Abstract
Although treatment of prostate cancer (PCa) has improved over the past several years, taxanes such as cabazitaxel remain the only form of effective chemotherapy that improves survival in patients with metastatic castration-resistant PCa. However, the effectiveness of this class of drugs has been associated with various side effects and drug resistance. We previously reported that fisetin, a hydroxyflavone, is a microtubule stabilizing agent and inhibits PCa cell proliferation, migration, and invasion and suggested its use as an adjuvant for treatment of prostate and other cancer types. In this study, we investigated the effect of fisetin in combination with cabazitaxel with the objective to achieve maximum therapeutic benefit, reduce dose and toxicity and minimize or delay the induction of drug resistance and metastasis. Our data show for the first time that a combination of fisetin (20 μM) enhances cabazitaxel (5nM) and synergistically reduces 22Rν1, PC-3M-luc-6, and C4-2 cell viability and metastatic properties with minimal adverse effects on normal prostate epithelial cells. In addition, the combination of fisetin with cabazitaxel was associated with inhibition of proliferation and enhancement of apoptosis. Furthermore, combination treatment resulted in inhibition of tumor growth, invasion and metastasis when assessed in two in-vivo xenograft mouse models. These results provide evidence that fisetin may have therapeutic benefit for patients with advanced PCa through enhancing the efficacy of cabazitaxel under both androgen-dependent and androgen-independent conditions. This study underscores the benefit of the combination of fisetin with cabazitaxel for the treatment of advanced and resistant PCa and possibly other cancer types.
Keywords: Fisetin, Cabazitaxel, combination therapy, microtubule, microtubule targeting agents
INTRODUCTION
Despite advances in screening and treatment, prostate cancer (PCa) remains a leading cause of death among American men (1). An estimated 180,890 new cases and 26,120 deaths are projected in 2016 (2). Androgen ablation therapy has been shown to be effective in regression of PCa; however the cancer eventually progresses to metastatic castration-resistant PCa (mCRPC), for which there is no cure. A wide range of novel therapies have been introduced clinically for treatment of mCRPC, including androgen synthesis inhibitors, immunotherapies, and microtubule targeting agents (MTAs) such as taxanes (3,4). In 2004, two clinical studies demonstrated a survival advantage of docetaxel (Taxotere) chemotherapy in these patients (5,6), setting a new standard of care and representing a significant milestone in the treatment of PCa (7). Consequently, docetaxel is the most commonly prescribed first-line chemotherapy for mCRPC. However, many patients have limited therapeutic options once tumors become refractory to docetaxel chemotherapy. In such cases no treatments improve survival. In 2010, the Food and Drug Administration (FDA) approved cabazitaxel (Jevtana®) as the second-line treatment for men with mCRPC, providing a new avenue for these patients during or after treatment with docetaxel chemotherapy (8). Even with cabaxitaxel therapy, most patients ultimately become chemoresistant and their treatment remains a major challenge. Thus, limited options for the management of advanced PCa call for new and more effective and improved treatment approaches. Combination chemotherapy is a widely used paradigm for improving efficacy of individual drugs in the management of numerous human malignancies. It is argued that management of cancer is more feasible and holds better promise with the use of drug combinations that can hit multiple targets (8–11). Combination chemotherapy has several advantages such as lower dose requirement which subsequently lessens the side effects and circumvents drug resistance; and inhibits or delays metastasis (12–18). Zhang et al (13) showed that genistein enhances the response to cabazitaxel treatment in mCRPC cells. Synergistic drug interactions act in concert to reduce long term colonogenic survival and inhibit oncogenic and metastatic pathways (19–21).
Fisetin, a dietary tetrahydroxyflavone that belongs to the flavonoid group of polyphenols, is present in many vegetables and fruits and has been found to inhibit multiple oncogenic pathways both in-vitro and in-vivo in many different types of cancer (22–24). The development of new agents such as fisetin could provide more effective therapeutic options for PCa patients. We previously reported that fisetin is a microtubule stabilizing agent that significantly inhibits PCa cell proliferation, migration, and invasion (25). Similarly, Haddad et al. (26) observed a decrease in proliferation with concomitant induction of apoptosis in PCa cells upon fisetin treatment. We further observed that fisetin inhibited mammalian target of rapamycin (mTOR) complexes 1 and 2 and suppressed Cap-dependent translation (27). In separate studies from our laboratory, we observed that fisetin acts as a dual inhibitor of PI3K/Akt and mTOR pathways in nonsmall lung cancer cells (28). This appears to be an exciting observation since both Akt and mTOR pathways are among the major signaling networks that have been implicated in advanced cancer. Using in silico modeling we showed that fisetin interacts with mTOR at two sites, thereby explaining its inhibitory effect on cellular growth and proliferation (28). NudC, a protein associated with the microtubule motor dynein/dynactin complex that regulates microtubule dynamics was inhibited by fisetin treatment (25). We have also shown that fisetin treatment in athymic nude mice implanted with AR-positive CWR22Rν1 human PCa cells, inhibited tumor growth and reduced serum PSA levels. Taking advantage of this finding, we showed that fisetin acts as an inhibitor of AR signaling and suggested that it could be a useful chemopreventive and chemotherapeutic agent against PCa (22). A recent study showed that the combination of paclitaxel and fisetin induces mitotic catastrophe and autophagic cell death in the in vitro model of A549 non-small cell lung cancer cells (29). We hypothesized that lower dose of fisetin will enhance the efficacy of cabazitaxel against advanced and metastatic human PCa cells. Many anti-tubulin agents are substrates of P-gp, a broad spectrum ATP-dependent efflux pump that reduces the efficacy of anticancer drugs. As a result, higher doses of these drugs are required to achieve adequate intracellular concentration in multidrug-resistant cancer cells.
The overall goal of this study was to determine if fisetin would increase the chemo-sensitivity of PCa cells to cabazitaxel for the purpose of reducing cabazitaxel dosage and toxicity and increasing its effectiveness in overcoming drug resistance. Combined fisetin and cabazitaxel treatment synergistically inhibited the growth of 22Rν1 cells, decreased the expression of proliferative markers (PCNA and Ki67) and the anti-apoptotic markers (Bcl-2) and induction of the apoptotic marker (Bax). In addition, fisetin and cabazitaxel treatment inhibited metastases of PC-3M-luciferase androgen independent cells.
MATERIALS & METHODS
Materials
Thiazolyl Blue Tetrazolium Blue (MTT) was purchased from Sigma-Aldrich (St. Louis, MO). Bax, Mcl-1, Bcl-2, Ki67 and PCNA antibodies, Annexin V-FITC staining kit were obtained from Cell signaling Technology (Danvers, MA). Anti-mouse and anti-rabbit secondary antibody horseradish peroxidase conjugate was obtained from Amersham Life Science Inc. (Arlington Height, IL). Fisetin was purchased from Sigma chemical Co. (St. Louis, MO). Cabazitaxel was purchased from LC laboratory (Woburn, MA). BCA protein assay kit was obtained from Pierce (Rockford, IL)
Cell Culture and Treatment
Human PCa PrEC, 22Rν1, C4-2, and lung A549 cells were obtained from ATCC (Manassas, VA) in August 2012 and authenticated using a multiplex PCR amplification Kit (PowerPlex 16 HS System, Promega, Madison, WI). PC-3M-luc-6 cells were obtained from PerkinElmer in 2013 (San Francisco, CA), used within six months and not further authenticated. NCI/ADR-RES cells were obtained from National Cancer Institute (Maryland, MI) in 2012 and were not authenticated further. The cells were cultured in RPMI 1640, F12K, or HBSS medium supplemented with 10% fetal bovine serum and 1% penicillin/streptomycin. The cells were maintained under standard cell culture conditions at 37 °C and 5% CO2 in a humid environment. Fisetin and cabazitaxel were dissolved in Dimethyl sulfoxide (stock concentration 10 mM). Cells (60–70 % monolayer confluent) were treated with fisetin and after 48 hours treated with cabazitaxel in complete growth medium.
