Abstract
Objective
Aging results in progressive changes in deglutitive functions, which may be due in part to alterations in muscle morphology and physiology. Mastication is a critical component of bolus formation and swallowing, but aging effects on masticatory function have not been well studied.
Study Design
The purpose of this study was to: 1) quantify the effects of aging on mastication; 2) determine the effects of tongue exercise on mastication in young adult and old rats. We hypothesized that there would be significant differences in mastication characteristics (number of bites, interval between bites, time to eat) as a function of age and that tongue exercise would resolve pre-exercise differences between age groups.
Methods
We expanded the established model of progressive, 8-week tongue exercise training to include a mastication measurement: acoustic recordings of vermicelli pasta biting from 17 old and 17 young adult rats, randomized into training and control groups.
Results
We found that: 1) mastication characteristics were impacted by age; specifically in older rats, time to eat and number of bites were increased and intervals between bites were decreased, suggesting increased oral motor processing requirements for bolus formation; 2) tongue exercise did not impact mastication behaviors in young adult or old rats.
Conclusion
Tongue exercise may not have been specific enough to mastication to result in behavioral changes or exercise dose may not have been sufficient. Nevertheless, results were noteworthy in expanding the established rat model of aging and have relevant clinical implications for future translation to human populations.
Keywords: deglutition, deglutition disorders, swallowing, mastication, dysphagia, rat, aging
Introduction
Age-related alterations in tongue and jaw muscles could underlie, in part, the functional changes in deglutition that are reported by elderly people 1,2. Sarcopenia, characterized by muscle atrophy and decreased strength, likely affects mastication efficiency and lingual strength and thus could be a contributing factor in deglutitive decline with age 3–7.
Chewing contributes to swallow initiation because sufficient food particalization leads to peak cohesion, which is necessary for bolus formation and safe passage quickly through the pharynx while bypassing the airway 4,8. The tongue has an important role in bolus formation and manipulation for saliva mixing and propulsion 2. Thus, age-related changes occurring during this critical introductory phase, including reduced masticatory and tongue strength, are likely to affect bolus formation and therefore swallowing 8–10. However, it is not known how reduced tongue muscle strength may contribute to insufficient formation, containment, and manipulation of a bolus during mastication, or if mastication can be improved in older individuals with tongue exercise.
The purpose of this study was to quantify age-related changes in mastication in a rat model. The following research aims were addressed: 1) Quantify age-related effects of mastication in Fischer 344/Brown Norway rats using vermicelli pasta biting to compare data from young adult and old rats; 2) Determine the effects of tongue exercise on mastication in young adult and old rats. We hypothesized that: 1) Measures of mastication would be impaired in old rats; 2) Tongue exercise would alter temporal aspects of masticatory behavior in both young adult and old rats by increasing tongue involvement in oral mastication processes and decreasing the burden on mastication muscles.
The rationale for these hypotheses is supported by the literature. It is well known that with increasing age there is a decline in the structure and function of muscles in the tongue and jaw, supporting our first hypothesis 3,7,11,12. Further, it has been shown that muscles of the jaw and tongue operate in synchrony during masticatory actions to prepare the bolus for transport 13–16. Accordingly, our second hypothesis is based on reasoning that increased tongue strength allows greater lingual support for mastication and thus allow improved temporal synchrony for lingual-masticatory muscle actions during chewing.
Materials and Methods
This study was performed in accordance with the PHS policy on care and use of laboratory animals, the NIH Guide for Care and Use of Laboratory Animals (8th edition), and the Animal Welfare Act. The University of Wisconsin School of Medicine and Public Health Animal Care and Use Committee approved the animal care and use protocol.
Animals
To complete this study, 34 male Fischer 344 Brown Norway rats were obtained the National Institute on Aging animal colony: 17 old (32 months) and 17 young adult (9 months). Rats in each group were randomly assigned to either a tongue exercise (9 old and young adult) or no-exercise control group (8 old and young adult).
Preparation
Rats were housed in pairs in polycarbonate cages on a reverse light-dark cycle (12:12-hours) to allow testing when rats are most active. During this acclimation period, rats were given pasta in their home cages daily (approximately 10–15 pieces per cage; for 2–4 consecutive days; Creamette ® brand, uncooked vermicelli pasta 1.5 mm).
Procedures
Pasta testing occurred over a 3-day maximum period; if a rat did not complete the task on the first day of testing, food restriction continued. The rat was trialed again the next day. Six pieces of uncooked vermicelli pasta were used per recording session and were placed in the front, left corner of the home cage. Pasta was 1.5 mm in diameter, cut into 7 cm-long pieces. An AT2020 cardioid condenser microphone (Audio-Technica, Machida, Tokyo, Japan) was suspended in the center of the cage top, approximately 6 inches from anywhere inside the chamber. Rat behavior was audio and video recorded (Sony HDR-PJ540; Kōnan Minato, Tokyo, Japan). Acoustic signals from pasta bites were processed with M Box Mini and ProTools LE (Avid Technology, Burlington, MA) software at a sampling rate of 44.1 kHz and 16-bit resolution and analyzed with a custom code written for MATlab (Natick, MA).
Tongue exercise was performed over an 8-week period as described previously 17. The tongue exercise apparatus operated under the computer control to dispensed a water reward if the preset force challenge was reached by tongue pressing (Matrix Product Development, Cottage Grove, WI). The sampling rate of the force transducer was 200 Hz with 0.2 g precision.
