Abstract
Low muscle oxidative capacity contributes to exercise intolerance in chronic obstructive pulmonary disease (COPD). Near-infrared spectroscopy (NIRS) allows non-invasive determination of the muscle oxygen consumption recovery rate constant (k), which is proportional to oxidative capacity assuming two conditions are met: 1) exercise intensity is sufficient to fully-activate mitochondrial oxidative enzymes; 2) sufficient O2 availability. We aimed to determine reproducibility (coefficient of variation, CV; intraclass correlation coefficient, ICC) of NIRS k assessment in the gastrocnemius of 64 participants with (FEV1 64±23%predicted) or without COPD (FEV1 98±14%predicted). 10–15s dynamic contractions preceded 6min of intermittent arterial occlusions (5–10s each, ~250mmHg) for k measurement. k was lower (P<0.05) in COPD (1.43±0.4min−1; CV=9.8±5.9%, ICC=0.88) than controls (1.74±0.69min−1; CV=9.9±8.4%; ICC=0.93). Poor k reproducibility was more common when post-contraction and deoxygenation were low, suggesting insufficient exercise intensity for mitochondrial activation and/or the NIRS signal contained little light reflected from active muscle. The NIRS assessment was well tolerated and reproducible for muscle dysfunction evaluation in COPD.
Keywords: Skeletal muscle, Mitochondria, Exercise intolerance, Oxygen consumption, Kinetics, Quality-control
1. INTRODUCTION
Chronic obstructive pulmonary disease (COPD) is characterized by dyspnea on exertion, with subsequent reduced exercise tolerance and quality of life. Skeletal muscle dysfunction is a systemic consequence of COPD that also contributes to increased morbidity and mortality in this population (Agustí et al., 2003; Casaburi, 2001; Decramer et al., 2008; Maltais et al., 2000, 2014; Nici, 2000; Vogiatzis and Zakynthinos, 2012; Wouters et al., 2002). Morphological and structural skeletal muscle alterations in COPD are especially prevalent in the locomotor muscles, and include atrophy and weakness, loss of type I fibers, loss of muscle oxidative capacity and mitochondrial dysfunction, among others (Allaire et al., 2004; Coronell et al., 2004; Couillard and Prefaut, 2005; Engelen et al., 2000; Gosker et al., 2002, 2007; Maltais et al., 2014; Picard et al., 2008; Whittom et al., 1998). Amelioration of these muscular alterations contributes to the substantial benefits of pulmonary rehabilitation in COPD patients (Maltais et al., 2014).
The prevalence and progression of the loss of muscle oxidative phenotype in relation to disease severity is still unclear, and this is partly because measurement of muscle oxidative capacity requires an invasive biopsy or complex 31P magnetic resonance spectroscopy assessments. In review, Meyer et al. (2013) showed that low muscle oxidative capacity and increased reactive oxygen species production was evident in skeletal muscle across all spirometric stages of COPD disease severity. Furthermore, Natanek et al. (2013) showed wide heterogeneity in quadriceps type I fiber expression in 114 COPD patients evenly distributed across GOLD stages 2–4. These findings demonstrate that muscle oxidative capacity appears to be highly variable across disease severity, which underscores the need for simple methods to assess changes in muscle oxidative capacity in COPD patients independent from systemic effects of the disease.
We aimed to address this using a non-invasive method based on near-infrared spectroscopy (NIRS; Motobe et al., 2004; Ryan et al., 2012). This technique provides measurement of the recovery rate constant (k) of muscle oxygen consumption , isolated from influences of circulatory or pulmonary function, and which is directly related to muscle oxidative capacity in single muscle fibers (r2=0.77; Wüst et al., 2013). Muscle k can be assessed by NIRS during ~6 minutes of recovery from brief contractions, using a series of intermittent arterial occlusions (5–10 s each); during occlusions, the rate of decline in the muscle tissue saturation index (TSI) is directly proportional to . This technique has been validated in young healthy subjects against phosphocreatine recovery kinetics and quadriceps muscle biopsy (Ryan et al., 2013, 2014). It has also been used to assess muscle oxidative capacity in spinal cord injury (Erickson et al., 2013), amyotrophic lateral sclerosis (Ryan et al., 2014) and chronic heart failure (Southern et al., 2014), among other conditions. However, to our knowledge, this technique has not been applied in COPD where muscle morphologic adaptations such as fat infiltration, fibrosis, inflammation, increased subcutaneous adipose, loss of type I fibers and mitochondrial density (Maltais et al., 2014) may hamper NIRS measurement of muscle oxidative capacity.
The method relies on two competing assumptions: 1) that exercise is sufficiently intense to maximally activate mitochondrial oxidative enzymes and elicit a sufficient increase in (Korzeniewski and Rossiter, 2015; Wüst et al., 2011, 2013); 2) that O2 delivery is not limiting to k (Haseler et al., 2004). This latter condition is especially important in COPD where poor systemic O2 delivery, muscle capillary rarefaction and brief arterial occlusions may combine to reduce TSI below some critical threshold, thereby slowing recovery kinetics. Test-retest reliability (intraclass correlation coefficient, ICC) of k in healthy subjects ranges from 0.26 to 0.68 (Ryan et al., 2012; Southern et al., 2014), and whether reliable measurements are possible in COPD is currently unknown. This is particularly important in relation to the expected effect magnitude of oxidative capacity loss in COPD (~10–50%; Meyer et al., 2013). Therefore, we aimed to determine the reliability of NIRS assessment of gastrocnemius muscle oxidative capacity in smokers with and without COPD. We hypothesized that test-retest variability in k would be sufficiently low to allow NIRS estimates of oxidative capacity to be a useful method to detect COPD-related loss. Secondly, we aimed to identify correlates of high variability in repeated k measurement, if it occurred. These correlates may provide a basis for quality control of the NIRS muscle assessment.
2. MATERIALS AND METHODS
2.1 Participants
Both smoking (Montes de Oca et al., 2008) and COPD (Maltais et al., 2014) have each been implicated in COPD-associated muscle dysfunction. Therefore, to account for the independent influence of smoking history, we sought current and former smokers with at least 10 pack-year smoking history to volunteer: 32 COPD patients (GOLD stage 1–4, defined by the criteria for the Global initiative for Chronic Obstructive Lung Disease) and 28 participants with normal spirometry (CON) (Table 1). This was an ancillary study of COPDGene (ClinicalsTrials.gov Identifier NCT00608764), for which a complete list of inclusion and exclusion criteria is given in Regan et al. (2010). Participants were informed about the procedures and risks associated with the study, and gave written informed consent. The study was approved by the Institutional Review Board of Los Angeles Biomedical Research Institute at Harbor-UCLA Medical Center, in accordance with the Declaration of Helsinki.
Table 1.
Participant characteristics
| COPD | CON | |
|---|---|---|
| Characteristics | ||
| N. | 28 | 28 |
| Age (yrs) | 65 (±8) | 60 (±7)* |
| Weight (kg) | 76 (±15) | 79 (±17) |
| Height (cm) | 171 (±11) | 170 (±8) |
| BMI (kg/m2) | 26 (±5) | 27 (±5) |
| Gender (M/F) | 17/11 | 16/12 |
| Race (AA/NHW) | 5/23 | 15/13 |
| FVC (L) | 3.3 (±0.9) | 3.5 (±0.8) |
| FEV1 (L) | 1.8 (±0.7) | 2.8 (±0.6)* |
| FEV1 %pred | 63.9 (±23.4) | 97.9 (±13.6)* |
| SpO2 (%) | 97 (±1.6) | 98 (±1.1) |
| GOLD stage N. (1/2/3/4) | 7/13/5/3 | |
| Resting Muscle Characteristics | ||
| Saturation (TSI) (%) | 66 (±6) | 68 (±5) |
| ATT (mm) | 2.3 (±1.9)¶ | 2.8 (±1.9)♮ |
Data are mean (±SD). CON = controls; BMI = body mass index; M = male; F = female; AA = African American; NHW = Non Hispanic White; FVC = forced vital capacity; FEV1 = forced expiratory volume in 1 second; SpO2 = arterial oxygen saturation; TSI = tissue saturation index; ATT = adipose tissue thickness
p ≤ 0.01 vs. COPD patients;
= COPD n=21;
= CON n=18
2.2 Protocol
Each participant visited the laboratory once, during which NIRS muscle oxidative capacity and spirometry tests were performed.