Cell Viability
Cells were seeded (6 × 104 cells/2 mL) in 12-well plates (24 hours), and treated with fisetin (0–40 μM) for 48 hours or cabazitaxel (0–40 nM) for 24 hours. For the combination treatment cells were incubated with fisetin for 48 hours and then with cabazitaxel for an additional 24 hours. Cell viability was determined by MTT according to the manufacturer’s protocol. Briefly, after treatment cells were then treated with media containing MTT solution (1 mg/mL) for 3–4 hours. Afterward, the MTT solution was removed, and the blue crystalline precipitate internalized by the cells was dissolved in DMSO. Finally, plates were placed in a plate reader to measure absorbance at 570 nm.
Colony Formation Assay
Cells were seeded (500–1000 cells/5 mL) in petri dish plates for 24 hours. Cells were treated with fisetin and after 48 hours with cabazitaxel and allowed to form colonies in 5–10 days. Colonies were fixed with glutaraldehyde (6.0% v/v), stained with crystal violet (0.5% w/v) and photographs were taken at 40x magnification.
Western blotting
For Immunoblotting, 30–40 μg protein was resolved over 8–12 % polyacrylamide gels and transferred to nitrocellulose membrane. The blot was incubated in blocking buffer (5% non-fat dry milk 1% tween 20 in 20 mM TBS, pH 7.6) for 1 hour at room temperature. The membrane incubated with appropriate monoclonal or polyclonal primary antibody in blocking buffer followed by incubation with anti-mouse or anti-rabbit secondary antibody horseradish peroxidase conjugate and detected by chemiluminescence and autoradiolography using Bio-Rad Gel-Doc (Hercules, CA).
Apoptosis assessment by Annexin V-FITC staining
The Annexin V-FITC staining kit was used to identify apoptotic cells. Cells were treated with fisetin for 48 hours and then with cabazitaxel for 24 hours, washed, centrifuged and the cell-pellet suspended in Annexin V-FITC binding buffer. Cell suspensions were then incubated with Annexin V-FITC conjugate and Propidium iodide solution. Data were collected on a Becton-Dickinson FACSCalibur (San Jose, CA) and analyzed in FlowJo, Version 9.7 (FLowJo, LLC, Ashland, OR).
In-vivo tumor xenograft study
Two xenograft mouse models were used to assess tumor growth, invasion and metastasis in-vivo. In the first model, a total of 24 athymic nude male mice 6–8 weeks old were injected subcutaneously with 1×106 22Rν1 cells. In the second model, 24 athymic nude male mice 6–8 weeks old were injected subcutaneously with 3×106 PC-3M-luc-6 cells. Two weeks later, tumor bearing mice were randomly divided into four groups (n=6) and treated IP with: 1) fisetin (20 mg/kg; 3 times/week); 2) cabazitaxel (5 mg/kg; once/week); 3) the combination of fisetin (20 mg/kg; 3 times/week) and cabazitaxel (5 mg/kg; once/week); or 4) vehicle (control).
Immunohistochemistry Analysis
Sections (5mm thick) were cut from paraffin-embedded tumor tissues. Immunostaining was performed using specific antibodies with appropriate dilutions. The slides were developed in diaminobenzidine and counter stained with a hematoxylin stain. The stained slides were dehydrated and mounted in permount and visualized on Nikon Eclipse IT system (Nikon Instruments, Inc.). Images were captured with an attached camera linked to a computer.
Bioluminescent imaging
In-vivo bioluminescent imaging (BLI) was performed with an IVIS Imaging System (Xenogen, San Francisco, CA). Animals were placed onto the warmed stage inside the camera box and received continuous exposure to 1–2% isoflurane to sustain sedation during imaging. Imaging times ranged from one second to 2 minutes, depending on the bioluminescence, and three to five mice were imaged at a time. Regions of interest (ROI) from displayed images were drawn around the tumor sites and quantified as photons/second using the Living Image software (Xenogen, San Francisco, CA). Background in-vivo bioluminescence was measured as approximately 1–2×105 photons/s for similarly sized ROIs at non-tumor sites of mice.
For ex-vivo imaging
150 mg/kg D-luciferin was injected into the mice immediately prior to necropsy. Animals were humanely sacrificed and tissues of interest were removed, placed into separate petri dish culture plate and imaged for 1 to 2 min. Tissues were subsequently fixed in 10% formalin (Sigma, St. Louis, Missouri) and prepared for standard histopathology evaluation.
Statistical analysis of the data
Results were analyzed using a two-way analysis of variance (ANOVA) to assess statistical significance and p values <0.05 were considered significant.
Synergistic quantification of drug combination
Drug interactions were analyzed by the combination index (CI) method developed by Chou (30) using the CompuSyn software (Biosoft, Cambridge, UK). CI < 0.9 indicates synergism, 1.1 additive and >1.1 antagonism.
RESULTS
Effect of treatment with fisetin, cabazitaxel and their combination on cell viability and colony formation in PCa cells
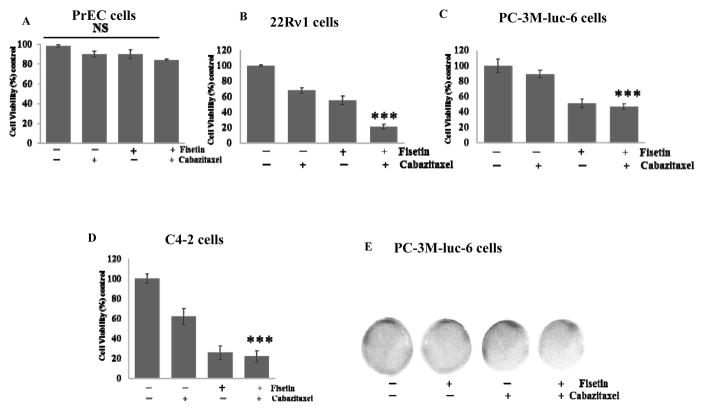
To determine whether fisetin enhances cabazitaxel sensitivity in PCa cells in-vitro, cell viability was determined using normal human prostatic epithelial cells (PrEC) and several PCa cell lines, including 22Rν1, PC3M-luc-6 and C4-2 cells. As shown in Figure 1A, fisetin (20 μM), cabazitaxel (5 nM) and their combination had minimal effects on the viability of normal PrEC. In contrast we observed 45%, 49% and 74% decreased cell viability with fisetin and 32%, 11% and 38% decreased cell viability with cabazitaxel in 22RRν1, PC-3M-luc-6 and C4-2 cells respectively. Fisetin and cabazitaxel in combination reduced viability by approximately 79%, 53% and 78% respectively (Fig. 1B–1D).
Figure 1. Effect of fisetin, cabazitaxel and their combination in-vitro.
Representative histogram images of PCa cells showing cell viability assessed by MTT assay with fisetin (20 μM), cabazitaxel (5nM) and the combination (fisetin 20 μM and cabazitaxel 5nM). A. PrEC cells. B. 22Rν1cells. C. PC-3M-luc-6 cells. D. C4-2 cells. E. Representative photograph showing the effect of fisetin (20μM) and cabazitaxel (5nM) treatment on growth of PC-3 cells investigated by monolayer colony formation assay. Data are shown as a mean ± SD of at least three independent experiments performed in triplicate; ***, p ≤ 0.002; NS = not significant.
A colonogenic assay was used for studying the effectiveness of fisetin, cabazitaxel and their combination on the survival and proliferation of PCa cells. Combined treatment with fisetin (20 μM) and cabazitaxel (5 nM) significantly suppressed colony formation compared to treatment with either agent alone (Fig. 1E). Isobologram analysis suggested that the interaction between fisetin (20 μM) and cabazitaxel (5 nM) was highly cell-dependent. For example, the synergism (CI < 1) was the most significant in 22Rν1 (CI = 0.45), whereas in PC3M (CI = 0.75) and in C4-2 cells (CI = 0.72) as shown in figure S1 and table 1. These observations demonstrate that fisetin synergistically and significantly enhanced the sensitivity of PCa cells to cabazitaxel treatment and inhibited cell viability and the long-term colonogenic growth of PCa cells.