On training days (5 days per week), exercise animals were placed in the training operandum and presented with water for 10-minutes of exercise. Force thresholds were calculated as a percentage of an individual rat’s estimated maximum press (EMP [g, converted to mN]) that was determined at baseline. These thresholds were incremented up every two weeks to model progressive resistance exercise. Force thresholds were set at 50% EMP for weeks 1 and 2, 60% EMP for weeks 3 and 4, 70% EMP for weeks 5 and 6, and 80% EMP for weeks 7 and 8. While in the operandum, the number of tongue presses above threshold was recorded as “participation” for each rat in the exercise-group for each training day. Post-exercise maximum tongue force (mN) values were obtained at the completion of 8 weeks of training (exercise group) or no training (control group).
Control animals were placed into the operandum and allowed to lick the disk no more than three times at no greater than 0.2g to participate in the training task. In this manner, control rats were water-restricted and trained to use the operandum to allow for baseline and 8-week tongue press recordings, but did not engage in tongue exercise. Pre-training pasta recording took place prior to the 8-week tongue exercise period. Post-training pasta recording took place within 2 days of last training day to combat detraining effects. A summary of pasta acclimation, pasta eating data collection, and tongue exercise is outlined in Table 1.
Table 1.
Description of acclimation, testing, and training of animals for both pasta biting and tongue training procedures.
| Stages of Training | |||
|---|---|---|---|
|
Week # |
Stage | Description | Measures |
| Week 1 | Acclimation | Animals housed on a reverse light-dark cycle; handled for seven days; pasta provided in home cages | None |
| Week 2 | Water Restriction & Pasta Testing Room Acclimation | Animal access to water progressively restricted; introduced to the pasta testing; pasta in their home cages | Pasta Acclimation |
| Week 3 | Tongue Training & Pasta Test Apparatus Acclimation & Food Restriction | Animals trained to lick the tongue force transducer at 0.2g; given pasta in the pasta data collection apparatus; access to food gradually restricted | Tongue Training & Pasta Apparatus Acclimation |
| Week 4 | Completion of Tongue Training Acclimation & Baseline EMP & Pre-Training Pasta Test Data Collection | Tongue training acclimation is completed; baseline EMP testing occurs for three days; each animal is trialed and recorded with six pieces of pasta each; total time for pasta testing occurs over three days maximum. | 1. Tongue Training Acclimation 2. Baseline EMP Calculation 3. Pre-Training Pasta Test Data Collection |
| Week 5–12 | Tongue Exercise Training | Over 8 weeks, force at which animals press to receive water reward is incremented up, based on EMP values; final week of training, animals are re-acclimated with pasta testing apparatus and given pasta in home cages | Tongue Exercise Training |
| Week 13 | Final EMP Testing Post-Training Pasta Test | Final EMP testing occurs for three days; each animal is trialed and recorded with six pieces of pasta each; total time for pasta testing occurs over three days maximum. | 1. Final EMP Testing 2. Post-Training Pasta Test |
EMP=Estimated Maximal Press
Analysis
Once biting data were collected, acoustic files were filtered to reduce noise using the WAVES “X-Noise” Noise Reduction plug-in and then exported in .wav format. These files were sampled at 44.1 kHz at 16-bit resolution and further analyzed using a computer program developed previously in MATlab 18. The measures quantified included: number of bites; mean intervals between bites; and average time taken to consume 1 pasta piece. Number of bites were averaged for each rat across 6 pasta pieces. Bite-like noises that occurred less than 10 ms apart were excluded because they exceeded previously determined rat biting speeds and were deemed extraneous noises 18.
All six pasta trials for each rat were digitally recorded, manually noise reduced, and segmented into separate digital files for each trial to allow further analysis. One of the investigators (BK) performed the noise reduction and file clipping into six pasta trials. A second blinded investigator (CD), performed noise reduction and clipping on 30% of the trials. Both investigators’ processed files were analyzed in two ways: (1) Custom-designed MATlab program provided the number of bites and the interbite interval; (2) ProTools provided time-to-eat using acoustic signals from the six individual pasta pieces. To determine reliability of each of these measurements between investigators, an intra-class correlation coefficient (ICC) was calculated for each of the three measures. The resultant ICCs ranged from 0.81–0.998, which indicated a high level of reliability of measurement.
A two-way analysis of variance (age X exercise) was performed using SAS software (SAS Institute Inc., Cary, NC). A critical α-level of ≤ 0.5 was used to determine statistical significance for tongue force training measures and characteristics of mastication. Levene’s Test of Homogeneity of Variance was used to evaluate equal variance within age groups and condition; in the case of unequal variance, rank-ordered data were used for group comparisons.