2.2.1 NIRS muscle oxidative capacity test
A wireless, portable, continuous-wave, spatially-resolved spectroscopy (SRS) NIRS device (PortaMon, Artinis, The Netherlands) was used to measure relative concentrations of deoxy-hemoglobin and deoxy-myoglobin (here termed HHb for simplicity) and oxy-hemoglobin and oxy-myoglobin (HbO2) in the tissues ~1.5 cm beneath the probe (interoptode distance was 3 cm). From these measurements relative changes in total hemoglobin and myoglobin (THb = HHb+HbO2) and the Hb difference (Hbdiff = HbO2−HHb) were calculated. In addition, the tissue saturation index (TSI, %) was measured using the SRS approach (using interoptode distances of 2–3 cm) (Ferrari et al., 2004).
A modified NIRS protocol based on Ryan et al. (2012) was used. The participant lay supine and the NIRS probe was wrapped in plastic film, placed longitudinally on the belly of the right medial gastrocnemius, and secured with an elastic bandage. A 13 × 85 cm rapid-inflation pressure-cuff (SC12D, Hokanson, USA) was placed on the proximal thigh of the same leg and attached to an electronically-controlled rapid cuff-inflator (E20, Hokanson, USA). A pad was placed under the ankle such that the lower leg and NIRS probe was suspended above the bed. During the ~30 min assessment, the participant was asked to relax and refrain from moving the leg except when instructed.
Initially, the participant was familiarized with the execution of cyclical plantar-flexion/relaxation exercise at ~1Hz, to activate the medial gastrocnemius against a manually applied resistance, and with the rapid-cuff inflation procedures. Repeated cuff inflations from low (~50 mmHg) to high (~250 mmHg) pressures were performed during this familiarization phase. Arterial occlusion was determined from a tolerated cuff-pressure within the range of 230–300 mmHg (236±17 mmHg) that resulted in HHb rise, HbO2 fall and approximately constant THb over ~15–20 s.
The measurement protocol began after 2–3 min of rest, where baseline TSI and SpO2 at a fingertip (Rad-5 Pulse Oximeter MasimoSET®, Masimo Co., Irvine, CA) were measured over 2 min. Subsequently, after having removed the pulse oximeter, the participant was instructed to execute 10–12 cycles of plantar-flexion exercise, followed immediately by arterial occlusion until a steady-state in TSI was reached (mean duration ~90 s; Figure 1). The cuff was then instantly deflated and muscle reoxygenation was recorded until a steady-state was reached (typically ~3 min). This procedure (the physiologic normalization, PN) identified the functional range of TSI under resting conditions from TSImin at the end of the sustained arterial occlusion to TSImax at the peak of the reactive hyperemia (Figure 1). Finally, the participant performed two oxidative capacity assessments. These consisted of: 1) cyclical plantar-flexion exercise to desaturate the muscle to a target of 50% of the PN amplitude (typically 10–15 s of contractions) (Hamaoka et al., 2007; McKully et al., 1994; Motobe et al., 2004; Ryan et al., 2012) (Figure 1); 2) a series of intermittent arterial occlusions (AO; 5 occlusions for 5 s, and 10 for 10 s, each separated by 5–20 s recovery). A single oxidative capacity assessment lasted ~6 minutes. The second repetition was conducted once a resting steady state was re-established (typically ~1 min).
Figure 1. Tissue saturation index (TSI, %) changes during the NIRS muscle assessment.
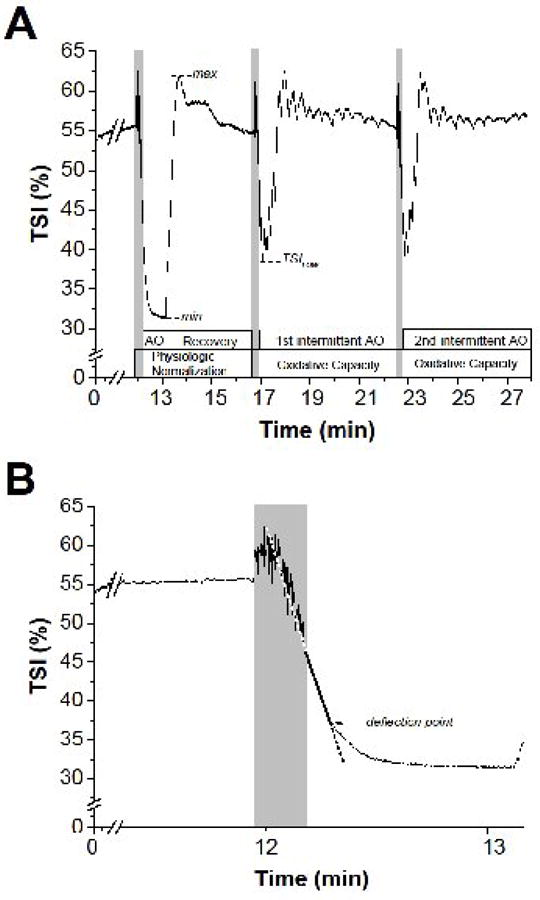
Panel A. Protocol phases, and the parameter calculated from the analyses of TSI signal changes, are indicated at the bottom of the graph. Grey shading indicates brief dynamic plantar-flexion exercise. AO = arterial occlusion; max and min = highest and lowest TSI values during the Physiologic Normalization (PN) phase; TSILOW = lowest saturation value reached during oxidative capacity assessments (for further details see Methods). Panel B. Expansion of panel A to illustrate the linear regression to determine the deflection point of muscle TSI (arrow) during the sustained arterial occlusion (AO). Grey shading indicates brief dynamic plantar-flexion exercise.
At the end of the procedure the skinfold at the NIRS site was measured to estimate adipose tissue thickness (ATT, mm) (Lange Skinfold Caliper, Beta Technology Inc., Santa Cruz, CA).
2.2.2 Spirometry
Approximately 15 minutes before spirometric testing, participants inhaled two puffs of metered dose albuterol sulfate (ProAir HFA, Teva Respiratory, Horsham, PA, USA). Spirometry was performed in accordance with the American Thoracic Society guidelines (Miller et al., 2005) using a dual beam Doppler ultrasound-based spirometer (EasyOne Pro, Ndd Medical, Zürich, Switzerland) (Regan et al., 2010). FEV1 and forced vital capacity (FVC) were measured from the greatest FEV1 and FVC over up to eight maximum expiratory maneuvers, where the greatest two measurements were within 150 mL.
2.3 Analyses
2.3.1 NIRS oxidative capacity test
During the repeated oxidative capacity tests, for each intermittent arterial occlusion the negative slope of TSI (%.s−1) was fitted by a linear function to estimate relative (Figure 2A,C). Note that during occlusion the rate of deoxygenation (the negative slope of TSI) is inversely proportional to , and is therefore reported below as a positive value (%.s−1). The exponential recovery rate constant (k, min−1) was estimated using non-linear least-squares regression (Figure 2B,D) (OriginPro v8.6, OriginLab Co., Northampton, USA) (Wüst et al., 2013).
