevented infection, as did a passive transfer of the antibodies before the challenge (9–12). In humans, broadly neutralizing antibodies were shown to have developed more rapidly and to higher titers in individuals with an acute resolving infection as compared to those with persisting infection (13–17). In the context of reinfection, it was shown that a subsequent exposure to HCV led to the development of cross-reactive antibodies, suggesting an improved humoral response (18). However, some reports suggest that the infection can be resolved in the absence of any detectable HCV-specific antibody responses in both chimpanzees and humans (19–21). In those studies, HCV viral loads were low, and HCV-specific T cell responses were detected suggesting that a very transient viremia might not be enough to prime HCV-specific antibody responses. Furthermore, examining immune responses in a cohort of women who spontaneously resolved a single source outbreak of HCV demonstrated that circulating HCV-specific antibodies were undetectable in many subjects 18–20 years after recovery (22). These opposing results have led to confusion regarding the contribution of HCV-specific antibodies to clearance of infection and highlight the need to obtain a better insight of the nature of humoral immune response during acute HCV infection.
Antigen-specific IgG-secreting memory B cell frequencies can be evaluated by bulk B cell stimulation coupled with enzyme-linked ImmunoSpot (ELISpot) assays (23). Although informative, this method does not allow for direct characterization or recovery of the HCV-specific B cells for downstream analyses. Alternatively, identification of antigen-specific B cells is possible using tetramers generated from biotinylated antigens coupled with fluorescently labeled streptavidin. Such tetramers have been shown to specifically identify and isolate tetanus toxoid-specific B cells from the blood of vaccinated donors (24). Similarly, HIV gp41 tetramers have been used to characterize HIV-specific B cells and their antibody repertoire during different stages of HIV infection (25).
Here we report the development of an HCV-specific B cell tetramer reagent composed of the ectodomain of HCV envelope glycoprotein E2 (J6 strain, genotype 2a). The specificity of this tetramer was validated using an E2-specific hybridoma cell line and PBMCs from subjects persistently infected with HCV of different genotypes. To better understand the kinetics of antigen-specific B cell responses during HCV infection, we performed a longitudinal study to directly visualize and quantify the frequency of E2-specific B cells in the peripheral blood of subjects progressing from an acute to chronic infection. Finally, as a proof of concept, this novel HCV E2 tetramer enabled us to isolate E2-specific class-switched memory B cells and perform B cell receptor (BCR) deep sequencing on two persistently HCV-infected subjects. The dominant repertoire profiles, skewed CDR3 length distributions and increased mutation frequencies all suggested that these cells were selected, expanded and had undergone affinity maturation processes.
Materials and Methods
Expression and purification of biotinylated E2 ectodomain
The pCMJJ4 vector served as the backbone for expression of the HCV E2 ectodomain (J6 genotype 2a strain, amino acids 384 to 664). The E2 ectodomain sequence was linked to a BirA substrate peptide (GLNDIFEAQKIEWHE) (BSP85), followed by a PreScission protease-cleavable protein-A (PA) tag (Fig. 1A). Lentiviruses were generated as described previously (26). Briefly, the E2 expression vector and accessory plasmids (psPAX2 and pMD2G) were introduced into HEK293T cells by calcium phosphate transfection. Supernatants containing lentiviruses were harvested 3 days later and were subsequently used to transduce HEK293T cells expressing the BirA enzyme, which biotinylates the BirA substrate peptide. Analysis of surface Thy1.1 expression was utilized to determine transduction efficiency. Cells expressing Thy1.1 were then inoculated into CELLine Flasks (Integra Biosciences, Hudson, NH) and supernatants were harvested every 7 days. The supernatants were clarified by centrifugation at 5000 x g for 30 min at 4°C and E2-biotin-Protein-A was purified by the use of an IgG FF affinity column (GE Healthcare, Atlanta, GA). The protein-A tag was removed by cleavage with 50:1 ratio of protein to PreScission protease (GE Healthcare, Atlanta, GA) overnight at 4°C. E2-biotin was subsequently purified with an IgG FF column (GE Healthcare, Atlanta, GA) to remove uncleaved E2-Protein-A and the Protein-A tag. Finally, a GSTrap FF column was used to remove PreScission protease (GE Healthcare, Atlanta, GA). As a control reagent, an HIV gp140 tetramer (clade C HIV-1, isolate DU422) was also generated using the same approach.
FIGURE 1. HCV envelope glycoprotein E2 monomer construct and purification.
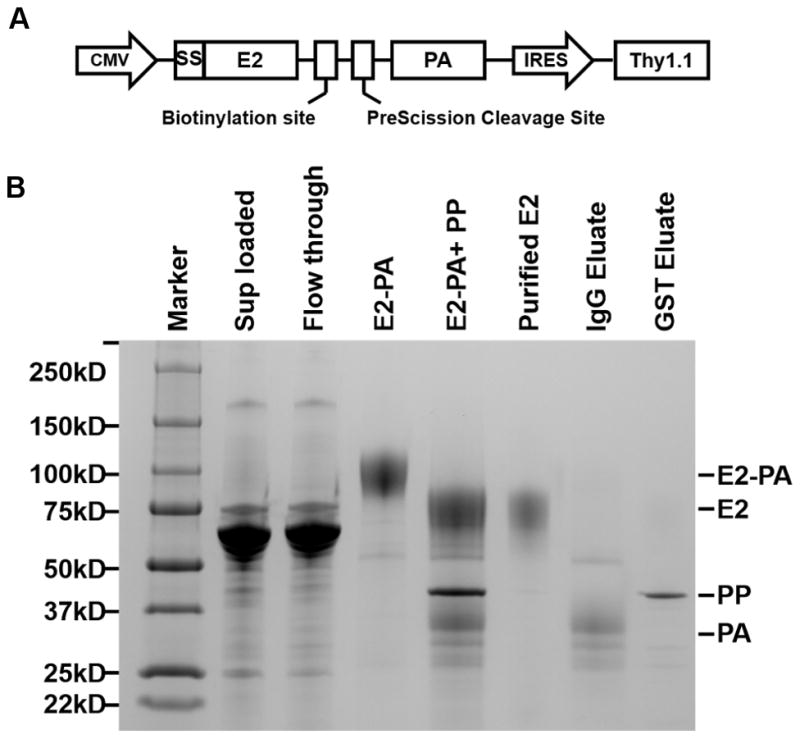
(A) Schematic diagram of the E2-Protein A (E2-PA) expression cassette in the pCMJJ4 vector. The ectodomain of E2 (amino acids 384 to 664; J6 (genotype 2a)) was cloned downstream of Prolactin signal sequence (SS) to promote targeting and trafficking through the secretory pathway. A BSP85 sequence was inserted downstream for site specific mono-biotinylation followed by a preScission cleavage site and a Protein A tag (PA) for affinity purification and elution of purified E2-biotin monomer. The reporter gene Thy1.1 expression was cloned under the control of an internal ribosome entry site (IRES).
(B) SDS-PAGE and Coomassie Blue staining showing E2 monomer purification steps as follows: Supernatants from HEK-293T cell lines expressing E2-biotin-Protein A were clarified by centrifugation (Sup loaded, lane 2) and applied to the resin. The column was extensively washed to remove unbound material (Flowthrough, lane 3). E2-biotin-Protein A was eluted off the column (E2-PA, lane 4) and incubated with PreScission protease (PP, lane 5). E2-biotin (lane 6) was further purified by removing uncleaved E2-biotin-Protein A and Protein A tag by IgG column and PreScission protease using GST column. The eluates from the IgG (lane 7) and GST columns (lane 8) are also shown.
Tetramer preparation and hybridoma staining
Biotinylated E2 monomers were incubated with either phycoerythrin (PE) labeled ExtrAvidin® (Sigma, St. Louis, MO) or allophycocyanin (APC) labeled streptavidin (Molecular Probes, Thermo Fisher Scientific, Rochester, NY) at a molar ratio of 4:1. Fluorescently labeled streptavidin reagent was added to the E2 monomer in 6 aliquots, each followed by an incubation of 10 min at room temperature (1 h total). Before hybridoma cell staining, tetramer preparations were centrifuged for 10 min at maximum speed to remove aggregates. Hybridoma cells were first stained with Ghost Dye™ Red 780 (Tonbo Biosciences, San Diego, CA) to exclude dead cells. Cells were washed and treated with BD FACS permeabilizing solution 2 for 10 min at room temperature. Cells were washed and incubated with 0.4μg E2 tetramers for 30 min at 4°C. Cells were washed and fixed in 1% formaldehyde in PBS before FACS analysis.
