Abstract
Chemotherapeutic resistance remains a challenge in the treatment of ovarian carcinoma, especially in recurrent disease. Despite the fact that most patients with newly diagnosed tumors attain complete remission following cytoreductive surgery and chemotherapy, ovarian carcinoma has a recurrence rate that exceeds 75%. The ATP Binding Cassette family G member 2 (ABCG2) efflux protein has been described as one mechanism that confers multiple drug resistance (MDR) to solid tumors and contributes to topotecan (TPT) resistance in ovarian carcinoma. In fact, one clinical trial demonstrated ABCG2 expression in all patients with primary or recurrent ovarian carcinoma. On the basis of our previous work, we hypothesized that three compounds (CID44640177, CID1434724, and CID46245505), which represent a new piperazine-substituted pyrazolo[1,5]pyrimidine substructure class of ABCG2-specific antagonists, would restore chemosensitivity to drug-resistant ovarian cancer in vitro and in vivo. In order to address the treatment difficulties associated with chemotherapeutic resistance in ovarian cancer, we combined each compound (CID44640177, CID1434724, and CID46245505) with TPT and administered the mixture to chemoresistant Igrov1/T8 ovarian cancer cells in vitro and Igrov1/T8 xenografts in CB-17 SCID mice. We found that only nanomolar concentrations of each ABCG2-inhibitor in combination with TPT were required to restore chemosensitivity to Igrov1/T8 cells in vitro. In vivo, substantial tumor reduction was achieved with each compound in 4 days, with CID1434724 causing the largest reduction in excess of 60%. No signs of secondary toxic effects were observed with the ABCG2 antagonists. These novel compounds should be viewed as promising drug candidates to reverse ABCG2-mediated chemoresistance.
Keywords: ABCG2, BCRP, ovarian cancer, multiple drug resistance, inhibitors, antagonists, murine model
Introduction
Epithelial ovarian cancer continues to be the most lethal gynecologic malignancy and chemotherapeutic resistance remains a major barrier to treatment. Unfortunately, most ovarian cancers are not diagnosed until advanced disease (Grade III and IV), and these patients only have a 5-year survival rate of approximately 20%.(1–4) Despite the fact that response rates to cytoreductive surgery and first-line chemotherapeutic treatments (platinum compounds and taxanes) approach 75%,(2) ovarian carcinoma has a recurrence rate that exceeds 75%.(1,5,6) In addition, recurrent tumors are characteristically more resistant to chemotherapy, with response rates only at approximately 20%.(1,2,7) Furthermore, the limited efficacy of chemotherapy in recurrent disease has been attributed to the development of multiple drug resistance (MDR).(1,3–5,7,8) Due to continued treatment difficulties encountered in drug resistant ovarian adenocarcinoma and resulting mortality, it is important to identify compounds that reverse chemotherapeutic resistance.
MDR may be mediated by a variety of mechanisms,(1,4,7) though the most significant and prevalent mechanism in ovarian cancer is cellular extrusion of chemotherapy by the ATP Binding Cassette (ABC) transporter proteins.(1,3,4,7) Although the family of ABC transporters is relatively large (48 ABC encoding genes(9)), only three transporters comprise the major mechanism of MDR in cancer: ABCB1, ABCC1, and ABCG2.(10–12) In ovarian cancer, some members of the ABCC subfamily have been implicated in platinum agent and taxane resistance, while ABCG2 has been demonstrated to confer topotecan (TPT) resistance.(1,8,13–15) Consistent with these cellular observations, one clinical trial demonstrated ABCG2 expression in all patients with primary or recurrent ovarian carcinoma.(16) In addition, a Gynecologic Oncology Group phase III trial investigating ABCB1, ABCC2, and ABCG2 expression on clinical outcomes in ovarian cancer revealed that women expressing the specific C421A ABCG2 polymorphism, approximately 20% of studied patients, had a shorter progression free survival time following chemotherapeutic treatment.(17) Therefore, ABCG2 plays an important clinical role in conferring MDR to ovarian cancer.
There are a variety of ABCG2 inhibitors that have been investigated in vitro and in vivo with many types of cancers exhibiting MDR. However, there are only limited ABCG2 antagonists that have been examined in solid tumors despite its clinical importance as an MDR mechanism.(18–24) Thus far, only Fumitremorgin C and Ko143 (a Fumitremorgin C analogue) have been investigated in preclinical animal studies utilizing topotecan for ovarian carcinoma.(18–20) Fumitremorgin C is neurotoxic, which hinders its clinical use.(19) Though low toxicity was observed with Ko143 when administered as oral doses in mice, the question of its potential toxicity in humans due to its relation to Fumitremorgin C is a possible hurdle in advancement to the clinic.(18) Another compound with some activity against ABCG2, WK-X-34, was tested in ABCB1 overexpressing, daunorubicin resistant ovarian cancer, but it appeared to reverse MDR mainly through its inhibition of ABCB1.(20) Furthermore, GF120918 (Elacridar), an ABCB1 inhibitor, was tested in mice using ovarian cancer cells resistant to doxorubicin and was later discovered to have some activity against ABCG2.(25) A single phase II clinical trial investigating inhibition of ABCG2 by Lapatinib in recurrent ovarian cancer has been performed.(16) However, there was no observed clinical benefit and the trial was canceled due to substantial adverse hematologic events. As a result, there is a critical need to identify inhibitors with low toxicity and high potency for ABCG2.
Although ABCG2 has a broad diversity of substrates, the most well known with relation to MDR in ovarian carcinoma are TPT, mitoxantrone, platinum agents, paclitaxel, and doxorubicin.(1,17) TPT is a second-line therapy used adjunctively with primary cytotoxic agents in ovarian carcinoma.(5) TPT is one of the most studied agents for treatment of relapsed ovarian carcinoma and according to present evidence there is no current difference in safety or effectiveness when compared to other second-line non-platinum chemotherapeutic regimens.(26,27) Therefore, TPT is a useful drug and its cellular resistance is valuable to examine as a model for ABCG2-mediated MDR in ovarian cancer.
Due to the importance of discovering new ABCG2 inhibitors, our study focused on investigating three novel ABCG2-inhibitors with similar chemical scaffolds for the treatment of MDR in ovarian cancer. Each compound was effective in reversing MDR in cell culture and in a murine ovarian carcinoma model with relatively low toxicity. Neither TPT nor ABCG2-inhibitor alone had a major effect on tumor response to treatment. However, when TPT was combined with low doses of an ABCG2 inhibitor we observed substantial tumor size reduction and necrosis. The median toxic doses of each ABCG2 inhibitor in vitro were all greater than 1μM. Additionally, our compounds appear to have low toxicity in vivo with a higher potency (each with sub-10nM median inhibitory concentrations) than other ABCG2 inhibitors previously described in ovarian cancer.(18–24) To determine the optimal administration route, we administered each ABCG2 antagonist in combination with TPT by intratumoral (IT), retro-orbital (RO), and intraperitoneal (IP) injection. When accounting for tumor necrosis and fibrosis along with tumor size reduction, we observed a significant decrease in tumor viability compared to the controls (p < 0.01). These studies are an important basis for transitioning this new class of ABCG2 inhibitors to the clinic as a potential remedy for MDR in ovarian cancer.
