Abstract
Statement of the Problem:
The usage of glass ionomer cements (GICs) restorative materials are very limited due to lack of flexural strength and toughness.
Purpose:
The aim of this study was to investigate the effect of using a leucite glass on a range of mechanical and optical properties of commercially available conventional glass ionomer cement.
Materials and Method:
Ball milled 45μm leucite glass particles were incorporated into commercial conventional GIC, Ketac-Molar Easymix (KMEm). The characteristics of the powder particles were observed under scanning electron microscopy. The samples were made for each experimental group; KMEm and lucite- modified Ketac-Molar easy Mix (LMKMEm) according to manufacturer’s instruction then were collected in damp tissue and stored in incubator for 1 hour. The samples were divided into two groups, one stored in distilled water for 24 hours and the others for 1 week.10 samples were made for testing biaxial flexural strength after 1 day and 1 week, with a crosshead speed of 1mm/min, calculated in MPa. The hardness (Vickers hardness tester) of each experimental group was also tested. To evaluate optical properties, 3 samples were made for each experimental group and evaluated with a spectrophotometer. The setting time of modified GIC was measured with Gillmore machine.
Result:
The setting time in LMKMEm was 8 minutes. The mean biaxial flexural strength was LMKMEm/ 1day: 24.13±4.14 MPa, LMKMEm/ 1 week: 24.22±4.87 MPa KMEm/1day:28.87±6.31 MPa and KMEm/1 week: 26.65±5.82 MPa which were not statistically different from each other. The mean Vickers hardness was LMKMEm: 403±66 Mpa and KMEm: 358±22 MPa; though not statistically different from each other. The mean total transmittance (Tt) was LMKMEm: 15.9±0.7, KMEm: 22.3±1.2, the mean diffuse transmittance (Td) was LMKMEm: 12.2±0.5, KMEm: 18.0±0.5 which were statistically different from each other.
Conclusion:
Leucite glass can be incorporated with a conventional GIC without interfering with setting time. Yet, it did not improve the mechanical and optical properties of the GIC.
Keywords: Dental material , Glass Ionomer Cement , Glass , Mechanical Phenomena , Optical Phenomena
Introduction
One major advantage of glass ionomer cements (GICs) as a restorative material is bonding to dental tissue and due to this, the prepared cavity for this material can be very conservative.[1] In addition to this, glass-ionomer has many other advantages such as fluoride release, coefficient of thermal expansion close to tooth structure, durable bond to tooth tissue and biocompatibility.[2-3] In spite of all these advantages, the clinical performance of the material are limited due to other drawbacks: low flexural and fracture toughness, early water sensitivity, low fatigue strength, high surface roughness and poor wear resistance.[2-4]
Many investigators have attempted to modify the formulation of GICs to overcome these problems. The modifications have been done on the powder and liquid composition and configuration. [2-6] Modification in powder was different in many aspects, from refinement of glass particle size to incorporation of different particles such as metal, ceramics and fibres. [1-2,7-14] Along with modifications in the glass powder of GICs, the matrix has been changed. Some of these modifications are: use of copolymer (carboxylic acid-itaconic acid), incorporation of tartaric acid in the liquid part, use of the copolymer with high molecular weight, concentration of poly acid, incorporation of freeze- dried acid copolymer in the cement powder and the use of N-vinylpyrrolidone containing polyacids (NVP).[15-16] Culberston et al.[7] showed that acrylic acid-itaconic acid- NVP (AA/IA/NVP) polymers with diverse molar ratio can produce GICs with increased mechanical properties.
Hydroxy apatite (HA) can react with GICs matrix by polyacid hydroxyl group, so incorporation of HA into GICs may improve physical properties and biocompatibility of set cement. Besides, it contributes to the bond strength of GICs due to a similar composition to tooth structure.[10] Lucas et al.[10] illustrated that incorporation of HA in GICs powder increased the fracture toughness significantly so it made more durable bond to dental tissues. Moreover, according to Moshavernia et al.[5] addition of nano HA enhanced the mechanical properties of commercial GIC Fuji II (Fuji II LC, GC Corp.; Tokyo, Japan) and it seems that the bonding property was also enhanced.
Micro-sized Yttria stabilized zirconia (YSZ) powder was added to Miracle Mix (GC America Inc.; Alsip, IL, USA) powder to improve the mechanical properties. The study showed that the uniform distribution of the glass and YSZ particle in the matrix, which ensured a high packing density of GICs, gave relatively high mechanical properties to YSZ GIC.[17] However, these two last modifications have been done as a patent and there are no commercial brands with these modifications.