Figure 2. Representative COPD (A, B) and control (C, D) participants’ responses during the muscle oxidative capacity assessment.
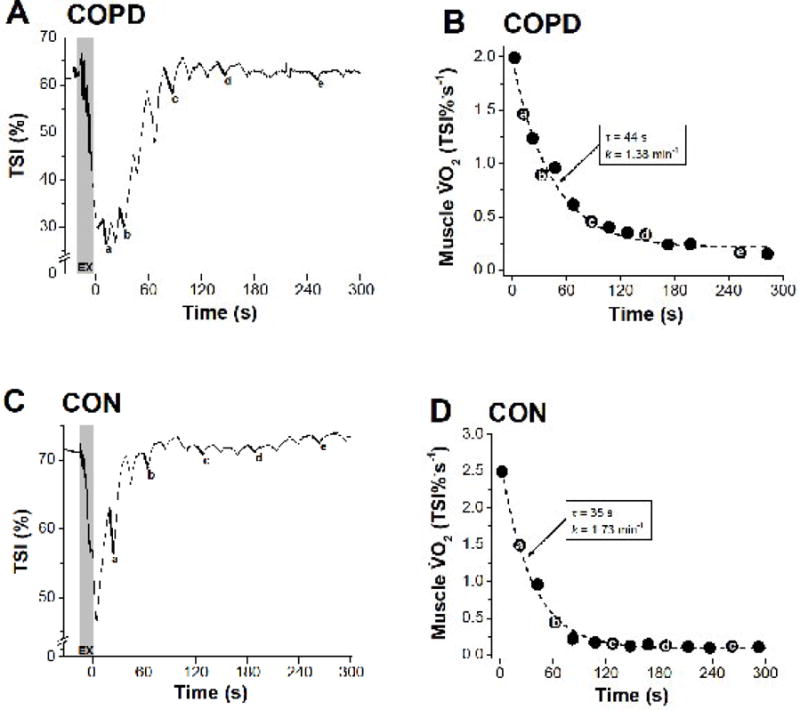
Panels A and C show the TSI profiles during dynamic exercise, and intermittent arterial occlusion during recovery. Panels B and D show the calculated muscle recovery profiles and kinetic fit (dashed line). The letters (a–e) are given to illustrate how the corresponding value is derived from respective TSI negative slopes during intermittent occlusions. The grey area (EX) indicates the brief dynamic exercise. τ (s) is the time constant determined by non-linear least-squares regression. k, is the rate constant, which is linearly related to muscle oxidative capacity (k = (1/τ).60, min−1).
2.3.2 NIRS quality control
Low test-retest variability (>1 SD) was used as the quality control criterion. For those tests with reproducibility outside 1 SD, the potential for limitations in O2 delivery and/or contraction-induced activation of mitochondrial oxidative phosphorylation during the oxidative capacity test were investigated to assess for physiologic contributors to test-retest variability. To determine a value of TSI during the sustained occlusion in the PN phase where the decline in TSI began to slow (a deflection in TSI; Figure 1B), a linear regression was applied from the onset of the sustained AO up to a point just before TSI deviated from linearity. This was investigated as a potential marker for the onset of O2 delivery limitation to during arterial occlusion. The lowest TSI (TSILOW) reached during each oxidative capacity test was recorded (both as an absolute muscle saturation and relative to the PN) and compared with the TSI deflection point (Figure 1). The increase in during contractions was estimated from the greatest recorded during the oxidative capacity test, and expressed in absolute units (%.s−1) and as a foldchange above the steady-state resting (measured at the end of the oxidative capacity test): a small increase or fold-change in may indicate insufficient contractile stimulus for mitochondrial oxidative phosphorylation and result in a low k.
2.3.3 Statistics
A Student’s paired t test was used to identify differences between COPD and CON. A Bland-Altman analysis for repeated measurements was used to assess the agreement between the two recovery k assessments (Bland and Altman, 1999). Coefficient of variation (CV) and intraclass correlation coefficient (ICC) were used to assess within-subject test-retest reproducibility. Variables correlated with the difference between repeated-measures of k (Δk) were sought by Spearman univariate linear regression analysis. Significant differences were accepted at P ≤ 0.05. Results are presented as mean ± SD, unless otherwise specified. A Shapiro-Wilk’s test (P ≥ 0.05) and visual inspection of the histograms, Q-Q plots and box plots were performed to determine normal distribution of k values for both COPD and CON groups (COPD, P > 0.45; CON, P > 0.06). Statistical analyses were performed using Prism v6.0f (GraphPad, San Diego, CA, USA) and SPSS v20 (IBM, Chicago, IL, USA).
3. RESULTS
3.1 Participant characteristics
Four COPD patients were unable to successfully complete the NIRS muscle protocol: two could not tolerate the sustained arterial occlusion for the PN, and the k could not be confidently resolved in one repeat of two other COPD patients. These 4 COPD patients were excluded from further analysis. Results are reported from 28 COPD and 28 normal spirometry CON participants. Two COPD patients required nasal cannula O2 during the visit (at 3–4 L.min−1). Participant characteristics are shown in Table 1. CON were younger than COPD (60 ± 7 vs. 65 ± 8 years, P < 0.05).
3.2 Muscle near-infrared spectroscopy
3.2.1 Resting muscle
Resting muscle TSI and ATT did not differ between COPD patients and CON (Table 1).
3.2.2 Physiologic normalization (PN)
In all COPD patients, PN ranged from a minimum of 22 % TSI to a maximum of 77 % TSI, with a mean range (max – min) of 32 ± 9 %. This was not different (P > 0.05) than CON: PN ranged 19 to 81 %, with a mean range of 32 ± 11 %.
3.2.3 Muscle oxidative capacity ( )
A total of 112 recovery kinetics assessments were performed for the study. On average, there was no difference between repeated k measurements within COPD or CON participants (Table 2). The individual test-retest reliability was not different between COPD (CV = 9.9%, ICC = 0.88) and CON (CV = 9.9%, ICC = 0.93) (Table 2, Figure 3). Power analyses (G*Power 3.1; Faul et al, 2007) revealed a 1−β = 0.81 for comparison of k between groups (the primary outcome). In all participants, Bland-Altman limit of agreement analysis revealed low mean bias (−0.03min−1), and 95% confidence intervals of −0.58, 0.64 min−1 (Figure 4). We could detect no order effect between repeats of k measurement (P = 0.24; 1-tailed t-test). On average, k was ~25% lower in COPD than CON (Table 2) and was diminished at all GOLD stages: CON, 1.74 ± 0.71 min−1 (n=28); GOLD 1, 1.45 ± 0.36 min−1 (n=7); GOLD 2, 1.48 ± 0.37 min−1 (n=13); GOLD 3, 1.22 ± 0.32 min−1 (n=5); and GOLD 4, 1.54 ± 0.41 min−1 (n=3).
Table 2.
Reproducibility and coefficient of variation (CV) of gastrocnemius muscle oxidative capacity assessed by near-infrared spectroscopy, in smokers with and without COPD.
| k (min−1) | CV | ICC | 1st vs. 2nd rep |
||
|---|---|---|---|---|---|
|
|
|||||
| 1st rep | 2nd rep | % | P | ||
| COPD | 1.45 (±0.38) | 1.42 (±0.36) | 9.85 | 0.88 | N.S. |
| CON | 1.75 (±0.70)* | 1.72 (±0.70) | 9.94 | 0.93 | N.S. |
Data are mean (±SD). CON = controls; k = proportional to muscle oxidative capacity; CV = coefficient of variation; ICC = intraclass correlation coefficient.
p≤ 0.05 vs. COPD patients
Figure 3. Muscle oxidative capacity (k) test-retest analyses.