Materials and Methods
Cell lines and Reagents
Igrov1 human ovarian carcinoma cells and an Igrov1-derived cell line overexpressing ABCG2, Igrov1/T8, were generously provided by Dr. Douglas Ross at the University of Maryland and were received in 2011. Cells were cultured in RPMI 1640 medium (Gibco, InVitrogen Corporation, Carlsbad, CA) supplemented with 10% fetal bovine serum (Gibco), 1% penicillin/streptomycin (Gibco), and 5 ng/L ciprofloxacin (Bayer Pharmaceuticals, Berkeley, CA) and maintained at 37°C in an incubator with an atmosphere of 95% air and 5% CO2. The Igrov1/T8 ABCG2 overexpressing phenotype was maintained by exposure to 950 nM topotecan hydrochloride hydrate (Sigma, Saint Louis, MO) for one hour per week on a day that the cells were not expanded. We synthesized and identified three ABCG2-selective efflux inhibitors, CID44640177, CID1434724, and CID46245505, by high-throughput flow cytometry as previously reported.(28,29) For the purposes of this manuscript we will refer to the ABCG2 inhibitors CID44640177, CID1434724, and CID46245505 as 177, 724, and 505, respectively.
Chemotherapeutic Resistance, Cytotoxicity, and Inhibitor Efficacy Assays
Drug resistance was quantified by comparing the percent confluency of Igrov1/T8 cells and Igrov1 parental cells maintained in 950 nM TPT and the effective concentration of topotecan (TPT) that resulted in 50% cell death (EC50) of Igrov1 parental and Igrov1/T8 cells. To determine that Igrov1/T8 cells were resistant to TPT, Igrov1 parental and T8 cells were plated at 50% confluency on day 0 in a concentration of 950 nM TPT. Percent confluency was estimated under light microscopy everyday for 14 days. Once the Igrov1/T8 cells grew to 100% confluency (day 7) they were split down to 50% confluency on that day to prevent overgrowth and cell death. A conversion factor of 2 was used for Igrov1/T8 confluency on days 7 to 14 for an estimate of continued cell growth. To determine the EC50 of resistant and parental cells, Igrov1 Parental and ABCG2-overexpressing Igrov1/T8 cells were separately placed into 6-well plates such that the total number of cells in each well was approximately 1.5 × 105 in a total volume of 2 ml (7.5 × 104 cells/ml). TPT was added to the Igrov1 parental and Igrov1/T8 wells at increasing concentrations from 0 to 3 μM and cell viability was determined on days 3 and 7. Similar experiments were used to evaluate the cytotoxicity and efficacy of each ABCG2-selective inhibitor by examining the median lethal dose (TD50) or the half-maximal inhibitory concentration (IC50) of each compound.(28)
Colony Formation Assay
This assay was performed according to the Soft Agar Assay described by Provost and Wallert laboratories (Minnesota State University, Moorhead, MN) with minor modifications. Igrov1 Parental and Igrov1/T8 cells were plated at 1,250 cells/well in 24-well plates. The cells were treated with TPT, 177, 724, or 505 alone and in combinations of ABCG2 inhibitor with varying concentrations of TPT. Cells were then incubated under standard cell culture conditions at 37°C and 5% CO2 for 48 hours. Next, the cells were washed in PBS, trypsinized, centrifuged, and re-plated on top of agar mixed in a half RPMI with 20% FBS and half 1% agarose solution according to the protocol. After 14 days, colonies consisting of 20 or more cells were counted following staining with 0.005% crystal violet. The combination index (CI) values for each TPT/inhibitor combination were calculated according to the formula below:
| [1] |
Where, I1 and I2 represent the concentration of two different compounds that individually achieve X% target inhibition and CI1 and CI2 represent the concentration of those same compounds that achieve X% target inhibition in combination.
Microarray
Total RNA was amplified and reverse transcribed to cDNA using an Ambion WT Expression kit (Ambion), which was then fragmented and labeled using the Affymetrix GeneChip WT Terminal Labeling Kit (Affymetrix).(30) The resulting cDNA was then hybridized to a Human Gene Array 1.1 ST Array (Affymetrix) and subsequently washed and stained. Each step was carried out according to the manufacturer's protocol. The microarray gene chips were scanned using an AffyMetrix GeneChip Scanner 3000 and the normalized data were analyzed using GeneSpring software (Agilent). The data discussed in this publication have been deposited in NCBI's Gene Expression Omnibus (31) and are accessible through GEO Series accession number GSE85582.
Quantitative Reverse-Transcriptase PCR (qRT-PCR) and DNA Gel Electrophoresis
Total RNA was extracted from ovarian carcinoma mice xenografts, mouse liver, and Igrov1/T8 and Igrov1 parental cells in culture using the Qiagen RNeasy Mini Kit (Qiagen Inc., Valencia, CA). Purified, DNase-1 treated total RNA was then reverse transcribed using the Retroscript RT Kit (Ambion). ABCG2 was quantified using the Light Cycler SYBR Green I Master Real-Time PCR Mix (Roche Applied Science) in a 30 μL reaction. The primer sequences used for gene amplification were (ABCG2 sense – 5’-TGGCTGTCATGGCTTCAGTA-3’ and antisense – 5’-GCCACGTGATTCTTCCACAA-3’).(31) cDNA from mouse skeletal muscle was used as a negative control while cDNA from cultured Igrov1/T8 cells was used as a positive control. GAPDH was used as an endogenous control. qRT-PCR reactions were carried out on the LightCycler 480 Real-Time PCR System (Roche Applied Science) in technical triplicates using previously reported cycling parameters.(32) The second derivative max method was used to determine crossing points (Cp) of each amplification curve.(33) qRT-PCR cDNA products were also examined by 2% agarose gel electrophoresis using a Bio-Rad Sub-Cell GT Cell (Bio-Rad) according to the manufacturer's instructions.
Flow Cytometry
We previously demonstrated that the JC-1 fluorochrome is a substrate of ABC transporters.(34) Igrov1 Parental and Igrov1/T8 cells were stained and prepared for flow cytometry using the JC-1 Mitochondrial Membrane Potential Detection Kit (Cell Technology, Mountain View, CA) according to the manufacturer's instructions. Compound 177, 724, or 505 was added to one vial with stained Igrov1 Parental and Igrov1/T8 cells immediately prior to flow cytometry. Cells were then run on a Becton Dickinson FACScan automated flow cytometer including negative and positive controls.
Mice, Tumor Growth, and Drug Administration
CB-17 SCID mice were obtained from Charles River Laboratories, Wilmington, MA, and were allowed to acclimate to the animal facility for one week prior to use in the study. The mice were kept on a 12-hour light and dark cycle and had free access to food and water. Mice were handled in accordance with the Guide for Care and Use of Laboratory Animals under the approval of the Institutional Animal Care and Use Committee (IACUC). Approximately 7.5 × 106 - 1 × 107 Igrov1 parental cells or Igrov1/T8 cells suspended in 200 μL matrigel (BD Biosciences, Two Oak Park, Bedford, MA) was injected into each hindlimb region of the mice. Both hindlimbs were injected in some mice, while others had only one hindlimb injected with cells. Tumors formed between 2 and 4 weeks following injection. At approximately 4 weeks when the tumors reached our established detectable size range, 50 mm3 – 250 mm3, the tumor xenografts were injected with TPT at 0 and 24 hours by three different administration routes: intra-tumoral (IT), retro-orbital (RO), and intra-peritoneal (IP). 177, 724, and 505 were also injected at 48 and 72 hours by one of the three administration routes to verify MDR and non-toxicity of the tumor xenografts. Subsequently, TPT combined with the reversal agent was administered every 24 hours for 4-5 days or until tumors regressed below our limit of detection (~15 mm3). The dosage of drug for each injection was determined based on analysis of in vitro EC50, TD50, and IC50 results. Tumor measurements were acquired using a Scienceware® Digi-Max™ slide caliper (Sigma-Aldrich) and tumor volume was calculated by the equation: L*(W2/2), where L is the length in the rostral-caudal direction and W is the width measured transversely.(19,28) Statistical significance was evaluated using a two-tailed t-test and reported as a p-value. Mice were then euthanized and any remaining tumor was cut into histological sections, stained with H&E, and verified by light microscopy. Fibrosis and/or necrosis of remaining tumor sections was quantifiably measured by two investigators, initially blinded to each other's results, using a Lovin Field Finder Micro Slide grid (Electron Microscopy Sciences, Hatfield, PA). An average of both investigators’ measurements was calculated for each drug therapy group and incorporated into the size reductions of the tumors.