Study participants
Study subjects were recruited among people who inject drugs (PWID5) participating in the Montreal Acute Hep C Cohort Study (HEPCO) as previously described (27) or presenting to the hepatology clinic of St-Luc Hospital as previously described (28). Acute infection was identified and followed as previously described (29). The estimated date of infection (EDI6) was defined as the median point between the last negative and the first positive HCV test. Chronic HCV infection was defined as a positive HCV RNA test at 6 months following the EDI. A total of 37 subjects were examined in a cross-sectional analysis: 31 chronic patients (HCV Ab +ve and HCV RNA +ve) and 6 spontaneous resolvers (HCV Ab +ve and HCV RNA −ve). In addition, 7 PWIDs were analyzed longitudinally at 3 key time points before, during and after acute HCV infection: baseline (negative both for HCV RNA and antibodies), late acute phase (5±2 months post EDI) and chronic phase (>12 months post EDI). Two control groups consisting of 7 healthy donors and 6 HCV naive PWID were also included. Participant’s demographics and clinical characteristics are listed in Table I. This study was approved by the Institutional Ethics Committee of the CRCHUM (protocols SL05.014 and SL05.025). All experiments were performed on cryopreserved peripheral blood mononuclear cells (PBMCs).
Table I.
Demographics and clinical characteristics of study subjects
| Acute to chronic HCV n=7 |
Chronic HCV n=24 |
Resolvers n=6 |
Naive PWID n=6 |
Healthy donors n=7 |
|
|---|---|---|---|---|---|
| Sex (M/F) | 7/0 | 18/6 | 4/2 | 5/1 | 4/3 |
| Median Age (years) | 29 | 43 | 26 | 27 | 32 |
| HCV genotype (1a/1b/2/3/3a/ND) | 1/6/−/−/−/− | 10/2/5/1/4/2 | 4/2/−/−/−/− | NA | NA |
| Median time point tested (years post infection) | NA | 4 | NA | NA | NA |
| Median time point tested (days post EDI) | Baseline: −46 Late acute: 155 Chronic HCV: 554 |
NA | 427 | NA | NA |
NA: not applicable
ND: not done
EDI: estimated date of infection
Human PBMC staining and Flow cytometry
PBMCs from healthy controls and HCV-infected individuals were thawed and blocked with RPMI supplemented with 20% heat-inactivated human serum and 5 μl of human Fc block/2×106 cells (BD Biosciences, Mississauga, ON) for 15 min at 4°C. Cells were then washed with FACS buffer (PBS 1X (Wisent), 1% Fetal bovine serum (FBS, Sigma, St. Louis, MO), 0.01% sodium azide (Thermo Fisher, Burlington, ON)) and stained with the tetramer for 30 min at room temperature. Cells were washed twice with FACS buffer and stained with either panel 1 (Figures 3 and Figure 4) or panel 2 (cell sorting) for 30 min at 4°C. Cells were washed again twice and fixed with 1% formaldehyde in PBS before FACS analysis. The following conjugated anti-human monoclonal antibodies were used in panel 1: CD3-Pacific Blue™ (clone UCHT1), CD14-V500 (clone M5E2), CD16-V500 (clone 3G8), CD19-Alexa Fluor® 700 (clone HIB19), CD27-APC-H7 (clone M-T271), IgM-BB515 (clone G20–127). Panel 2 contained all panel 1 antibodies with the addition of CD10-BV605 (clone HI10a) and CD21-PE-Cy™7 (clone B-ly4). All antibodies were obtained from BD Biosciences (Mississauga, ON). Live cells were identified using LIVE/DEAD® fixable aqua dead cell stain kit (Molecular Probes™, Thermo Fisher Scientific, Burlington, ON). Multiparameter flow cytometry was performed at the flow cytometry core of the CRCHUM using a BD LSRII instrument and cell sorting was completed using a BD Aria IIIu instrument, both equipped with violet (405 nm), blue (488 nm), yellow-green (561nm) and red (633 nm) lasers and FACSDiva version 8.0.1 (BD Biosciences, Mississauga, ON). FCS data files were analyzed using FlowJo version 10.0.8 for Mac (Tree Star, Inc., Ashland, OR). Fluorescence minus one controls (FMOs) were used to set the gate for CD27 and CD10. B cell gating included the selection of live cells that are also CD14−, CD16− and CD3− (Fig. 3A).
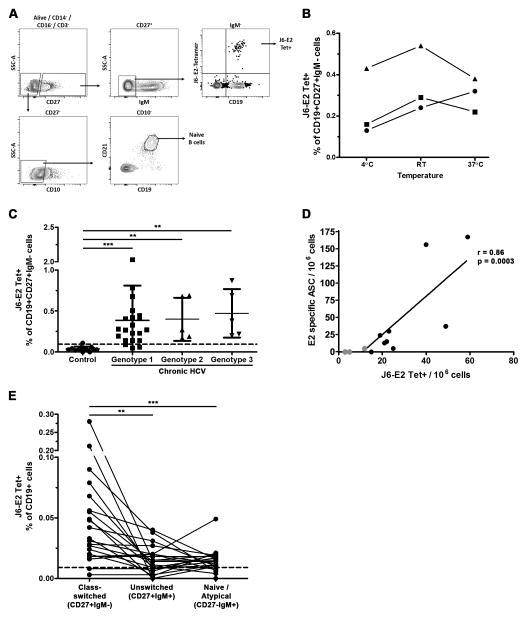
FIGURE 3. Identification of HCV E2-specific class-switched memory B cells in the peripheral blood of chronic HCV participants using J6-E2 tetramer.
(A) Gating strategy. Cells were first gated on live single CD14−, CD16−, CD3− lymphocytes (not shown). Naive B cells were defined as CD27−, CD10−, CD19+ and CD21hi. Class-switched memory B cells were defined as CD27+, IgM− and CD19+. HCV E2-specifc cells were identified using J6-E2 tetramer. Positive tetramer gate was set relative to background staining observed on CD19- B cells.
(B) Tetramer staining was performed at three different temperatures for three independent samples. Room temperature staining was selected and used throughout the study.
(C) Cumulative flow cytometry data for ex vivo staining of PBMCs using J6-E2 tetramer and B cell markers in uninfected controls (healthy donors and naive PWID combined; n=13) and chronic HCV participants (n=31). Each dot represents the percentage of tetramer positive cells within class-switched memory B cells (CD19+CD27+IgM−) from one subject. Lines represent mean in all groups and error bar represent the standard deviation (SD). Threshold (dotted line) of detection was set at 0.095% (mean detection from uninfected controls + 2 SD).
(D) Correlation between tetramer staining and IgG B cell ELISpot (n=12). Tetramer positive cells were expressed as number of positive cell / 1×106 PBMCs. E2 specific IgG ELISpot data was expressed as the number of E2 specific antibody secreting cells (ASC) / 1×106 PBMCs. Spearman r and p values are indicated.
(E) Cumulative flow cytometry data for genotype 1 samples (n=21) of tetramer positive populations in different B cell subsets. Class-switched memory B cells were identified as CD19+CD27+IgM−; unswitched memory B cells were identified as CD19+CD27+IgM+ and naive/atypical B cells were identified as CD19+CD27−IgM+. Mann-Whitney U test for (C) and (E): **, p<0.01; ***, p<0.0001.
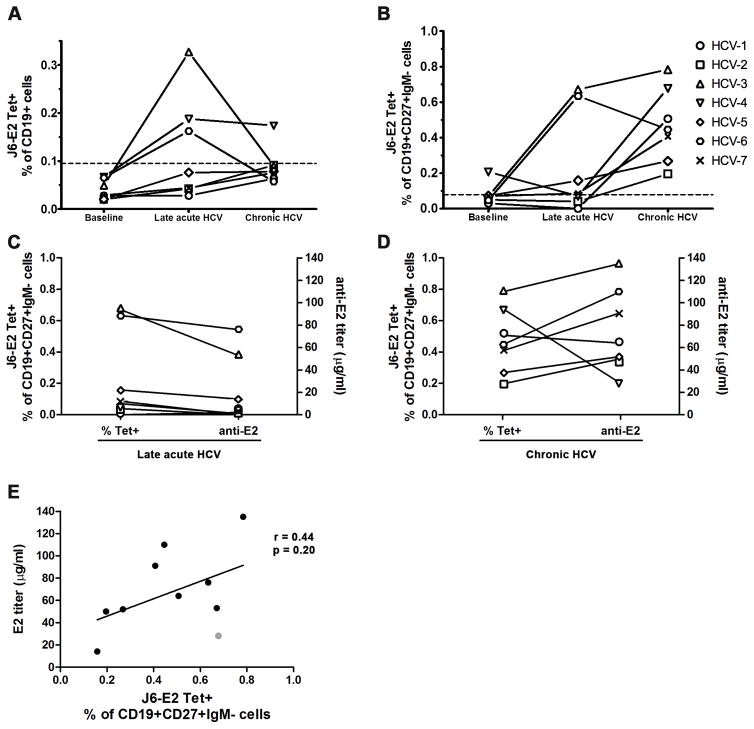
FIGURE 4. Longitudinal analysis of HCV E2-specific B cells during acute HCV infection progressing to chronicity.