High viscosity conventional GICs like Ketac-Molar-easy mix (3M ESPE I.D. No.70201119107, Germany), and Fuji IX (GC America Inc.; Alsip, IL, USA) have been introduced recently, designed as an alternative to amalgam for posterior preventive restorations. These show significant improvements in compressive strength and fracture toughness.[15-18] They can be used as a core build up material under a crown, permanent filling for primary teeth and class I restorations in non-occluded regions.[15,19] Nevertheless, they are not suitable for high stress areas and still their main problems are low fracture toughness and flexural strength compared to other restorative materials.[18]
The aim of this study was to evaluate physical and mechanical properties of a conventional glass ionomer material after substitution a leucite glass for the acid soluble glass in the powder of this material.
Materials and Method
The material used in this study was a conventional glass ionomer material, Ketac-Molar Easymix (KMEm) (3M ESPE I.D. No.70201119107, Germany), shade (A3). The principle composition of the powder is aluminum-calcium-lanthanum-fluorosilicate glass and the liquid is an aqueous solution of polycarbonic acid (a copolymer from acrylic and maleic acid) and tartaric acid.
In order to modify the GIC powder, a glass powder with a composition of a leucite glass ceramic (PS4) which was made by a PhD student in Sheffield University Dental School Adult Dental care, was added to KMEm glass ionomer powder. The modified glass powder was ball milled for 10 min and 400 rpm in ball milling machine (Retsch GmbH; PM 100, Germany) to reduce the particles size. To achieve a uniform size of glass filler the leucite glass ceramic was sieved manually with a 45μ sieve (Retsch GmbH; Germany), thus most of the glass had a particle size less than 45 μm. The leucite glass ceramic was added to the glass-ionomer powder in 20 vol%. This addition has been done manually by dry mixing on a paper plate using a metal spatula until an even distribution was achieved.
The original and modified glass powder was observed under scanning electron microscope (SEM) (X1-20 SEM, Holland).
A silicone rubber mould was used for fabrication of the samples. The samples were made in a disc with a thickness of 2.5mm and diameter of 12mm.Mixing of the modified powder and the cement liquid was carried out using a metal spatula according to manufacturer’s instructions. The mixing time did not exceed 1 min and working time was 3-3.5 min. The cement paste was carried into the mould by metal spatula and pressed using a glass slab. After the material was set, the specimens were collected in a damp tissue and were stored in an incubator (LEEC Compact Incubator LEEC Limited; Nottingham, UK) at a temperature of 37ºC for 1 hour. This was followed by storing the specimens in distilled water for 24 hours. For the biaxial flexural strength test, the specimens were ground and polished down to 600 grit using SiC (Buehler-Met; Metallographic Grinding paper, UK) paper. The final dimensions of specimens were measured with digital micrometer with a precision of 0.01 mm on four central axes and thickness of all samples was 2±0.2mm. For the hardness test, the specimens were ground and polished down to 1μm using SiC papers and diamond paste.
Three samples were made for each experimental group for spectrophotometer test in the silicon rubber mold. The samples were made with a thickness of 2mm and diameter of 16mm. Mixing the modified powder and the cement liquid was carried out by using a metal spatula according to manufacturer's instructions. The mixing time did not exceed 1 min and working time was 3-3.5 min. The cement paste was carried into the mould by metal spatula and was pressed using a glass slab. After the materials were set, the specimens were collected in damp tissue and were stored in an incubator at a temperature of 37ĉ for 1 hour. After that the specimens were stored in distilled water for 24 hours. The specimens were ground and polished down to 600 grit using SiC papers. The thickness of specimens was measured with digital micrometer with a precision of 0.01 mm on three central axes and it was 1±0.1 mm. The specimens were stored in distilled water until the spectrophotometer test was carried out.
The setting time of modified glass ionomer was measured with Gillmore needle apparatus (Impact Test Equipment Ltd; Scotland, UK) and digital timer. The Gilmore needle works on the principle of two different sized needles with different weight being placed onto the surface of setting cement. Initially a large needle (10 mm) with a weight of (28g) is applied and the working time is determined when the needle no longer indents the surface. A second needle with a smaller diameter (1mm) and larger weight (400g) is then applied to the surface and time when this needle no longer indents the surface is designated as the setting time. Both tests were carried out at ambient temperature 21ºC.
To evaluate flexural strength, ten discs (12mm diameter and 2±0.2 thickness) were made for each experimental group. The biaxial flexural strength was measured after 1day and 1 week.