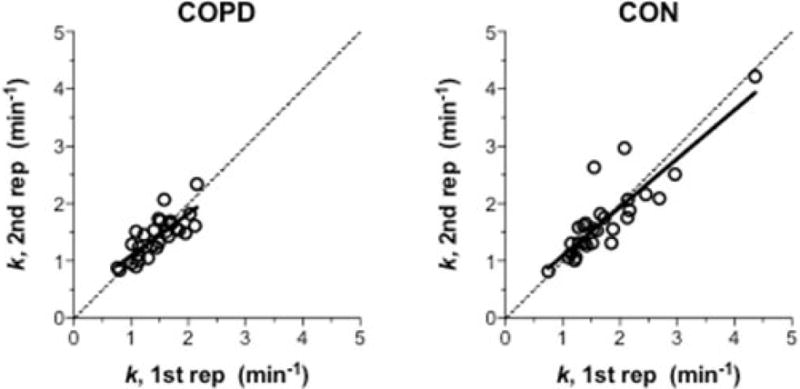
Comparison of muscle k inferred from the two repetitions of muscle recovery kinetics in COPD (n = 28) and controls, CON (n = 28). Continuous line is the linear regression. Dotted line is the line of identity (x = y).
Figure 4. Bland-Altman plot of the agreement between repeated measurements of muscle recovery rate constant (k).
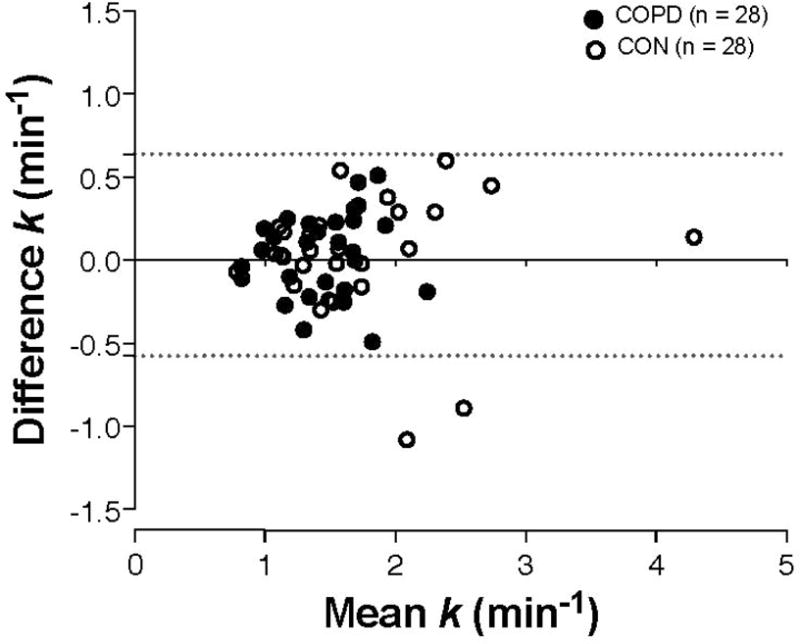
Closed symbols are COPD patients (n = 28) and open symbols are controls (CON, n = 28). Horizontal dashed lines indicate the 95% limits of agreement (range −0.58, 0.64 min−1).
3.2.4 NIRS test quality control
During the oxidative capacity test, the lowest TSI (TSILOW) in both repeats was typically achieved within the first or second AO (e.g., see Figure 1). In both COPD and CON, TSILOW averaged ~47% absolute (Table 3), equivalent to ~32% and ~29% of the PN range respectively. On average, the TSI deflection point occurred at 46 ± 9 % in COPD and 46 ± 11 % in CON. This meant that, typically (66% of tests), TSILOW was greater than TSI deflection point. In 38 tests (27%) TSILOW was below TSI deflection point.
Table 3.
Near-infrared spectroscopy tissue saturation index (TSI) variables of the gastrocnemius during post-contraction intermittent arterial occlusion. Mean and range of the saturation nadir (TSILOW, %) and relative peak muscle oxygen consumption ( , %.s−1) during repeated tests in smokers with and without COPD.
| 1st repetition
|
2nd repetition
|
CV
|
||||
|---|---|---|---|---|---|---|
| TSILOW % |
% s−1 |
TSILOW % |
% s−1 |
TSILOW % |
% |
|
| COPD | 47.5 (30.4 – 65.9) |
1.38 (0.66 – 3.18) |
46.7 (26.2 – 65.2) |
1.51 (0.37 – 3.74) |
3.92 | 16.11 |
| CON | 47.4 (6.5 – 67.8) |
1.71 (0.20 – 10.60) |
42.2 (12.6 – 69.8) |
1.49 (0.20 – 6.55) |
5.25 | 17.65 |
Data are mean (range). CON = controls; TSILOW = lowest value reached by tissue saturation index during the arterial occlusions series; = muscle oxygen consumption
In COPD, the peak during the oxidative capacity test was 1.38±0.59 %.s−1 and 1.51±0.88 %.s−1 respectively for the first and second repetitions (equivalent to a 14±7 and 16±13 fold increase above the recovery steady-state, 0.12±0.12 %.s−1). Peak values during the oxidative capacity test in CON were 1.71±1.89 %.s−1 and 1.49±1.17 %.s−1 respectively for the first and second repeat, equivalent to a 18±12 and 16±11 fold increase above resting (0.10±0.05 %.s−1), and were not different compared with COPD (P = 0.68).
Variables predictive of poor reproducibility were sought as potential quality control indices for the NIRS oxidative capacity test. Univariate linear regression analysis revealed that variability in repeated k measurements, assessed from the difference between the two k values (Δk), was positively correlated with k (r2 = 0.17; P ≤ 0.001): meaning faster kinetics were related to greater variably of measurement. However, other variables hypothesized to explain variability in k, including those expected to contribute to limitations in activation, O2 delivery, or NIRS signal sensitivity, such as minimum TSI during PN and TSILOW, age and resting TSI, PN maximum value and fold change, did not show a strong association with Δk (P > 0.10).
We investigated the characteristics of poorly-reproducible tests where Δk exceeded 1 SD (equal to the mean effect size for COPD; Δk > 0.3 min−1): thirteen participants (5 COPD, 8 CON) exceeded this variability threshold. These unreliable tests were characterized by a low (TSI = 1.15 ± 0.44 %.s−1) and poor exercise deoxygenation (e.g. a high TSILOW value of 53.5 ± 5.8 %). Six of these participants had large adipose layer (ATT = 8.3 ± 3.0 mm) and six had high skin melanin, each likely limiting the volume of the muscle interrogated by the NIRS probe. In all these 13 participants, the lowest TSI during the test (TSILOW) was below the TSI deflection point, suggesting that low O2 availability was not associated with muscle oxidative capacity assessment reliability. Excluding tests on the basis of Δk > 0.3 min−1 improved k measurement reliability (CV = 7.0±4.3%, ICC = 0.98, n = 43).
4. DISCUSSION
This is the first study to measure locomotor muscle oxidative capacity from recovery rate constant (k) in a large group of smokers with or without COPD, using a non-invasive, relatively simple, short-duration assessment by NIRS. Fifty-six out of 60 participants (93%) tolerated the NIRS assessment and returned interpretable results. Overall these data showed: there was no mean bias between test-retest repeats of gastrocnemius k measurement by NIRS in both COPD patients and age-similar smokers without airflow obstruction; that individual test-retest reproducibility was high (CV = 9.9%, ICC = 0.9); and that k averaged 25 % less in COPD compared to smokers with normal spirometry. Despite known muscle morphologic adaptations including increased fat and fibrotic infiltration, inflammation, loss of type I fibers and mitochondrial density (Maltais et al., 2014), our findings support that the NIRS assessment is a reliable method to detect COPD-related loss of muscle oxidative capacity.