J6-E2 tetramer positive B cells were detected longitudinally in individuals that developed a chronic HCV infection (n=7) as described in Figure 3. Three key time points were tested: Baseline (> 1.5 months prior to estimated date of infection (EDI)); Late acute (5±2 months post EDI); and Chronic HCV (>12 months post EDI). Each dot represents the percentage of tetramer positive cells from one subject in total CD19+ B cells (A) and in class-switched memory B cells (CD19+CD27+IgM−) (B). Dotted line (A–B) represents the threshold for tetramer positive signal.
(C–D) Plasma anti-E2 IgG titers were measured by ELISA and compared to the tetramer frequencies for the late acute time point (C) and Chronic HCV time point (D) (both n=7). (E) Correlation between anti-E2 titers and J6-E2 tetramer positive frequencies from class-switched memory B cells. Spearman r and p values are indicated. ELISAs were done in three independent experiments and the results are shown from one representative experiment in duplicate.
E2 ELISA
HCV E2 glycoprotein (30) (1 μg/ml in 0.1 M Na2CO3 buffer) was used to coat 96 well flat bottom immuno plates (Nalgene Nunc, Thermo Fisher Scientific, Rochester, NY) overnight at 4°C. Coated plates were washed twice with phosphate-buffered saline plus 0.05% Tween 20 (PBS-T) and then blocked with 10% normal goat serum in PBS-T (Jackson ImmunoResearch, West Grove, PA) for 1 h at 37°C. Human plasma samples from HCV-1 to HCV-7 as well as three healthy controls were added to the plates (10 fold serial dilutions in binding buffer (0.1% normal goat serum in PBS-T)) for 90 min at room temperature and the plates were washed eight times with PBS-T. Then, 0.1 μg/ml biotinylated anti-human IgG mAbs MT178/145, (Mabtech, Cincinnati, OH) diluted in binding buffer was added to each well for 90 min at room temperature and plates were washed eight times with PBS-T. Streptavidin-HRP (Mabtech, Cincinnati, OH) diluted 1:5000 in binding buffer was added to plates for 45 min at room temperature and plates were washed eight times with PBS-T. Tetramethylbenzidine substrate (BD, Franklin Lakes, NJ) was added to develop color according to manufacturer. Absorbance (450 nm) was measured using a Versamax microplate reader and SoftMaxPro software (Molecular Devices, Sunnyvale, CA). Standard curves were done using serial dilutions of E2 monoclonal antibody 2C1, goat anti-mouse IgG, biotin conjugate (Invitrogen, Thermo Fisher Scientific, Waltham, MA). The assay was quantified Elisaanalysis.com software. Three independent experiments were performed in duplicates.
Human IgG ELISpot
B cell ELISpot was performed with the human IgG ELISpot kit (Mabtech, Cincinnati, OH) according to the manufacturer instruction and as described by Jahnmatz et al (31). Briefly, PBMCs were thawed and rested for 1h at 37°C, 5% CO2 in R10 (RPMI, supplemented with 10% FBS). Cell were stimulated with 1μg/ml R848 and 10ng/ml rhIL-2 in AIM-V supplemented with 10% FBS (AIM-V-FBS) in a 24 wells plate at 2×106 cells / well or left unstimulated for 72 h at 37°C, 5% CO2. Cells were washed 3 times and plated in duplicates in PVDF MSIPS4W10 ELISpot plates (EMD Millipore, Etobicoke, ON) that were previously coated with anti-IgG (MT91/145, Mabtech, Cincinnati, OH) O/N at 4°C, washed and blocked with AIM-V-FBS for 1h at 37°C, 5% CO2. Plates were incubated 18h at 37°C, 5% CO2. Plates were washed 9 times with PBS-T. Total IgG response was detected with anti-IgG-Biotin (MT78/145) and HCV specific antibody response was detected with J6-E2-Biotin, for 2h at room temperature. Plates were washed 9 times and incubated with streptavidin-ALP (Alkaline phosphatase, 1:1000, Mabtech, Cincinnati, OH) for 1 h at room temperature. Plates were washed seven times with PBS-T, three times with PBS and one time with water. Spots were developed with the AP conjugate substrate kit (BioRad, Montreal, QC) for 4 min in the dark, according to manufacturer instructions. Plates were extensively washed with tap water, dried O/N and spots were counted with Immunospot plate reader (CTL, Shaker Heights, OH) and normalized to antibody-secreting cells (ASC) / 1×106 PBMC.
Purification and sorting of tetramer positive class-switched memory B cells and naive B cells
PBMCs were thawed and total B cells were purified by negative selection using the MACS Pan B cell Isolation Kit (Miltenyi Biotec Inc, Auburn, CA) according to the manufacturer’s protocol. Tetramer staining and cell surface staining for CD3, CD10, CD14, CD16, CD19, CD21, CD27 and IgM were performed as described above. Tetramer positive class-switched memory B cells (CD19+CD27+IgM−) and naive B cells (CD19+CD27-CD10-CD21hi) were FACS sorted with an Aria IIIu flow cytometer (BD Biosciences, Mississauga, ON).
B cell receptor sequencing
Sorted cells were frozen and shipped to Adaptive Biotechnologies (Seattle, WA) for genomic DNA extraction and B cell receptor (BCR) heavy chain (IGH) deep sequencing. Lists of unique CDR3 sequences (clonotypes) and their frequency within the repertoire were obtained for each sample. The raw data can be accessed at https://clients.adaptivebiotech.com/pub/boisvert-2016-JI. Data were filtered to remove out of frame sequences and sequences with stop codons within the CDR3 region. Clonotypes with sequence counts equivalent to or less than the average count per cell were also removed from the analysis. Data were analyzed using the ImmunoSEQ™ analysis platform. To study the dominance profile, clonotypes were classified into 4 groups according to their frequency within the repertoire. The first group was composed of dominant clonotypes that were each present at a frequency >1% of the repertoire. The second group comprised sub-dominant clonotypes, with frequencies between 0.1% and 1% of the repertoire. The third group was made of low abundance clonotypes with frequencies between 0.05% and 0.1% of the repertoire. Finally, the fourth group contained clonotypes of lowest abundance with frequencies of <0.05% of the repertoire. The CDR3 region length in amino acids (AA) was determined and the distribution of the frequency of each length was analyzed. Very short (<7 AA) and very long (>31 AA) CDR3 lengths were rare and omitted from the analysis. V gene mutation frequency of each clonotype sequence was calculated as a percentage and represents the number of substitutions / 100 base pairs compared to germline sequence according to the IMGT database (32).
Statistics
Differences between groups were analysed using the Mann-Whitney U test. Correlations were analysed by Spearman test using GraphPad Prism version 5.0 (La Jolla, CA).
Results
Generation of HCV E2-specific B cell tetramers
There are currently no reagents available to directly examine HCV-specific B cells. Thus, we utilized the ectodomain of HCV envelope glycoprotein E2 (amino acids 384 to 664) derived from genotype 2a (J6 strain) to develop a novel B cell tetramer capable of identifying HCV-specific B cells from the peripheral blood of infected individuals. The J6 genotype 2a strain was chosen because its ectodomain was previously successfully expressed and crystalized to resolve the E2 protein structure diffracted to 2.4 A° resolution (30). This protein was also shown to be properly folded when expressed in HEK293T cells (33). A cartoon diagram for the generation of the E2 expression vector is shown in Fig. 1A. The insertion of the biotinylation site BSP85 sequence enabled site-specific mono-biotinylation while the addition of a Protein A tag allowed for affinity purification. Biotinylated E2 monomers were produced in a lentivirus-transduced HEK293T cells as described in the Materials and Methods. Monomer purity and size were confirmed by SDS-PAGE (Fig. 1B). Purified E2-PA (90 kDa; Lane 4), was cleaved by PreScission protease (PP, 46 kDa) to remove the protein A tag (PA 30 kDa; Lane 5), and E2 monomer (expected size 60 KDa) was purified by passage over IgG then GST columns (Lanes 6–8). Since the purified E2 monomers corresponded to the expected size, they were used to generate tetramers by incubation with fluorophore-conjugated streptavidin or ExtrAvidin® at a molar ratio of 4:1.