The thickness of each specimen was measured in the centre and three peripheral points using a digital micrometer before testing. Flexural property was quantified by biaxial flexural strength test (LLOYD Instrument, LRX 103648, Farham, UK) at a crosshead speed of 1 mm/min (Figure 1). The flexural strength was obtained by measuring the load at fracture in Newton and then calculated in MPa.
Figure1.

Arrangement for biaxial flexural strength test (Universal testing machine; LLOYD Instrument, Farham, UK).
Ten discs were made for each experimental group (N=10). The discs were mounted on the Vickers hardness machine (Vickers Hardness VX Series Tester) and indented with Vickers diamond pyramid at 1 Kg load and the resulting diagonal length was measured. The Vickers hardness in MPa is then obtained using the following equation: HV=1.854 P/d2
Where p is the applied load in Newtons and d is the mean diagonal length in mm.[21]
Three sample of each group (N=3) were analyzed by Perkin Elmer Lambda 2 spectrophotometer with an integrating sphere sensor (Figure 2). The spectral range of the colorimeter was between 380 and 700 nm at 1 nm interval. The data were analyzed by UV WinLab (version 2) software. One-way ANOVA followed by Tukey's pairwise comparison was used for analyzing the data.
Figure2.
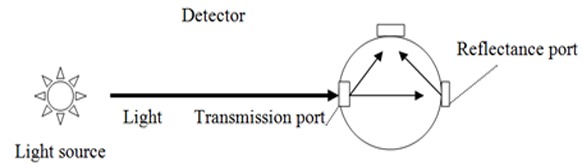
Schematic picture of the mechanism of light transmittance detection through the integrating sphere.
Result
Characterizations of powders
The SEM of glass powder in KMEm (3M ESPE, I.D. No.70201119107, Germany) (control) and LMKMEm (experimental) groups are illustrated in Figures 3 and 4
Figure3.

The SEM micrograph of Leucite modified Ketac-Molar Easymix glass which was ball milled with 10 min rotating time, 400 rpm, Biomedical Science Department, Sheffield University, 2009.
Figure4.

The SEM micrograph of Ketac-Molar Easymix glass powder, Biomedical Science Department, Sheffield University, 2009.
The particle size in LMKME was larger as a result of adding 45μm Leucite glass ceramic. However the particle size of the powder was significantly decreased after ball milling and it can be clearly seen in Figure 5.
Figure5.
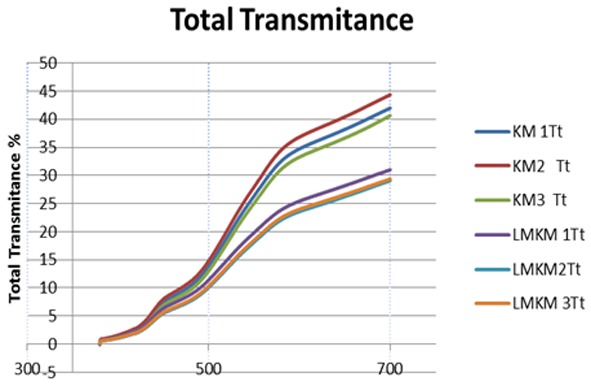
Graph representing comparison of total transmittance (Tt) curve of each sample in KMEm (KM) as control group and LMKMEm (LMKM) as experimental group.
Setting time analysis
The setting time in KMEm GICs (3M, ESPE, I.D. No.70201119107, Germany) is 7 min. Adding 20 vol% leucite glass ceramic to the powder of KMEm GICs increased the setting time. After ball milling, the setting time was decreased to 8 min which was very close to original cements.
Biaxial Flexural Strength
The mean biaxial flexural strength of the control (KMEm; 3M, ESPE, I.D. No.70201119107, Germany) and experimental (LMKMEm) groups is summarized in Table 1.
Table 1.
Summary of result of biaxial flexural strength of two glass ionomer cement and storage time (MPa).
| Materials and storage time | Mean (MPa) | Min-Max | SD (MPa) | N |
|---|---|---|---|---|
| KMEm,1 day | 28.87 | 19.2-37.1 | 6.31 | 10 |
| LMKMEm,1 day | 24.13 | 17.4-31.5 | 4.14 | 10 |
| KMEm,1 week | 26.65 | 17.6-36.1 | 5.82 | 10 |
| LMKMEm,1 week | 24.22 | 18.6-32.7 | 4.87 | 10 |
KMEm= Ketac-Molar Easymix (Control group), $LMKMEm= Leucite Modified Ketac-Molar Easymix(Experimental group)
KMEm after 1 day storage recorded a mean biaxial flexural strength of 28.87 MPa, which was the highest. After 1 week storage, the biaxial flexural strength decreased in this group and a mean of 26.65 MPa was recorded for this group. However the difference was not significant. (p> 0.05) For LMKMEm, the biaxial flexural strength has not changed after 1 week storage and a mean of 24.13 MPa and 24.22 MPa was recorded for 1day and 1 week storage respectively.