Results
Age Effects
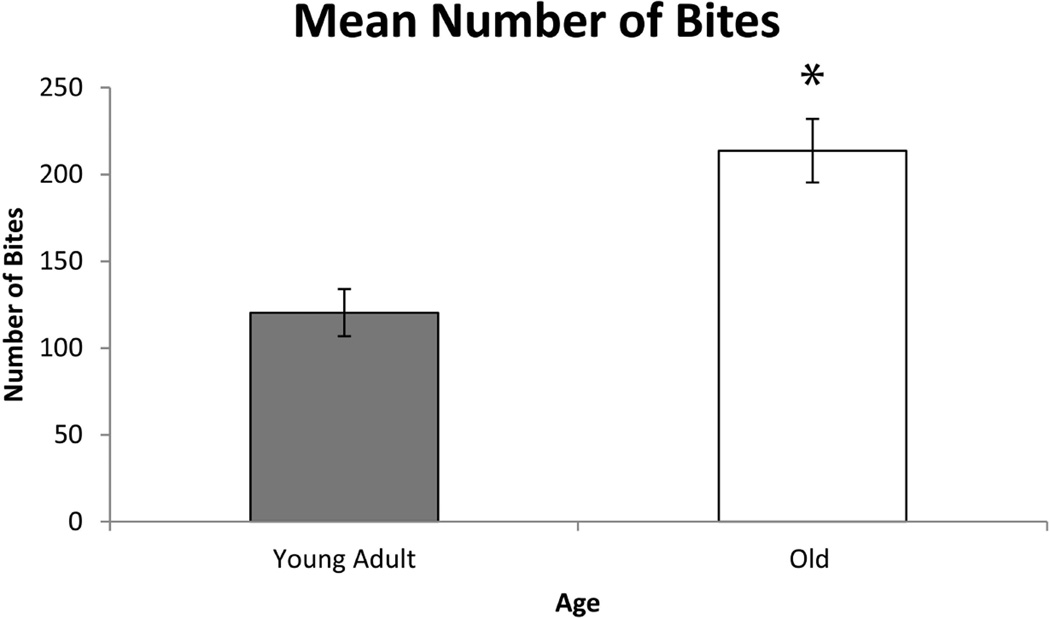
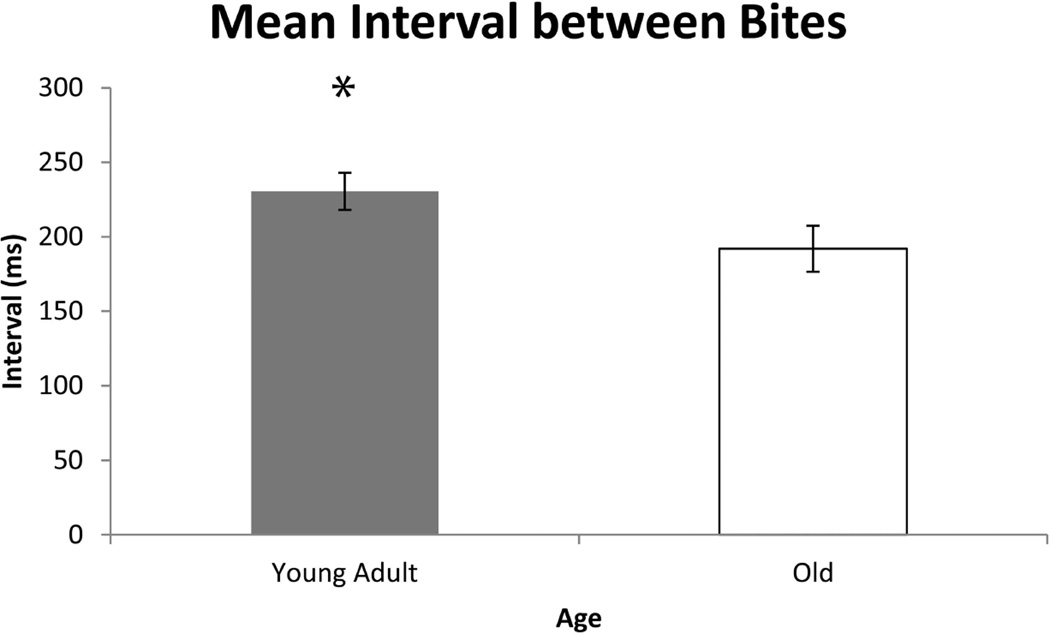
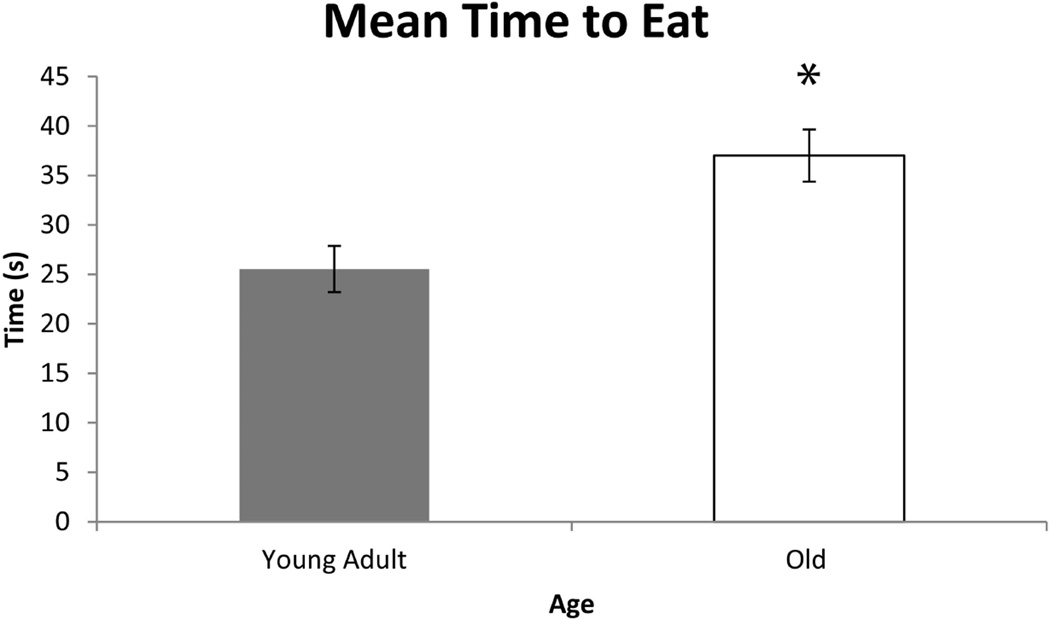
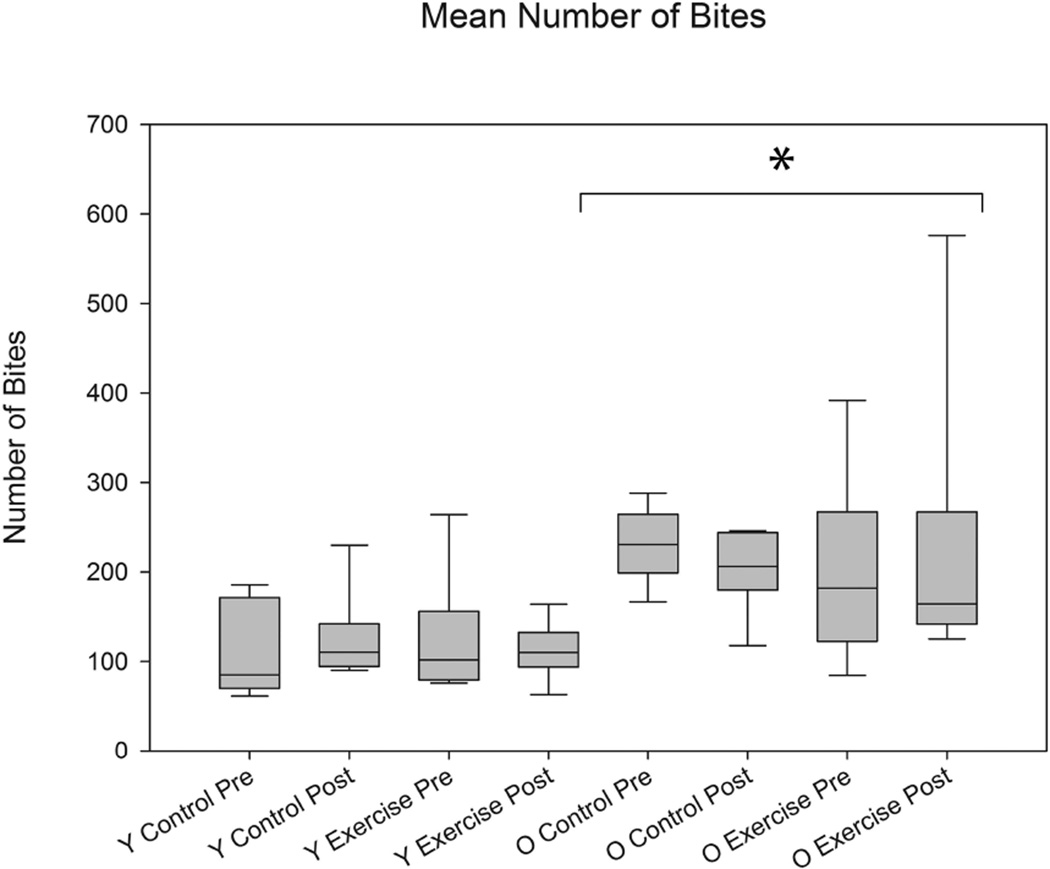
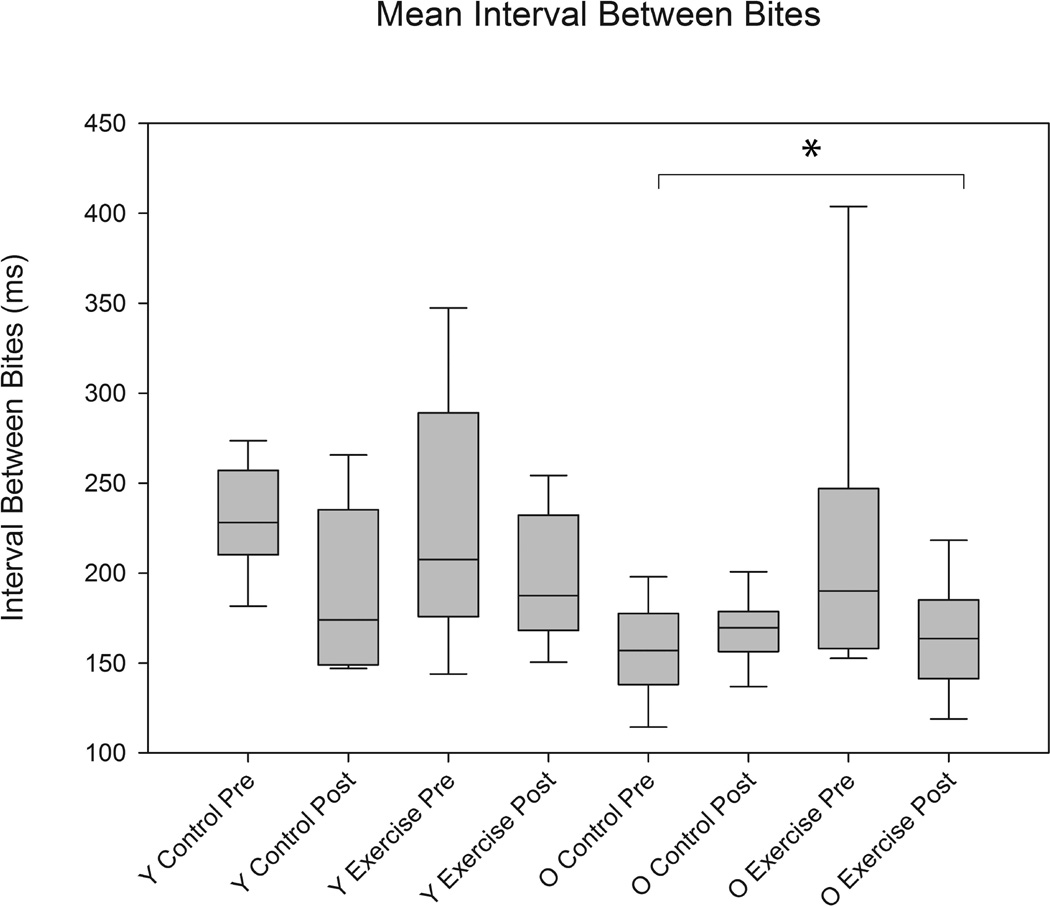
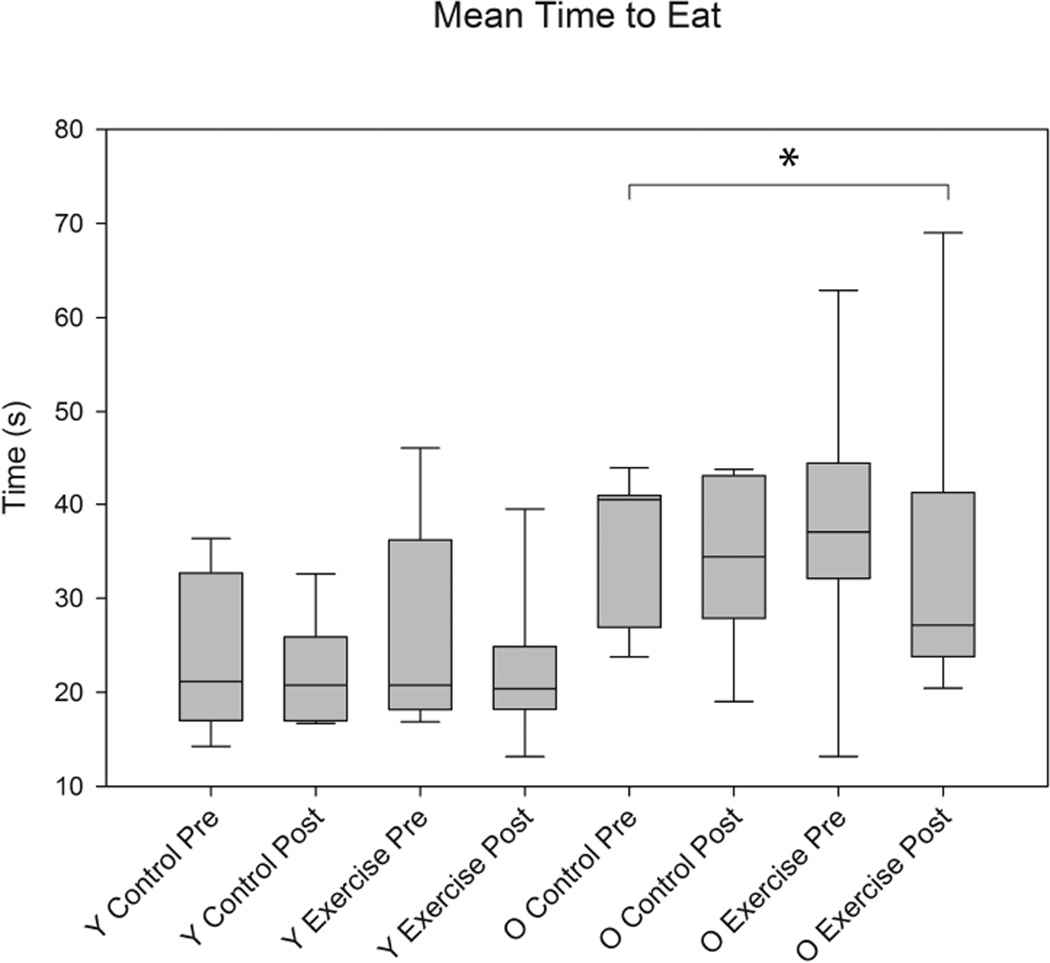
As shown in Figures 1, 2, and 3, significant differences were found in biting characteristics between young adult and old adult rats at baseline. Specifically, significant main effects for age were found in the absence of interaction effects. The old rat group had a greater number of bites (F1,30 = 5.86 , p= 0.0003) and shorter interval times between bites (F1,30 = 3.01 , p= 0.03 ), but on average, ate more slowly than the young adult group (F1,30 = 3.53, p= 0.003). Significant age group differences were not found in variability in number of bites and variability in interval times between bites (F1,30 = 1.02 , p= 0.09; F1,30 = 0.96, p= 0.4, respectively). However, variability in average time to eat was significantly increased in the old group (F1,30 = 2.65, p= 0.02).
Fig. 1.
The average number of bites was significantly greater in the old rat group vs. the young adult rat group at baseline (pre-training) (F1,30 = 3.01, p= 0.03). Error bars=Standard Error
Fig. 2.
The average interval (ms) between bites was significantly greater in the young adult rat group vs. the old rat group at baseline (pre-training) (F1,30 = 3.01, p= 0.0333). Error bars=Standard Error
Fig. 3.