Results
Quantification of topotecan (TPT) resistance in Igrov1 cells
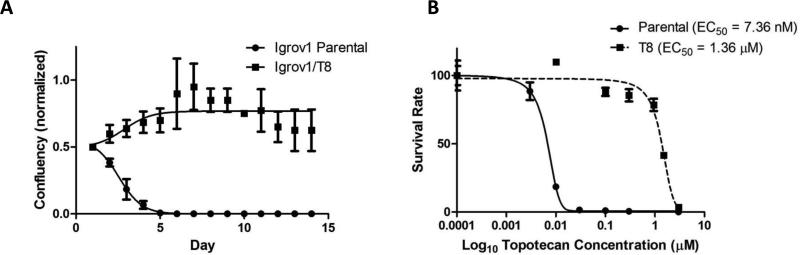
The cell line Igrov1 was previously made drug resistant to Asta Z and Adriamycin through sequential passage in increasing doses of those drugs and shown to be resistant to TPT by Maliepaard, et al.(35,36) In order to quantify the degree to which Igrov1/T8 was resistant to TPT, we performed two sets of experiments. First, we compared the growth of Igrov1/T8 versus the drug sensitive parental line, Igrov1, in a fixed concentration of TPT over a two-week period (Fig. 1A). In a concentration of 950nM TPT, cells were plated at 50% confluency and over 14 days Igrov1/T8 cells grew while the parental line showed 100% cell death by day 5. In the second set of experiments, we determined the concentration at which 50% of cells would die (EC50) after 7 days of culture using different concentrations of TPT (Fig. 1B). This analysis showed an EC50 of 1.4 μM for the drug resistant Igrov1/T8 in comparison to 7.4 nM for the Igrov1 parental cell line, a 189-fold difference.
Fig. 1. Growth and survival of Igrov1 and Igrov1/T8 cells in the presence of TPT.
(A) Igrov1 and Igrov1/T8 cells were incubated in the presence of 950 nM TPT and percent confluency was estimated under light microscopy over a period of 14 days. The resistant cells were split on day 7 due to cellular overgrowth. The average values with standard deviation are shown; n=3. (B) Igrov1 and Igrov1/T8 cells were incubated in the presence of 0 nM, 3 nM (Igrov1), 10 nM, 30 nM, 100 nM, 300 nM, 1 μM (Igrov1/T8), and 3 μM concentration of TPT. The cells in each concentration were counted on day 7 and the percent viability of the cells at each concentration was calculated normalizing to the cell count at 0 nM TPT. The EC50 was extrapolated by fitting the data to a sigmoidal dose response curve. Average values with standard deviation are shown; n=2.
TPT drug resistance is mediated by ABCG2
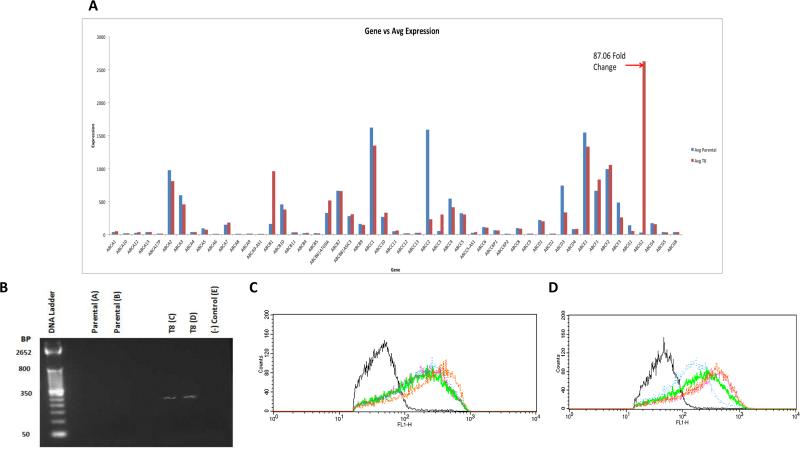
To determine the mechanism of drug resistance, several experiments were performed. First, we examined the expression of all known ABC proteins in both the Igrov1 parental and Igrov1/T8 drug resistant cell lines using microarray analysis (Fig. 2A). Comparison of the array data indicated that ABCG2 was the most differentially expressed, having an 87-fold increase in Igrov1/T8 expression in comparison to Igrov1. This differential expression was confirmed by RT-PCR (Fig. 2B).
Fig. 2. ABCG2 Expression and Functional Efflux of Igrov1 and Igrov1/T8 Cells.
(A) Gene chip expression. Total RNA was isolated from Igrov1 and Igrov1/T8 cell lies and was hybridized to a human gene chip array. Expression of the known ABC transporters was examined for each cell line. The ABC expression profile of Igrov1 Parental and Igrov1/T8 cells is shown. (B) Real-time PCR. Igrov1 and Igrov1/T8 total RNA was reverse transcribed using ABCG2 primers and the cDNA products were amplified by qRT-PCR. cDNA products were examined using DNA gel electrophoresis. Double distilled water with primers was used as the negative control. Fluorescence corresponding to ABCG2 cDNA is shown. (C) (D) Functional analysis of ABCG2 activity. Igrov1 (C) and Igrov1/T8 (D) cells were loaded with JC-1 and examined for whether JC-1 was extruded in the presence and absence of 177, 724, and 505. ABCG2 inhibitors 177, 724, and 505 were added to cells stained with JC-1 and are represented by the orange, green, and pink lines, respectively. Cells loaded with the JC-1 stain alone (blue) were compared to cells alone without JC-1 (black).
We then performed a functional efflux assay using JC-1, a fluorochrome that is extruded through ABCG2.(34) The relative fluorescence corresponding to the presence (right shift) or absence (left shift) of JC-1 was measured using flow cytometry. Accordingly, ABCG2 Igrov1 parental cells had a higher amount of fluorescence than ABCG2 overexpressing Igrov1/T8 cells, indicating retention of JC-1 in Igrov1 parental cells and extrusion of JC-1 in Igrov1/T8 cells. Following the addition of each ABCG2 antagonist, JC-1 retention increased inside the Igrov1/T8 cells. Fluorescence of the Igrov1 parental cells was essentially unaffected by the presence of any of the ABCG2 inhibitors. These results indicate that ABCG2 efflux activity is reversed by the presence of 177, 724, and 505 (Fig. 2C and D).