One-way ANOVA followed by Tukey’s pairwise comparison showed no significant statistical difference between the control and experimental groups (p> 0.05).
Vickers Hardness
The calculated Vickers hardness values are summarized in Table 2.
Table 2.
Summary of the calculated Vickers hardness in two glass ionomer cements groups in MPa.
| Materials | Mean (MPa) | Min-Max | SD (MPa) | N |
|---|---|---|---|---|
| KMEm (Control group) | 358 | 325-399 | 22 | 10 |
| LMKMEm (Experimental group) | 403 | 275-533 | 66 | 10 |
LMKMEm GIC recorded a mean Vickers hardness of 402 MPa which is higher than KMEm GIC with 358 MPa. One-way ANOVA followed by Tukey’s pairwise comparison showed no significant statistical difference between the control and experimental groups (p> 0.05).
Optical properties
Three optical properties of two glass ionomers were analyzed in this study. The result is graphically presented in Figures 6, 7
Figure6.
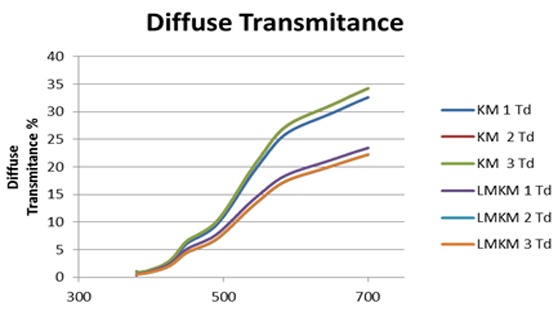
Graph representing comparison of diffuse transmittance (Td) curve of each sample in KM (KMEm) as control group and LMKM (LMKMEm) as experimental group.
Figure7.
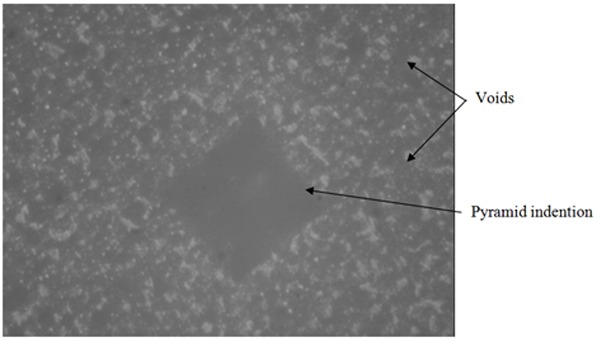
The pyramid indention of indenter of Vickers hardness test machine in Ketac Molar Easymix (KMEm), control group sample.
The means of 22.3 for total transmittance (Tt) and 18 for diffuse transmittance (Td) and 4.3 for direct transmittance (Tdir) were recorded for KMEm GIC (control group) and the means of Tt, Td and Tdir were recorded 15.9, 12.2 and 3.7 respectively for LMKMEm GIC (experimental group). A decrease in the mean of all optical values was observed in the experimental group according to these data.
One-way ANOVA followed by Tukey’s pairwise comparison showed significant statistical difference of Tt, Td between the control and experimental groups (p< 0.05). Regarding Tdir, one-way ANOVA followed by Tukey’s pairwise showed no statistical difference between the control and experimental groups (p> 0.05).
Discussion
In this experimental study, 20% volume fraction of a conventional GIC (like KMEm) was replaced with non -reactive leucite glass to try and produce a conventional GIC with improved mechanical properties. Leucite glass was chosen to add to the powder because it is a strong glass with an affinity to poly acrylic acid.[22]
Simultaneous with preparing and working with glass ionomer formulation, it is really critical to consider working and setting time.[7] Substitution of reactive powder with non-reactive produced a modified conventional glass ionomer (LMKMEm) with significantly increased setting time (14 minute). The proper setting time for restorative GIC is 6-8 minutes.[23] So to overcome this problem, the powder was ball milled to produce finer grain size to reduce the setting time. Finer grain size can speed up the setting reaction because of greater surface.[16]
Modification of conventional glass ionomer with leucite glass was a novel approach. Hence the proper milling time and rotating speed for ball milling of the powder had to be done by trial and error. The powder was ball milled with increasing time till the mixing cement had set in proper setting time and was ended up by 10 minute rotating time and 400 rotating speed. The SEM micrographs clearly showed finer grain size of the LMKMEm GICs powder after ball milling (Figures 3, 4). Regarding manipulation properties, LMKMEm- GICs has a setting time of 8 minute which is a proper setting time for restorative GIC. Additionally, the LMKMEm GICs powder is mixed to the liquid as the same amount as the KMEm-GICs powder and the viscosity of the paste is the same as well. (Figure 8)
Figure8.