Validation of E2 tetramers using E2-specific hybridoma
We first sought to confirm that the E2 tetramer reagent could specifically recognize antibodies targeting the E2 glycoprotein ectodomain. E2 tetramer reactivity was validated by two approaches. First, anti-mouse Ig BD flow cytometry compensation beads (CompBeads) were incubated with either 2C1 monoclonal antibody that recognize HCV E2 glycoprotein, or 1D6 monoclonal antibody that recognize HIV gp120 (34). Next, the antibody-coated beads were incubated with E2 or gp140 tetramers, and the tetramer APC fluorescence intensity was analyzed by flow cytometry. The E2 tetramer was able to recognize the CompBeads that were coated with the E2 specific monoclonal antibody 2C1 (Fig. 2A, left). This interaction was specific, as the E2 tetramer could not recognize the HIV antibody 1D6 and the HIV-specific gp140 tetramer did not recognize the beads coated with the anti-E2 2C1 antibody. The gp140 tetramer did, however, recognize the beads containing the gp120 specific antibody 1D6 (Fig. 2A, right). We next asked whether the HCV-E2 tetramer could directly recognize hybridoma cells that produce anti-HCV E2 monoclonal antibodies. To test this, the E2 tetramer was used in an intracellular staining of the hybridoma cell line (2C1) that produces a monoclonal antibody to J6-E2 protein (Fig. 2B, top). The E2 tetramer stained the 2C1 hybridoma but did not recognize the hybridoma that produces the HIV envelope protein gp140-specific antibody (1G12) (Fig. 2B, bottom). Together these data suggest that the E2 tetramer could specifically recognize antibodies targeting the HCV E2 glycoprotein.
FIGURE 2. Specificity of J6-E2 tetramers.
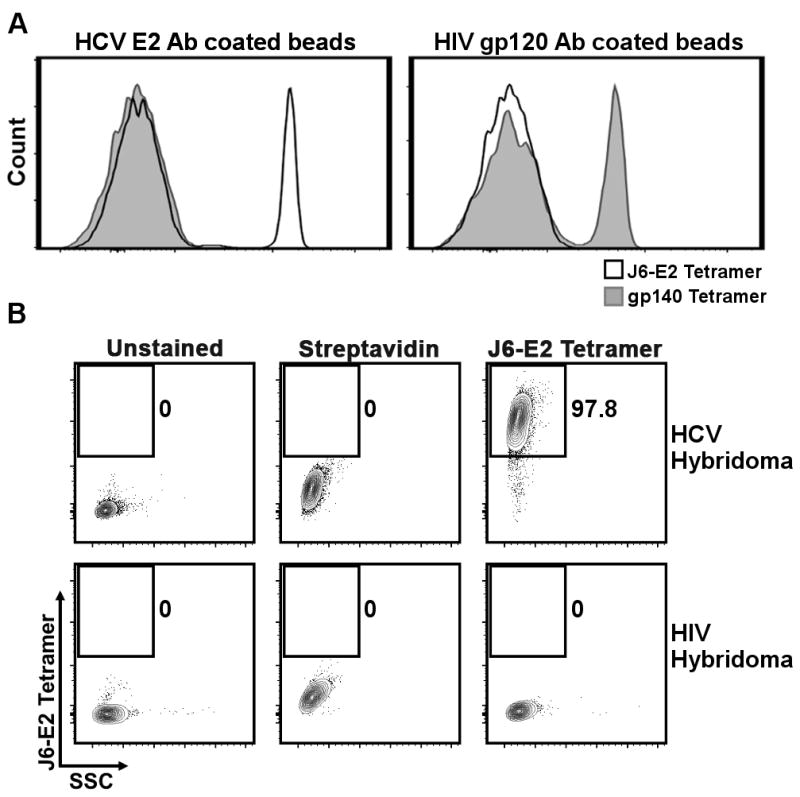
(A) Representative flow cytometry histogram of CompBeads coated with either the E2-specific monoclonal antibodies 2C1 (left panel) or the HIV gp120-specific monoclonal antibody ID6 (right panel) stained with J6-E2 (black line) or gp140 (grey shaded) tetramers. APC fluorescence intensity was measured and is represented as relative count for each sample.
(B) Representative flow cytometry plot of the hybridoma cell lines 2C1 (HCV E2 specific) (top) and 1G12 (HIV gp140 specific) stained with J6-E2 tetramers (right panels). Unstained hybridomas (left panels) and hybridomas stained with APC conjugated streptavidin (middle panels) were used as controls. Numbers denote frequencies of live tetramer-positive cells.
Successful identification of HCV E2-specific B cells in chronic HCV subjects
Next, we evaluated the capacity of the E2 tetramer to detect HCV-specific B cells in HCV-infected individuals. Tetramer and surface staining were performed on PBMC to identify HCV E2-specific class-switched memory B cells (CD19+CD27+IgM−) in subjects with established chronic HCV infection (HCV RNA +ve for >1 year, genotypes 1, 2 or 3; n=21/5/5 respectively). A representative gating strategy is presented in Fig. 3A. The tetramer positive gate was set relative to the background staining observed on CD19− cells. Tetramer staining was evaluated at three temperatures: 4°C, room temperature and 37°C. The staining was most efficient at room temperature, enabling detection of more HCV specific B cells (Fig. 3B), with minimal background on control samples as compared to 37°C (data not shown). Within the total class-switched memory B cell compartment, the average frequency of HCV-specific B cells was 0.40% (range 0.05–2.03%) (Fig. 3C). The mean background binding from healthy donors (HDs, n=7) and HCV negative people who inject drugs (PWID) (n=6) was 0.04%, which when combined, ranged from 0–0.11%. The threshold of detection was set at 0.095% (mean frequency + 2 standard deviations (SD) of HDs and naive PWID) and HCV E2-specific tetramer-positive populations were detected in 28/31 of chronic HCV participants.
Sensitivity of the tetramer detection was compared to the detection of HCV-specific B-cells using an IgG ELISpot assay. As shown in Fig. 3D, there was a significant correlation between the number of E2 specific antibody secreting cells (ASCs) and the number of tetramer positive cells (Spearman r = 0.86; p = 0.0003). In addition, the ELISpot assay showed that in the three samples for which we could not detect a tetramer positive population, E2 specific ASCs were not detected or were barely detectable (grey dots, Fig. 3D).
Analyses of other B cell subsets (unswitched memory B cells (CD27+IgM+) and naive/atypical B cells (CD27-IgM+)) in genotype 1 samples showed that the tetramer positive cells were detectable in both populations (Fig. 3E). However, in the majority of cases (15/21, p<0.01) we detected more HCV-specific cells in the class-switched memory B cell subset. The remaining six samples had an equivalent frequency of tetramer positive cells in the unswitched memory population. There were also 5 samples where the frequency of naive/atypical B cells was equivalent or higher than the frequency of class-switched memory B cells but the overall frequency of tetramer positive B cells was quite low and did not allow for a more detailed comparison of these two subsets. None of the three samples that were below the threshold of detection in class-switched memory B cells had a significant tetramer positive population in the unswitched or naive/atypical memory B cells. Collectively, these results suggest that the J6 E2 tetramer can be used to successfully identify HCV-specific B cells in infected participants and that HCV E2-specific B cells are present in the majority of individuals with chronic infection.
Limited detection of HCV E2-specific B cells during acute HCV infection
Identification of HCV-specific B cells during acute HCV infection would enable the characterization of early changes in that population leading to the development of the antibody response. We thus examined the longitudinal frequency of HCV E2-specific B cells in acutely infected participants who went on to develop persistent HCV infection (n=7). During the late acute phase of infection (5±2 months, EDI), E2 tetramer positive cells were detected in approximately half of the samples (3/7) within both the total CD19+ B cell population (Fig. 4A) and the class-switched memory B cell population (CD19+CD27+IgM−) (Fig. 4B). All participants tested (7/7) developed a tetramer positive population within the class-switched memory B cell population at their latest follow-up chronic time point (>12 months). These results suggest that at least in some participants the development of the HCV-specific B cell response was significantly delayed. ELISA assay was performed with the plasma samples from the same participants during both the late acute and chronic time points. At the late acute time point, we detected E2 specific antibodies only in the three samples with a tetramer positive population (Fig. 4C). All samples showed seroconversion at the chronic time point (Fig. 4D). For the majority of the samples, there was an observable correlation between the E2 antibody titers and the frequency of tetramer positive class-switched memory B cells (Fig. 4E). The only exception was sample HCV-4 where we detected a high frequency of tetramer positive cells but very low anti-E2 antibody titers (grey dot in Fig. 4D).