Novel chemosensitizers reverse ABCG2-mediated drug resistance in vitro
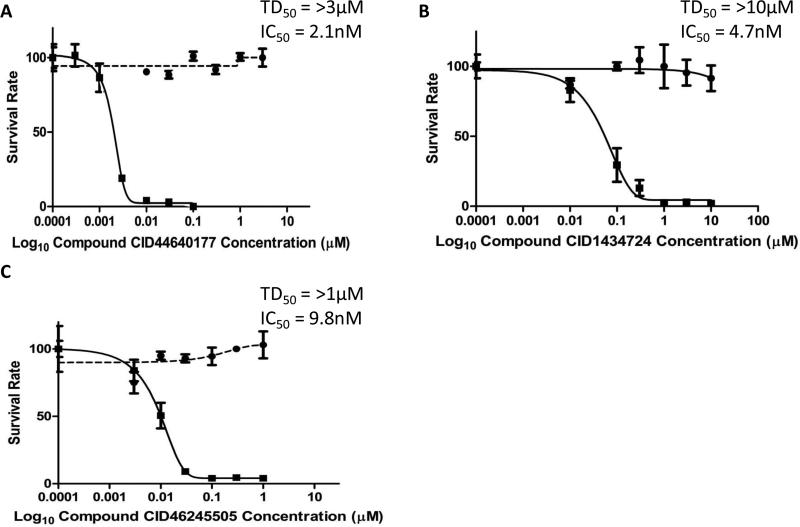
We have previously identified three potential compounds (177, 724, and 505) that inhibit ABCG2 activity.(28,29) In order to determine if these compounds restored chemosensitivity to the drug resistant cells, we measured the cell viability of Igrov1/T8 cells in the presence of increasing concentrations of these chemosensitizers and a fixed concentration of 100 nM TPT (Fig. 3). This concentration of TPT, if used alone, results in 100% cell death of the parental cell line, but has no effect on the survival of the chemoresistant Igrov1/T8 cell line. 177, 724, and 505 showed an ability to restore chemosensitivity as measured by the concentration at which there was 50% cell death (half maximal inhibitory concentration; IC50). The doses of each ABCG2 inhibitor were escalated to a point where near 100% cell death was observed after 7 days. Compound 177 was the most potent ABCG2 antagonist resulting in an IC50 of 2.1 nM. Although, compounds 724 and 505 had similar potency indicated by their IC50's of 4.7 nM and 9.8 nM, respectively.
Fig. 3. Half Maximal Inhibitory Concentrations (IC50) for ABCG2 Antagonists in the Presence of TPT.
Igrov1/T8 cells were incubated with varying amounts of 177 (A), 724 (B), or 505 (C) and 100 nM TPT (–). The median toxic dose (TD50) was also reported by incubating Igrov1/T8 cells in 177 (A), 724 (B), or 505 (C) alone (--). Cell viability and the IC50 were determined on day 7. Average values with standard deviation are shown, n = 2.
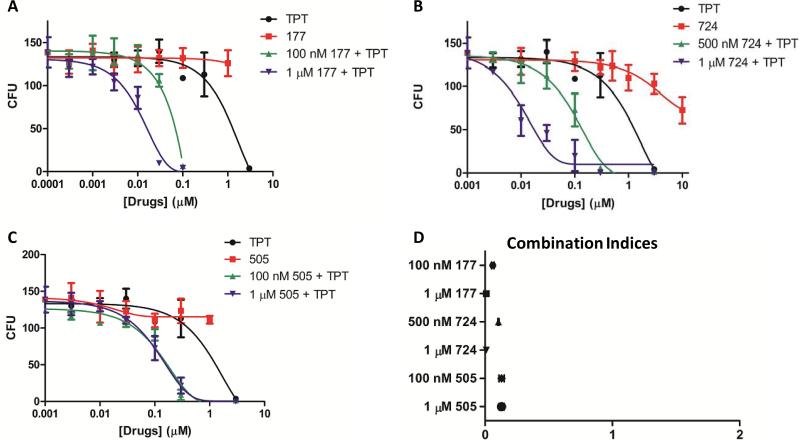
Additionally, we performed a colony formation assay with chemoresistant Igrov1/T8 cells pre-treated with TPT in combination with an ABCG2 inhibitor for 48 hours (Fig. 4). TPT in combination with compound 177, 724, or 505 resulted in fewer colonies at lower concentrations of TPT than TPT alone (4-91 colonies vs 109 colonies at 100 nM TPT). Therefore, ABCG2 inhibitors lower the dose of TPT required to inhibit the growth of TPT-resistant Igrov1/T8 cells. Combination index is typically used to demonstrate that two drugs work synergistically. In our case, the compounds are blocking the ability of a cell to exude TPT. Therefore, our combination index values indicate the relative effectiveness of these compounds to reverse chemoresistance. Combination index values were calculated for each combination of TPT and ABCG2 inhibitor and all were less than 1, indicating their ability to restore chemosensitivity by inhibiting ABCG2 (Fig. 4). Non-resistant Igrov1 cells grew few to no colonies in the presence of greater than or equal to 10 nM TPT alone and in combination with 177, 724, or 505, whereas exposure to 177, 724, or 505 alone resulted in up to 203 viable colonies which decreased to lows of 120, 11, and 112 colonies when exposed to the highest concentrations of 177, 724, and 505 administered: 1 μM, 10 μM, and 1 μM, respectively (data not shown).
Fig. 4. Colony Formation and Combination Indices Following Pre-treatment of Igrov1/T8 Cells with TPT and ABCG2 Inhibitors.
Igrov1/T8 cells were pre-treated with varying concentrations of TPT and 177 (A), 724 (B), or 505 (C) for 48 hours. The cells were then plated on agar and incubated. After 14 days, the colonies consisting of 20 or more cells were counted (n = 3). The combination index values for each combination were calculated (D).
In order to insure that our results were not due to toxicity produced by increasing doses of the chemosensitizer, we also examined the cell viability over a range of chemosensitizer concentrations and determined that the concentrations at which there was 50% cell death (e.g. median toxic dose; TD50) was greater than the concentrations examined. In all cases the TD50 was greater than 1 μM and at least 100-fold the IC50, indicating that the chemosensitizers were non-toxic (data not shown).
Chemosensitizers have potent in vivo effects
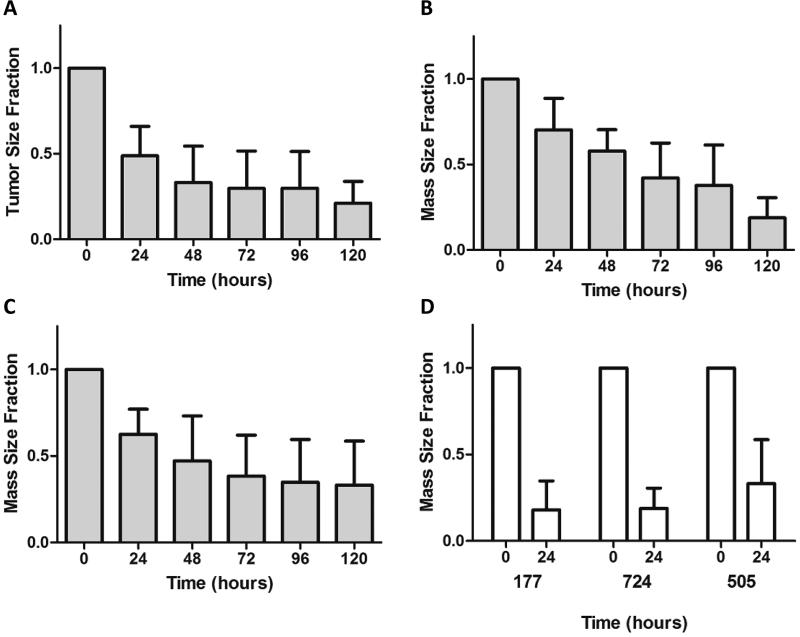
As a first step to demonstrate that these compounds would have efficacy in vivo, chemosensitizers and TPT were administered conjunctively by intratumoral (IT) injection over a 5-day period in a murine ovarian cancer xenograft model.(19) By this time, an average reduction in tumor size in excess of 60% was measured in all cases (Fig. 5). When 177, 724, and 505 were injected alone everyday for 3 days at therapeutic concentrations (100 nM, 500 nM, and 100 nM, respectively), they did not have any effect on tumor size (data not shown). Additionally, the mice did not exhibit any significant weight changes or other observable toxic side effects over the course of therapy with the ABCG2 inhibitors, TPT, or their combination.