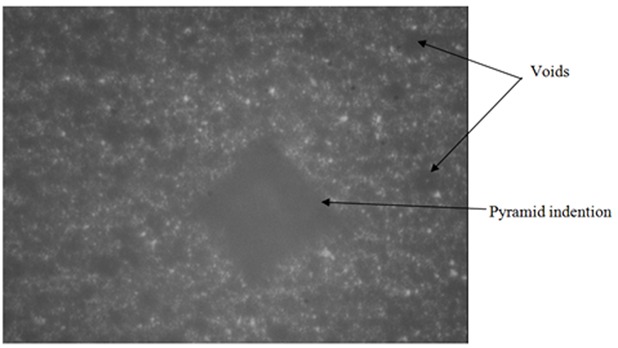
The pyramid indention of indenter of Vickers hardness test machine in Leucite Modified Ketac Molar Easymix (LMKMEm) experimental group sample.
In this study, flexural strength test was used to evaluate the mechanical strength of the new cement. Mechanical strength of many brittle dental materials such as cements is evaluated by tensile rather than compressive strength. Crack propagation is the main reason for the failure of these materials which is favored by tensile rather than compressive loading. Prosser et al.[24] have recommended that the flexural strength is the most appropriate measurement method for strength evaluation of GICs. They elucidated that, as the fracture would occur in atomic level in GICs matrix by tensile or shear failure; compressive strength basically had no meaning.[24]
Although tensile strength is the best method to estimate the mechanical properties of GICs, direct measurement of tensile strength for brittle material has technical problems. Tensile strength measurement by loading in compression (diametrical tensile strength) is only valid if a material does not have significant plastic deformation. So, diametrical tensile strength test usually gives higher values to polyacrylic acid base cements which have some degrees of plasticity. According to these reasons, it has been suggested that the flexural strength tests are the most practical and reliable way for evaluation of tensile strength of brittle materials. However, measurement of compressive and diametrical tensile strength has been reported for GICs, recently.[25]
The results in Table 1 show that the mechanical properties of KMEm have not improved with this modification. A mean biaxial flexural strength of 24.13±4.14 was recorded for LMKMEm after 1 day storage in distilled water. In comparison it was 28.87±6.31 MPa for KMEm. However this difference was not statistically significant.
It is suggested that different microstructure and variation in composition may have affected the mechanical properties of the GICs.[25] The smaller particle size, smaller and lesser voids and proper bonding of glass fillers and matrix in KMEm GIC appear to have resulted in higher flexural strength. The somewhat larger particle size, lack of proper bond between the filler and matrix and larger and more voids in LMKMEm GIC have caused opposite trends value for flexural strength. Mitsuhashi et al.[3] suggested that the direct tensile strength of resin modified GIC increased significantly by reduction of filler particle size to 5μm. They claimed that the particle size in KMEm -GIC powder is mainly less than 9.6μm, which means that 90% of the particle having a diameter of less than 9.6μm. Additionally 50% of the glass particles are 2.8μm or smaller.[3] Moreover, it has been reported that proper bonding between filler particles and glass matrix is contributing to mechanical properties of set cement by modifying the character of local stress and crack propagation in the matrix.[2] As a result of improper bond between the leucite glass particles and matrix of GIC, the large leucite glass particles might act as a void and contribute to crack propagation in LMKMEm GIC. It can be another reason for decreasing the flexural strength in LMKMEm GIC. Treatment of leucite glass particles with a coupling agent might improve the bonding of this glass particle to the matrix and consequently can improve the mechanical properties of GIC. However further research is necessary to establish this.