Table 1.
Combination Index (CI) to assess the degree of drug combination using the CompuSyn software
| Cell Lines | Treatment | Parameters | CI Value | DRUI Value | ||
|---|---|---|---|---|---|---|
| Dm | m | r | ||||
| 22Rν1 | Fisetin | 27.0305 | −0.7615±0.17257 | −0.9753 | 8.34449 | |
| Cabazitaxel | 7.71559 | −2.1193±0.18013 | −0.9964 | 2.96809 | ||
| Combination (4000:1) | 0.45676 | |||||
| PC-3 | Fisetin | 0.24692 | −0.353±0.01560 | −0.9990 | 1.35079 | |
| Cabazitaxel | 0.02675 | −0.1611 | −1.0000 | 158.195 | ||
| Combination (4000:1) | 0.74663 | |||||
| C4-2 | Fisetin | 3.62164 | −0.9983±0.21078 | −0.9784 | 1.39825 | |
| Cabazitaxel | 0.39547 | −0.2726±0.10660 | −0.9313 | 141.146 | ||
| Combination (4000:1) | 0.72226 | |||||
Fisetin (20 μM), cabazitaxel (5nM) and the combination (Fisetin 20 μM and cabazitaxel 5nM) synergistically inhibit cell viability in 22Rν1, PC-3M and C4-2 cell lines. Slope (m), correlation coefficients (r), and median-effect dosages (Dm) from median-effect plots, combination indexes (CI) and dose-reduction indexes (DRI) for fisetin alone, cabazitaxel alone, and fisetin and cabazitaxel combination treatment.
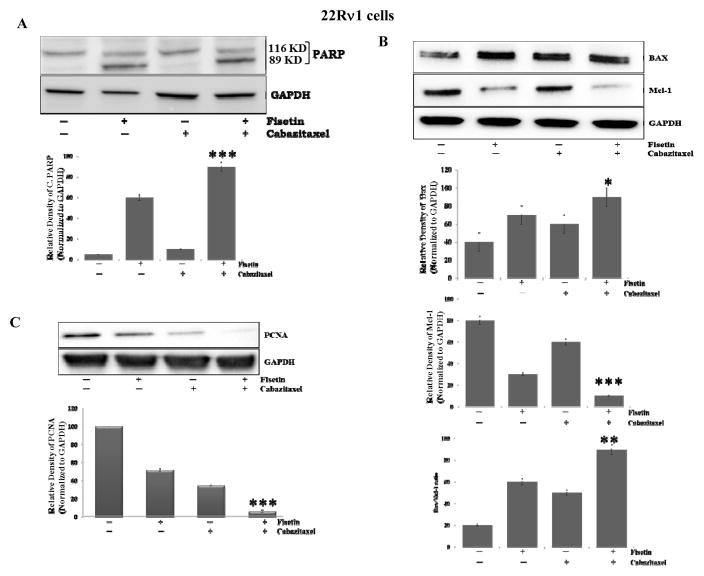
Effect of treatment with fisetin, cabazitaxel and their combination on cellular proliferation and apoptosis in PCa 22Rν1 cells
To investigate whether apoptosis is induced by the combination of fisetin and cabazitaxel, we examined the activation of poly ADP-ribose polymerase (PARP), a key effector molecule of apoptosis. Our results showed that fisetin and cabazitaxel, alone increased cleavage of PARP, whereas the combination significantly (p ≤ 0.0001) increased the expression of cleavage of PARP compared with the control, or each agent alone (Fig. 2A). Furthermore, to understand the molecular basis of the effects of the combination, we studied several molecules involved during the initiation and execution of apoptosis. Levels of pro-apoptotic Bax were increased by fisetin and cabazitaxel and this increase was augmented by the combination treatment (Fig. 2B). At the same time, levels of anti-apoptotic Mcl-1 decreased significantly (p = 0.0005). Modulation in the expression of Bax and Mcl-1 by the combination resulted in an increased Bax: Mcl-1 ratio in a way that favored apoptosis (p = 0.001, fig. 2B). These data indicate that the combination was significantly effective than fisetin, cabazitaxel alone in induction of apoptosis.
Figure 2. Effect of treatment with fisetin, cabazitaxel and their combination on markers of proliferation and apoptosis.
A–C. Representative blots showing the effect of fisetin (20 μM), cabazitaxel (5nM) and the combination (fisetin 20 μM and cabazitaxel 5nM) treatment on, cleavage of PARP, BAX, Mcl-1 and PCNA proteins respectively in 22Rν1cells. Data are shown as representative of three independent experiments. GAPDH served as a loading control. Intensities of the bands were measured using densitometry and values first normalized to respective GAPDH and then reported below each gel as relative to control. Data are shown as a mean ± and SD of at least three independent experiments performed in triplicate; *, p ≤ 0.05**, p ≤ 0.005, ***, p ≤ 0.0005.
Overexpression of proliferating cell nuclear antigen (PCNA) and Ki67 are considered prognostic biomarkers for various types of cancers including PCa. Thus, to determine the potency and putative anti-proliferative effect of fisetin, cabazitaxel and the combination treatment in-vitro, we used PCNA protein expression as a marker. The level of PCNA expression was inhibited in the combination treatment compared with the effects of each agent alone (Fig. 2C).
Effect of treatment with fisetin, cabazitaxel and their combination on the growth of 22Rν1 tumors in athymic nude mice and on overall survival
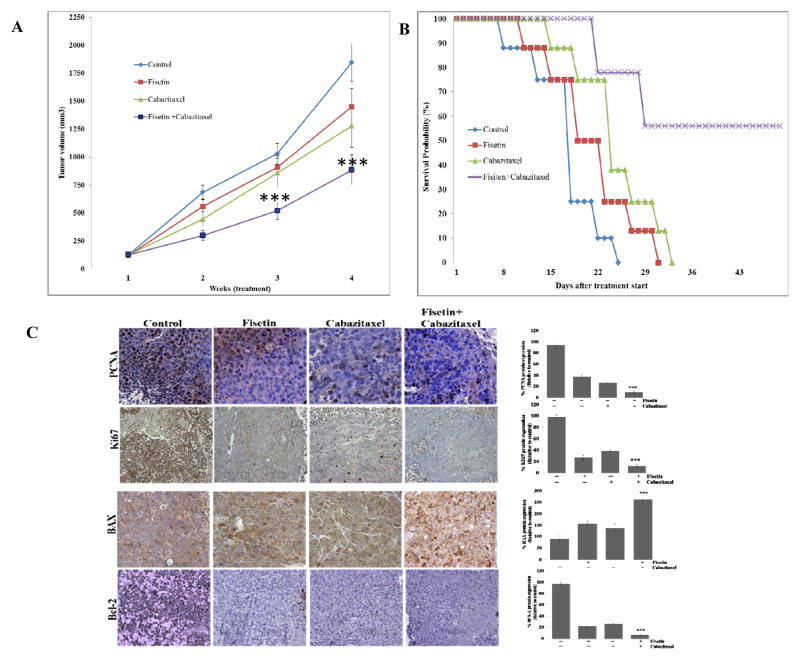
We next evaluated the effect of fisetin, cabazitaxel and the combination on tumor growth in a xenograft mouse model. We observed that treatment with fisetin alone resulted in 22% inhibition of tumor growth; cabazitaxel treatment alone resulted in 31% inhibition whereas fisetin and cabazitaxel in combination resulted in 53% inhibition of tumor growth compared with the control group (Fig. 3A).
Figure 3. Effect of treatment with fisetin, cabazitaxel and their combination on tumor growth and metastasis in mice implanted with 22Rν1 cells.