The average time to eat a pasta piece (s) was significantly longer in the old rat group vs. the young adult rat group at baseline (pre-training) (F1,30 = 3.53, p= 0.0034). Error bars=Standard Error
Exercise Effects
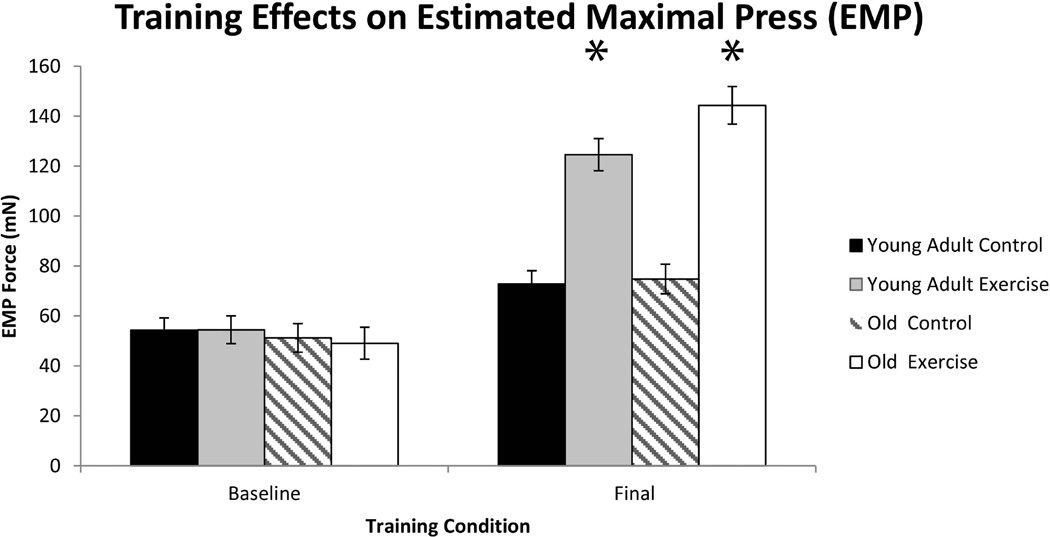
Significant main effects for exercise were found in tongue forces. That is, there were significantly greater tongue forces (mN) in the tongue exercise group versus the control group at the completion of 8 weeks of tongue exercise (F1,30 = 19.64, p= <0.0001; Figure 4). There were no significant effects for age. However, tongue exercise was not associated with any significant differences in mastication characteri;stics (Figures 5, 6, and 7).
Fig. 4.
Estimated Maximal Press (EMP) was significantly increased in the exercise training group vs. the control group following 8 weeks of tongue exercise training (F1,30 = 19.64, p= <0.0001)
Fig. 5.
There were no significant differences with training in the average number of bites between control and exercise animals (F1,30 = 0.89, p= 0.67). Age differences were still significantly different for average number of bites post training (F1,30 = 3.82, p= 0.0026). Y= Young Adult; O= Old
Fig. 6.
There were no significant differences with training in the average interval between bites (ms) between control and exercise animals (F1,30 = 1.89, p= 0.18). Age differences were still significantly different for average interval between bites (ms) post training (F1,30 = 1.75, p= 0.05). Y= Young Adult; O= Old
Fig. 7.
There were no significant differences with training in the average time to eat (s) between control and exercise animals (F1,30 = 0.15, p= 0.52). Age differences were still significantly different for average time to eat (s) post training (F1,30 = 4.04, p= 0.0016). Error bars=Standard Error. Y= Young Adult; O= Old
Discussion
Our hypotheses were: (1) that measures of mastication would be impaired in old rats, and (2) that temporal aspects of mastication would change as a result of exercise. The results of our experiments supported the first, but not the second, hypothesis. We found that the old rat group took longer to eat a piece of dried pasta, used a greater number of bites, and had a decreased time interval between bites when compared with the young adult group. Thus, the old rat group ate more slowly overall than young adult group and demonstrated less efficient oral processing by requiring a greater number of bites per piece of pasta. Accordingly, measures of mastication were significantly different between age groups and offered support for our hypothesis of impaired mastication characteristics in old rats. However, tongue exercise did not significantly alter mastication characteristics in the young adult or old groups.
Reports of age-related alterations in cranial muscle structure and physiology in humans are in agreement with our findings of impaired mastication characteristics in the old rat group. In humans, the masseter and temporalis muscles had reduced cross sectional area and density as a function of aging 7. Electromyographic (EMG) recordings in these muscles demonstrated age-related physiological changes, including decreased muscle activity 19. Furthermore, with harder foods, the number of masticatory cycles was increased in older adults 5. Taken together, our findings and those reported previously in humans suggest that with increasing age there is a change in masticatory function with a greater number of bites required used for oral processing and bolus formation, possibly due to underlying changes in muscle morphology and physiology.
Reductions in muscle activation or strength may be task dependent in elderly people. Upon sustained contraction of temporalis and masseter muscles, older people had greater EMG activity than young participants, but these higher EMG levels were not maintained by the elderly participants during chewing 11. That is, during masticatory activities, the older individuals had less EMG activity in these same muscles 11. Therefore, while masticatory strength may be maintained for static contraction, muscle activation and strength may be compromised during chewing. However, an alternative explanation may be that age-related reductions in cranial muscle endurance, rather than strength, may account for altered muscle activation and mastication patterns. One recent report argued that the increased number of masticatory cycles observed in older humans served as a compensatory function 20. These prior reports support the behavioral results of the current study. Given the correspondence of behavioral findings in old rats and elderly humans, the use of a rat model is justified in future investigations of morphology and physiology in the muscles of mastication. Future research that examines muscle fiber type characteristics and contractile properties in muscles of mastication is required to address the distinction between strength and endurance alterations for chewing behaviors. Furthermore, it is not known if age-related morphological, physiological, and behavioral changes in mastication characteristics impact swallowing efficiency and safety.