Fig. 5. Intratumoral Injection of TPT in Combination with 177, 724, or 505 into Igrov1/T8 Xenografts.
Igrov1/T8 cells were injected into the hind limbs of CB-17 SCID mice.(19) Once the cells had grown to the appropriate size range (50-250 mm3), TPT in conjunction with (A) 177, (B) 724, or (C) 505 was injected intratumorally. The size of the tumor mass was measured every 24 hours over a period of 120 hours. Average values with standard deviation for each compound are shown, n = 5. (D) The size of the tumor mass immediately prior to the first injection (0 hours) and the size at 120 hours were compared.
IT and IP administrations are the most effective routes
Since chemosensitizers administered in combination with TPT were effective at reducing tumor size, we then sought to determine the most effective route of administration. We administered chemosensitizer combined with TPT by IT, RO, and IP injection everyday for 5 days. IT and IP routes of administration showed the most effective tumor size reductions by day 5 ranging from 62-66% and 16-69%, respectively (Table 1), with the most tumor reduction achieved by administration of 724 by IT and 505 by IP.
Table 1.
Average mass reduction of Igrov1/T8 xenografts after various routes of combination drug administration with and without accounting for fibrosis
| Administration Route | Dosage |
N | Average Mass Reduction after 96 hours (%) | SEM | P-value | |||
|---|---|---|---|---|---|---|---|---|
| TPT | CID44640177 | CID1434724 | CID46245505 | |||||
| IT | 100 nM | 100 nM | 9 | 64 | 8.01 | <0.01 | ||
| 100 nM | 500 nM | 8 | 66 | 8.83 | <0.01 | |||
| 100 nM | 100 nM | 6 | 62 | 8.54 | <0.01 | |||
| RO | 100 nM | 1 μM | 4 | 13 | 4.52 | 0.04 | ||
| 100 nM | 1 μM | 4 | 14 | 15.92 | 0.42 | |||
| 100 nM | 1 μM | 5 | 2 | 7.31 | 0.78 | |||
| IP | 300 nM | 100 nM | 5 | 16 | 1.08 | <0.01 | ||
| 300 nM | 500 nM | 3 | 52 | 11.06 | 0.01 | |||
| 300 nM | 100 nM | 3 | 69 | 10.33 | <0.01 | |||
| Administration Route | Dosage |
N | Average Reduction after 96 hours adjusted for Fibrosis of Remaining Mass (%) | SEM | P-value | |||
|---|---|---|---|---|---|---|---|---|
| TPT | CID44640177 | CID1434724 | CID46245505 | |||||
| IT | 100 nM | 100 nM | 8 | 71 | 7.24 | <0.01 | ||
| 100 nM | 500 nM | 8 | 80 | 7.69 | <0.01 | |||
| 100 nM | 100 nM | 8 | 72 | 6.11 | <0.01 | |||
| RO | 100 nM | 1 μM | 4 | 51 | 4.98 | <0.01 | ||
| 100 nM | 1 μM | 5 | 35 | 9.67 | 0.01 | |||
| 100 nM | 1 μM | 5 | 27 | 11.24 | 0.04 | |||
| IP | 300 nM | 100 nM | 5 | 51 | 7.44 | <0.01 | ||
| 300 nM | 500 nM | 3 | 77 | 4.86 | <0.01 | |||
| 300 nM | 100 nM | 3 | 74 | 10.66 | <0.01 | |||
In order to verify that residual masses were tumor and/or fibrosis, we examined each tumor histologically. When viewed by light microscopy, all residual masses contained substantial necrosis and fibrosis in addition to some tumor. The most substantial amount was seen in the IT injection group, although the largest amount of change in tumor size reduction was seen in the RO group (2-14% to 27-51%) (Table 1). We also normalized mass reduction for fibrosis and/or necrosis and found that the amount of remaining tumor was significantly less than what was measured grossly, especially by the RO and IP administration routes. By this analysis, all routes of administration resulted in significant tumor reduction (all p-values < 0.05) with compound 724 resulting in the most reduction by the IT and IP administration, at 80% and 77%, respectively.
Discussion
MDR remains a large barrier to improved patient outcomes in ovarian cancer. ABCG2 contributes to MDR by active efflux of chemotherapeutic substrates, including TPT, and is preferentially overexpressed in TPT-resistant ovarian carcinoma more than 1000-fold compared to other ABC transporters.(1,13) The importance of ABCG2 in ovarian cancer is further emphasized by the fact that certain polymorphisms lead to a shorter progression-free survival time, although the functional effects of these polymorphisms remain unknown.(17) This indicates that ABCG2 expression may play an important role in clinical outcomes and survival, making it an important target for pharmacotherapy in MDR ovarian cancer.
In the present study, we examined the role of three novel piperazine-substituted pyrazolo[1,5]pyrimidine substructure ABCG2 antagonists that we previously identified(28,29) and their role in restoring chemosensitivity to MDR ovarian cancer. Compounds 177, 724, and 505 were found to effectively inhibit ABCG2-mediated efflux of TPT in ovarian cancer cells with sub-10nM IC50s, suggesting that each compound is a potent ABCG2-antagonist drug candidate. The three other ABCG2 inhibitors, Fumitremorgin C, Ko143, and GF120918, that have been investigated in TPT-resistant ovarian cancer in vivo all have IC50's near the micromolar range.(18,19) Following the administration of our ABCG2 antagonists with TPT by varying routes, TPT-resistant ovarian carcinoma xenografts demonstrated significant tumor reduction (p < 0.05). In our initial observations, only the size of the mass was recorded. However, substantial tumor necrosis and fibrosis was observed on histology and taken into account when quantifying overall tumor reduction, leading to much larger overall reductions than could be grossly observed.
The relative IC50's of each drug tested here were similar, but showed greater differences in their in vivo potencies. All were in the sub-ten nanomolar range and effectively inhibited ABCG2-mediated efflux of TPT, but their potencies in vivo did not directly correlate with those observed in vitro. We used a sigmoidal curve fit by a nonlinear regression technique to calculate the IC50 values, which closely reflects the behavior of biological dose-response systems.(37,38) Although small inherent error exists in this model, it may be improved by increasing the number of data points. IC50 values may not have a one-to-one correspondence in vivo due to potential nonlinear pharmacokinetics, extensive first pass metabolism, or pharmacodynamics that alter bioavailability.(39) Multiple studies have reported differences between in vitro and in vivo efficacy of antitumor drugs in ovarian cancer and ABCG2-mediated resistance.(40–42) Predicting the correlation between in vitro and in vivo activity remains challenging due to these potential confounding factors, and the variation in potency in vivo that was observed in this study is likely related to pharmacokinetics, pharmacodynamics, or other issues beyond their IC50's.(43) While a full biological analysis and comparison of the isogenic cell lines would provide additional information into other potential molecular differences, our study was focused on the effects of our most promising compounds.
Compounds 177, 724, and 505 did not appear to promote overt toxicity. The TD50 values from our previous work(28,29) suggest that these compounds were suitable for use in vivo because of their apparent low toxicity at the doses used for administration. No matter how high we escalated the dose of compound 177, 724, and 505 alone, we did not see any tissue necrosis or death. Additionally, we did not observe any tremors or convulsions in the mice that would have been suggestive of neurotoxicity as was previously reported with fumitremorgin C.(18) We did not observe any significant weight loss and histological evaluation of the liver, heart, kidneys, and bone marrow did not show any signs of necrosis, suggesting the absence of any significant systemic side effects. Thus, these compounds appear to carry a low toxicity risk, which is important when considering their addition to a relatively toxic chemotherapeutic regimen.