A. Line graph showing tumor volume growth determined by weekly measurements. Each value in the graph is the mean ± SE from six mice. ***, p ≤ 0.0005 was considered as significant. B. Line graph showing survival analysis. C. At the end of study, tumors were harvested from mice, subjected to IHC for proliferation (anti-ki67) and (anti-PCNA) and apoptosis (anti- BAX) and (anti-Bcl-2). Left, representative photomicrographs of Ki67, PCNA, BAX and Bcl-2-stained tumor section (40x). Right, quantitation of Ki67, PCNA, BAX and Bcl-2; ***, p ≤ 0.0001.
The outcome of any chemotherapeutic agent is evaluated on the basis of its ability to increase overall survival. We, therefore, evaluated the effect of the combination on overall survival in mice over the course of treatment. Compared with the expected median survival of 55 days in the combination treatment group, cabazitaxel and fisetin alone resulted in median life expectancy of 33 and 31 days respectively (Figure 3B). This significant increase in overall survival clearly suggests that the combination possesses high potential for PCa survival.
To explore cellular mechanisms that could account for the tumor inhibition by the combination, we assessed tumor cell proliferation using PCNA and Ki67 expression and apoptosis using Bax and Bcl-2. Cabazitaxel alone decreased PCNA, and Ki67 by 47%, increased Bax by 75% and decreased Bcl-2 by 55% in the 22Rν1 xenografts (Fig. 3C). The combination reduced PCNA and Ki67 expression by 75% and Bcl-2 by 88 %, whereas it increased Bax expression by 80% (Fig. 3C). Furthermore, these findings suggest that fisetin and cabazitaxel inhibit tumor growth through a combination of decreasing proliferation and inducing apoptosis. Taken together, in-vivo studies indicate that combined treatment with low dose of fisetin and cabazitaxel is more effective in suppressing tumor growth than either agent alone.
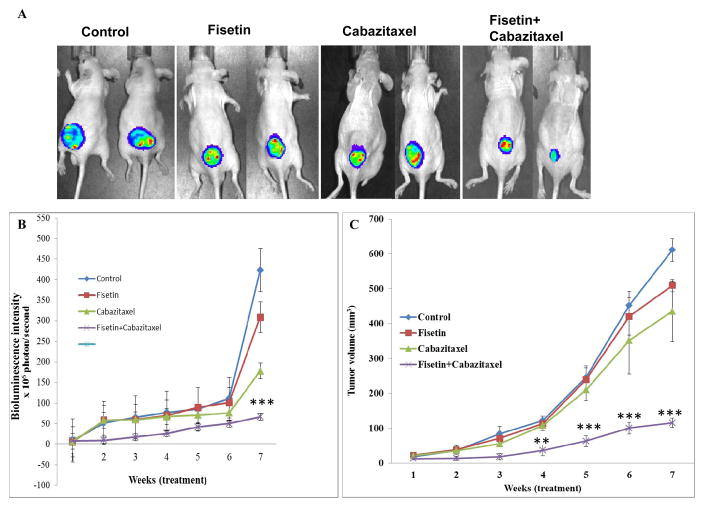
Effect of treatment with fisetin, cabazitaxel and their combination on the growth of metastatic PC-3M-luc-C6 tumors in athymic nude mice
To precisely monitor the effects of fisetin, cabazitaxel and the combination on the growth and metastasis of PC-3M-luciferase cells in live mice, cells were implanted subcutaneously in 24 mice. Three days after PC-3M cell implantation, mice were randomly divided into four groups. Treatments were given up to 7 weeks and stopped 24 h before euthanizing the animals. No apparent toxicity was observed in any of the combination treated mice as observed using the body weight (data not shown). Combination treatment with fisetin and cabazitaxel significantly (p = 0.0001) inhibited tumor growth compared to control and individual treatment groups (Fig. 4A and B). We observed only a 9 fold increase in tumor volume in the combination group over 7 weeks, while fisetin or cabazitaxel alone resulted in 22, 20 fold increase respectively, and tumor volume in the control group increased by 32 fold. In addition, we also observed that treatment with fisetin and cabazitaxel alone resulted in 18% and 29% inhibition in tumor growth respectively. However, combination of fisetin and cabazitaxel resulted in 81% inhibition in tumor growth. Overall, the effects of the combined treatments was very significant than control or each agent alone (Fig. 4C).
Figure 4. Effect of treatment with fisetin, cabazitaxel and their combination on tumor growth in a subcutaneous PC3M-luc-6 xenograft model.
A. Representative bioluminescence of PC3M-luc-6 tumor-bearing mice at the 7-week of treatments. B. ROI intensity as determined by bioluminescence. Each value in the graph is the Mean ± SE from six mice. ***, p ≤ 0.0005 was considered as significant. C. Line graph showing tumor volume growth determined by weekly measurements. Each value in the graph is the mean ± SE from six mice. ***, p ≤ 0.0001 was considered as significant.
To determine the effects of fisetin, cabazitaxel alone and the combination treatment on PCa metastasis, we excised distant organs (lymph nodes, liver, kidneys, lungs, and heart) from each group and subjected them to ex-vivo imaging. Mice from the control group showed high incidence of metastasis into distant organs, which was reduced in fisetin, cabazitaxel alone and in the combination treated mice. As shown in Figure S2, increased bioluminescence signal intensity was observed in the lymph nodes, liver, kidneys, lungs and heart (Figure S2A) of control mice, which was significantly decreased in the excised liver (p = 0.05) from the combination treated mice (Figure S2B), but not significantly decreased in lymph nodes and lungs.
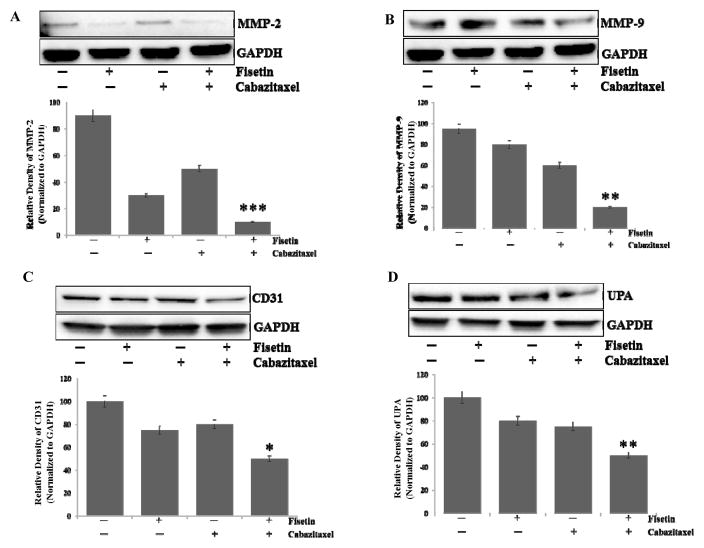
Activation of matrix metalloproteinase (MMPs), urokinase plasminogen activator (uPA), and angiogenic factors (VEGF and CD31) are shown to promote PCa metastasis into distant organs (31–33). A possibility was explored whether fisetin, cabazitaxel, alone and in combination inhibit the expression of MMP2, MMP9 and CD31 in PC-3M-luc-6 cells. Western blot analysis indicated a significant decrease in MMP2, MMP9 expression in PC-3M-luc-6 cells with combination treatment (Fig. 5A and B). Similarly, combination treatment resulted in decreased expression of CD31 (Fig. 5C). We further examined the effect of combination treatment on other metastatic markers such as uPA and observed a decrease in expression in the combination treated PC-3M-luc-6 cells (Fig. 5D).
Figure 5. Effect of treatment with fisetin, cabazitaxel and their combination on metastasis and angiogenesis in a subcutaneous PC3M-luc-6 xenograft model.
