Abstract
Introduction:
Non Hodgkin lymphoma-Diffuse large B cell lymphoma (DLBC) is composed of more varieties of one disease. Analysis and understanding of a wide range of characteristics of the disease, which include: clinical, immunohistochemical, cytogenetic and molecular characteristics may improve treatment results.
Aim:
achieving the estimated three-year survival and influence of IRF/MUM1 expression to three-year survival.
Material and methods:
A study was retrospective–prospective, patients were followed for seven years a period of dine. The study included 60 patients de novo DLBCL. Age was 18-72 years old, the average age 45 years, male 31 (51,7%) and female 29 (48.3%). Median follow-up was 47 months (3-91 months). To determine differentiation immunophenotype antibodies those were used anti-CD20, anti-CD10, anti-Bcl-6, IRF-4/MUM1, CD 138.
Results:
Included the GCB type was 65%. Impact prognostic index IPI>2 GBC vs non GBC p=0,038 X2. Statistically significant difference was confirmed compared to the IPI> 2 to 3 year OS p<0,0005 X2. Significantly longer three-year survival was provided in the group GCB 36 (92,3%) vs. non GCB 8 (38,1%) p=0,003 X2. Clinical and immunohistochemical factors showed a significant impact to three-year survival by univariate: LDH p=0,005, MUM1 p=0,003, while CD10 p=0,069 was confirmed on the level of borderline impact. Using multivariate analysis, expression MUM1 has the greatest impact p<0.0005 OR=0.083 (95% CI 0.23-0.303) on the disease outcome – three-year survival.
Conclusion:
expression MUM1 >25% has the greatest impact on the disease outcome – three-year survival.
Keywords: DLBCL, MUM1 expression, three-year survival
1. INTRODUCTION
According to the latest classification of the World Health Organization Classification of Tumors published 2008th in Lyon, molecular, biological and clinical studies have recognized within Non Hodgkin lymphoma - Diffuse large B cell lymphoma (DLBC) morphological, molecular and immunohistochemical subgroups and entities (1). Despite great progress in treatment of DLBCL in last 20 years of immunochemotherapy era, failure free survival (FFS) remains around 50% with a particularly poor prognosis for those who have not been treated with immunochemotherapy and autologous transplantation.
In current practice immunochemotherapy (R-CHOP) is considered the standard first-line treatment of NHL built of CD20 + B cells, and thus DLBCL. Although the R-CHOP is the best existing first-line therapy for DLBCL, it is curative in about half of patients, while in others it does not achieve a permanent and/or complete remission. Approximately 30-40% of patients responds very well to treatment and live long, while 60-70% die from the disease. Patients with high and intermediate IPI (> 2) have a high rate of relapsing. In these patients, refractory disease requires more aggressive therapeutic approach. Advances in treatment can potentially be achieved using autologous peripheral blood stem cell transplantation (APBSCT), but it is accompanied by an increased rate of mortality and delayed improvement of survival (2).
Therefore, first-line therapy requires further training, which includes understanding the pathophysiological mechanisms of the disease, distinguishing patients with favorable between those with unfavorable characteristics, in which the standard therapeutic approach is not sufficient. It should provide proper patient selection and choice of adequate treatment, which is important in achieving a better and longer therapeutic response, reducing morbidity and treatment costs, avoiding disability and other late effects of treatment, including secondary malignancies.
If we go back to the fact that the NHL is composed of more varieties of one disease, since progress can only be achieved from a critical analysis and understanding of a wide range of characteristics of the disease, which include: clinical, immunohistochemical, cytogenetic and molecular characteristics. It is appropriate to assume that a good choice of therapy, with the definition of prognostic indicators, may improve treatment results.
Prognosis and predictions of NHL is represented by clinical factors expressed by International Prognostic Index (IPI). However, it is evident that clinically and pathologically standard parameters themselves are not sufficient. Biological properties of the tumor largely determine its clinical behavior, indicate prognosis and treatment outcome. It can identify new “targets” that will target the so-called biological therapy.