The BCR repertoire of HCV E2-specific class-switched memory B cells is focused
As a proof of concept, we sequenced the B cell receptor (BCR) of naive and HCV-specific B cells of two subjects to analyze the characteristics of B cell antigenic selection and maturation process within the repertoire. The selection and amplification of B cell clonotypes lead to a focussing of the BCR repertoire with fewer clones detected and a dominant profile emerging. J6-E2 tetramer positive class-switched memory B cells (CD19+CD27+IgM−) from subjects HCV-8 and HCV-9 were sorted at their latest follow-up time point (≥5 years) and deep sequenced for the BCR Ig heavy chain (IGH). Naive B cells (CD19+CD27-CD10-CD21hi) were also sorted and used as a control for the unselected BCR repertoire. Sample information, including the number of sorted cells and the number of sequencing reads, are listed in Table II. The resulting list of unique clonotypes were divided into 4 groups based on their frequency within the repertoire: (i) dominant clonotypes each representing ≥1% of the repertoire; (ii) sub-dominant clonotypes representing 0.1–1% of the repertoire; (iii) low abundance clonotypes representing 0.05–0.1% of the repertoire; and (iv) lowest abundance clonotypes, with frequencies of <0.05% of the repertoire. As expected, the BCR repertoire of naive B cells showed no selection of dominant clonotypes and very few sub-dominant clonotypes (Fig. 5, left pie charts). For both subjects, most of the naive repertoire (>90%) was composed of the lowest abundance clonotypes. In sharp contrast, the repertoire of HCV E2-specific tetramer positive class-switched memory B cells was focused in both subjects where most of the clonotypes identified (90–99%) were dominant (Fig. 5, middle pie charts). Participant HCV-9 possessed 16 clonotypes totalling 99% of the B cell repertoire, whereas the repertoire of participant HCV-8 was composed of 37 clonotypes, totaling 90% of the total repertoire. The most dominant clonotype for participant HCV-9 (IGHV03-D05-J06-01) represented 42% of the repertoire while the most dominant clonotype for participant HCV-8 (IGHV04-D02-02J05-01) represented only 6% of the repertoire (Fig. 5, right pie charts). Together, these results demonstrate that the J6-E2 tetramer can be used to identify, select and sort HCV-specific B cells for downstream analyses. Further, the focused repertoire from both HCV-infected subjects suggest antigen-specific selection and/or expansion of this B cell population.
Table II.
BCR sequencing summary
| Sample | HCV status | Time point tested (estimated) | Sorted cells | Sorted cell number | Productive sequences Total | Productive sequences Unique | Clonality |
|---|---|---|---|---|---|---|---|
| HCV-8 | Chronic HCV | 5 years | Naive B cells* | 40 000 | 414 673 | 9 611 | 0.037 |
| HCV E2- specific B cells** | 250 | 4 643 | 32 | 0.355 | |||
| HCV-9 | Chronic HCV | 6 years | Naive B cells | 40 000 | 996 999 | 11 927 | 0.029 |
| HCV E2- specific B cells | 220 | 10 814 | 62 | 0.089 |
Naive B cells are CD19+CD27-CD10-CD21hi
HCV E2 specific B cells are class-switched memory B cells (CD19+CD27+IgM−)
FIGURE 5. Dominance profile of BCR repertoires from HCV E2-specific and naive B cells.
Naive B cells (CD19+CD27-CD10-CD21hi) and J6-E2 tetramer specific class-switched B cells (CD19+CD27+IgM−) from two HCV chronic subjects (HCV-8 (top) and HCV-9 (bottom)) were sorted and B cell receptors (BCR) heavy chain (IGH) deep sequenced as described in Materials and Methods. Unique sequences (clonotypes) were stratified into 4 groups according to their frequency within the repertoire: dominant clonotypes (black, frequency ≥1%); sub-dominant clonotypes (dark grey, frequency 0.1–1%); low abundance clonotypes (light grey; frequency 0.05–0.1%); lowest abundance clonotypes (white; frequency ≤0.05%). The percentage of each category is indicated in the pie charts and the numbers in brackets represent the number of unique clonotypes forming each category. The frequencies of individual clonotypes within the dominant category (HCV E2-specific samples) are shown in the subdivided pie charts on the right.
Distinct CDR3 length distribution in HCV E2-specific class-switched memory B cells compared to naive B cells profile
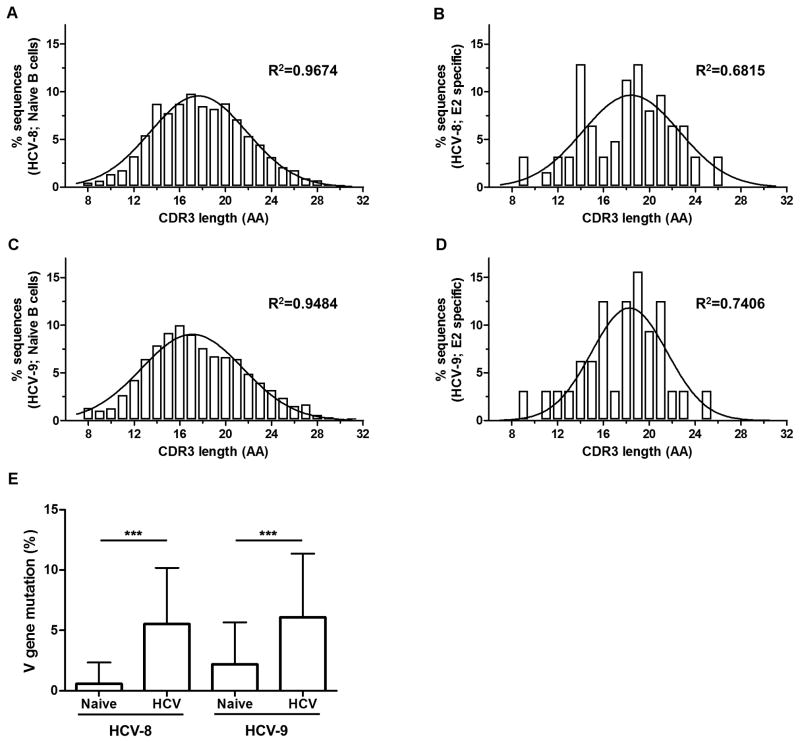
In a naive B cell repertoire, it is estimated that CDR3 lengths have a normal Gaussian-like distribution with no particular selection or amplification of any clonotype (35). However, in an antigen-specific population, particular clonotypes are selected and go through the process of affinity maturation leading to the enrichment and dominance of certain CDR3 lengths (35). We analyzed the distribution of CDR3 lengths for both the naive and E2-specific sorted samples. As demonstrated in Fig. 6 A and C, the CDR3 lengths within the naive samples showed a normal bell shape distribution in both subjects (R2 > 0.94). In contrast, the CDR3 length distributions from E2-specific B cells were skewed in both subjects (Fig. 6 B, D; R2 < 0.75). In participant HCV-8, lengths of 14, 18 and 19 amino acids were highly enriched (Fig. 6B). Likewise, CDR3 lengths of 16 and 18–21 amino acids were enriched in participant HCV-9 (Fig. 6D). These results provide additional evidence that E2-tetramer positive cells are indeed antigen-specific and have undergone specific selection and expansion during HCV infection.
FIGURE 6. Skewed CDR3 length distributions and increased mutation frequency in the HCV E2-specific BCR repertoire as compared to naive B cells.
Distribution of amino acid (AA) lengths of CDR3 regions presented as percentage of sequences from the total repertoire. Naive B cell samples from HCV-8 (A) and HCV-9 (C) subjects showed a normal Gaussian-like distribution. HCV E2-specific class-switched memory B cells from HCV-8 (B) and HCV-9 (D) subjects had a skewed distribution divergent from the Gaussian-like shape. R2 values of Gaussian non-linear regression are shown for each sample. (E) Mutation frequencies within the V gene segment of CDR3 regions for all samples represented as the number of substitution / 100 bp compared to germline sequence according to the IMGT database. ***, p<0.0001 (Mann-Whitney U test).
Increased mutation frequency in CDR3 regions from HCV E2-specific class-switched memory B cells compared to naive B cells
During the process of affinity maturation, the variable region of the BCR undergoes somatic hypermutation, followed by selection of clones with the highest affinity to the antigen (36). There was a statistically significant increase in the V gene mutation frequency from HCV E2-specific samples compared to the corresponding naive B cell samples in both subjects (Fig. 6E). There were no differences in the average mutation frequencies between the HCV E2-specific HCV-8 and HCV-9 samples. This suggests that HCV E2-specific class-switched memory B cells underwent affinity maturation and accumulated a significant number of mutations that were specific for each participant.
Discussion
The importance of B cell-mediated antibody responses in spontaneous clearance of primary HCV infection is controversial. In chimpanzees, no correlation could be established between the antibody response and spontaneous viral clearance (37). In humans, a study from a cohort of a single-source outbreak of HCV infection showed that the rapid development of neutralizing antibodies correlated with spontaneous clearance (15). However, other reports described cases where HCV infection was spontaneously resolved without the detection of HCV-specific antibodies in humans (19–22). The development of new methods enabling characterization of HCV-specific B cells in acutely infected patients is needed to establish better understanding of the correlates of the humoral immune response associated with spontaneous resolutions of HCV infection. Generation of B cell tetramer is an important strategy because it enables deeper characterization of the B cell sub population that are involved, as well as their activation level or exhaustion status. Previously, a gp41 B cell tetramer was successfully used to study the development of the antibody response in the context of HIV infection (25). In the current study, we used the ectodomain of HCV glycoprotein E2 from a genotype 2a strain (J6) to develop a biotinylated monomer that could be used to generate a tetramer specifically recognizing HCV E2-specific B cells (Fig. 1). Our reagent was first validated using E2 specific antibodies and hybridoma cells producing E2 specific antibodies (Fig. 2).