The biaxial flexural strength of KMEm had decreased after 1 week storage in distilled water. Although this reduction was not significant, it was an interesting finding. In this GIC, 5% dried polycarbonate (a copolymer from acrylic and maleic acid) is incorporated into the powder. This leads to greater overall acid concentration in the cement which increases cross-linking and improves mechanical values without a considerable increasing in the initial viscosity. It was reported that GICs based on copolymer of acrylic and maleic acid demonstrate deterioration in flexural strength after water storage.[26] It seems in the glass ionomers with polyacrylic matrix compressive and flexural strength increases after storage in the water, whereas that with copolymers acid does not.[27] Generally, the mechanical strength of GIC increases with time. This has been attributed to the maturation of the cement matrix due to further cross-linking because of the formation of new aluminum and calcium polyacrylates and the slow build up of a silica matrix from acid solution of glass particles.[26]
The fracture mode of flexural strength samples was also different in KMEm and LMKMEm. All the samples in LMKMEm (experimental groups) regardless to the storage time have been fractured into two pieces. While in KMEm (control groups) a few samples have been fracture into three pieces. It can be attributed to higher flexural strength of control groups comparing to experimental groups.
In this study, traditional Vickers hardness was used to test the hardness of KMEm-GIC and LMKMEm-GIC. As it was mentioned in the literature review, this was not the best method for testing hardness of GICs because this material is a heterogeneous, biphasic and weak. The applying load was 1 kg which might be very heavy for these materials. But it was the only available method for testing the hardness of the new material.
According to the result in Table 2, the Vickers hardness of LMKMEm was 403±66 MPa which was higher than KMEm (358 ± 22). This greater value could be attributed to use of extremely hard leucite glass fillers; however, this difference was not statistically significant.
Guggenberger et al.[28] reported that the hardness of KMEm reached the level of hybrid composite with the value of 420 MPa. In their study they did not mention the test method they used. Their result was represented in a diagram without showing the standard deviation. According to product pamphlet from ESPE, the surface hardness of KMEm GIC was recorded 420±82 MPa with DIN 53456 standard methods.
The result of the above mentioned study is different from the outcome of the present study. The difference in the hardness value can be explained by using different testing methods and numerous porosities in our samples.
The reading of indention was really difficult in this study due to indistinct border of pyramid in the micrograph of Vickers hardness tester (Figures 9 and 10). This phenomenon was explained by Yap AU. et al.[29] They suggested that the pile- up of the material around the indenter due to plasticity of the material is the main cause of this problem. Therefore, to record exact mechanical properties from instrumented indention, a good understanding of the relationship between indentational load and the true area is necessary. [29]
The optical properties of new GIC along with the mechanical properties were evaluated. Table 3 shows the mean Tt of 22.3±1.2 and Td of 18.0±0.5 for KME m-GIC compared to 15.9±0.7 and 12.2±0.5 respectively for LMKMEm GIC. A reduction in Tt value and Td value was observed in the LMKMEm GIC which was statistically significant p< 0.05. According to this result LMKMEM GIC is more opaque comparing to KMEm GIC. One of the reasons for opacity of GIC is the mismatch of refractive index of the fillers and matrix.[30] Hence, this reduction can be attributed to more porosity and imperfection in LMKMEM GIC which can increase the refractive index of the matrix.
Table 3.
Summary of calculated three optical properties in two glass-ionomer cements.
| Optical properties | Materials | Tt | Td | Tdir |
|---|---|---|---|---|
| KMEm (Control group) | 22.3±1.2 | 18.0±0.5 | 4.3±1.3 | |
| LMKMEm (Experimental group) | 15.9±0.7 | 12.2±0.5 | 3.7±0.2 | |
Filler composition and content also can change the optical properties of direct restorative materials.31 By very careful evaluation of Figure 6 and Figure 7, a shifting down of Tt and Td can be observed in LMKMEm GIC. The curvature of Tt and Td is the same in both materials but it seems that the curve shifted down in LMKMEm GIC group. It showed that color does not change in the new material because light absorption did not change in all reflected wavelengths. The leucite glass has low refractive index so this opacity could be attributed to different refractive indices of leucite glass and glass ionomer matrix.
Using leucite glass composition with refractive index closer to the matrix might preclude this problem and more lucent GICs could be produced. However, KMEm is a posterior restorative material and improvement in mechanical properties might have priority compared to optical properties.
The samples with numerous porosities were the prominent problem in this study. The depth and size of the voids is related to microstructure of the GIC. Some factors such as size and shape of fillers, wet ability of fillers and mixing procedure can affect the number of porosity.[25] Mechanical mixing and using angled nozzle for accurate placement can produce samples with less porosity.
In this study, new GIC with proper manipulation properties was produced successfully. The mechanical properties of the new material have not improved as a result of problems which were mentioned in the discussion. However, this study could be a good basic study for future investigations.