We also aimed to identify correlates of tests with reproducibility that lay outside 1 SD of the distribution of all tests, as potential features for quality control. 13 of 56 participants (5 COPD, 8 CON) showed a high variability in k (Δk > 0.3 min−1, which was the mean effect size of COPD). Poor reproducibly was not associated with presence of COPD. Poor reproducibility was explained principally by a small increase in and only modest deoxygenation during contractions. This suggests that insufficient contractile stimulus for mitochondrial activation and/or that the NIRS signal contained little light reflected from active muscle (large adipose layer in n=6, and high skin melanin content in n=6), may contribute to poor test quality. These findings indicate that a poor quality tests are related to an insufficient increase in during contractions, and not to the presence of an O2 delivery limitation, as might be anticipated in COPD.
4.1 Muscle oxidative capacity in COPD
We found that k in the gastrocnemius skeletal muscle was, on average, 25 % less (range ~12–30%) in COPD than smokers of a similar age but without pulmonary obstruction. This average is consistent with the ~10–50% lower muscle oxidative enzyme activity (e.g. citrate synthase) or oxidative capacity observed in quadriceps biopsy samples from COPD patients compared with controls (Meyer et al., 2013), suggesting that the NIRS test provides a relevant non-invasive alternative to invasive biopsy assessments (Ryan et al., 2014). It is of note that k in our control group was 25% lower than non-smoking control participants in other studies using the same NIRS methods (non-smoker gastrocnemius k = ~2 min−1 equivalent to an oxidative capacity of ~250 pmol.s−1.mg dry weight−1; Ryan et al., 2014). However, those studies almost exclusively included young participants aged 24–27 years, while our smokers ranged 49–77 years. Whether the apparently low muscle oxidative capacity in our study relates to the effects of long-term smoking, or alternatively to age or physical inactivity remains to be determined.
While our study was not sufficiently powered to detect differences across disease severity, one outlier of the three GOLD 4 patients (k = 2.12 min−1) likely skewed a general trend for a progressive decline in oxidative capacity across GOLD severity classifications 2–4. Even so, the distribution of k values among the 28 COPD patients suggests that low k may occur at any GOLD stage, and therefore muscle dysfunction may not be solely associated with the disease severity in COPD (Maltais et al., 2014; Wagner, 2006). The precise etiologies resulting in loss of muscle oxidative capacity in COPD awaits further research. Nevertheless, the 93% tolerability and good test-retest agreement of the NIRS test in COPD prove the feasibility and reliability of this approach for discriminating patients with poor muscle function i.e. gastrocnemius k value lower than the 2 min−1 reference for a healthy adult. Because the NIRS test is non-invasive, it may provide the opportunity for muscle assessment in large-cohort studies, which are needed to better identify the complex multifactorial etiology of muscle oxidative dysfunction in COPD.
4.2 Variables influencing reproducibility of the NIRS muscle oxidative capacity test
The NIRS assessment of muscle oxidative capacity relies on the observed linear proportionality between the recovery rate constant (k) of cellular and cellular oxidative capacity i.e. the of the muscle cells investigated (Wüst et al., 2013). The primary predictor in our study of poor k reproducibility was a high value for k (r2 = 0.17; P ≤ 0.001). This is likely a simple reflection of the limited ability to accurately model the recovery rate constant with a limited number of measurements when kinetics are rapid. Nevertheless, this does not limit the ability of the test to detect abnormally low muscle oxidative capacity, which is the primary aim for studies of COPD patients or other conditions of chronic inactivity or disease.
The relationship between cellular recovery k and oxidative capacity in muscle is predictable based on first order rate reaction kinetics (Voet and Voet, 2004), as long as O2 concentration in muscle mitochondria remains non-limiting. However, recent studies suggest that control of oxidative phosphorylation in skeletal muscle in humans is not first order, and that allosteric or ‘each step’ activation of mitochondrial oxidative pathways is required to activate the enzymes limiting cellular (Korzeniewski and Rossiter, 2015; Wüst et al., 2011). Thus, accurate measurement of oxidative capacity by NIRS relies on competing demands to achieve a sufficiently high level of muscle activity and to release mitochondrial oxidative enzyme regulation, but to limit muscle activity to a sufficiently low level such that muscle mitochondrial O2 delivery does not become a limiting variable. Failure to meet either of these conditions would result in an erroneously low measurement of muscle oxidative capacity by NIRS.
Using single muscle fibers from the frog suspended in a medium containing a high O2 concentration, Wüst et al. (2013) observed that, unlike poorly-oxidative fibers, recovery k of highly-oxidative fibers was dependent on the frequency of stimulation of the preceding contractions. A contractile protocol sufficient to elicit ~50% was required to release oxidative enzyme regulation to allow recovery k to become proportional to cellular oxidative capacity. The duration required of this contractile protocol was not assessed. The human medial gastrocnemius, the site of NIRS probes in our study, expresses a mixed fiber type distribution and contains both poorly and highly-oxidative muscle fibers. Therefore, a low response during muscle contractions would be predictive of a poor quality assessment and result in an erroneously low oxidative capacity. Consistent with this notion, in 13 of our participants the NIRS test results were poorly reproducible and in these there was a tendency (P = 0.20) for a low exercise-induced increase in . This highlights the importance of ensuring high-intensity muscle contractions during the NIRS oxidative capacity assessment for validity of the test.
As stated previously, another condition that must be satisfied for validity of the NIRS test is non-limiting mitochondrial O2 delivery. Using 31P magnetic resonance spectroscopy to determine gastrocnemius PCr recovery kinetics from plantar flexion exercise (a proxy for k), Haseler et al. (2004) showed in young sedentary subjects that PCr recovery was not limited by O2 delivery under normoxic conditions. However, during hypoxic gas breathing, PCr recovery kinetics were slowed. It should be pointed out that the duration of exercise was 6 min, and therefore and muscle deoxygenation were likely far greater than those observed in our study where exercise was limited to ~15 s. Nevertheless, were TSI to be driven below some limiting value, recovery k may become limited by O2 delivery in vivo, which would result in an erroneously low k. While the brief exercise in the NIRS test does not strain central cardiac or pulmonary limits for O2 delivery, age-or disease-related chronic adaptations, such as muscle capillary rarefaction, inflammation, anemia or reduced muscle myoglobin (Maltais et al., 2014), have the potential to limit muscle mitochondrial O2 concentration in COPD and therefore invalidate the NIRS assessment. However, we found that the lowest value of TSI measured during the NIRS assessment (TSILOW; typically reached during the 1st or 2nd post-exercise arterial occlusion) was not related to poor test-retest reliability of k. This suggests that outlying low k values are not consequent to O2 delivery limitation, at least down to TSILOW values of ~30% of the physiologic range.
4.3 Quality control of the muscle NIRS oxidative capacity assessment
In part, the beauty of the NIRS test of muscle oxidative capacity is that it relies on kinetics, and therefore does not require calibrated measurements. For this reason, one aim of this study was to identify variables that could be used as markers of quality control. We proposed to identify features within any tests that showed poor reproducibility. The strongest correlate of variability in repeated k measurements (Δk) was the value of k itself, which does not provide a basis for quality control. To our surprise, however, we found no correlation between Δk and proposed quality assessments (e.g. increase in increase during contractions or low TSI during occlusions). This may reflect the overall strong test-retest reproducibility in COPD patients and controls. We therefore identified 13 participants in whom variability exceeded 1 SD (Δk > 0.3 min−1). Of these 13, there was a high prevalence of large ATT, high skin melanin content, low increase in and a small exercise-induced deoxygenation. While these features alone do not form the basis of quality control, they highlight that patient physical characteristics limiting reflected light from active muscle tissue are likely partly responsible for reducing reproducibility of k measurements. Alternative NIRS systems, such as high-power time-resolved (TRS) NIRS, allow deeper penetration into muscle during rest and exercise (Okushima et al., 2015), and therefore may increase the reliability of recovery kinetic assessment in these patients.