List of biomarkers with potential prognostic and predictive significance of Diffuse large B cell lymphoma (DLBCL) is huge (3). Based on differentiation cell type determined by immunohistochemical technique and fluorescence in situ hybridization (FISC) by two groups of DLBCL are determined: germinal center B cell type like (GCB) DLBCL with identification of two cell type: bcl-6 + / CD10 + / MUM1-/CD138- and bcl-6 + /–/ CD10-/-MUM1 / CD138 -, which has a favorable prognosis, and activated B cell type (ABC) DLBCL immunophenotype bcl-6 + /-CD10-/ MUM1 + / CD138-., which has a poor prognosis with a small third untested group with a poor prognosis, which is considered unique with ABC (4). However, the prognostic value of some immunohistochemical characteristics of DLBCL is fully defined.
The 2008 WHO classification of tumors of hematopoietic and lymphoid tissues along with data from a study by Rosenwald, Coloma, Hans, Hummel, and al (5-8) point out that the cases with the expression of CD10 cells are considered GCB type, as well as cases that were CD10-, bcl6 +, MUM1-. All other cases are considered non-GCB type. Hans’s algorithm uses three antibodies: CD10, MUM1 and BCL6 on which form non GBC and GBC subgroups.
Today, the determination of cell type differentiation is an important part of the diagnostic work-up of DLBCL with the recent studies testing and the importance of molecular markers involving FOXP1 and GCET1, whose significance is tested in many studies (9, 10).
In addition, noteworthy is the importance of the other biological characteristics of the B cell NHL as well as understanding of programmed cell death-apoptosis.
Understanding the complexity of carcinogenesis has a positive impact on finding the right option in choosing the right therapy for the treatment and improving the lives of patients.
Recognition of apoptotic deregulation as a fundamental element in the development of cancer today is the most important guide in the study of cancer and finding targeted therapeutic options.
2. MATERIALS AND METHODS
The study was clinical retrospective-prospective. Patients were followed in relation to the clinical characteristics and the data of histopathologic diagnosis until the completion of the study. In this study we analyzed 60 patients who had been diagnosed de-novo diffuse large B cell lymph (DLBCL) and who were treated and followed up at the Hematology Clinic, University Clinical Center of Sarajevo. Median follow-up was 47 months (3-91 months). At the end of the study 44 (73.35%) patients were alive. Patients were divided into two groups: the origin of germinal center - GCB and non germinal center - non GCB. According to the latest WHO classification in relation to subtypes and entities, the study included patients who belonged: DLBCL NOS with subtype T-rich and entities: Mediastinal large B cell lymphoma 3 patients and ALK positive DLBCL 1 patient. The study included patients aged 18-72 years.
It was a homogeneous group of patients in comparison to the first line of treatment. In the first-line treatment patients received immunochemotherapy per protocol R-CHOP (rituximab 375mg/m2 iv day 1 + CHOP / day 1 Cyclophosphamide 750 mg/m2 iv, 50mg/m2iv Doxorubicin, Oncovin max. 2 mg / iv, 1–5th day Prednisone 100 mg per os).
Radiotherapy was administered at: bulky, extra nodal sites and the residual mass.
Post-treatment restaging consisted of a repetition of earlier pathological tests and / or biopsy.
Response was assessed according to conventional criteria (normalization of metabolic tests and the absence of previously existing tumor mass).
Biopsy material was first analyzed in several different centers to diagnose DLBCL using the following markers: CD20, bcl-2, bcl-6, cyclin D1, very rare CD10, according to the indications: CD5, bcl-1, CD3, CD30, S- 100, CK-HMW, CK/AE1/AE3, CK, CD79alfa, CD15, ALK, EMA, LCA/CD45, CD43, TTF-1, vimentin, TDT, CD99, CD23, cap, lambda, synoptophysin, CK7, NSE, HMB45, desmin, ASMA
Additional immunohistochemical staining were performed at the Institute of Pathology and Cytology of Clinical Center of University of Sarajevo on the same histopathological material. Biopsy samples were further analyzed by immunohistochemistry for the markers: BCL6, CD10, IRF/MUM1, CD138 at the Institute of Pathology, Clinical Center University of Sarajevo.