Targeting ABCG2 represents a less-studied mechanism for restoration of chemosensitivity in MDR ovarian cancer. Currently, only about 20% of recurrent tumors respond to second-line therapy.(1,2,7) Despite the fact that ABCG2 has been well described as the key mechanism for resistance of ovarian cancer to TPT,(1,13,44) relatively few attempts to develop drugs at this important target have been made. Moreover, the compounds that have been created and tested in ovarian cancer have not progressed, typically due to toxicity.(16,18–20,45) Both Fumitremorgin C and its analogue, Ko143, were discovered to inhibit ABCG2 and tested in ovarian carcinoma murine models. However, Fumitremorgin C is neurotoxic in vivo and therefore not suitable for use in the clinic.(19) Though no toxicity has been described with Ko143, it is structurally similar to Fumitremorgin C and concern for in vivo toxicity may hinder its clinical use.(18) The only compounds inhibiting ABCG2 efflux that have been tested in clinical trials for ovarian cancer are GF120918 (Elacridar) and Lapatinib.(16,45) Elacridar was found to increase TPT bioavailability, however it was not shown to affect tumor burden in its phase I trial. Lapatinib, which is primarily a tyrosine kinase inhibitor with some effect on inhibiting ABCG2-mediated efflux, has been well-studied with its potential to reverse MDR.(16,32,46) Lapatinib is currently the only compound affecting ABCG2 activity that has been examined in phase II clinical trial for recurrent ovarian cancer.(16) However, the trial was canceled due to substantial adverse hematologic events and no observed benefit. Furthermore, antitumor effects of ABCG2 antagonists, Elacridar and WK-X-34, explored in ovarian cancer were both found to act primarily through inhibition of ABCB1 (P-gp).(20,22,25) While both ABCB1 and ABCC1 are important in mediating MDR, they do not play as large a role in TPT-resistant ovarian cancer as does ABCG2. Even though the compounds in this study have some activity toward ABCB1 at high concentrations, the concentrations used in the present study were far below these levels and the compounds would thus be expected to be specific for ABCG2. Though ABCB1 has a higher level of expression in the drug-resistant Igrov1/T8 cell line as compared to the Igrov1 cell line, we previously reported that compounds 177, 724, and 505 are more selective for ABCG2 over ABCB1 using a JC-1 fluorescent dye substrate marker.(28,29) Therefore, the increased level of expression of ABCB1 is not expected to account for the chemoreversal observed. Since ABCB1 helps to protect a variety of tissues, such as the bone marrow stem cells, from the toxic adverse effects of chemotherapeutics, ABCG2-selective inhibitors may be preferred in some clinical applications.
Notably, our studies compare different routes of administration and their effect on reduction in tumor growth. While route of administration may have a substantial effect on tumor growth inhibition, this level of comparison is not frequently reported.(47) Of seventeen in vivo studies investigating potential ABCG2 inhibitors for various types of cancer, only three compared two different routes of administration, namely oral (PO) to either IV or IP.(18,25,48) Furthermore, no studies investigating ABCG2 in vivo have compared the antitumor effects of three different routes of administration. In our formal comparison of IT, IP, and RO routes of administration the greatest antitumor effect was achieved by IT and IP. Only one study has compared PO with IP using Ko143 as the ABCG2 antagonist and did not report differences in the antitumor effect, but only described that Ko143 was nontoxic by both routes and enhanced TPT bioavailability by PO.(18) This group also described that oral bioavailability of TPT was enhanced with PO GF120918 administration. These results suggest that the PO route may lead to predominant inhibition of intestinal ABCG2, with less of the drug available for targeting ABCG2 overexpression in the tumor. Therefore, IP may be a preferred route to PO for reversing chemoresistance. It is well known that material delivered via IP injection is absorbed much slower than by IV injection,(47) and in peritoneal malignancies, such as ovarian carcinoma, this allows the tumor to be exposed to the drug therapy for a longer period of time with less systemic effects. Consequently, first pass metabolism might not play as large of a role with IP injections, whereas the first pass metabolism seen with the PO route might still affect drug efficacy. On the other hand, our study used subcutaneous hindlimb xenografts for enhanced accessibility and ease of tumor measurements during the treatment course. Therefore, altered pharmacokinetics, pharmacodynamics, or first pass metabolism might have contributed to the differences that we observed. Alternatively, lower doses of inhibitor reaching the tumor xenograft following systemic absorption may have been sufficient to result in tumor death. In general, a more dramatic effect is seen with IP administration in the treatment of ovarian cancer when compared to IV.(49–51) Thus, IP remains the administrative route of choice in ovarian cancer and is a reasonable route of administration to use for the compounds described here.
Each compound presented here appears suitable for GMP-based preclinical studies and first in human studies due to their high in vivo potency, low IC50's, and low toxicity. All compounds were able to effectively reverse ABCG2-mediated TPT-resistance with compound 724 resulting in the largest tumor reduction by both IT and IP routes of administration. However, IP administration would be the most useful since IT administration is not clinically practical. Future studies should focus on development of compound 724 for use in human clinical trial with recurrent ovarian carcinoma failing second-line treatment, which encompasses approximately 80% of women with recurrent disease.(1,2,7) Due to its unique structure and action against ABCG2, the addition of compound 724 to a chemotherapeutic regimen for TPT-resistant ovarian cancer may allow for increased rates of clinical remission and progression-free survival in this lethal disease.
Acknowledgements
The University of Kansas Specialized Chemistry Center synthesized compounds CID44640177, CID1434724, and CID46245505 for our previous project and these were used in the present study.
Financial Support: National Institutes of Health Clinical and Translational Science Award grant 5UL1TR000041-05, R.S. Larson
Footnotes
The authors have no potential conflicts of interest.