Next, using this novel E2 tetramer, we successfully identified antigen-specific B cells in HCV-infected subjects (Fig. 3–4). The most frequent genotype in the Montreal HEPCO cohort is genotype 1. Despite high variability of sequences between genotypes (identity <70%), especially in the hypervariable regions of E2 (HVR1-3), exposed conserved regions are present and have been shown to be important for the binding of E2 protein with the co-receptor CD81 protein on the cell surface and subsequent viral entry (38, 39). Cross-reactive human antibodies have been described that bind various E2 proteins from multiple HCV genotypes (40–42). We tested two different E2 tetramers that were derived from genotype 1 (H77 strain) and genotype 2 (J6 strain) with PBMC samples obtained from genotype 1, 2 and 3 infected participants. The staining of antigen-specific B cell with the J6-E2 tetramer was far more specific as compared to the H77-E2 tetramer (data not shown). The H77-E2 tetramer staining had increased background levels and less intensity in signal. As a result, all experiments were done using the J6-E2 tetramer. With this tetramer, E2 specific class-switched memory B cells were detected in the majority of chronically infected individuals (18/21 for genotype 1 and 5/5 for both genotypes 2 and genotype 3, Fig. 3C). Therefore, the usage of a different E2 genotype (J6, 2a) did not affect binding of antigen-specific B cells from patients with different infection genotypes. Furthermore, the sensitivity of the tetramer was comparable to the B cell ELISpot assay as shown in Fig. 3D.
However, in a longitudinal analysis, tetramer positive populations could only be detected in approximately half of the tested individuals during the late acute HCV infection (Fig. 4A, B). This was in agreement with the anti-E2 titer, measured by ELISA where only the samples with a positive tetramer population exhibited a detectable anti-E2 titer (Fig. 4C). This is consistent with previous reports showing a delayed antibody response during acute infection. It suggests that at least in some patients, the development and/or expansion of HCV E2-specific B cells is hindered, which may be a factor that contributes to the establishment of a chronic infection. It is also possible that early HCV-specific B cells are of lower affinity and thus are unable to bind the E2 tetramer efficiently. To expand this analysis, in a preliminary experiment, we were able to detect HCV-specific B cells in 3 out of 6 subjects after spontaneous resolution of HCV infection with a mean frequency of 0.3% (range: 0.15% to 0.49%) of class-switched memory B cells (1 year post EDI, data not shown). This is also consistent with a previous report that showed antibody responses during HCV infection are usually of low titer, and decline rapidly after spontaneous resolution (7). Analysis of the E2 tetramer positive population frequency and function longitudinally during earlier time points in a larger cohort of participants with different infection outcomes may provide more detailed insight into the role of these antigen-specific B cells in determining the course and outcome of HCV infection. We focussed our analysis on class-switched memory B cells for this pilot project. This new tool however, will also allow us to characterize the phenotype of HCV specific B cells in greater detail. In the genotype 1 chronically infected subjects, we could also detect HCV specific unswitched memory B cells (CD27+IgM+), and to a lower extent naive/atypical B cells (CD27-IgM+), but the frequency of HCV specific class-switched memory B cells was certainly higher in majority of samples analyzed (15/21, Fig. 3E). Future studies will be needed to address this question in more detail, as well as to characterize the activation and/or exhaustion profiles of HCV-specific B cells.
HCV E2-specific class-switched memory B cells were sorted using the E2 tetramer for BCR deep sequencing. In two independent subjects, the HCV-specific BCR repertoire was focused, suggestive of antigen driven selection. Moreover, we have shown that the normal Gaussian-like distribution of CDR3 lengths was skewed in both participants analyzed, suggesting an amplification and/or selection of specific clonotypes (35, 43). It is also postulated that the affinity maturation leads to shorter CDR3 length in antigen-specific B cells (44). However, we did not observe any differences between the average CDR3 length of naive B cells and HCV E2-specific cells in the two subjects analysed thus far (data not shown). Also, the accumulation of mutations in the CDR3 sequence is indicative of affinity maturation process (36). Our analysis showed an increased mutation frequency in HCV-specific BCRs compared to naive receptors. Together these results suggest that sorted E2-specific class-switched memory B cells were indeed antigen-specific, selected, expanded and accumulated mutations during the affinity maturation process.
The BCR repertoire of bulk memory and naive B cells was previously investigated during HCV infection (45). However, in this study total memory B cells as opposed to antigen-specific cells were analyzed. It was shown that the gene usage was distinct between those who spontaneously resolved the infection versus those who were chronically infected. Also, the clonality of the repertoire was greater in resolving infection compared to chronically infected individuals. Phylogenetic analysis demonstrated tight clustering of a limited number of related B cell clonotypes in resolvers compared to a more dispersed pattern in chronically infected individuals, suggestive of an increased clonal selection in the resolvers. Finally, in the same report, the CDR3 length distribution was particularly skewed in BCRs of subjects who resolved the infection with a single CDR3 length being the dominant clone compared to BCRs obtained from persistent infection where the deviation from the Gaussian-like distribution was less apparent. Utilizing HCV E2-specific tetramers will now enable us to investigate the evolution of the repertoire in HCV-specific B cells, in particular, the association of distinct BCR gene family usage among individuals who spontaneously clear versus those that progress to chronicity. One limitation of this approach is the low frequency of HCV-specific B cells. Nevertheless, given the advent of sensitive technologies, we are hopeful that the longitudinal comparisons of the BCR repertoire at the single cell level from subjects with different infection outcomes will be feasible in the near future, as this important tool will allow for a better characterization of HCV-specific B cells.
B cell disorders such as mixed cryoglobulinemia and non-Hodgkin lymphomas are complications associated with chronic HCV infection (46). It has been demonstrated that clonal B cell expansion in the liver is associated with these extra-hepatic manifestations (47). Furthermore, E2-specific B cells isolated from an asymptomatic HCV patient used the VH01-69 gene (48) that is associated with B cell lymphomas in chronic HCV (49). B cells were also implicated in liver fibrosis, where they have been shown to be activated, produce inflammatory cytokines and constitutively secrete IgG (50). Isolation and characterization of HCV-specific B cells in individuals with such phenotypes will provide a better insight into the role of B cells in these aberrant manifestations and may provide better B cell-based immunotherapies. Little is known about the interaction between B cells and CD4 helper T cells, specifically the follicular helper T cells (Tfh) during an acute and chronic HCV infection. A recent report demonstrated that HCV specific Tfh cells had an activated phenotype during the acute phase of HCV infection, with an increased expression of ICOS, which correlated with antibody production (51). Future studies combining HCV specific B cell visualization (together with subset identification, activation and exhaustion markers) with detailed analyses of the neutralizing antibody repertoire and Tfh development and function should elucidate the importance of this interaction and how it influences the generation of virus-specific neutralizing antibodies and infection outcome prognoses.
Acknowledgments
We thank all of the donors who participated in this study. Authors wish to thank Dominique Gauchat of the flow cytometry core of the CRCHUM for technical help with cell sorting experiments.
Footnotes
Grant support
We acknowledge the NIH support (PHS grants R01AI070101, R01AI124680, R01AI126890 and R21AI118337 to A.G and ORIP/OD P51OD011132 (formerly NCRR P51RR000165) to the Yerkes National Primate Research Center and the Canadian Institutes of Health Research (CIHR) (MOP-133680), Alberta Innovates Health Solutions, the Fonds de recherche du Québec – Santé (FRQS) AIDS and Infectious Diseases Network (Reseau FRQS SIDA-MI). MB is supported by postdoctoral fellowships from the American Liver Foundation, the Fonds de recherche du Québec – Santé (FRQS) and the Canadian Network on Hepatitis C (CanHepC). NHS is supported by a Chercheur Boursier salary award from the FRQS.
HCV abbreviation: hepatitis C virus
DAA abbreviation: direct acting antivirals
PWID abbreviation: people who inject drugs
EDI abbreviation: estimated date of infection
Authors’ contributions
MB and WZ designed and performed experiments, analyzed data and wrote the manuscript. EE performed experiments. NB and JPV provided subject samples and JB recruited and followed subjects and provided clinical data. JM provided the E2 and gp140 plasmids. NHS and AG obtained funds, designed experiments, analyzed data and wrote the manuscript.