Representative blots showing the effect of fisetin and cabazitaxel treatment on metastasis. A. Anti-MMP-2. B. Anti-MMP-9. Angiogenesis, C. Anti-CD31 activity and D. Anti-UPA. Data are shown as representative of three independent experiments. GAPDH served as a loading control. Bands were measured using densitometry and values first normalized to respective GAPDH bands and then reported below each gel as relative to control.
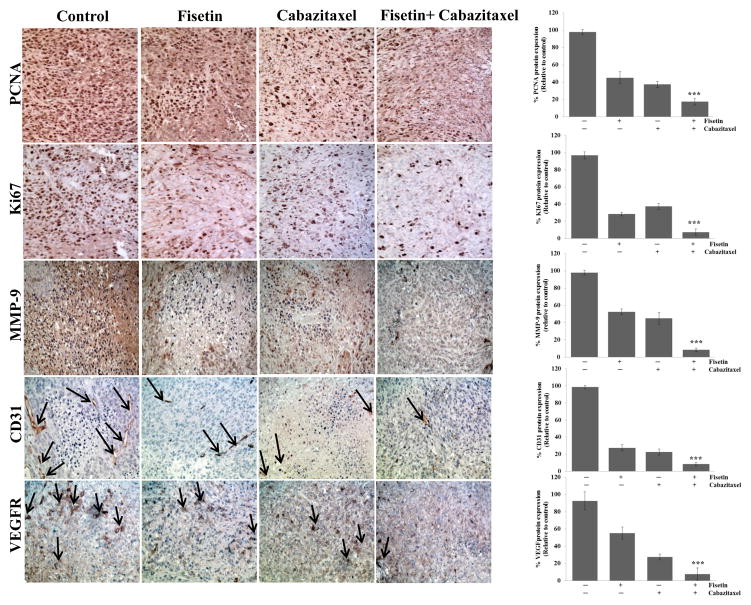
Effect of treatment with fisetin, cabazitaxel and their combination on the protein expression of PCNA and Ki-67, MMP-9, CD31 and VEGF in metastatic PC-3M-luc-6 tumors established in athymic nude mice
To further explore cellular mechanisms that could account for the antitumor effects, we assessed effects on vascularity and angiogenesis using CD31 and VEGF as markers respectively. Cabazitaxel alone decreased PCNA and Ki-67 by 40% and 42.5% respectively, and decreased microvessel density by 84.4% and angiogenesis by 85% in the PC-3M-luc-C6 xenografts (Fig. 6). The combination reduced PCNA and Ki-67 expression by 60% and 68.3% respectively and microvessel density by 88.5% and angiogenesis by 90%. These results indicate that the combination had a greater effect on these parameters than either agent alone.
Figure 6. Effect of treatment with fisetin, cabazitaxel and their combination on proliferation, apoptosis, and tumor angiogenesis in a subcutaneous PC3M-luc-6 xenograft model.
At the end of study, tumors were harvested from mice, subjected to IHC for proliferation (anti-ki67 and anti-PCNA); metastasis (anti-MMP-9); angiogenesis (anti-CD31) and (anti-VEGF). Left, representative photomicrographs of Ki67, PCNA, MMP-9, CD31 and VEGF-stained tumor section (x 40). Right, quantitation of Ki67, PCNA, MMP-9, CD31 and VEGF; **, p ≤ 0.02, ***, p ≤ 0.001.
The matrix metalloproteinases (MMPs) are an important component of cell invasion capable of degrading a range of extracellular matrix proteins allowing cancer cells to immigrate and invade. Accordingly, we examined proteins that enhance the metastatic process. Fisetin and cabazitaxel alone decreased the expression of MMP-9 protein by 45% and 50% respectively (Fig. 6), whereas, the combination treatment decreased the expression of MMP-9 protein by 90%. These findings suggest that the combination inhibited tumor growth in a multi-pronged manner through decreasing proliferation, inducing apoptosis and blocking tumor angiogenesis and metastasis.
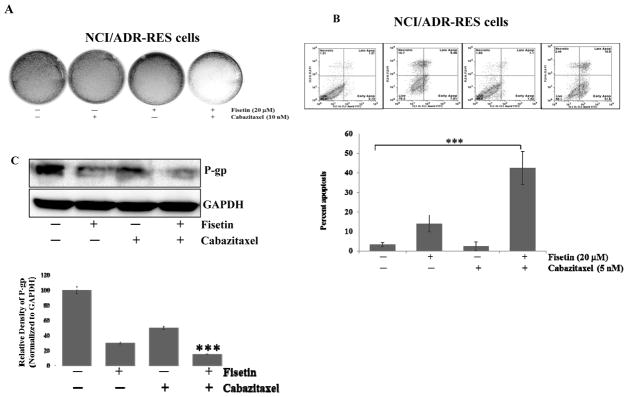
Effect of treatment with fisetin, cabazitaxel and their combination on cellular proliferation and apoptosis in drug resistant NCI/ADR-RES cells
One of the widely investigated mechanisms of taxane resistance is increased efflux of drug via the P-glycoprotein (gp) pump. Cancer cells with multi-drug resistance (MDR1) gene expression reduce the intracellular concentration of taxanes by increasing drug efflux through P-gp. We examined the susceptibility to fisetin and cabazitaxel treatment in a P-gp-overexpressing NCI/ADR-RES cell line. We previously reported (25) that treatment of NCI/ADR-RES cells with fisetin results in decreased cell viability and colony formation. We investigated whether fisetin could sensitize this resistant cancer cell line to cabazitaxel treatment. As shown in figure 7A, combined fisetin and cabazitaxel treatment significantly inhibited colony formation of these cells as compared with either agent alone.
Figure 7. Effect of treatment with fisetin, cabazitaxel and their combination on on colony formation, apoptosis and P-gp in drug resistant NCI/ADR-RES cells.
A. Representative photographs showing the effect of fisetin treatment on NCI/ADR-RES cell growth investigated by monolayer colony formation assay. B. The induction of apoptosis in NCI/ADR-RES cells was assessed by flow cytometry using a FITC conjugated Annexin V (x-axis) marker coupled with propidium iodide (PI, y-axis) exclusion, below, quantitation of apoptosis ***, p ≤ 0.001. C. Representative blots showing the effect of fisetin and cabazitaxel treatment on P-gp activity. Data are shown as representative of three independent experiments. GAPDH served as a loading control. Bands were measured using densitometry and values first normalized to respective GAPDH bands and then reported below each gel as relative to control. Data are shown as representative of three independent experiments for each assay.
We compared the induction of apoptosis by fisetin (20 μM), cabazitaxel (5 nM), and the two drugs in the combination in NCI/ADR-RES cells. Cells treated with fisetin and then cabazitaxel were more sensitive and underwent apoptosis (42.5%) more than cells that were treated with cabazitaxel (3.37%) or fisetin (14%) alone (Fig. 7B), these results support the suggestion that fisetin is a key contributor to the cytotoxic effects of cabazitaxel treatment in NCI/ADR-RES cells. Fisetin decreased the expression of P-gp, which would suggest decreased ability of these cells to actively efflux drugs. These findings highlight the novel effect of fisetin as a microtubule-stabilizing agent and provide evidence that fisetin could be further developed to sensitize resistant cancer cells and used in combination with cabazitaxel for therapy of PCa and potentially other cancer types. Regulation of P-gp is critical to the maintenance of intracellular concentration of taxanes and regulating drug efflux. We asked whether fisetin treatment down regulated P-gp expression in response to cabazitaxel treatment. Marked down regulation of P-gp protein is shown in figure 7C in response to the combination treatment of the NCI/ADR-RES cells. These data support the conclusion that fisetin treatment renders NCI/ADR-RES cells more sensitive to cabazitaxel treatment.
DISCUSSION
Taxanes have proven to be one of the best anti-tumor agents in the clinic. Their enhanced clinical activity against a variety of human malignancies has encouraged scientists to continue investigation of this class of drugs. However, their inherent shortcomings in terms of both toxicity and resistance quickly become apparent. Continued investigation into the development and discovery of new drugs, and exploring new treatment strategies that reduce side effects and circumvent drug resistance may provide more effective therapeutic options for cancer patients. Considering these facts we believe that natural agents from dietary sources such as fisetin could offer a safe and effective alternative to existing repertoire of microtubule-targeting agents in combination with standard of care.