The sections were incubated with primary antibody, including:
Anti-CD20 (1:150, clone L26, DakoCytomation, Glostrup, Denmark),
Anti-CD10 (1:150, clone 56C6, Novocastra Laboratories, Newcastle, Tyne, UK),
Anti-Bcl-6 (1:40, clone PG.B6p, DakoCytomation, Glostrup, Denmark),
CD138 (1:10 dilution, Clone AM 411-10 M, BIOGENEX, CA USA,
IRF/MUM1 (1:40 dilution, clone sc 6059, Santa Cruz Biotechnology, INC, CA, USA),
The project provides visualization which was performed with EnVision® method (DakoCytomation, Glostrup, Denmark) with the manufacturer’s instructions. Appropriate positive and negative controls were used.
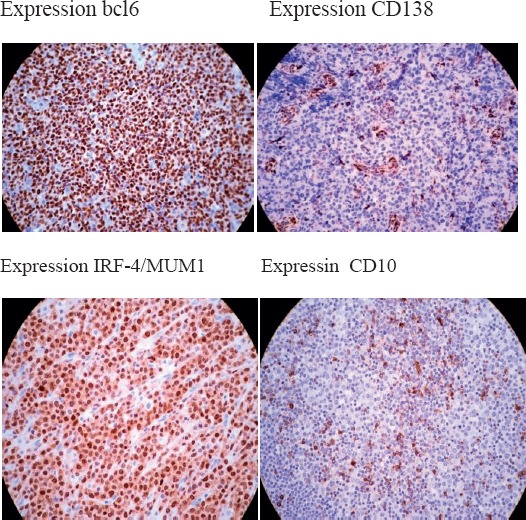
Bcl-6 and CD10 was quantified using the H score (histo-score) system, according to the method described by McCarty et al. Positive expression of the MUM1 and CD138 was considered when more than 25% neoplastic cells. Microscopy was performed on a microscope ZEISS Scope A1. Microscopy preparation had next appearance:
Statistical analysis:
When it comes to statistical analysis we used univariate methods for evaluation of significant difference (X2 test, binary logistic regression analysis). We assessed the overall survival with Kaplan-Meier methods and unstratified long-rank test. We used a multivariate backward Wald model to assess the significance for the efficacy variables and to establish th Odds ratio (OR) and 95% CI for each subgroup. P<0.05 was considered as significant
3. RESULTS
This study included 60 patients diagnosed with de novo diffuse large B cell lymphoma (DLBCL). The age of the respondents was 18-72 years and the average age prevalence was 45 years old. We analyzed 31 (51.7%) males, 29 (48.3%) were women.
Responses of total period of monitoring
During the period of examination, with 60 patients who were treated by immunochemotherapy and who had DLBCA, complete remission 47 (78,3%), PR-partial remission 8 (13,3%), PB-progressive disease 5 (8,3) was achieved. Statistically significant difference was confirmed compared to the IPI> 2 (low: high) 39 (65%) vs 21 (35%) x2 p= 0.014, clinical stage I/II vs III/IV x2 26 (43.3%) vs 36 (56.7%) p<0.0005, ECOG >27 (11.7%) vs 53 (88.3%) p=0.008 and level LDH normal vs. increased 38(63,3%)vs 22 (36,7%) p=0.003 compared to achieve first complete remission.
Difference in survival length of the examinees with MUM1>25% is statistically significant χ2 (Mantel-Cox)=19.2 p<0.0005. Examinees with MUM1>25% live shorter (23 months; 95%(16-29 months) comparing to examinees with MUM<25% who live 37 months in average; 95% (34-40 months).
Analysis risk factor to three years survival
Using Binary Logistic Regressive Analysis it is confirmed: significant differences are not confirmed ages p=0.903 OR 0.956 (0.465-1.966), gender p=0.322 OR 0.593 (0.211-1.667) but there is significant differences ECOG >2 p=0.002 OR 6.390 (2.022-20.194) and level LDH p=0.005 OR 4.66 (1.586-13.698) to three year survival.