Based on our findings and experience, a few considerations emerge to inform quality control of the NIRS oxidative capacity test. First, the current best method of quality control is to perform the measurement twice in the same visit. We propose that poorly-reproducible tests, where Δk > 0.3 min−1, be repeated to reduce the influence of outlying results, and repeated measurements averaged. Careful attention should be made to the NIRS probe placement, to ensure that a muscle region is chosen that both minimizes the skinfold under the probe and maximizes the sampling of active muscle during contractions and recovery. Doppler ultrasound, skinfold calipers, muscle palpation during contraction and/or surface EMG may help to identify optimal NIRS probe placement.
Exercise stimuli that result in a small increase and small reduction in saturation were associated with poor test reliability. Therefore, our data suggest that the risk to NIRS test validity of under-stimulating the muscle during dynamic contractions is greater than the risk of O2 limiting deoxygenation caused by contractions that are too intense or sustained. Thus, ensuring that the exercise-induced desaturation reaches a value of ~30–50% of the physiologic range (PN) helps in test quality assurance. This can be confirmed in real time by monitoring the TSI response to contractions and adjusting the intensity and/or duration of exercise (i.e. extending the duration beyond the ~10–15 s we used here) to achieve the desaturation target. This, of course, requires knowledge in the PN range prior to the oxidative capacity test, which is a modification to the protocol of Ryan et al. (2012) where the PN range measurement is performed last. This has the concern that a period of ischemia (even briefly) may affect mitochondrial function and therefore influence the assumptions inherent in the measurement of oxidative capacity from recovery kinetics e.g. an ischemic preconditioning effect (Crisafulli et al., 2011). Our findings, however, that muscle k in older controls (~1.75 min−1) and COPD (~1.45 min−1) were consistent with that predicted from biopsy studies (Meyer et al., 2013) suggest that brief ischemia does not harm the validity of the test.
4.4 Clinical implications
Low muscle oxidative capacity is associated with exercise intolerance in COPD and therefore may contribute to reduce physical activity and quality of life in these patients (Maltais et al., 2014; Meyer et al., 2013). We studied the gastrocnenius muscle as a primary locomotor muscle for walking, and which is also extensively activated during standing and in sway. Rehabilitation (cycling and walking) is known to ameliorate oxidative capacity deficits in quadriceps (Maltais et al., 1996) and is associated with a reduction of dyspnea and leg fatigue symptoms during exercise in COPD. Therefore, reliable measurements of skeletal muscle structure and function, independent of disease-related impairments in pulmonary function or muscle blood flow, are of crucial importance to monitor the peripheral consequences of COPD. This reliable, non-invasive, short-duration, relatively inexpensive and well-tolerated assessment of oxidative capacity in COPD muscle may also enable a better targeting to therapeutic strategies to improve physical activity, exercise tolerance and quality of life in these patients.
4.5 Additional considerations
Our approach focused on biological variability, in that test-retest precision of k was assessed in smokers with and without COPD with the same day and session, and the NIRS probe was not removed from the muscle between repeated tests. Southern et al. (2014) assessed the test-retest variability between consecutive days in young healthy participants, an approach that included the combined effects of both methodological and biological variability. This may partly explain why variability of k assessment in our study (ICC range 0.88–0.93; CV = 9.9 %) was higher than Southern et al. (2014) (ICC range 0.26–0.59; CV = 10.6 %). In addition, Southern et al. (2014) assessed reliability in 15 participants whereas our study investigated 56. This difference also contributes to the greater ICC found in our study. Nevertheless, our data show that the approach is reliable in COPD and older controls where muscle quality is reduced compared with young subjects.
Furthermore, Southern et al. (2013) found greater day to day test-retest variability of the NIRS oxidative capacity test when using self-metered exercise (specifically, exercise against elastic resistance bands) compared with monitored exercise using a custom-built plantar flexion ergometer. We were specifically interested in assessing a pragmatic approach to the NIRS oxidative capacity test using self-metered exercise in order to assess the applicability of the assessment in the clinical setting without the requirement for additional specialized equipment. We used a manually-applied resistance to plantar flexion administered by the same researcher in all participants. The intensity of the 10–15 contractions was assessed indirectly by feel and also monitored in the TSI trace in real time. This meant that the operator could instruct the participant to alter the intensity or duration of contractions during the test itself to achieve an ‘optimal’ deoxygenation signal (and, by implication, response). We believe that this approach might have advantages over delivery of a standardized metered exercise dose in patients with chronic disease where there is wide variability in O2 delivery and O2 utilization responsiveness. Our high ICC values for k support this suggestion. It also suggests that our pragmatic approach provides a reliable assessment of muscle oxidative capacity suitable for clinical research or routine assessment in COPD patients.
4.6 Limitations
There are few limitations to report for this study. The CON group were slightly, but significantly younger than the COPD patients (Table 1). It is possible that the younger age in CON may contribute to the mean effect size of k in COPD (−0.3 min−1). Nevertheless, the average difference in age was small (5 years on an average age of 65 years), and is not the only variable that affects muscle oxidative capacity. For example, physical activity, occupation, drug therapies and comorbidities are expected to play a significant role in the etiology of loss of muscle oxidative capacity in COPD (Maltais et al., 2014; Wagner, 2006).
Two COPD patients used nasal cannula O2 delivery during the NIRS assessment. However, these participants’ data lay well within the range of the group as a whole (Δk was −0.05 and −0.13 min−1). We could identify no additional reason to treat these data differently from the group. In addition, while baseline arterial O2 saturation was measured by pulse oximetry, we found no influence of SpO2 on the reproducibility of k. The brief single leg plantar-flexion contractions were insufficient to alter SpO2 and we found no differences in muscle HbO2 among the 13 participants with poor reproducibility of k and the rest of the group. We therefore believe that arterial oxygenation did not influence our results. However, this should be monitored in future studies to ensure that the assumption of constant SpO2 is met.
Finally, we did not assess oxidative capacity from muscle biopsy in this study in relation to NIRS measures in these COPD patients. By their nature, NIRS assessment and muscle biopsy sample different muscle regions, complicating direct comparison. Assessment of quadriceps oxidative capacity by NIRS and biopsy was previously established in healthy young subjects (r > 0.6; Ryan et al., 2014). Validation of accuracy of the NIRS assessment of muscle oxidative capacity in COPD therefore awaits further investigation.