In line with this, our study was designed to investigate the effect of a combination of fisetin and cabazitaxel to achieve maximum therapeutic benefit with the intention that this approach would eventually reduce drug dose and toxicity, and minimize or delay the induction of drug resistance. In this study, we demonstrate the effect of fisetin alone, and in combination with cabazitaxel in-vitro in cell culture and in-vivo in a subcutaneous xenograft mouse model. Combination-mediated antitumor activity was associated with both inhibition of proliferation and promotion of apoptosis. Furthermore, combination resulted in inhibition of tumor invasion and metastasis. These results provide evidence that (i) fisetin may have therapeutic benefit for patients with PCa through enhancing the efficacy of cabazitaxel, (ii) fisetin enhances the efficacy of cabazitaxel-induced apoptosis in androgen-dependent and androgen-independent PCa cells and (iii) several potential cellular and molecular mechanisms inhibit the proliferation of tumor, block tumor angiogenesis, coupled with induction of apoptosis.
The addition of fisetin to cabazitaxel resulted in enhanced anti-cancer activity in both 22Rν1and C4-2 cells and the metastatic PC-3M-luc-C6 cells. Taxanes are known to mediate their anti-cancer activity through 2 key mechanisms: (i) the inhibition of microtubule depolymerization and (ii) the attenuation of the anti-apoptotic activity of BCL-2 and BCL-XL (34). Cabazitaxel-mediated inhibition of microtubule depolymerization induces cell-cycle arrest at G2/M phase. We reported that fisetin is a microtubule stabilizing agent that binds to β-tubulin and significantly affects microtubule dynamics (25). In addition, we reported that fisetin inhibits cellular proliferation, migration and invasion of PCa cells (25). This suggested that combination of two agents may complement each other and result in enhanced anti-cancer activity. We concluded from our docking analysis that fisetin localizes within the taxol binding pocket (25). It remains unclear whether fisetin competes with other taxanes binding to microtubules. Further elucidation of these differences will validate these findings. Although both agents enhanced the polymerization of purified tubulin, their mechanisms of action differ. Fisetin acts as a potent anti-proliferative agent, whereas, cabazitaxel is a potent apoptotic agent. A combination of both agents induced an enhanced effect. This implies that fisetin or cabazitaxel have additional targets that are independent of tubulin binding. This finding is in agreement with a previous study that observed synergistic interaction between taxol and discodermolide in four human carcinoma cell lines. However, in that study both agents were microtubule stabilizing agents; formed polymers that are stable to cold and calcium; and caused cell cycle arrest. In addition, discodermolide competitively inhibits the binding of paclitaxel to tubulin polymers (35).
Tumor growth is composed of a balance between cell proliferation and cell apoptosis. These activities are regulated by many factors such as the BCL-2 family, Bax and PARP. It is well-known that fisetin interacts with many different signaling cascades to regulate critical cellular processes (24,36). Fisetin has been reported to induce cell-cycle arrest (37), induce apoptosis via inhibiting anti-apoptotic proteins, and impair phosphorylation of MEK and AKT in various cancer cells (23). Several preclinical and clinical trials have reported on the promising combined treatment of microtubule-stabilizing agents with mTOR inhibitors, and reported enhanced activity linked to increased tumor cell apoptosis (38–41). A study reported that the addition of a LY294002, a PI3K inhibitor, to tamoxifen and everolimus, an mTOR inhibitor, improved the antitumor effect compared with tamoxifen alone or the other two agents in combination. The triple treatment had the greatest efficacy in inhibiting MCF-7 tumor growth and angiogenesis (42). Likewise, fisetin is known as a dual inhibitor of both PI3K/Akt pathways (28), and also an inhibitor of the mTOR pathway (22,37). Our results suggest that the anti-cancer effects of the combination treatment in PCa involve multiple mechanisms that culminate in an overall inhibition of tumor growth through both inhibition of proliferation and induction of apoptosis. In terms of affecting apoptosis, our results showed that fisetin and cabazitaxel combination is associated with an increased pro-apoptotic/anti-apoptotic ratio that favors apoptosis.
Multiple signaling pathways provide crosstalk between the epithelial and the stromal compartments to enhance tumor growth, including androgen receptor (AR) signaling. Disrupting this “two-compartment” crosstalk has led to the development of drugs that target tumor stromal elements in addition to the cancer cells. Impairment of microtubule function leads to decreased AR nuclear translocation (43). This mechanism is independent of taxane-induced mitotic arrest and could provide an alternative mechanism of drug action that could explain its clinical activity.
Our laboratory also reported fisetin inhibits AR signaling in androgen-dependent PCa cells (22). The strong in-vivo treatment responses observed in our study demonstrate that the combination inhibited AR signaling, that is known to promote tumor growth. Chien CS et al reported that fisetin has inhibitory effects on the adhesion, migration, and invasion ability of a highly metastatic PC-3 cells. Further results from this study demonstrated that fisetin inhibited MMP-2 and MMP-9 expression and activity and through suppressing PI3K/Akt and JNK signaling pathways. This suggested that fisetin can serve as a potential candidate for treating cancer metastasis (44).
Tumor-induced angiogenesis is a major contributor to tumor growth. Therefore, blocking angiogenesis is an archetypal stromal-targeting strategy that has proven to be successful in treating a variety of different metastatic tumor types (45). Study by Broggini-Tenzer. et al demonstrated that specific down-regulation of proangiogenic VEGF signaling by mTOR inhibition or VEGF neutralization with clinically approved agents strongly resensitized patupilone-resistant lung-adenocarcinoma cell-derived tumors to patupilone (14). Likewise, taxane-resistant colon carcinomas, which are MDR overexpressing, were resensitized upon VEGF deprivation (46). Similarly, fisetin has been reported to inhibit the angiogenesis of many tumors (47). Our results suggest that fisetin and cabazitaxel treatment reduce microvessel density through down regulation of VEGF and CD31 activity. We speculate that fisetin could have inhibited angiogenesis through decreasing NF-kB signaling, which is known to contribute to angiogenesis. This is consistent with previous results from our laboratory that showed that fisetin induced apoptosis and decreased invasion of chemoresistant AsPC-1 pancreatic cancer cells through suppression of DR3-mediated NF-κB activation (48). In addition, our laboratory also provided evidence that fisetin can induce apoptosis and suppress the growth of colon cancer cells by inhibition of COX2 and Wnt/EGFR/NF-κB-signaling pathways (49). Another study reported that fisetin was potent in suppressing tumor necrosis factor (TNF)-induced NF-κB activation. The expression of NF-κB-regulated gene products involved in anti-apoptosis such as Bcl-2, Bcl-xL, XIAP and Survivin, proliferation such as cyclin D1, c-Myc, COX-2; invasion such as MMP-9 and angiogenesis such as VEGF were also down-regulated by fisetin (50). Our observations support that the combination treatment affects tumor vasculature. Therefore, the suppression of tumor growth and metastasis occurred not only through the inhibition of proliferative activity of tumor cells but also through inhibition of microvessel density. These experiments have several limitations. We used subcutaneous not orthotropic injection to model soft tissue growth. Although orthotropic injection is ideal, it is challenging to model, as mice have four biologically different and anatomically separated lobes of prostate. It is not clearly defined how well any of them recapitulates the human prostate.
In Summary, this research identified that fisetin enhanced cabazitaxel-mediated cytotoxicity suggesting that fisetin could be developed as a safe and effective agent to improve cabazitaxel chemotherapy in PCa and potentially other cancer types.
Supplementary Material
Acknowledgments
Financial support: This work was supported by the United States Public Health Service Grants R01CA160867 and R01CA160867S1 to HM
We would like to thank the Biological Testing Branch of National Cancer Institute for providing the drug resistant NCI/ADR-RES cell line.