Using Binary Logistic Regressive Univariate analysis significant differences is confirmed in the expression of MUM1 p=0.003 OR 0.082 (0.16-0.430) to three year survival in GBC vs. non GBC group in DLBCL. Statistically significant difference is confirmed in the expression of CD10 at the level of borderline impact of p<0.069 OR 2.331 (0.936–5.808) to three-year survival. Significant differences is not confirmed in the expression of BCL6 p=0.916 OR 0.005 (0.000-1.817) and expression of CD138 p=0.444 OR 0.453 (0.059-3.446) to three year survival.
Expression of CD138 was marginally positive (=25% positive) in 6 patients.
Influence of independent clinical and immunophenotype factors on three-year survival was examined using Multivariate Cox’s Regression Method Backward Wald. Independent factors showed statistically significant influence of ECOG>2 p=0.002, LDH p=0.005, MUM1 p=0.003 and IPI>2 p<0.0005.
Using Multivariate Cox’s Regression Method on three-year survival, MUM1 has the strongest influence p<0.0005 OR=0,083 (0.23-0.303), then LDH p=0.002 OR=5.8 (19.3-17.5) (Table 2).
Table 2.
Univariate and multivariate analysis risk factor three-year overall survival
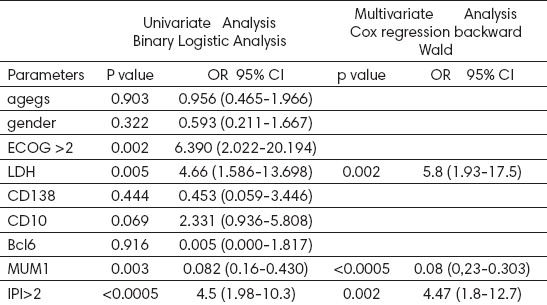
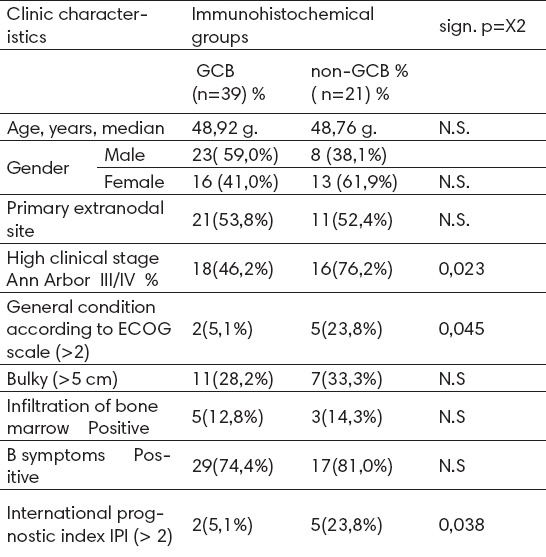
Table 1.
Clinical features DLBCL according to immunohistochemical profile
In comparison to three-year survival, immunohistochemical features in relation to expression bcl6, CD10, CD138 i MUM1, only expression MUM1 had a significant independent influence. Expressions bcl6 and Cd138 did not have significant influence to three-year survival DLBCL in the immunochemotherapy era. The influence of CD10 expression is confirmed on the bordering significant level p=0.069.
4. DISCUSSION
In the presented study the relationship between clinical status and immunohistochemical profiles has been analyzed in the expression: Bcl-6/CD10 / MUM1/CD138 of diffuse large B-cell lymphoma.
Group GCB which has similar subtypes with B cell origin of germinative center was correlated with group non-GCB, which has subtypes of non germinative center. Immunohistochemical groups were comparable with data from the last sub-classification nomenclature of lymphoma (1).
At the molecular level there are two large groups of patients with DLBCL: germinal center B cell type like (GCB) DLBCL, which has a favorable prognosis, and activated B cell type (ABC) DLBCL, which has a poor prognosis, with a third small untested group with poor prognosis, which is considered unique with ABC. In previous studies these two groups were analyzed (4-7).