5. CONCLUSION
We found that a non-invasive NIRS-based assessment of oxidative capacity of gastrocnemius muscle was well tolerated and reliable in middle-aged to elderly smokers with or without COPD. Our data were consistent with direct assessment of muscle citrate synthase activity or oxidative capacity from biopsy studies (Meyer et al., 2013) in that gastrocnemius k (a direct correlate of muscle oxidative capacity) was 25% lower in COPD than in smoker controls without pulmonary obstruction. We found high test-retest reliability of the NIRS oxidative capacity test in both COPD (CV = 9.9%; ICC = 0.88) and CON groups (CV = 9.9%; ICC = 0.93). Our attempts to identify objective markers of NIRS test quality were less successful: nevertheless, performance of repeated assessments in the same visit can identify outlying results, and these were associated with small and deoxygenation responses during dynamic contractions, and participants with a large skinfold or high skin melanin. Together these data suggest that poor-quality assessments occur when the exercise stimulus is insufficient for mitochondrial activation and/or the NIRS signal contains little light reflected from active muscle. Low post-contraction TSI was unrelated to NIRS test reliability, suggesting that O2 supply is sufficient for NIRS test validity, at least down to TSI of ~30% of the individuals physiologic range. Therefore it is recommended to err towards a more intense exercise rather than the maintenance of a high muscle oxygenation to optimize NIRS assessment of muscle oxidative capacity. Our findings support the reliability of non-invasive muscle oxidative capacity assessment by NIRS in COPD, which may be helpful to track the efficacy of interventions in COPD such as pulmonary rehabilitation that are designed to redress skeletal muscle dysfunction.
HIGHLIGHTS.
NIRS assessment of muscle oxidative capacity is well tolerated in COPD patients
NIRS muscle outcomes were highly reproducible in smokers with and without COPD
Skeletal muscle oxidative capacity was ~25% lower in COPD than smoker controls
Non-invasive NIRS reduces the need for biopsy to track muscle dysfunction in COPD
Acknowledgments
We would like to thank all the participants for the time and dedication to the study, and the members of Rehabilitation Clinical Trials Center at Los Angeles Biomedical Research Institute for their support.
SUPPORT
Swiss National Science Foundation grant P300P3_151705 to Adami Alessandra; NIH HL089856 and HL089897; Pulmonary Education Research Foundation (Fellowship to AA).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICTS OF INTEREST
No conflicts to declare.
Contributor Information
Alessandra Adami, Email: aadami@labiomed.org.
Robert Cao, Email: rcao@labiomed.org.
Janos Porszasz, Email: porszasz@ucla.edu.
Richard Casaburi, Email: casaburi@ucla.edu.
References
- 1.Agustí AG, Noguera A, Sauleda J, Sala E, Pons J, Busquets X. Systemic effects of chronic obstructive pulmonary disease. Eur Respir J. 2003;21(2):347–360. doi: 10.1183/09031936.03.00405703. [DOI] [PubMed] [Google Scholar]
- 2.Allaire J, Maltais F, Doyon JF, Noël M, LeBlanc P, Carrier G, Simard C, Jobin J. Peripheral muscle endurance and the oxidative profile of the quadriceps in patients with COPD. Thorax. 2004;59(8):673–678. doi: 10.1136/thx.2003.020636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bland JM, Altman DG. Measuring agreement in method comparison studies. Statistical Methods in Medical Research. 1999;8:135–160. doi: 10.1177/096228029900800204. [DOI] [PubMed] [Google Scholar]
- 4.Casaburi R. Skeletal muscle dysfunction in chronic obstructive pulmonary disease. Med Sci Sports Exerc. 2001;33(7Suppl):S662–S670. doi: 10.1097/00005768-200107001-00004. [DOI] [PubMed] [Google Scholar]
- 5.Coronell C, Orozco-Levi M, Méndez R, Ramírez-Sarmiento A, Gáldiz JB, Gea J. Relevance of assessing quadriceps endurance in patients with COPD. Eur Respir J. 2004;24(1):129–136. doi: 10.1183/09031936.04.00079603. [DOI] [PubMed] [Google Scholar]
- 6.Couillard A, Prefaut C. From muscle disuse to myopathy in COPD: potential contribution of oxidative stress. Eur Respir J. 2005;26(4):703–719. doi: 10.1183/09031936.05.00139904. [DOI] [PubMed] [Google Scholar]
- 7.Crisafulli A, Tangianu F, Tocco F, Concu A, Mameli O, Mulliri G, Caria MA. Ischemic preconditioning of the muscle improves maximal exercise performance but not maximal oxygen uptake in humans. J Appl Physiol. 2011;111(2):530–536. doi: 10.1152/japplphysiol.00266.2011. [DOI] [PubMed] [Google Scholar]
- 8.Decramer M, Rennard S, Troosters T, Mapel DW, Giardino N, Mannino D, Wouters E, Sethi S, Cooper CB. COPD as a lung disease with systemic consequences–clinical impact, mechanisms, and potential for early intervention. COPD. 2008;5(4):235–256. doi: 10.1080/15412550802237531. [DOI] [PubMed] [Google Scholar]
- 9.Engelen MP, Schols AM, Does JD, Wouters EF. Skeletal muscle weakness is associated with wasting of extremity fat-free mass but not with airflow obstruction in patients with chronic obstructive pulmonary disease. Am J Clin Nutr. 2000;71(3):733–738. doi: 10.1093/ajcn/71.3.733. [DOI] [PubMed] [Google Scholar]
- 10.Erickson ML, Ryan TE, Young HJ, McCully KK. Near-infrared assessments of skeletal muscle oxidative capacity in persons with spinal cord injury. Eur J Appl Physiol. 2013;113(9):2275–2283. doi: 10.1007/s00421-013-2657-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Faul F, Erdfelder E, Lang AG, Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39(2):175–191. doi: 10.3758/bf03193146. [DOI] [PubMed] [Google Scholar]
- 12.Ferrari M, Mottola L, Quaresima V. Principles, techniques, and limitations of near infrared spectroscopy. Can J Appl Physiol. 2004;29(4):463–487. doi: 10.1139/h04-031. [DOI] [PubMed] [Google Scholar]
- 13.Gosker HR, van Mameren H, van Dijk PJ, Engelen MP, van der Vusse GJ, Wouters EF, Schols AM. Skeletal muscle fibre-type shifting and metabolic profile in patients with chronic obstructive pulmonary disease. Eur Respir J. 2002;19(4):617–625. doi: 10.1183/09031936.02.00762001. [DOI] [PubMed] [Google Scholar]
- 14.Gosker HR, Zeegers MP, Wouters EF, Schols AM. Muscle fibre type shifting in the vastus lateralis of patients with COPD is associated with disease severity: a systematic review and meta-analysis. Thorax. 2007;62(11):944–949. doi: 10.1136/thx.2007.078980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hamaoka T, McKully KK, Quaresima V, Yamamoto K, Chance B. Near-infrared spectroscopy/imaging for monitoring muscle oxygenation and oxidative metabolism in healthy and diseased humans. J Biomed Opt. 2007 doi: 10.1117/1.2805437. Doi:12:062105-1-062105-16. [DOI] [PubMed] [Google Scholar]
- 16.Haseler LJ, Lin AP, Richardson RS. Skeletal muscle oxidative metabolism in sedentary humans: 31P-MRS assessment of O2 supply and demand limitations. J Appl Physiol. 2004;97(3):1077–1081. doi: 10.1152/japplphysiol.01321.2003. [DOI] [PubMed] [Google Scholar]