Footnotes
Conflict of interest: All authors declare no conflict of interest
References
- 1.Brawley OW. Prostate cancer epidemiology in the united states. World journal of urology. 2012;30:195–200. doi: 10.1007/s00345-012-0824-2. [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA: a cancer journal for clinicians. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 3.Yap TA, Zivi A, Omlin A, de Bono JS. The changing therapeutic landscape of castration-resistant prostate cancer. Nature reviews Clinical oncology. 2011;8:597–610. doi: 10.1038/nrclinonc.2011.117. [DOI] [PubMed] [Google Scholar]
- 4.Mukhtar E, Adhami VM, Mukhtar H. Targeting microtubules by natural agents for cancer therapy. Molecular cancer therapeutics. 2014;13:275–84. doi: 10.1158/1535-7163.MCT-13-0791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tannock IF, de Wit R, Berry WR, Horti J, Pluzanska A, Chi KN, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. The New England journal of medicine. 2004;351:1502–12. doi: 10.1056/NEJMoa040720. [DOI] [PubMed] [Google Scholar]
- 6.Petrylak DP, Tangen CM, Hussain MH, Lara PN, Jr, Jones JA, Taplin ME, et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. The New England journal of medicine. 2004;351:1513–20. doi: 10.1056/NEJMoa041318. [DOI] [PubMed] [Google Scholar]
- 7.Pienta KJ, Smith DC. Advances in prostate cancer chemotherapy: A new era begins. CA: a cancer journal for clinicians. 2005;55:300–18. doi: 10.3322/canjclin.55.5.300. quiz 23-5. [DOI] [PubMed] [Google Scholar]
- 8.Paller CJ, Antonarakis ES. Cabazitaxel: A novel second-line treatment for metastatic castration-resistant prostate cancer. Drug design, development and therapy. 2011;5:117–24. doi: 10.2147/DDDT.S13029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Narayanan BA, Narayanan NK, Pittman B, Reddy BS. Regression of mouse prostatic intraepithelial neoplasia by nonsteroidal anti-inflammatory drugs in the transgenic adenocarcinoma mouse prostate model. Clinical cancer research: an official journal of the American Association for Cancer Research. 2004;10:7727–37. doi: 10.1158/1078-0432.CCR-04-0732. [DOI] [PubMed] [Google Scholar]
- 10.McCarty MF. Targeting multiple signaling pathways as a strategy for managing prostate cancer: Multifocal signal modulation therapy. Integrative cancer therapies. 2004;3:349–80. doi: 10.1177/1534735404270757. [DOI] [PubMed] [Google Scholar]
- 11.Mukhtar H, Ahmad N. Cancer chemoprevention: Future holds in multiple agents. Toxicology and applied pharmacology. 1999;158:207–10. doi: 10.1006/taap.1999.8721. [DOI] [PubMed] [Google Scholar]
- 12.Adhami VM, Malik A, Zaman N, Sarfaraz S, Siddiqui IA, Syed DN, et al. Combined inhibitory effects of green tea polyphenols and selective cyclooxygenase-2 inhibitors on the growth of human prostate cancer cells both in vitro and in vivo. Clinical cancer research: an official journal of the American Association for Cancer Research. 2007;13:1611–9. doi: 10.1158/1078-0432.CCR-06-2269. [DOI] [PubMed] [Google Scholar]
- 13.Zhang S, Wang Y, Chen Z, Kim S, Iqbal S, Chi A, et al. Genistein enhances the efficacy of cabazitaxel chemotherapy in metastatic castration-resistant prostate cancer cells. The Prostate. 2013;73:1681–9. doi: 10.1002/pros.22705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Broggini-Tenzer A, Sharma A, Nytko KJ, Bender S, Vuong V, Orlowski K, et al. Combined treatment strategies for microtubule stabilizing agent-resistant tumors. Journal of the National Cancer Institute. 2015:107. doi: 10.1093/jnci/dju504. [DOI] [PubMed] [Google Scholar]
- 15.Velmurugan B, Mani A, Nagini S. Combination of s-allylcysteine and lycopene induces apoptosis by modulating bcl-2, bax, bim and caspases during experimental gastric carcinogenesis. European journal of cancer prevention: the official journal of the European Cancer Prevention Organisation. 2005;14:387–93. doi: 10.1097/00008469-200508000-00012. [DOI] [PubMed] [Google Scholar]
- 16.Tortora G, Caputo R, Damiano V, Caputo R, Troiani T, Veneziani BM, et al. Combined targeted inhibition of bcl-2, bcl-xl, epidermal growth factor receptor, and protein kinase a type i causes potent antitumor, apoptotic, and antiangiogenic activity. Clinical cancer research: an official journal of the American Association for Cancer Research. 2003;9:866–71. [PubMed] [Google Scholar]
- 17.Khor TO, Keum YS, Lin W, Kim JH, Hu R, Shen G, et al. Combined inhibitory effects of curcumin and phenethyl isothiocyanate on the growth of human pc-3 prostate xenografts in immunodeficient mice. Cancer research. 2006;66:613–21. doi: 10.1158/0008-5472.CAN-05-2708. [DOI] [PubMed] [Google Scholar]
- 18.Yamamoto Y, Lin PJ, Beraldi E, Zhang F, Kawai Y, Leong J, et al. Sirna lipid nanoparticle potently silences clusterin and delays progression when combined with androgen receptor cotargeting in enzalutamide-resistant prostate cancer. Clinical cancer research: an official journal of the American Association for Cancer Research. 2015;21:4845–55. doi: 10.1158/1078-0432.CCR-15-0866. [DOI] [PubMed] [Google Scholar]
- 19.Weiss LM, Hugle M, Romero S, Fulda S. Synergistic induction of apoptosis by a polo-like kinase 1 inhibitor and microtubule-interfering drugs in ewing sarcoma cells. International journal of cancer. 2016;138:497–506. doi: 10.1002/ijc.29725. [DOI] [PubMed] [Google Scholar]
- 20.Cui D, Dai J, Keller JM, Mizokami A, Xia S, Keller ET. Notch pathway inhibition using pf-03084014, a gamma-secretase inhibitor (gsi), enhances the antitumor effect of docetaxel in prostate cancer. Clinical cancer research: an official journal of the American Association for Cancer Research. 2015;21:4619–29. doi: 10.1158/1078-0432.CCR-15-0242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Babcook MA, Shukla S, Fu P, Vazquez EJ, Puchowicz MA, Molter JP, et al. Synergistic simvastatin and metformin combination chemotherapy for osseous metastatic castration-resistant prostate cancer. Molecular cancer therapeutics. 2014;13:2288–302. doi: 10.1158/1535-7163.MCT-14-0451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khan N, Asim M, Afaq F, Abu Zaid M, Mukhtar H. A novel dietary flavonoid fisetin inhibits androgen receptor signaling and tumor growth in athymic nude mice. Cancer research. 2008;68:8555–63. doi: 10.1158/0008-5472.CAN-08-0240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Adhami VM, Syed DN, Khan N, Mukhtar H. Dietary flavonoid fisetin: A novel dual inhibitor of pi3k/akt and mtor for prostate cancer management. Biochemical pharmacology. 2012;84:1277–81. doi: 10.1016/j.bcp.2012.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mukhtar E, Adhami VM, Khan N, Mukhtar H. Apoptosis and autophagy induction as mechanism of cancer prevention by naturally occurring dietary agents. Current drug targets. 2012;13:1831–41. doi: 10.2174/138945012804545489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mukhtar E, Adhami VM, Sechi M, Mukhtar H. Dietary flavonoid fisetin binds to beta-tubulin and disrupts microtubule dynamics in prostate cancer cells. Cancer letters. 2015;367:173–83. doi: 10.1016/j.canlet.2015.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Haddad AQ, Fleshner N, Nelson C, Saour B, Musquera M, Venkateswaran V, et al. Antiproliferative mechanisms of the flavonoids 2,2′-dihydroxychalcone and fisetin in human prostate cancer cells. Nutrition and cancer. 2010;62:668–81. doi: 10.1080/01635581003605524. [DOI] [PubMed] [Google Scholar]