Because of its expensive technology molecular analysis is more rarely used than immunohistochemical analysis related to CD10, bcl-6, MUM1 CD138.11 in some studies (12-14). Whether is this a good division is still not clear.
Rate of GCB defined immunohistochemical phenotype is variable from study to study (18% in the study Borovečki to 49% in the study Paepe et al) (15).
In our study, the group A (GCB) was 39 (65%) patients in group B (non-GCB) 21 (35%) patients. Study was preceded by a pilot study, “Prognostic and predictive significance of CD10 expression in diffuse large B cell lymphoma” (2007), which confirmed the significant impact of CD10 expression in relation to the achievement of CR1. Abstract of the study is published in the journal Leukemia Research - Clinical and Laboratory studies (16).
Analysis of clinical response
Median follow-up was 47 months (3-91 month), which is satisfactory considering the fact that relapse usually occurs within the first two years after our initial treatment. We analyzed 60 patients with DLBCL (median age was 45 with most patients in the age group 46-65 years, significantly impacts of age on the results of this study was not confirmed. We analyzed 31 (51.7%) males, 29 (48.7%) were women. Good response to first-line therapy is confirmed with the achievement of CR1 in 78.3% of investigated, which is comparable with the results of reference centers. A study by the 2007th published Coiffier (17). The difference in achieving CR1 can be explained by the fact that the study included a small group of subjects in which more patients belonging to group A (GCB) 39 (65%). Statistically significant difference was confirmed compared to the IPI> 2 (low: high) 39(65%) vs 21 (35%) χ2 p= 0.014, clinical stage I/II vs III/IV χ2 26 (43.3%) vs 36 (56.7%) p<0.012 and ECOG>27 (11.7%) vs. 53 (88.3%) p=0.008 compared to achieve first complete remission. Statistically significant difference confirmed IPI >2 (low: high) χ2=15,345 p<0.0005 in achieving three-year survival. With Univariate Binary Logistic Regression Analysis it is confirmed statistically significant influence of IPI>2 p<0.0005 OR= 4.5 95% CI (1.98-10.3) to three year survival (Table 2).
Using Univariate Binary Logistic Regression Analysis it is confirmed that the age and sex of the examinees do not have impact to three-year survival: ages p=0.903 OR 0.956 (0.465-1.966), gender p=0.322 OR 0.593 (0.211-1.667), with patients with DLBCL, while ECOG>2 has statistically significant influence to three-year survival p=0.002 OR=6.390 (2.022-20.194) as well as the level LDH p=0.005 OR=4.6) in the expression of MUM1 p=0.003 OR 0.082 95%CI (0.16-0.430 1.5-13.6) (Table 2). Using backward Wald multiple regression analysis on clinical and immunophenotypic features in 60 DLBCL patients in relation to three-year overall survival, the significant impact of MUM1 expression is confirmed p<0.0005 OR=0.083(0.23-0.303), then LDH p=0.002 OR=5.8 (1.93-17.5) and IPI p=0.002 OR=4.47 95%CI (1.8-12.7) (Figure 1A). Kaplan-Meier curve in the group GBC/MUM–the survival average 37.5 months 95%CI (34-40) months vs. nonGBC/MUM1+ 23,1 months 95% CI (16.3-29.8) months. Difference in the average of survival between these two groups is statistically significant p<0,0005. In the group GCB survival is longer (Figure 1B). During the period of follow-up the total three-year survival in the group GCB was longer at the level of significance of p<0.0005. In our study, data obtained in relation to the First Complete Remission and three-year overall survival suggest better sensitivity to immunochemotherapy in the first line treatment of DLBCL in GCB group.
Figure 1A.