Conclusion
Within the limitations of the present study, the addition of leucite glass into the KMEm GIC produced a modified conventional GIC with reasonable setting time and manipulation properties. The addition of leucite glass to the KMEm GIC did not improve the bi-axial flexural strength and hardness of the material.
The addition of leucite glass to the KMEm GIC cement produced a new material with a higher opacity comparing to the original material.
Acknowledgement
This article is based on thesis submitted to the Academic Unit of Restorative dentistry, University of Sheffield, Sheffield, UK in fulfillment of the requirements for master degree. Special gratitude is forwarded to Prof. Richard Van Noort, for his expert advice and academic devotion.
Conflict of Interest:The authors of this manuscript certify that they have no conflict of interest.
References
- 1.Nagaraja Upadhya P, Kishore G. Glass ionomer cement: The different generations. Trends Biomater Artif Organs. 2005; 18:158–165. [Google Scholar]
- 2.Hammouda IM. Reinforcement of conventional glass-ionomer restorative material with short glass fibers. J Mech Behav Biomed Mater. 2009; 2: 73–81. doi: 10.1016/j.jmbbm.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 3.Mitsuhashi A, Hanaoka K, Teranaka T. Fracture toughness of resin-modified glass ionomer restorative materials: effect of powder/liquid ratio and powder particle size reduction on fracture toughness. Dent Mater. 2003; 19: 747–757. doi: 10.1016/s0109-5641(03)00022-8. [DOI] [PubMed] [Google Scholar]
- 4.Ana ID, Matsuya S, Ohta M, Ishikawa K. Effects of added bioactive glass on the setting and mechanical properties of resin-modified glass ionomer cement. Biomaterials. 2003; 24: 3061–3067. doi: 10.1016/s0142-9612(03)00151-0. [DOI] [PubMed] [Google Scholar]
- 5.Moshaverinia A, Ansari S, Moshaverinia M, Roohpour N, Darr JA, Rehman I. Effects of incorporation of hydroxyapatite and fluoroapatite nanobioceramics intoconventional glass ionomer cements (GIC) Acta Biomater. 2008; 4: 432–440. doi: 10.1016/j.actbio.2007.07.011. [DOI] [PubMed] [Google Scholar]
- 6.Smith DC. Development of glass-ionomer cement systems. Biomaterials. 1998; 19: 467–478. doi: 10.1016/s0142-9612(97)00126-9. [DOI] [PubMed] [Google Scholar]
- 7.Culberston BM. Glass-ionomer dental restoratives. Prog Polym Sci. 2001; 26:577–604. [Google Scholar]
- 8.McLean JW. Glass-ionomer cements. Br Dent J. 1988; 164: 293–300. doi: 10.1038/sj.bdj.4806434. [DOI] [PubMed] [Google Scholar]
- 9.Lohbauer U, Walker J, Nikolaenko S, Werner J, Clare A, Petschelt A, Greil P. Reactive fibre reinforced glass ionomer cements. Biomaterials. 2003; 24: 2901–2907. doi: 10.1016/s0142-9612(03)00130-3. [DOI] [PubMed] [Google Scholar]
- 10.Lucas ME, Arita K, Nishino M. Tough-ness, bonding and fluoride-release properties of hydroxyapatite-added glassionomer cement. Biomaterials. 2003; 24: 3787–3794. doi: 10.1016/s0142-9612(03)00260-6. [DOI] [PubMed] [Google Scholar]
- 11.Kim DA, Abo-Mosallam HA, Lee HY, Kim GR, Kim HW, Lee HH. Development of a novel aluminum-free glass ionomer cement based onmagnesium/strontium-silicate glasses. Mater Sci Eng C Mater Biol Appl. 2014; 42: 665–671. doi: 10.1016/j.msec.2014.06.006. [DOI] [PubMed] [Google Scholar]
- 12.Shinonaga Y, Arita K, Nishimura T, Chiu SY, Chiu HH, Abe Y, et al. Effects of porous-hydroxyapatite incorporated into glass-ionomer sealants. Dent Mater J. 2015; 34: 196–202. doi: 10.4012/dmj.2014-195. [DOI] [PubMed] [Google Scholar]
- 13.Lohbauer U. Dental Glass Ionomer Cements as Permanent Filling Materials?- Properties, Limitations and Future Trends. Materials. 2010; 3: 76–96. [Google Scholar]
