Abstract
Background:
The incidence of cardiac morbidity and mortality is high in patients treated with hemodialysis (HD). The aim of this study was to evaluate the relationship between HD and the echocardiographic findings in patients with chronic kidney disease (CKD).
Methods:
Between 2012 and 2014, 150 patients with CKD. The echocardiographic data were done based on American Society of Cardiology (ASE). Measurement method for Ejection Fraction was E balling and for Diastolic Function was Tissue Doppler. Anemia, thyroid conditions and dialysis through an arteriovenous fistula or permanent catheter of dialysis for the patients are not considered.
Results:
The mean age at diagnosis for the patients was 57.8 years, 52.7% were males. Out of 150 patients, 112 patients (74.7%) had diabetes and 117 patients (78%) had a history of hypertension. The prevalence of all echocardiographic findings was more after the first dialysis compared with before the first dialysis in diabetic patients (P<0.05), but in non-diabetic patients, was not for the tricuspid valve stenosis, impaired right ventricular volume, systolic dysfunction and pulmonary hypertension (P>0.05).
Conclusions:
According to the findings of this study, seems that more accurate selection of patients for dialysis, paying special attention to hemodynamic change during dialysis, patient education about diet and better control of uremia and diabetes is essential.
Keywords: Hemodialysis, Chronic kidney disease, Echocardiography
1. INTRODUCTION
Chronic kidney disease (CKD), which prevalence is still growing worldwide, confers a higher risk of coronary artery disease (CAD), chronic heart failure (CHF) and/or death independently of conventional cardiovascular risk factors (1, 2). Tens of millions of persons worldwide have combined cardiovascular disease (CVD) and CKD (3). The risk of CVD in patients with chronic renal disease appears to be far greater than in the general population (4). The incidence of cardiac morbidity and mortality is high in patients treated with hemodialysis (HD) (5). Cardiovascular disease is the principal cause of mortality in patients with renal failure and left ventricular (LV) abnormalities that are adverse prognostic indicators for cardiovascular outcome (6). Dialysis patients who develop cardiac failure have a poor prognosis. HD-induced myocardial stunning driven by ischemia is a recognized complication of HD, which can be ameliorated by HD techniques that improve hemodynamic (7). End-stage renal disease (ESRD) associates with numerous changes in cardiac structure and function that may account for the sustained high prevalence of morbidity and mortality from cardiovascular disease, particularly ischaemic heart disease and heart failure (8).
The aim of this study was to evaluate the relationship between HD and the echocardiographic findings in patients with advanced renal failure in the west of Iran.
2. MATERIALS AND METHODS
In an analytical and cross-sectional study between 2012 and 2014 that was approved by the Ethics Committee of Kermanshah University of Medical Sciences, 150 patients with chronic kidney disease (CKD) (disease signs were confirmed for them clinically) referred to Emam Reza Hospital, Kermanshah, Iran. We checked the general characteristics such as age, sex, hypertension and diabetes in the patients. The echocardiographic information was checked in all patients before the first HD and after one year. The echocardiographic data were done based on American Society of Cardiology (ASE). Measurement method for Ejection Fraction was E balling and for Diastolic Function was Tissue Doppler. Anemia, thyroid conditions and HD through an arteriovenous fistula or permanent catheter of HD for the patients are not considered.
Inclusion criteria: Advanced kidney disease, having of complete echocardiographic information and the similarity of effects on echocardiographic findings, such as drugs of Inotrope.
Exclusion criteria: Having of incomplete echocardiographic information, effective treatment measures, such as CABG and valve repair.
Statistical analysis
For analyzing of data, was used SPSS version 20 software (SPSS, Inc., Chicago, USA). The T-test was done for means and Chi-square test for other values. P-values <0.05 were considered statistically significant.
3. RESULTS
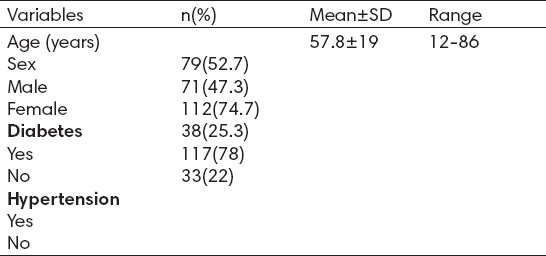
The mean age at diagnosis for the patients was 57.8 years (range, 12 to 86 year), 79 patients (52.7%) were males (Table 1). Out of 150 patients, 112 patients (74.7%) had diabetes and 117 patients (78%) had a history of hypertension.
Table 1.