- 17.Korzeniewski B, Rossiter HB. Each-step activation of oxidative phosphorylation is necessary to explain muscle metabolic kinetic responses to exercise and recovery in humans. J Physiol. 2015;593(24):5255–5268. doi: 10.1113/JP271299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maltais F, Decramer M, Casaburi R, Barreiro E, Burelle Y, Debigaré R, Dekhuijzen PN, Franssen F, Gayan-Ramirez G, Gea J, Gosker HR, Gosselink R, Hayot M, Hussain SN, Janssens W, Polkey MI, Roca J, Saey D, Schols AM, Spruit MA, Steiner M, Taivassalo T, Troosters T, Vogiatzis I, Wagner PD. ATS/ERS Ad Hoc Committee on Limb Muscle Dysfunction in COPD. An official American Thoracic Society/European Respiratory Society statement: update on limb muscle dysfunction in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2014;189(9):e15–e62. doi: 10.1164/rccm.201402-0373ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maltais F, LeBlanc P, Jobin J, Casaburi R. Peripheral muscle dysfunction in chronic obstructive pulmonary disease. Clin Chest Med. 2000;21(4):665–677. doi: 10.1016/s0272-5231(05)70176-3. [DOI] [PubMed] [Google Scholar]
- 20.Maltais F, LeBlanc P, Simard C, Jobin J, Bérubé C, Bruneau J, Carrier L, Belleau R. Skeletal muscle adaptation to endurance training in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1996;154(2 Pt 1):442–447. doi: 10.1164/ajrccm.154.2.8756820. [DOI] [PubMed] [Google Scholar]
- 21.McKully KK, Iotti S, Kendrick K, Wang Z, Posner JD, Leigh J, Jr, Chance B. Simultaneous in vivo measurements of HbO2 saturation and PCr kinetics after exercise in normal humans. J Appl Physiol. 1994;77(1):5–10. doi: 10.1152/jappl.1994.77.1.5. [DOI] [PubMed] [Google Scholar]
- 22.Meyer A, Zoll J, Charles AL, Charloux A, de Blay F, Diemunsch P, Sibilia J, Piquard F, Geny B. Skeletal muscle mitochondrial dysfunction during chronic obstructive pulmonary disease: central actor and therapeutic target. Exp Physiol. 2013;98(6):1063–1078. doi: 10.1113/expphysiol.2012.069468. [DOI] [PubMed] [Google Scholar]
- 23.Miller MR, Crapo R, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, Enright P, van der Grinten CP, Gustafsson P, Jensen R, Johnson DC, MacIntyre N, McKay R, Navajas D, Pedersen OF, Pellegrino R, Viegi G, Wanger J. ATS/ERS Task Force. General considerations for lung function testing. Eur Respir J. 2005;26(1):153–161. doi: 10.1183/09031936.05.00034505. [DOI] [PubMed] [Google Scholar]
- 24.Montes de Oca M, Loeb E, Torres SH, De Sanctis J, Hernández N, Tálamo C. Peripheral muscle alterations in non-COPD smokers. Chest. 2008;133(1):13–18. doi: 10.1378/chest.07-1592. [DOI] [PubMed] [Google Scholar]
- 25.Motobe M, Murase N, Osada T, Homma T, Ueda C, Nagasawa T, Kitahara A, Ichimura S, Kurosawa Y, Katsumura T, Hoshika A, Hamaoka T. Noninvasive monitoring of deterioration in skeletal muscle function with forearm cast immobilization and the prevention of deterioration. Dyn Med. 2004;3(1):2. doi: 10.1186/1476-5918-3-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Natanek SA, Gosker HR, Slot IG, Marsh GS, Hopkinson NS, Man WD, Tal-Singer R, Moxham J, Kemp PR, Schols AM, Polkey MI. Heterogeneity of quadriceps muscle phenotype in chronic obstructive pulmonary disease (Copd); implications for stratified medicine? Muscle Nerve. 2013;48(4):488–497. doi: 10.1002/mus.23784. [DOI] [PubMed] [Google Scholar]
- 27.Nici L. Mechanisms and measures of exercise intolerance in chronic obstructive pulmonary disease. Clin Chest Med. 2000;21(4):693–704. doi: 10.1016/s0272-5231(05)70178-7. [DOI] [PubMed] [Google Scholar]
- 28.Okushima D, Poole DC, Rossiter HB, Barstow TJ, Kondo N, Ohmae E, Koga S. Muscle deoxygenation in the quadriceps during ramp incremental cycling: Deep vs. superficial heterogeneity. J Appl Physiol. 2015;119(11):1313–1319. doi: 10.1152/japplphysiol.00574.2015. [DOI] [PubMed] [Google Scholar]
- 29.Picard M, Godin R, Sinnreich M, Baril J, Bourbeau J, Perrault H, Taivassalo T, Burelle Y. The mitochondrial phenotype of peripheral muscle in chronic obstructive pulmonary disease: disuse or dysfunction? Am J Respir Crit Care Med. 2008;178(10):1040–1047. doi: 10.1164/rccm.200807-1005OC. [DOI] [PubMed] [Google Scholar]
- 30.Regan EA, Hokanson JE, Murphy JR, Make B, Lynch DA, Beaty TH, Curran-Everett D, Silverman EK, Crapo JD. Genetic epidemiology of COPD (COPDGene) study design. COPD. 2010;7(1):32–43. doi: 10.3109/15412550903499522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ryan TE, Erickson ML, Brizendine JT, Young HJ, McCully KK. Noninvasive evaluation of skeletal muscle mitochondrial capacity with near-infrared spectroscopy: correcting for blood volume changes. J Appl Physiol. 2012;113(2):175–183. doi: 10.1152/japplphysiol.00319.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ryan TE, Southern WM, Reynolds MA, McCully KK. A cross-validation of near-infrared spectroscopy measurements of skeletal muscle oxidative capacity with phosphorus magnetic resonance spectroscopy. J Appl Physiol. 2013;115(12):1757–1766. doi: 10.1152/japplphysiol.00835.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ryan TE, Brophy P, Lin CT, Hickner RC, Neufer PD. Assessment of in vivo skeletal muscle mitochondrial respiratory capacity in humans by near-infrared spectroscopy: a comparison with in situ measurements. J Physiol. 2014;592(15):3231–3241. doi: 10.1113/jphysiol.2014.274456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Southern WM, Ryan TE, Reynolds MA, McCully KK. Reproducibility of near-infrared spectroscopy measurements of oxidative function and postexercise recovery kinetics in the medial gastrocnemius muscle. Appl Physiol Nutr Metab. 2014;39(5):521–529. doi: 10.1139/apnm-2013-0347. [DOI] [PubMed] [Google Scholar]
- 35.Vogiatzis I, Zakynthinos S. Factors limiting exercise tolerance in chronic ling diseases. Comprehensive Physiology. 2012;2(3):1779–1817. doi: 10.1002/cphy.c110015. [DOI] [PubMed] [Google Scholar]
- 36.Wagner PD. Skeletal muscles in chronic obstructive pulmonary disease: deconditioning, or myopathy? Respirology. 2006;11(6):681–686. doi: 10.1111/j.1440-1843.2006.00939.x. [DOI] [PubMed] [Google Scholar]
- 37.Whittom F, Jobin J, Simard PM, Leblanc P, Simard C, Bernard S, Belleau R, Maltais F. Histochemical and morphological characteristics of the vastus lateralis muscle in patients with chronic obstructive pulmonary disease. Med Sci Sport Exerc. 1998;30(10):1467–1474. doi: 10.1097/00005768-199810000-00001. [DOI] [PubMed] [Google Scholar]
- 38.Wouters EF. Chronic obstructive pulmonary disease. 5: systemic effects of COPD. Thorax. 2002;57(12):1067–1070. doi: 10.1136/thorax.57.12.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wüst RC, van der Laarse WJ, Rossiter HB. On-off asymmetries in oxygen consumption kinetics of single Xenopus laevis skeletal muscle fibres suggest higher-order control. J Physiol. 2013;591(3):731–744. doi: 10.1113/jphysiol.2012.241992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wüst RC, Grassi B, Hogan MC, Howlett RA, Gladden LB, Rossiter HB. Kinetic control of oxygen consumption during contractions in self-perfused skeletal muscle. J Physiol. 2011;589:3995–4009. doi: 10.1113/jphysiol.2010.203422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Voet D, Voet JG. Rates of Enzymatic Reactions. In: Voet D, Voet JG, editors. Biochemistry. third. John Wiley and Sons; Hoboken: 2004. pp. 472–482. [Google Scholar]