Tongue exercise had a negligible impact on masticatory characteristics in young and old rats in our study. It was reasonable to hypothesize that tongue exercise would alter mastication characteristics in our old subjects because previous studies have demonstrated the physiological link between chewing and tongue movements. While licking and tongue protrusion are components of food acquisition, tongue exercise alone may not be specific enough to impact chewing. Perhaps an exercise task that specifically targeted the muscles of the jaw may have had greater behavioral outcomes. However, because this study did not examine morphological or physiological variables within the muscles of mastication, we do not know if this particular tongue exercise program had an effect at the muscle level. It is possible that these muscles were affected by tongue exercise, but perhaps the effect was not substantial enough to result in improved chewing behaviors. It may be possible that a tongue exercise program of greater duration or intensity may have led to behavioral changes in mastication. Future studies should examine the issues of specificity of training and tongue exercise dose.
There are two potential limitations to this research study. First, the study design required that post-training mastication measures were made in exercise group rats following the final tongue training session. Therefore, it is possible that rats in the exercise group were fatigued at the time of post-training pasta testing, which may have obscured training outcomes in mastication. Second, we chose not to record onset and offset timing of mastication muscles using electromyographic (EMG) recordings to allow study of natural behaviors in awake animals. We did not use EMG because we were concerned that the instrumentation would interfere with or otherwise affect natural chewing behaviors.
Other sources of causation for the differences in our groups must be considered. Dry uncooked vermicelli pasta was selected as the stimulus for mastication testing because bites are easily recorded using sound equipment and pasta appears palatable to the rats 18,21. However, previous studies in humans have found that as food hardness varies, chewing also varies, with harder foods requiring more cycles of mastication than softer foods 5,10,22,23. Additionally, dentition is known to impact mastication in aging humans 10,24. Therefore, future studies should incorporate multiple textures to determine how dentition status and stimulus parameters may affect mastication cycles. Furthermore, sensory or systemic changes in central pattern generator capacity for completing mastication tasks as a result of aging have yet to be examined.
The differences in oral processing between rats and humans are important in interpreting our results and relating them to clinical populations. In rats, feeding occurs in three separate phases: ingestion, mastication, and the act of swallowing 25. Prior to mastication, ingestion involves the movement of the food from the environment into the oral cavity by way of chipping small pieces from the food and collecting these pieces on the tongue and posterior oral cavity for further processing during the mastication phase 25. In rats, the swallow is initiated when an adequate well-chewed bolus is formed and does not usually interrupt the chewing cycle 25. This oral pocketing prior to initiation of chewing in rats is in contradistinction to human oral processing because humans take varying sized bites, dependent upon food type and individual preference, collect the bolus in the oral cavity or pharynx until the swallow is initiated, marking a temporary cessation in chewing 26. Although these processes differ in sequence and timing, the biomechanics and muscular physiology used to operate the muscles of mastication to create and form a bolus are still comparable between the rat and the human. Thus, the rat provides us with a model that is readily available for study and comparison.
This study examined mastication in isolation. However, because mastication is only one intricate part of the entire process of deglutition, there are many other interrelated components for consideration in future studies. That is, it is important to examine mastication as a component of the larger process of deglutition.
Conclusions
This work expanded the established rat model of aging to include functional mastication endpoints. With aging, rats ate more slowly with a greater number of bites. Thus, age affected efficiency of mastication. While tongue force increased with exercise, there were no significant associations between tongue force increases and mastication characteristics. Data from this work can be combined with future assays of muscle structure and physiology to formulate hypotheses that can be tested in human clinical trials. Continued study is necessary and relevant for the development of clinical practices to improve masticatory function as a critical component of deglutition.
Acknowledgments
The author acknowledges the valuable contributions of Allison Schaser, Carissa Deleeuw, Heidi Kletzien, Jackie Weycker, Jared Cullen, John Russell, John Szot, Kellie Bowen, and Glen Leverson. The guidance of my MS committee, Drs. Nadine Connor, Michelle Ciucci, and Katherine Hustad is also gratefully acknowledged.
Funding: This study was funded by the National Institute on Deafness and Other Communication Disorders (R01DC005935, R01DC008149).
Footnotes
Compliance with Ethical Standards for Research involving Animals: This study was performed in accordance with the PHS policy on care and use of laboratory animals, the NIH Guide for Care and Use of Laboratory Animals (8th edition), and the Animal Welfare Act. The University of Wisconsin School of Medicine and Public Health Animal Care and Use Committee approved the animal care and use protocol.
Conflict of Interest: The authors declare that they have no conflict of interest.
Financial Disclosures: The authors declare that they have no financial disclosures.