References
- 1.Suryaprasad AG, White JZ, Xu F, Eichler BA, Hamilton J, Patel A, Hamdounia SB, Church DR, Barton K, Fisher C, Macomber K, Stanley M, Guilfoyle SM, Sweet K, Liu S, Iqbal K, Tohme R, Sharapov U, Kupronis BA, Ward JW, Holmberg SD. Emerging epidemic of hepatitis C virus infections among young nonurban persons who inject drugs in the United States, 2006–2012. Clin Infect Dis. 2014;59:1411–1419. doi: 10.1093/cid/ciu643. [DOI] [PubMed] [Google Scholar]
- 2.Schinazi R, Halfon P, Marcellin P, Asselah T. HCV direct-acting antiviral agents: the best interferon-free combinations. Liver Int. 2014;34(Suppl 1):69–78. doi: 10.1111/liv.12423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Simmons B, Saleem J, Hill A, Riley RD, Cooke GS. Risk of Late Relapse or Reinfection With Hepatitis C Virus After Achieving a Sustained Virological Response: A Systematic Review and Meta-analysis. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2016;62:683–694. doi: 10.1093/cid/civ948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Swadling L, Capone S, Antrobus RD, Brown A, Richardson R, Newell EW, Halliday J, Kelly C, Bowen D, Fergusson J, Kurioka A, Ammendola V, Del Sorbo M, Grazioli F, Esposito ML, Siani L, Traboni C, Hill A, Colloca S, Davis M, Nicosia A, Cortese R, Folgori A, Klenerman P, Barnes E. A human vaccine strategy based on chimpanzee adenoviral and MVA vectors that primes, boosts, and sustains functional HCV-specific T cell memory. Science translational medicine. 2014;6:261ra153. doi: 10.1126/scitranslmed.3009185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cashman SB, Marsden BD, Dustin LB. The Humoral Immune Response to HCV: Understanding is Key to Vaccine Development. Frontiers in immunology. 2014;5:550. doi: 10.3389/fimmu.2014.00550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Law JL, Chen C, Wong J, Hockman D, Santer DM, Frey SE, Belshe RB, Wakita T, Bukh J, Jones CT, Rice CM, Abrignani S, Tyrrell DL, Houghton M. A hepatitis C virus (HCV) vaccine comprising envelope glycoproteins gpE1/gpE2 derived from a single isolate elicits broad cross-genotype neutralizing antibodies in humans. PloS one. 2013;8:e59776. doi: 10.1371/journal.pone.0059776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Logvinoff C, Major ME, Oldach D, Heyward S, Talal A, Balfe P, Feinstone SM, Alter H, Rice CM, McKeating JA. Neutralizing antibody response during acute and chronic hepatitis C virus infection. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:10149–10154. doi: 10.1073/pnas.0403519101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Netski DM, Mosbruger T, Depla E, Maertens G, Ray SC, Hamilton RG, Roundtree S, Thomas DL, McKeating J, Cox A. Humoral immune response in acute hepatitis C virus infection. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2005;41:667–675. doi: 10.1086/432478. [DOI] [PubMed] [Google Scholar]
- 9.Farci P, Shimoda A, Wong D, Cabezon T, De Gioannis D, Strazzera A, Shimizu Y, Shapiro M, Alter HJ, Purcell RH. Prevention of hepatitis C virus infection in chimpanzees by hyperimmune serum against the hypervariable region 1 of the envelope 2 protein. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:15394–15399. doi: 10.1073/pnas.93.26.15394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morin TJ, Broering TJ, Leav BA, Blair BM, Rowley KJ, Boucher EN, Wang Y, Cheslock PS, Knauber M, Olsen DB, Ludmerer SW, Szabo G, Finberg RW, Purcell RH, Lanford RE, Ambrosino DM, Molrine DC, Babcock GJ. Human monoclonal antibody HCV1 effectively prevents and treats HCV infection in chimpanzees. PLoS pathogens. 2012;8:e1002895. doi: 10.1371/journal.ppat.1002895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Law M, Maruyama T, Lewis J, Giang E, Tarr AW, Stamataki Z, Gastaminza P, Chisari FV, Jones IM, Fox RI, Ball JK, McKeating JA, Kneteman NM, Burton DR. Broadly neutralizing antibodies protect against hepatitis C virus quasispecies challenge. Nature medicine. 2008;14:25–27. doi: 10.1038/nm1698. [DOI] [PubMed] [Google Scholar]
- 12.Vanwolleghem T, Bukh J, Meuleman P, Desombere I, Meunier JC, Alter H, Purcell RH, Leroux-Roels G. Polyclonal immunoglobulins from a chronic hepatitis C virus patient protect human liver-chimeric mice from infection with a homologous hepatitis C virus strain. Hepatology. 2008;47:1846–1855. doi: 10.1002/hep.22244. [DOI] [PubMed] [Google Scholar]
- 13.Dowd KA, Netski DM, Wang XH, Cox AL, Ray SC. Selection pressure from neutralizing antibodies drives sequence evolution during acute infection with hepatitis C virus. Gastroenterology. 2009;136:2377–2386. doi: 10.1053/j.gastro.2009.02.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Osburn WO, Snider AE, Wells BL, Latanich R, Bailey JR, Thomas DL, Cox AL, Ray SC. Clearance of hepatitis C infection is associated with the early appearance of broad neutralizing antibody responses. Hepatology. 2014;59:2140–2151. doi: 10.1002/hep.27013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pestka JM, Zeisel MB, Blaser E, Schurmann P, Bartosch B, Cosset FL, Patel AH, Meisel H, Baumert J, Viazov S, Rispeter K, Blum HE, Roggendorf M, Baumert TF. Rapid induction of virus-neutralizing antibodies and viral clearance in a single-source outbreak of hepatitis C. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:6025–6030. doi: 10.1073/pnas.0607026104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Raghuraman S, Park H, Osburn WO, Winkelstein E, Edlin BR, Rehermann B. Spontaneous clearance of chronic hepatitis C virus infection is associated with appearance of neutralizing antibodies and reversal of T-cell exhaustion. The Journal of infectious diseases. 2012;205:763–771. doi: 10.1093/infdis/jir835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Esteban-Riesco L, Depaulis F, Moreau A, Bacq Y, Dubois F, Goudeau A, Gaudy-Graffin C. Rapid and sustained autologous neutralizing response leading to early spontaneous recovery after HCV infection. Virology. 2013;444:90–99. doi: 10.1016/j.virol.2013.05.037. [DOI] [PubMed] [Google Scholar]
- 18.Osburn WO, Fisher BE, Dowd KA, Urban G, Liu L, Ray SC, Thomas DL, Cox AL. Spontaneous control of primary hepatitis C virus infection and immunity against persistent reinfection. Gastroenterology. 2010;138:315–324. doi: 10.1053/j.gastro.2009.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thimme R, Bukh J, Spangenberg HC, Wieland S, Pemberton J, Steiger C, Govindarajan S, Purcell RH, Chisari FV. Viral and immunological determinants of hepatitis C virus clearance, persistence, and disease. Proc Natl Acad Sci U S A. 2002;99:15661–15668. doi: 10.1073/pnas.202608299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Post JJ, Pan Y, Freeman AJ, Harvey CE, White PA, Palladinetti P, Haber PS, Marinos G, Levy MH, Kaldor JM, Dolan KA, Ffrench RA, Lloyd AR, Rawlinson WD, Hepatitis CI G. Transmission in Prisons Study. Clearance of hepatitis C viremia associated with cellular immunity in the absence of seroconversion in the hepatitis C incidence and transmission in prisons study cohort. The Journal of infectious diseases. 2004;189:1846–1855. doi: 10.1086/383279. [DOI] [PubMed] [Google Scholar]
- 21.Meyer MF, Lehmann M, Cornberg M, Wiegand J, Manns MP, Klade C, Wedemeyer H. Clearance of low levels of HCV viremia in the absence of a strong adaptive immune response. Virology journal. 2007;4:58. doi: 10.1186/1743-422X-4-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Takaki A, Wiese M, Maertens G, Depla E, Seifert U, Liebetrau A, Miller JL, Manns MP, Rehermann B. Cellular immune responses persist and humoral responses decrease two decades after recovery from a single-source outbreak of hepatitis C. Nature medicine. 2000;6:578–582. doi: 10.1038/75063. [DOI] [PubMed] [Google Scholar]
- 23.Sugalski JM, Rodriguez B, Moir S, Anthony DD. Peripheral blood B cell subset skewing is associated with altered cell cycling and intrinsic resistance to apoptosis and reflects a state of immune activation in chronic hepatitis C virus infection. Journal of immunology. 2010;185:3019–3027. doi: 10.4049/jimmunol.1000879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Franz B, May KF, Jr, Dranoff G, Wucherpfennig K. Ex vivo characterization and isolation of rare memory B cells with antigen tetramers. Blood. 2011;118:348–357. doi: 10.1182/blood-2011-03-341917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morris L, Chen X, Alam M, Tomaras G, Zhang R, Marshall DJ, Chen B, Parks R, Foulger A, Jaeger F, Donathan M, Bilska M, Gray ES, Abdool Karim SS, Kepler TB, Whitesides J, Montefiori D, Moody MA, Liao HX, Haynes BF. Isolation of a human anti-HIV gp41 membrane proximal region neutralizing antibody by antigen-specific single B cell sorting. PloS one. 2011;6:e23532. doi: 10.1371/journal.pone.0023532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Naldini L, Blomer U, Gallay P, Ory D, Mulligan R, Gage FH, Verma IM, Trono D. In vivo gene delivery and stable transduction of nondividing cells by a lentiviral vector. Science. 1996;272:263–267. doi: 10.1126/science.272.5259.263. [DOI] [PubMed] [Google Scholar]