References
- 1.Januchowski R, Wojtowicz K, Sujka-Kordowska P, Andrzejewska M, Zabel M. MDR gene expression analysis of six drug-resistant ovarian cancer cell lines. Biomed Res Int. 2013;2013:241763. doi: 10.1155/2013/241763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tewari KS, Mehta RS, Burger RA, Yu I-R, Kyshtoobayeva AS, Monk BJ, et al. Conservation of in vitro drug resistance patterns in epithelial ovarian carcinoma. Gynecol Oncol. 2005;98:360–8. doi: 10.1016/j.ygyno.2005.04.036. [DOI] [PubMed] [Google Scholar]
- 3.Materna V, Pleger J, Hoffmann U, Lage H. RNA expression of MDR1/P-glycoprotein, DNA-topoisomerase I, and MRP2 in ovarian carcinoma patients: correlation with chemotherapeutic response. Gynecol Oncol. 2004;94:152–60. doi: 10.1016/j.ygyno.2004.03.035. [DOI] [PubMed] [Google Scholar]
- 4.Yakirevich E, Sabo E, Naroditsky I, Sova Y, Lavie O, Resnick MB. Multidrug resistance-related phenotype and apoptosis-related protein expression in ovarian serous carcinomas. Gynecol Oncol. 2006;100:152–9. doi: 10.1016/j.ygyno.2005.08.050. [DOI] [PubMed] [Google Scholar]
- 5.Bookman MA. First-line chemotherapy in epithelial ovarian cancer. Clin Obstet Gynecol. 2012;55:96–113. doi: 10.1097/GRF.0b013e31824b45da. [DOI] [PubMed] [Google Scholar]
- 6.Morgan RJ, Alvarez RD, Armstrong DK, Burger RA, Chen L, Copeland L, et al. Ovarian cancer, version 2.2013. J Natl Compr Canc Netw. 2013;11:1199–209. doi: 10.6004/jnccn.2013.0142. [DOI] [PubMed] [Google Scholar]
- 7.Arts HJ, Katsaros D, de Vries EG, Massobrio M, Genta F, Danese S, et al. Drug resistance-associated markers P-glycoprotein, multidrug resistance-associated protein 1, multidrug resistance-associated protein 2, and lung resistance protein as prognostic factors in ovarian carcinoma. Clin Cancer Res. 1999;5:2798–805. [PubMed] [Google Scholar]
- 8.Bagnoli M, Beretta GL, Gatti L, Pilotti S, Alberti P, Tarantino E, et al. Clinicopathological impact of ABCC1/MRP1 and ABCC4/MRP4 in epithelial ovarian carcinoma. Biomed Res Int. 2013;2013:143202. doi: 10.1155/2013/143202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Honorat M, Falson P, Terreux R, Di Pietro A, Dumontet C, Payen L. Multidrug resistance ABC transporter structure predictions by homology modeling approaches. Curr Drug Metab. 2011;12:268–77. doi: 10.2174/138920011795101804. [DOI] [PubMed] [Google Scholar]
- 10.Szakács G, Paterson JK, Ludwig JA, Booth-Genthe C, Gottesman MM. Targeting multidrug resistance in cancer. Nat Rev Drug Discov. 2006;5:219–34. doi: 10.1038/nrd1984. [DOI] [PubMed] [Google Scholar]
- 11.Robey RW, Massey PR, Amiri-Kordestani L, Bates SE. ABC transporters: unvalidated therapeutic targets in cancer and the CNS. Anticancer Agents Med Chem. 2010;10:625–33. doi: 10.2174/187152010794473957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rottenberg S, Borst P. Drug resistance in the mouse cancer clinic. Drug Resist Updat. 2012;15:81–9. doi: 10.1016/j.drup.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 13.Maliepaard M, van Gastelen MA, de Jong LA, Pluim D, van Waardenburg RC, Ruevekamp-Helmers MC, et al. Overexpression of the BCRP/MXR/ABCP gene in a topotecan-selected ovarian tumor cell line. Cancer Res. 1999;59:4559–63. [PubMed] [Google Scholar]
- 14.Stacy AE, Jansson PJ, Richardson DR. Molecular pharmacology of ABCG2 and its role in chemoresistance. Mol Pharmacol. 2013;84:655–69. doi: 10.1124/mol.113.088609. [DOI] [PubMed] [Google Scholar]
- 15.Nakanishi T, Ross DD. Breast cancer resistance protein (BCRP/ABCG2): its role in multidrug resistance and regulation of its gene expression. Chin J Cancer. 2012;31:73–99. doi: 10.5732/cjc.011.10320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weroha SJ, Oberg AL, Ziegler KLA, Dakhilm SR, Rowland KM, Hartmann LC, et al. Phase II trial of lapatinib and topotecan (LapTop) in patients with platinum- refractory/resistant ovarian and primary peritoneal carcinoma. Gynecol Oncol. 2011;122:116–20. doi: 10.1016/j.ygyno.2011.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tian C, Ambrosone CB, Darcy KM, Krivak TC, Armstrong DK, Bookman MA, et al. Common variants in ABCB1, ABCC2 and ABCG2 genes and clinical outcomes among women with advanced stage ovarian cancer treated with platinum and taxane-based chemotherapy: a Gynecologic Oncology Group study. Gynecol Oncol. 2012;124:575–81. doi: 10.1016/j.ygyno.2011.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Allen JD, van Loevezijn A, Lakhai JM, van der Valk M, van Tellingen O, Reid G, et al. Potent and specific inhibition of the breast cancer resistance protein multidrug transporter in vitro and in mouse intestine by a novel analogue of fumitremorgin C. Mol Cancer Ther. 2002;1:417–25. [PubMed] [Google Scholar]
- 19.Garimella TS, Ross DD, Eiseman JL, Mondick JT, Joseph E, Nakanishi T, et al. Plasma pharmacokinetics and tissue distribution of the breast cancer resistance protein (BCRP/ABCG2) inhibitor fumitremorgin C in SCID mice bearing T8 tumors. Cancer Chemother Pharmacol. 2005;55:101–9. doi: 10.1007/s00280-004-0866-2. [DOI] [PubMed] [Google Scholar]
- 20.Jekerle V, Klinkhammer W, Scollard DA, Breitbach K, Reilly RM, Piquette-Miller M, et al. In vitro and in vivo evaluation of WK-X-34, a novel inhibitor of P-glycoprotein and BCRP, using radio imaging techniques. Int J Cancer. 2006;119:414–22. doi: 10.1002/ijc.21827. [DOI] [PubMed] [Google Scholar]
- 21.Jekerle V, Klinkhammer W, Reilly RM, Piquette-Miller M, Wiese M. Novel tetrahydroisoquinolin-ethyl-phenylamine based multidrug resistance inhibitors with broad-spectrum modulating properties. Cancer Chemother Pharmacol. 2007;59:61–9. doi: 10.1007/s00280-006-0244-3. [DOI] [PubMed] [Google Scholar]
- 22.Maliepaard M, van Gastelen MA, Tohgo A, Hausheer FH, van Waardenburg RC, de Jong LA, et al. Circumvention of breast cancer resistance protein (BCRP)- mediated resistance to camptothecins in vitro using non-substrate drugs or the BCRP inhibitor GF120918. Clin Cancer Res. 2001;7:935–41. [PubMed] [Google Scholar]
- 23.Juvale K, Stefan K, Wiese M. Synthesis and biological evaluation of flavones and benzoflavones as inhibitors of BCRP/ABCG2. Eur J Med Chem. 2013;67:115–26. doi: 10.1016/j.ejmech.2013.06.035. [DOI] [PubMed] [Google Scholar]
- 24.Qadir M, O'Loughlin KL, Fricke SM, Williamson NA, Greco WR, Minderman H, et al. Cyclosporin A is a broad-spectrum multidrug resistance modulator. Clin Cancer Res. 2005;11:2320–6. doi: 10.1158/1078-0432.CCR-04-1725. [DOI] [PubMed] [Google Scholar]
- 25.Hyafil F, Vergely C, Du Vignaud P, Grand-Perret T. In vitro and in vivo reversal of multidrug resistance by GF120918, an acridonecarboxamide derivative. Cancer Res. 1993;53:4595–602. [PubMed] [Google Scholar]
- 26.Peng LH, Chen XY, Wu TX. Topotecan for ovarian cancer. Cochrane Database Syst Rev. 2008:CD005589. doi: 10.1002/14651858.CD005589.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ahmad T, Gore M. Review of the use of topotecan in ovarian carcinoma. Expert Opin Pharmacother. 2004;5:2333–40. doi: 10.1517/14656566.5.11.2333. [DOI] [PubMed] [Google Scholar]