- 14.Moshaverinia A, Roohpour N, Chee WL, Schricker SR. A review of powder modifications in conventional glass- ionomer dental cements. J Mater Chem. 2011; 21: 1319–1328. [Google Scholar]
- 15.Major C. Advance in Glass-Ionomer Cements. 1th ed. Berlin, Chicago: Quintessence Publishing Co; 1999. p. 97. [Google Scholar]
- 16.Alan D, Wilson J. Glass-Ionomer Cement. 1th ed. Chicago: Quintessence books; 1988. p. 247. [Google Scholar]
- 17.Gu YW, Yap AUJ, Cheang P, Koh YL, Khor KA. Development of zirconia-glass ionomer cement composites. J Non-Crystalline Solids. 2005; 351: 508–514. [Google Scholar]
- 18.Hickel R, Dasch W, Janda R, Tyas M, Anusavice K. New direct restorative materials. FDI Commission Project. Int Dent J 1998; 48: 3–16. doi: 10.1111/j.1875-595x.1998.tb00688.x. [DOI] [PubMed] [Google Scholar]
- 19.Peez R, Frank S. The physical-mechanical performance of the new Ketac Molar Easymix compared tocommercially available glass ionomer restoratives. J Dent. 2006; 34: 582–587. doi: 10.1016/j.jdent.2004.12.009. [DOI] [PubMed] [Google Scholar]
- 20.Moshaverinia A, Ansari S, Movasaghi Z, Billington RW, Darr JA, Rehman IU. Modification of conventional glass-ionomer cements with N-vinylpyrrolidone containing polyacids, nano-hydroxy and fluoroapatite to improve mechanical properties. Dent Mater. 2008; 24: 1381–1390. doi: 10.1016/j.dental.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 21.Pittayachawan P, McDonald A, Petrie A, Knowles JC. The biaxial flexural strength and fatigue property of Lava Y-TZP dental ceramic. Dent Mater. 2007; 23: 1018–1029. doi: 10.1016/j.dental.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 22.Jivraj SA, Kim TH, Donovan TE. Selection of luting agents, part 1. J Calif Dent Assoc. 2006; 34: 149–160. [PubMed] [Google Scholar]
- 23.Robert G. Craig JM. Powers. Restorative Dental Materials. 11th ed. St Louis, Missouri: Mosby; 2002. p. 92. [Google Scholar]
- 24.Prosser HJ, Powis DR, Wilson AD. Glass-ionomer cements of improved flexural strength. J Dent Res. 1986; 65: 146–148. doi: 10.1177/00220345860650021101. [DOI] [PubMed] [Google Scholar]
- 25.Xie D, Brantley WA, Culbertson BM, Wang G. Mechanical properties and microstructures of glass-ionomer cements. Dent Mater. 2000; 16: 129–138. doi: 10.1016/s0109-5641(99)00093-7. [DOI] [PubMed] [Google Scholar]
- 26.Khouw-Liu VH, Anstice HM, Pearson GJ. An in vitro investigation of a poly(vinyl phosphonic acid) based cement with four conventional glass-ionomer cements. Part 1: Flexural strength and fluoride release. J Dent 1999; 27: 351–357. doi: 10.1016/s0300-5712(98)00061-x. [DOI] [PubMed] [Google Scholar]
- 27.Williams JA, Billington RW. Changes in compressive strength of glass ionomer restorative materials with respect to time periods of 24 h to 4 months. J Oral Rehabil. 1991; 18: 163–168. doi: 10.1111/j.1365-2842.1991.tb00044.x. [DOI] [PubMed] [Google Scholar]
- 28.Guggenberger R, May R, Stefan KP. New trends in glass-ionomer chemistry. Biomaterials. 1998; 19: 479–483. doi: 10.1016/s0142-9612(97)00127-0. [DOI] [PubMed] [Google Scholar]
- 29. Yap AU, Wang X, WU X, Chung SM. Comparative hardness and modulus of tooth-colored restoratives: a depth-sensing micro in dentation study. Biomaterials. 2004;25: 2179–285. doi: 10.1016/j.biomaterials.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 30.Noort RV. Introduction to Dental Materials. 3th ed. Sheffield UK: Mosby Elsevier; 2007. p. 90. [Google Scholar]
- 31. Engqvist H, Lööf J, Uppström S, Phaneuf MW, Jonsson JC, Hermansson L, et al. Transmittance of a bioceramic dental restorative material based on calciumaluminate. J Biomed Mater Res B Appl Biomater. 2004;69: 94–98. doi: 10.1002/jbm.b.20042. [DOI] [PubMed] [Google Scholar]