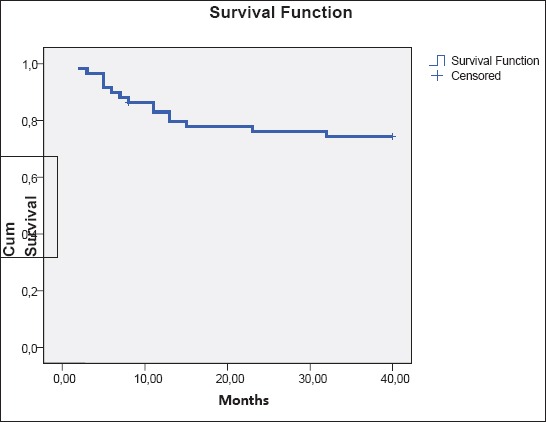
Kaplan-Meier curves for three year overall survival
Figure 1B.
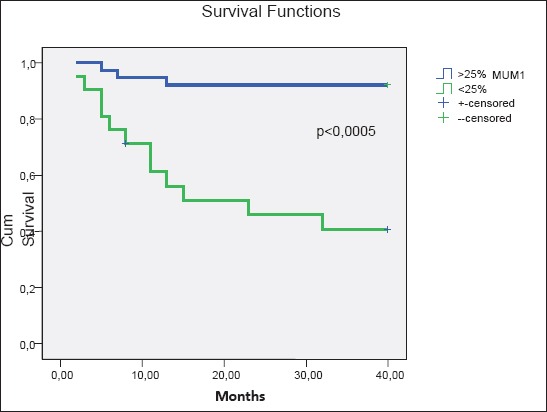
Kaplan-Meier curves for overall survival (3 year):. expression MUM1
Results of immunohistochemical analysis
In this study we found the expression of bcl-6, CD10 and MUM1, with two separate immunophenotypic groups: GCB and non-GCB, comparable with those given in the new WHO classification of tumors of hematopoietic and lymphoid tissues 2008 (1) and data by Rosenwald, Coloma and Hans (5-7), who point out that the cases with the expression of CD10 cells are considered GCB type, as well as cases that were CD10-, bcl6 +, MUM1. Expression of CD138 was 25% in 6 patients and statistically significant impact of expression CD138 was not proved in this study p=0.444 OR 0.453 (0.059-3.446).
In this study, the expression of bcl-6 (weak vs. moderate + high) was not confirmed with significant difference in the groups GBC vs. non-GBC p=0.588 X2 = 0.003 and consequently not found significant effect of bcl-6 in response to the application of immunochemotherapy.
Effect of bcl-6 expression as a predictor of longer OS in DLBCL with IPI was confirmed in the era before immunotherapy, as confirmed in their study by Berglund M (18). With application of immunochemotherapy predictive role of blc6 expression is impaired. Some studies have confirmed the loss of the positive prognostic impact of Bcl-6 in the group of patients treated with anti-CD20 antibody in comparison with the patients treated only with chemotherapy. Explanation may perhaps be found in the mechanism of increased chemo-sensitivity of lymphoma in the use of immunotherapy with chemotherapy as anti CD20-blocks anti-apoptotic effect of some of the mediator, such as bcl-2 and bcl-6, or NFkB system (19-23).
High expression of CD10 is confirmed in group GCB X2 =21.538 p <0.0005 where it reached a better response to therapy compared to CR. In relation to three-year survival the expression of CD10 was confirmed with impact on borderline impact X2 p=0.069 OR 2.331 (0.936-5.808). Expression of MUM1 was> 25% in group non-GCB, and <25% in group GCB, as well as in the study of Alizadah (4). Difference in the survival length examinees with expression MUM1 >25% vs. <25% is statistically significant χ2 (Mantel-Cox)=19,2 p<0,0005. The examinees with >25% MUM1 live shorter (23 months; 95% (16-29 months), compared with examinees with <25% MUM1 who live in average 37; 95% (34-40 months) (Figure 1B). High expression of MUM1 is confirmed in group non-GCB, which has reduced sensitivity, poorer response to treatment, in relation to the achievement of CR1 and OS in the application of immunochemotherapy with DLBCL. Our results in relation to impact expression MUM1 on three-year survival are comparable with the study of author Berglund M (18). He states that the expression of bcl-6 and CD10 are better predictors of response and that expression of MUM1 is not associated with a good prognosis. Results of this study are comparable with the results of the above authors in relation to expression of MUM1 and partially CD10, while not comparable to the results related to the expression of bcl-6. The data obtained in our study confirmed that immunohistochemical profile affects the achievement of three-year survival with significant influence of expression MUM1 and borderline impact expression of CD10 and in applying immunohemotherapy in treating DLBCL. Good predictors of response are confirmed in the application of immunochemotherapy: expression of MUM1-, which is comparable with the results obtained by Muris JJ (24) which in his study concludes that the strongest predictors are CD10 expression, MUM1, bcl-2 and IPI.