- 28.Strouse JJ, Ivnitski-Steele I, Khawaja HM, Perez D, Ricci J, Yao T, et al. A selective ATP-binding cassette subfamily G member 2 efflux inhibitor revealed via high-throughput flow cytometry. J Biomol Screen. 2013;18:26–38. doi: 10.1177/1087057112456875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Strouse JJ, Ivnitski-Steele I, Njus HM, Foutz TD, Yao T, Weiner WS, et al. Selective Efflux Inhibition of ATP-binding Cassette Sub-family G Member 2. Probe Reports from the NIH Molecular Libraries Program [Internet] National Center for Biotechnology Information (US); Bethesda (MD): 2010. [2015 Sep 17]. Available from: http://www.ncbi.nlm.nih.gov/books/NBK133425/ [PubMed] [Google Scholar]
- 30.Estes DA, Lovato DM, Khawaja HM, Winter SS, Larson RS. Genetic alterations determine chemotherapy resistance in childhood T-ALL: modelling in stage- specific cell lines and correlation with diagnostic patient samples. British Journal of Haematology. 2007;139:20–30. doi: 10.1111/j.1365-2141.2007.06763.x. [DOI] [PubMed] [Google Scholar]
- 31.Edgar R, Domrachev M, Lash AE. Gene expression omnibus: NCBI gene expression and hybridization array data repository. Nucleic Acids Res. 2002;30:207–10. doi: 10.1093/nar/30.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dai C, Tiwari AK, Wu C-P, Su X-D, Wang S-R, Liu D, et al. Lapatinib (Tykerb, GW572016) reverses multidrug resistance in cancer cells by inhibiting the activity of ATP-binding cassette subfamily B member 1 and G member 2. Cancer Res. 2008;68:7905–14. doi: 10.1158/0008-5472.CAN-08-0499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Trujillo KA, Hines WC, Vargas KM, Jones AC, Joste NE, Bisoffi M, et al. Breast field cancerization: isolation and comparison of telomerase-expressing cells in tumor and tumor adjacent, histologically normal breast tissue. Mol Cancer Res. 2011;9:1209–21. doi: 10.1158/1541-7786.MCR-10-0424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Strouse JJ, Ivnitski-Steele I, Waller A, Young SM, Perez D, Evangelisti AM, et al. Fluorescent substrates for flow cytometric evaluation of efflux inhibition in ABCB1, ABCC1, and ABCG2 transporters. Anal Biochem. 2013;437:77–87. doi: 10.1016/j.ab.2013.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bénard J, Da Silva J, De Blois MC, Boyer P, Duvillard P, Chiric E, et al. Characterization of a human ovarian adenocarcinoma line, IGROV1, in tissue culture and in nude mice. Cancer Res. 1985;45:4970–9. [PubMed] [Google Scholar]
- 36.Maliepaard M, Scheffer GL, Faneyte IF, van Gastelen MA, Pijnenborg AC, Schinkel AH, et al. Subcellular localization and distribution of the breast cancer resistance protein transporter in normal human tissues. Cancer Res. 2001;61:3458–64. [PubMed] [Google Scholar]
- 37.Lyles RH, Poindexter C, Evans A, Brown M, Cooper CR. Nonlinear model-based estimates of IC(50) for studies involving continuous therapeutic dose-response data. Contemp Clin Trials. 2008;29:878–86. doi: 10.1016/j.cct.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Parellada J, Guinea M. Flavonoid Inhibitors of Trypsin and Leucine Aminopeptidase: A Proposed Mathematical Model for IC50 Estimation. J Nat Prod. 1995;58:823–9. doi: 10.1021/np50120a001. [DOI] [PubMed] [Google Scholar]
- 39.Qiu Y, Li X, Duan JZ. Influence of drug property and product design on in vitro-in vivo correlation of complex modified-release dosage forms. J Pharm Sci. 2014;103:507–16. doi: 10.1002/jps.23804. [DOI] [PubMed] [Google Scholar]
- 40.Zhou X-F, Yang X, Wang Q, Coburn RA, Morris ME. Effects of dihydropyridines and pyridines on multidrug resistance mediated by breast cancer resistance protein: in vitro and in vivo studies. Drug Metab Dispos. 2005;33:1220–8. doi: 10.1124/dmd.104.003558. [DOI] [PubMed] [Google Scholar]
- 41.Stakleff KS, Sloan T, Blanco D, Marcanthony S, Booth TD, Bishayee A. Resveratrol exerts differential effects in vitro and in vivo against ovarian cancer cells. Asian Pac J Cancer Prev. 2012;13:1333–40. doi: 10.7314/apjcp.2012.13.4.1333. [DOI] [PubMed] [Google Scholar]
- 42.Holschneider CH, Johnson MT, Knox RM, Rezai A, Ryan WJ, Montz FJ. Bullatacin-- in vivo and in vitro experience in an ovarian cancer model. Cancer Chemother Pharmacol. 1994;34:166–70. doi: 10.1007/BF00685935. [DOI] [PubMed] [Google Scholar]
- 43.Wienkers LC, Heath TG. Predicting in vivo drug interactions from in vitro drug discovery data. Nat Rev Drug Discov. 2005;4:825–33. doi: 10.1038/nrd1851. [DOI] [PubMed] [Google Scholar]
- 44.Jia P, Wu S, Li F, Xu Q, Wu M, Chen G, et al. Breast cancer resistance protein– mediated topotecan resistance in ovarian cancer cells. International Journal of Gynecological Cancer. 2005;15:1042–8. doi: 10.1111/j.1525-1438.2005.00260.x. [DOI] [PubMed] [Google Scholar]
- 45.Kuppens IELM Witteveen EO, Jewell RC Radema SA, Paul EM Mangum SG, et al. A phase I, randomized, open-label, parallel-cohort, dose-finding study of elacridar (GF120918) and oral topotecan in cancer patients. Clin Cancer Res. 2007;13:3276–85. doi: 10.1158/1078-0432.CCR-06-2414. [DOI] [PubMed] [Google Scholar]
- 46.Ma S, Hu Y, Wang F, Huang Z, Chen Y, Wang X, et al. Lapatinib antagonizes multidrug resistance-associated protein 1-mediated multidrug resistance by inhibiting its transport function. Mol Med. 2014;20:390–9. doi: 10.2119/molmed.2014.00059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Turner PV, Brabb T, Pekow C, Vasbinder MA. Administration of Substances to Laboratory Animals: Routes of Administration and Factors to Consider. J Am Assoc Lab Anim Sci. 2011;50:600–13. [PMC free article] [PubMed] [Google Scholar]
- 48.Yamazaki R, Nishiyama Y, Furuta T, Hatano H, Igarashi Y, Asakawa N, et al. Novel acrylonitrile derivatives, YHO-13177 and YHO-13351, reverse BCRP/ABCG2-mediated drug resistance in vitro and in vivo. Mol Cancer Ther. 2011;10:1252–63. doi: 10.1158/1535-7163.MCT-10-0874. [DOI] [PubMed] [Google Scholar]
- 49.De Cesare M, Calcaterra C, Pratesi G, Gatti L, Zunino F, Mènard S, et al. Eradication of ovarian tumor xenografts by locoregional administration of targeted immunotherapy. Clin Cancer Res. 2008;14:5512–8. doi: 10.1158/1078-0432.CCR-08-0445. [DOI] [PubMed] [Google Scholar]
- 50.Shah DK, Veith J, Bernacki RJ, Balthasar JP. Evaluation of combined bevacizumab and intraperitoneal carboplatin or paclitaxel therapy in a mouse model of ovarian cancer. Cancer Chemother Pharmacol. 2011;68:951–8. doi: 10.1007/s00280-011-1566-3. [DOI] [PubMed] [Google Scholar]
- 51.Milenic DE, Wong KJ, Baidoo KE, Nayak TK, Regino CAS, Garmestani K, et al. Targeting HER2: a report on the in vitro and in vivo pre-clinical data supporting trastuzumab as a radioimmunoconjugate for clinical trials. MAbs. 2010;2:550–64. doi: 10.4161/mabs.2.5.13054. [DOI] [PMC free article] [PubMed] [Google Scholar]