The characteristics of patients (n=150)
There were significant differences between before the first HD and after one year for all the echocardiographic findings in Table 2. Prevalence of the echocardiographic findings was more after the first HD compared with before the first HD in all patients (P<0.05).
Table 2.
The echocardiographic findings of the patients, before and after the first hemodialysis (n=150)
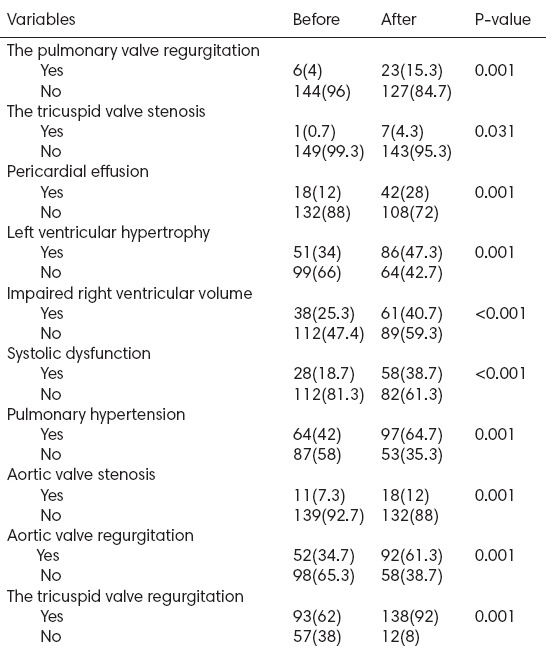
Table 3 shows the echocardiographic findings for 112 diabetic patients, before the first HD and after one year. Prevalence of the echocardiographic findings was more after the first HD compared with before the first HD in diabetic patients (P<0.05).
Table 3.
The echocardiographic findings of diabetic patients, before and after the first hemodialysis (n=112)
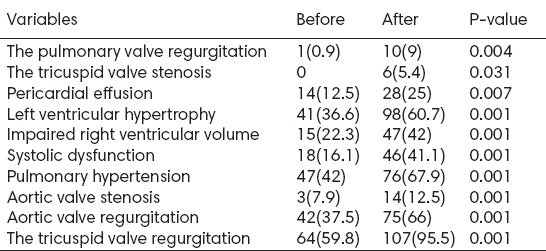
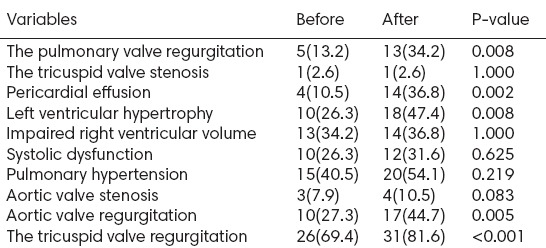
Table 4 shows the echocardiographic findings for 38 non-diabetic patients, before the first HD and after one year. The prevalence of the echocardiographic findings was more after the first HD compared with before the first HD in non-diabetic patients (P<0.05), exception for the tricuspid valve stenosis, impaired right ventricular volume, systolic dysfunction and pulmonary hypertension that had no significant differences before and after the first HD (P>0.05).
Table 4.
The echocardiographic findings of non-diabetic patients, before and after the first hemodialysis (n=38)
4. DISCUSSION
In this study, we checked the relationship between HD (before the first dialysis compared with after one year) and the echocardiographic findings in patients with CKD. In addition, we divided the patients into two groups (diabetic and non-diabetic patients) and then compared the echocardiographic findings before the first dialysis compared with after one year.
CVD is the leading cause of mortality in uremic patients. A lot of cross-sectional studies in dialysis patients reported that traditional CVD risk factors such as hypertension and hypercholesterolemia had low predictive power, while markers of inflammation and malnutrition were highly correlated with cardiovascular mortality (9). On the other hand, patients with CKD have increased risks of accelerated atherosclerosis, nonfatal myocardial infarction, congestive heart failure, atrial and ventricular arrhythmias, and cardiac death and also present difficult scenarios in using conventional cardioprotective therapy (3). CKD accelerates the course of coronary artery disease, independent of conventional cardiac risk factors (10). Vascular calcification (VC) is highly prevalent and rapidly progressive in high stage CKD (11). In CKD patients, LV diastolic dysfunction occurs frequently and is associated with heart failure (HF) and higher mortality (12). Cardiomyopathy in chronic uremia results from pressure and volume overload. The former causes concentric LV hypertrophy, results of hypertension and aortic stenosis, and is also associated with diabetes mellitus and anemia. The clinical consequences of cardiomyopathy include heart failure, ischaemic heart disease, dialysis hypotension, and arrhythmias. The adverse impact of ischaemic heart disease is probably mediated through the development of cardiac failure (13). The results indicate that LV diastolic dysfunction as revealed by the increased peak velocity of atrial filling reflects arterial stiffening in type 2 diabetic (T2D) patients. In addition, myocardial wall thickening at the LV outflow tract reflects not only arterial stiffening but also carotid atherosclerosis. Therefore, these abnormal echocardiographic findings of LV diastolic dysfunction and myocardial wall thickening may be useful markers of the presence of progressive arteriosclerosis in T2D patients (14).