Levels of Evidence: Animal Study-NA
References
- 1.Roy N, Stemple J, Merrill RM, Thomas L. Dysphagia in the elderly: preliminary evidence of prevalence, risk factors, and socioemotional effects. Ann Otol Rhinol Laryngol. 2007;116:858–865. doi: 10.1177/000348940711601112. [DOI] [PubMed] [Google Scholar]
- 2.Schindler JS, Kelly JH. Swallowing disorders in the elderly. Laryngoscope. 2002;112:589–602. doi: 10.1097/00005537-200204000-00001. [DOI] [PubMed] [Google Scholar]
- 3.Porter MM, Vandervoort AA, Lexell J. Aging of human muscle: structure, function and adaptability. Scand J Med Sci Sports. 1995;5:129–142. doi: 10.1111/j.1600-0838.1995.tb00026.x. [DOI] [PubMed] [Google Scholar]
- 4.Saitoh E, Shibata S, Matsuo K, Baba M, Fujii W, Palmer JB. Chewing and food consistency: effects on bolus transport and swallow initiation. Dysphagia. 2007;22:100–107. doi: 10.1007/s00455-006-9060-5. [DOI] [PubMed] [Google Scholar]
- 5.Peyron MA, Blanc O, Lund JP, Woda A. Influence of age on adaptability of human mastication. J Neurophysiol. 2004;92:773–779. doi: 10.1152/jn.01122.2003. [DOI] [PubMed] [Google Scholar]
- 6.Nicosia MA, Hind JA, Roecker EB, et al. Age effects on the temporal evolution of isometric and swallowing pressure. J Gerontol A Biol Sci Med Sci. 2000;55:M634–M640. doi: 10.1093/gerona/55.11.m634. [DOI] [PubMed] [Google Scholar]
- 7.Newton JP, Abel EW, Robertson EM, Yemm R. Changes in human masseter and medial pterygoid muscles with age: a study by computed tomography. Gerodontics. 1987;3:151–154. [PubMed] [Google Scholar]
- 8.Prinz JF, Lucas PW. An optimization model for mastication and swallowing in mammals. Proc Biol Sci. 1997;264:1715–1721. doi: 10.1098/rspb.1997.0238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Logemann JA. Effects of aging on the swallowing mechanism. Otolaryngol Clin North Am. 1990;23:1045–1056. [PubMed] [Google Scholar]
- 10.Woda A, Mishellany A, Peyron MA. The regulation of masticatory function and food bolus formation. J Oral Rehabil. 2006;33:840–849. doi: 10.1111/j.1365-2842.2006.01626.x. [DOI] [PubMed] [Google Scholar]
- 11.Galo R, Vitti M, Santos CM, Hallak JE, Regalo SC. The effect of age on the function of the masticatory system--an electromyographical analysis. Gerodontology. 2006;23:177–182. doi: 10.1111/j.1741-2358.2006.00113.x. [DOI] [PubMed] [Google Scholar]
- 12.Newton J, Yemm RA, Menhinick S. Changes in human jaw muscles with age and dental state. 1993;10:16–22. doi: 10.1111/j.1741-2358.1993.tb00074.x. [DOI] [PubMed] [Google Scholar]
- 13.Hiiemae KM, Hayenga SM, Reese A. Patterns of tongue and jaw movement in a cinefluorographic study of feeding in the macaque. Arch Oral Biol. 1995;40:229–246. doi: 10.1016/0003-9969(95)98812-d. [DOI] [PubMed] [Google Scholar]
- 14.Naganuma K, Inoue M, Yamamura K, Hanada K, Yamada Y. Tongue and jaw muscle activities during chewing and swallowing in freely behaving rabbits. Brain Res. 2001;915:185–194. doi: 10.1016/s0006-8993(01)02848-7. [DOI] [PubMed] [Google Scholar]
- 15.Hori K, Ono T, Nokubi T. Coordination of tongue pressure and jaw movement in mastication. J Dent Res. 2006;85:187–191. doi: 10.1177/154405910608500214. [DOI] [PubMed] [Google Scholar]
- 16.Kakizaki Y, Uchida K, Yamamura K, Yamada Y. Coordination between the masticatory and tongue muscles as seen with different foods in consistency and in reflex activities during natural chewing. Brain Res. 2002;929:210–217. doi: 10.1016/s0006-8993(01)03392-3. [DOI] [PubMed] [Google Scholar]
- 17.Connor NP, Russell JA, Wang H, Jackson MA, Mann L, Kluender K. Effect of tongue exercise on protrusive force and muscle fiber area in aging rats. J Speech Lang Hear Res. 2009;52:732–744. doi: 10.1044/1092-4388(2008/08-0105). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kane JR, Ciucci MR, Jacobs AN, et al. Assessing the role of dopamine in limb and cranial-oromotor control in a rat model of Parkinson's disease. J Commun Disord. 2011;44:529–537. doi: 10.1016/j.jcomdis.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cecilio FA, Regalo SC, Palinkas M, et al. Ageing and surface EMG activity patterns of masticatory muscles. J Oral Rehabil. 2010;37:248–255. doi: 10.1111/j.1365-2842.2010.02051.x. [DOI] [PubMed] [Google Scholar]
- 20.Palinkas M, Cecilio FA, Siessere S, et al. Aging of masticatory efficiency in healthy subjects: electromyographic analysis--Part 2. Acta Odontol Latinoam. 2013;26:161–166. [PubMed] [Google Scholar]
- 21.Tennant KA, Asay AL, Allred RP, Ozburn AR, Kleim JA, Jones TA. The vermicelli and capellini handling tests: simple quantitative measures of dexterous forepaw function in rats and mice. J Vis Exp. 2010 doi: 10.3791/2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fucile S, Wright PM, Chan I, Yee S, Langlais M-E, Gisel EG. Functional Oral-Motor Skills: Do They Change with Age? doi: 10.1007/PL00009571. [DOI] [PubMed] [Google Scholar]
- 23.Fucile S, Wright PM, Chan I, Yee S, Langlais M-E, Gisel EG. Functional Oral-Motor Skills: Do They Change with Age? Dysphagia. 1998;13:195–201. doi: 10.1007/PL00009571. [DOI] [PubMed] [Google Scholar]
- 24.Jaradeh S. Neurophysiology of swallowing in the aged. Dysphagia. 1994;9:218–220. doi: 10.1007/BF00301913. [DOI] [PubMed] [Google Scholar]
- 25.Hiiemäe KM, Ardran GM. A cinefluorographic study of mandibular movement during feeding in the rat (Rattus norvegicus) Journal of Zoology, London. 1968;154:139–154. [Google Scholar]
- 26.Palmer JB, Rudin NJ, Lara G, Crompton AW. Coordination of mastication and swallowing. Dysphagia. 1992;7:187–200. doi: 10.1007/BF02493469. [DOI] [PubMed] [Google Scholar]