- 27.Grebely J, Morris MD, Rice TM, Bruneau J, Cox AL, Kim AY, McGovern BH, Shoukry NH, Lauer G, Maher L, Lloyd AR, Hellard M, Prins M, Dore GJ, Page K, In CSG. Cohort profile: the International Collaboration of Incident HIV and Hepatitis C in Injecting Cohorts (InC3) Study. Int J Epidemiol. 2013;42:1649–1659. doi: 10.1093/ije/dys167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Badr G, Bedard N, Abdel-Hakeem MS, Trautmann L, Willems B, Villeneuve JP, Haddad EK, Sekaly RP, Bruneau J, Shoukry NH. Early interferon therapy for hepatitis C virus infection rescues polyfunctional, long-lived CD8+ memory T cells. Journal of virology. 2008;82:10017–10031. doi: 10.1128/JVI.01083-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kared H, Fabre T, Bedard N, Bruneau J, Shoukry NH. Galectin-9 and IL-21 mediate cross-regulation between Th17 and Treg cells during acute hepatitis C. PLoS pathogens. 2013;9:e1003422. doi: 10.1371/journal.ppat.1003422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Khan AG, Whidby J, Miller MT, Scarborough H, Zatorski AV, Cygan A, Price AA, Yost SA, Bohannon CD, Jacob J, Grakoui A, Marcotrigiano J. Structure of the core ectodomain of the hepatitis C virus envelope glycoprotein 2. Nature. 2014;509:381–384. doi: 10.1038/nature13117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jahnmatz M, Kesa G, Netterlid E, Buisman AM, Thorstensson R, Ahlborg N. Optimization of a human IgG B-cell ELISpot assay for the analysis of vaccine-induced B-cell responses. Journal of immunological methods. 2013;391:50–59. doi: 10.1016/j.jim.2013.02.009. [DOI] [PubMed] [Google Scholar]
- 32.Yousfi Monod M, Giudicelli V, Chaume D, Lefranc MP. IMGT/JunctionAnalysis: the first tool for the analysis of the immunoglobulin and T cell receptor complex V-J and V-D-J JUNCTIONs. Bioinformatics. 2004;20(Suppl 1):i379–385. doi: 10.1093/bioinformatics/bth945. [DOI] [PubMed] [Google Scholar]
- 33.Whidby J, Mateu G, Scarborough H, Demeler B, Grakoui A, Marcotrigiano J. Blocking hepatitis C virus infection with recombinant form of envelope protein 2 ectodomain. Journal of virology. 2009;83:11078–11089. doi: 10.1128/JVI.00800-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dickey C, Ziegner U, Agadjanyan MG, Srikantan V, Refaeli Y, Prabhu A, Sato A, Williams WV, Weiner DB, Ugen KE. Murine monoclonal antibodies biologically active against the amino region of HIV-1 gp120: isolation and characterization. DNA and cell biology. 2000;19:243–252. doi: 10.1089/104454900314519. [DOI] [PubMed] [Google Scholar]
- 35.Miqueu P, Guillet M, Degauque N, Dore JC, Soulillou JP, Brouard S. Statistical analysis of CDR3 length distributions for the assessment of T and B cell repertoire biases. Molecular immunology. 2007;44:1057–1064. doi: 10.1016/j.molimm.2006.06.026. [DOI] [PubMed] [Google Scholar]
- 36.De Silva NS, Klein U. Dynamics of B cells in germinal centres. Nature reviews Immunology. 2015;15:137–148. doi: 10.1038/nri3804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bassett SE, Thomas DL, Brasky KM, Lanford RE. Viral persistence, antibody to E1 and E2, and hypervariable region 1 sequence stability in hepatitis C virus-inoculated chimpanzees. Journal of virology. 1999;73:1118–1126. doi: 10.1128/jvi.73.2.1118-1126.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lavie M, Sarrazin S, Montserret R, Descamps V, Baumert TF, Duverlie G, Seron K, Penin F, Dubuisson J. Identification of conserved residues in hepatitis C virus envelope glycoprotein E2 that modulate virus dependence on CD81 and SRB1 entry factors. Journal of virology. 2014;88:10584–10597. doi: 10.1128/JVI.01402-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Owsianka AM, Timms JM, Tarr AW, Brown RJ, Hickling TP, Szwejk A, Bienkowska-Szewczyk K, Thomson BJ, Patel AH, Ball JK. Identification of conserved residues in the E2 envelope glycoprotein of the hepatitis C virus that are critical for CD81 binding. Journal of virology. 2006;80:8695–8704. doi: 10.1128/JVI.00271-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kong L, Giang E, Robbins JB, Stanfield RL, Burton DR, Wilson IA, Law M. Structural basis of hepatitis C virus neutralization by broadly neutralizing antibody HCV1. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:9499–9504. doi: 10.1073/pnas.1202924109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Keck Z, Wang W, Wang Y, Lau P, Carlsen TH, Prentoe J, Xia J, Patel AH, Bukh J, Foung SK. Cooperativity in virus neutralization by human monoclonal antibodies to two adjacent regions located at the amino terminus of hepatitis C virus E2 glycoprotein. Journal of virology. 2013;87:37–51. doi: 10.1128/JVI.01941-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hadlock KG, Lanford RE, Perkins S, Rowe J, Yang Q, Levy S, Pileri P, Abrignani S, Foung SK. Human monoclonal antibodies that inhibit binding of hepatitis C virus E2 protein to CD81 and recognize conserved conformational epitopes. Journal of virology. 2000;74:10407–10416. doi: 10.1128/jvi.74.22.10407-10416.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mc Guire PJ, Cunningham-Rundles C, Ochs H, Diaz GA. Oligoclonality, impaired class switch and B-cell memory responses in WHIM syndrome. Clinical immunology. 2010;135:412–421. doi: 10.1016/j.clim.2010.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Galson JD, Clutterbuck EA, Truck J, Ramasamy MN, Munz M, Fowler A, Cerundolo V, Pollard AJ, Lunter G, Kelly DF. BCR repertoire sequencing: different patterns of B-cell activation after two Meningococcal vaccines. Immunology and cell biology. 2015;93:885–895. doi: 10.1038/icb.2015.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Racanelli V, Brunetti C, De Re V, Caggiari L, De Zorzi M, Leone P, Perosa F, Vacca A, Dammacco F. Antibody V(h) repertoire differences between resolving and chronically evolving hepatitis C virus infections. PloS one. 2011;6:e25606. doi: 10.1371/journal.pone.0025606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gill K, Ghazinian H, Manch R, Gish R. Hepatitis C virus as a systemic disease: reaching beyond the liver. Hepatology international. 2015 doi: 10.1007/s12072-015-9684-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sansonno D, Lauletta G, De Re V, Tucci FA, Gatti P, Racanelli V, Boiocchi M, Dammacco F. Intrahepatic B cell clonal expansions and extrahepatic manifestations of chronic HCV infection. European journal of immunology. 2004;34:126–136. doi: 10.1002/eji.200324328. [DOI] [PubMed] [Google Scholar]
- 48.Chan CH, Hadlock KG, Foung SK, Levy S. V(H)1–69 gene is preferentially used by hepatitis C virus-associated B cell lymphomas and by normal B cells responding to the E2 viral antigen. Blood. 2001;97:1023–1026. doi: 10.1182/blood.v97.4.1023. [DOI] [PubMed] [Google Scholar]
- 49.Ivanovski M, Silvestri F, Pozzato G, Anand S, Mazzaro C, Burrone OR, Efremov DG. Somatic hypermutation, clonal diversity, and preferential expression of the VH 51p1/VL kv325 immunoglobulin gene combination in hepatitis C virus-associated immunocytomas. Blood. 1998;91:2433–2442. [PubMed] [Google Scholar]
- 50.Thapa M, Chinnadurai R, Velazquez VM, Tedesco D, Elrod E, Han JH, Sharma P, Ibegbu C, Gewirtz A, Anania F, Pulendran B, Suthar MS, Grakoui A. Liver fibrosis occurs through dysregulation of MyD88-dependent innate B-cell activity. Hepatology. 2015;61:2067–2079. doi: 10.1002/hep.27761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Raziorrouh B, Sacher K, Tawar RG, Emmerich F, Neumann-Haefelin C, Baumert TF, Thimme R, Boettler T. Virus-Specific CD4+ T Cells Have Functional and Phenotypic Characteristics of Follicular T-Helper Cells in Patients With Acute and Chronic HCV Infections. Gastroenterology. 2016;150:696–706. e693. doi: 10.1053/j.gastro.2015.11.005. [DOI] [PubMed] [Google Scholar]