One study (11), showed for the first time that microcirculatory dysfunction is specifically associated with a VC. Furthermore, both VC and microcirculatory dysfunction are associated with atherosclerosis, arterial stiffness, autonomic dysfunction and LV hypertrophy as well as all cause and cardiovascular mortality (11, 15, 16). It has been reported that ischemia maintained for several hours by subtotal coronary occlusion can also cause LV dysfunction that persists for days after the release of the occlusion and restoration of myocardial blood flow. Stunning has also been demonstrated in conscious dogs with subtotal coronary obstruction in which ischemia was induced by muscular exercise (17). HD-induced myocardial stunning is common, and may contribute to the development of heart failure and increased mortality in HD patients. After 12 months, both groups of patients (with and without evidence of HD-induced regional wall motion abnormalities) exhibited a significant deterioration in SBP during HD. Conventional HD exerts a significant acute cardiovascular stress, the exact consequences of which are poorly understood. This study supports the contention that subclinical myocardial ischemia is commonly precipitated by dialysis (18).
The absence of sinus rhythm was an important risk indicator for all-cause death and cardiovascular events in patients with T2D on HD. LV hypertrophy was predictive of stroke and sudden death (19). Compared with non-diabetics, diabetics had a 48% significant increase in the primary endpoint, a 56% significant increase in cardiovascular death, a 30% significant increase in myocardial infarction, a 39% significant increase in stroke, and a 206% significant increase in hospitalization for congestive heart failure (20). In our study, there was no significant different between before and after the first dialysis in non-diabetic patients compared with diabetic patients in some of CVD factors. Therefore, diabetics are at more risk for CVD.
5. CONCLUSION
Diabetes is a risk factor for CVD. Also, according to the findings of this study, seems that more accurate selection of patients for dialysis, paying special attention to hemodynamic change during dialysis, patient education about diet and better control of uremia and diabetes is essential.
Footnotes
• Conflict of interest: none declared.
REFERENCES
- 1.Athyros VG, Katsiki N, Tziomalos K, Gossios TD, Theocharidou E, Gkaliagkousi E, et al. GREACE Study Collaborative Group Statins and cardiovascular outcomes in elderly and younger patients with coronary artery disease: a post hoc analysis of the GREACE study. Arch Med Sci. 2013;9(3):418–26. doi: 10.5114/aoms.2013.35424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Franczyk-Skóra B, Gluba A, Banach M, Rysz J. Treatment of non-ST-elevation myocardial infarction and ST-elevation myocardial infarction in patients with chronic kidney disease. Arch Med Sci. 2013;9(6):1019–27. doi: 10.5114/aoms.2013.39792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McCullough PA. Cardiorenal risk: an important clinical intersection. Rev Cardiovasc Med. 2002;3(2):71–6. [PubMed] [Google Scholar]
- 4.Foley RN, Parfrey PS, Sarnak MJ. Epidemiology of cardiovascular disease in chronic renal disease. J Am Soc Nephrol. 1998;9(12 Suppl):S16–23. [PubMed] [Google Scholar]
- 5.Bos WJ, Bruin S, van Olden RW, Keur I, Wesseling KH, Westerhof N, et al. Cardiac and hemodynamic effects of hemodialysis and ultrafiltration. Am J Kidney Dis. 2000;35(5):819–26. doi: 10.1016/s0272-6386(00)70250-2. [DOI] [PubMed] [Google Scholar]
- 6.Stewart GA, Mark PB, Johnston N, Foster JE, Cowan M, Rodger RS, et al. Determinants of hypertension and left ventricular function in end stage renal failure: a pilot study using cardiovascular magnetic resonance imaging. Clin Physiol Funct Imaging. 2004;24(6):387–93. doi: 10.1111/j.1475-097X.2004.00583.x. [DOI] [PubMed] [Google Scholar]