- 27.Suh Y, Afaq F, Khan N, Johnson JJ, Khusro FH, Mukhtar H. Fisetin induces autophagic cell death through suppression of mtor signaling pathway in prostate cancer cells. Carcinogenesis. 2010;31:1424–33. doi: 10.1093/carcin/bgq115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Khan N, Afaq F, Khusro FH, Mustafa Adhami V, Suh Y, Mukhtar H. Dual inhibition of phosphatidylinositol 3-kinase/akt and mammalian target of rapamycin signaling in human nonsmall cell lung cancer cells by a dietary flavonoid fisetin. International journal of cancer. 2012;130:1695–705. doi: 10.1002/ijc.26178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Klimaszewska-Wisniewska A, Halas-Wisniewska M, Tadrowski T, Gagat M, Grzanka D, Grzanka A. Paclitaxel and the dietary flavonoid fisetin: A synergistic combination that induces mitotic catastrophe and autophagic cell death in a549 non-small cell lung cancer cells. Cancer cell international. 2016;16:10. doi: 10.1186/s12935-016-0288-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chou TC. Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharmacological reviews. 2006;58:621–81. doi: 10.1124/pr.58.3.10. [DOI] [PubMed] [Google Scholar]
- 31.Aalinkeel R, Nair BB, Reynolds JL, Sykes DE, Mahajan SD, Chadha KC, et al. Overexpression of mmp-9 contributes to invasiveness of prostate cancer cell line lncap. Immunological investigations. 2011;40:447–64. doi: 10.3109/08820139.2011.557795. [DOI] [PubMed] [Google Scholar]
- 32.Shariat SF, Roehrborn CG, McConnell JD, Park S, Alam N, Wheeler TM, et al. Association of the circulating levels of the urokinase system of plasminogen activation with the presence of prostate cancer and invasion, progression, and metastasis. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2007;25:349–55. doi: 10.1200/JCO.2006.05.6853. [DOI] [PubMed] [Google Scholar]
- 33.Boxler S, Djonov V, Kessler TM, Hlushchuk R, Bachmann LM, Held U, et al. Matrix metalloproteinases and angiogenic factors: Predictors of survival after radical prostatectomy for clinically organ-confined prostate cancer? The American journal of pathology. 2010;177:2216–24. doi: 10.2353/ajpath.2010.091190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pienta KJ. Preclinical mechanisms of action of docetaxel and docetaxel combinations in prostate cancer. Seminars in oncology. 2001;28:3–7. doi: 10.1016/s0093-7754(01)90148-4. [DOI] [PubMed] [Google Scholar]
- 35.Martello LA, McDaid HM, Regl DL, Yang CP, Meng D, Pettus TR, et al. Taxol and discodermolide represent a synergistic drug combination in human carcinoma cell lines. Clinical cancer research: an official journal of the American Association for Cancer Research. 2000;6:1978–87. [PubMed] [Google Scholar]
- 36.Syed DN, Adhami VM, Khan N, Khan MI, Mukhtar H. Exploring the molecular targets of dietary flavonoid fisetin in cancer. Seminars in cancer biology. 2016 doi: 10.1016/j.semcancer.2016.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Khan N, Afaq F, Syed DN, Mukhtar H. Fisetin, a novel dietary flavonoid, causes apoptosis and cell cycle arrest in human prostate cancer lncap cells. Carcinogenesis. 2008;29:1049–56. doi: 10.1093/carcin/bgn078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Miller K, Wang M, Gralow J, Dickler M, Cobleigh M, Perez EA, et al. Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. The New England journal of medicine. 2007;357:2666–76. doi: 10.1056/NEJMoa072113. [DOI] [PubMed] [Google Scholar]
- 39.Faried LS, Faried A, Kanuma T, Nakazato T, Tamura T, Kuwano H, et al. Inhibition of the mammalian target of rapamycin (mtor) by rapamycin increases chemosensitivity of caski cells to paclitaxel. European journal of cancer. 2006;42:934–47. doi: 10.1016/j.ejca.2005.12.018. [DOI] [PubMed] [Google Scholar]
- 40.Zhou Q, Wong CH, Lau CP, Hui CW, Lui VW, Chan SL, et al. Enhanced antitumor activity with combining effect of mtor inhibition and microtubule stabilization in hepatocellular carcinoma. International journal of hepatology. 2013;2013:103830. doi: 10.1155/2013/103830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sun JM, Kim JR, Do IG, Lee SY, Lee J, Choi YL, et al. A phase-1b study of everolimus plus paclitaxel in patients with small-cell lung cancer. British journal of cancer. 2013;109:1482–7. doi: 10.1038/bjc.2013.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen X, Zhao M, Hao M, Sun X, Wang J, Mao Y, et al. Dual inhibition of pi3k and mtor mitigates compensatory akt activation and improves tamoxifen response in breast cancer. Molecular cancer research: MCR. 2013;11:1269–78. doi: 10.1158/1541-7786.MCR-13-0212. [DOI] [PubMed] [Google Scholar]
- 43.Madan RA, Pal SK, Sartor O, Dahut WL. Overcoming chemotherapy resistance in prostate cancer. Clinical cancer research: an official journal of the American Association for Cancer Research. 2011;17:3892–902. doi: 10.1158/1078-0432.CCR-10-2654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chien CS, Shen KH, Huang JS, Ko SC, Shih YW. Antimetastatic potential of fisetin involves inactivation of the pi3k/akt and jnk signaling pathways with downregulation of mmp-2/9 expressions in prostate cancer pc-3 cells. Molecular and cellular biochemistry. 2010;333:169–80. doi: 10.1007/s11010-009-0217-z. [DOI] [PubMed] [Google Scholar]
- 45.Kerbel R, Folkman J. Clinical translation of angiogenesis inhibitors. Nature reviews Cancer. 2002;2:727–39. doi: 10.1038/nrc905. [DOI] [PubMed] [Google Scholar]
- 46.Laplante A, Demeule M, Murphy GF, Beliveau R. Interaction of immunosuppressive agents rapamycin and its analogue sdz-rad with endothelial p-gp. Transplantation proceedings. 2002;34:3393–5. doi: 10.1016/s0041-1345(02)03658-8. [DOI] [PubMed] [Google Scholar]
- 47.Bhat TA, Nambiar D, Pal A, Agarwal R, Singh RP. Fisetin inhibits various attributes of angiogenesis in vitro and in vivo--implications for angioprevention. Carcinogenesis. 2012;33:385–93. doi: 10.1093/carcin/bgr282. [DOI] [PubMed] [Google Scholar]
- 48.Murtaza I, Adhami VM, Hafeez BB, Saleem M, Mukhtar H. Fisetin, a natural flavonoid, targets chemoresistant human pancreatic cancer aspc-1 cells through dr3-mediated inhibition of nf-kappab. International journal of cancer. 2009;125:2465–73. doi: 10.1002/ijc.24628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Suh Y, Afaq F, Johnson JJ, Mukhtar H. A plant flavonoid fisetin induces apoptosis in colon cancer cells by inhibition of cox2 and wnt/egfr/nf-kappab-signaling pathways. Carcinogenesis. 2009;30:300–7. doi: 10.1093/carcin/bgn269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sung B, Pandey MK, Aggarwal BB. Fisetin, an inhibitor of cyclin-dependent kinase 6, down-regulates nuclear factor-kappab-regulated cell proliferation, antiapoptotic and metastatic gene products through the suppression of tak-1 and receptor-interacting protein-regulated ikappabalpha kinase activation. Molecular pharmacology. 2007;71:1703–14. doi: 10.1124/mol.107.034512. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.