- 7.Selby NM, Burton JO, Chesterton LJ, McIntyre CW. Dialysis-induced regional left ventricular dysfunction is ameliorated by cooling the dialysate. Clin J Am Soc Nephrol. 2006;1(6):1216–25. doi: 10.2215/CJN.02010606. [DOI] [PubMed] [Google Scholar]
- 8.Parfrey PS, Harnett JD, Barre PE. The natural history of myocardial disease in dialysis patients. J Am Soc Nephrol. 1991;2(1):2–12. doi: 10.1681/ASN.V212. [DOI] [PubMed] [Google Scholar]
- 9.Himmelfarb J, Stenvinkel P, Ikizler TA, Hakim RM. The elephant in uremia: oxidant stress as a unifying concept of cardiovascular disease in uremia. Kidney Int. 2002;62(5):1524–38. doi: 10.1046/j.1523-1755.2002.00600.x. [DOI] [PubMed] [Google Scholar]
- 10.Yerkey MW, Kernis SJ, Franklin BA, Sandberg KR, McCullough PA. Renal dysfunction and acceleration of coronary disease. Heart. 2004;90(8):961–6. doi: 10.1136/hrt.2003.015503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goodman WG, Goldin J, Kuizon BD, Yoon C, Gales B, Sider D, et al. Coronary-artery calcification in young adults with end-stage renal disease who are undergoing dialysis. N Engl J Med. 2000;342(20):1478–83. doi: 10.1056/NEJM200005183422003. [DOI] [PubMed] [Google Scholar]
- 12.Kim MK, Kim B, Lee JY, Kim JS, Han BG, Choi SO, et al. Tissue Doppler-derived E/E’ratio as a parameter for assessing diastolic heart failure and as a predictor of mortality in patients with chronic kidney disease. Korean J Intern Med. 2013;28(1):35–44. doi: 10.3904/kjim.2013.28.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.London GM, Parfrey PS. Cardiac disease in chronic uremia: pathogenesis. Adv Ren Replace Ther. 1997;4(3):194–211. doi: 10.1016/s1073-4449(97)70029-3. [DOI] [PubMed] [Google Scholar]
- 14.Masugata H, Senda S, Yoshikawa K, Yoshihara Y, Daikuhara H, Ayada Y, et al. Relationships between echocardiographic findings, pulse wave velocity, and carotid atherosclerosis in type 2 diabetic patients. Hypertens Res. 2005;28(12):965–71. doi: 10.1291/hypres.28.965. [DOI] [PubMed] [Google Scholar]
- 15.Chesterton LJ, Sigrist MK, Bennett T, Taal MW, McIntyre CW. Reduced baroreflex sensitivity is associated with increased vascular calcification and arterial stiffness. Nephrol Dial Transplant. 2005;20(6):1140–7. doi: 10.1093/ndt/gfh808. [DOI] [PubMed] [Google Scholar]
- 16.Haydar AA, Hujairi NM, Covic AA, Pereira D, Rubens M, Goldsmith DJ. Coronary artery calcification is related to coronary atherosclerosis in chronic renal disease patients: a study comparing EBCT-generated coronary artery calcium scores and coronary angiography. Nephrol Dial Transplant. 2004;19(9):2307–12. doi: 10.1093/ndt/gfh120. [DOI] [PubMed] [Google Scholar]
- 17.Braunwald E, Rutherford JD. Reversible ischaemic left ventricular dysfunction: evidence for the “hibernating myocardium”. J Am Coll Cardiol. 1986;8(6):1467–70. doi: 10.1016/s0735-1097(86)80325-4. [DOI] [PubMed] [Google Scholar]
- 18.Burton JO, Jefferies HJ, Selby NM, McIntyre CW. Hemodialysis-induced cardiac injury: determinants and associated outcomes. Clin J Am Soc Nephrol. 2009;4(5):914–20. doi: 10.2215/CJN.03900808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krane V, Heinrich F, Meesmann M, Olschewski M, Lilienthal J, Angermann C, et al. Electrocardiography and outcome in patients with diabetes mellitus on maintenance hemodialysis. Clin J Am Soc Nephrol. 2009;4(2):394–400. doi: 10.2215/CJN.02020408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Redon J, Mancia G, Sleight P, Schumacher H, Gao P, Pogue J, et al. Safety and efficacy of low blood pressures among patients with diabetes. Subgroup analyses from the ONTARGET (ONgoing Telmisartan Alone and in combination with Ramipril Global Endpoint Trial) J Am Coll Cardiol. 2012;59(1):74–83. doi: 10.1016/j.jacc.2011.09.040. [DOI] [PubMed] [Google Scholar]