The results of multiple regression analysis
Using Multivariate Cox’s Regression backward Wald, impact of independent clinical and immunophenotype factors on three-year survival was examined. Independent factors showed statistically significant influence univariately ECOG>2 p=0,002, LDH p=0,005, MUM1 p=0,003. Using multiple regression analysis of clinical and immunophenotypic features DLBCL in relation to three-year overall survival, the significant impact of MUM1 expression is confirmed p<0,0005 OR=0,083 (0,23-0,303), then LDH p=0,002 OR=5,8 (1,93-17,5) (Table 2). Pre-treatment prognostic and predictive value of immunohistochemically defined GCB and non GCB group within DLBCL was confirmed significant independent by univariate analysis and by multivariate analysis effect of the expression of MUM1.
The results of multiple regression analysis, the independent influence of MUM1 expression confirm significant impact of the planned three-year survival rate, where the impact associated with immunohistochemical markers of GCB and non GCB was analyzed and in which the analysis confirmed significant difference in the achievement of CR1 and OS.
5. CONCLUSIONS
Immunohistochemical profile in the expression: Bcl-6/CD10/ MUM1/ has a significant impact on therapeutic response DLBCL in relation to three year overall survival when applied immunochemotherapy. The significant impact of MUM1>25% expression is confirmed and particular influence the expression of CD10 to three-year survival. In this study significant impact to three-year survival of expression bcl6 in the era of immunochemotherapy in DLBCL was not confirmed.
Footnotes
• Author’s contribution: All authors contributed in all phases of preparing this article. Final proof reading was made by first author.
• Conflict of interest: none declared.
REFERENCES
- 1.Swerdlow SH, et al. WHO Classification of Tumours of Haematopoietic and Lynphoid Tissues. Int. Agency for Research on Cancer. 2008:233–68. [Google Scholar]
- 2.Donnall E, Karl GB, Stephan JF. Autolous Hematopoetic Cell Transplantation for Non Hodgkin lymphoma. Hematopoietic cell transplantation. 2000:939–48. [Google Scholar]
- 3.Brenner S. The genetics of behavior. Brit Med Bull. 1973;29:269. doi: 10.1093/oxfordjournals.bmb.a071019. [DOI] [PubMed] [Google Scholar]
- 4.Alizadeh AA, Eisen MB, Davis RE, et al. Distinct types of diffuse large B-cell lymphoma identified by gene expression profiling. Nature. 2000;403:503–11. doi: 10.1038/35000501. [DOI] [PubMed] [Google Scholar]
- 5.Rosenwald A, Wright G, Chan WC, et al. The use of molecular profiling to predict survival after chemotherapiy for diffuse large B-cell lymphoma. N Engl J Med. 2002;346:1937–47. doi: 10.1056/NEJMoa012914. [DOI] [PubMed] [Google Scholar]
- 6.Colomo L, Lopez-Guillermo A, Perales M, Rives S, Martinez A, Bosch F, Colomer D, Falini B, Montserrat E, Campo E. Clinical impact of the differentiation profile assessed by immunophenotyping in patients with diffuse large B-cell lymphoma. Blood. 2003 Jan 1;101(1):78–84. doi: 10.1182/blood-2002-04-1286. [DOI] [PubMed] [Google Scholar]
- 7.Hans C, et al. Confirmation of the molecular classification of difuze large B-cell lymphoma by immunohistochemistry using a tissue microrray. Blood. 2000;103:275–82. doi: 10.1182/blood-2003-05-1545. [DOI] [PubMed] [Google Scholar]
- 8.Hummel M, et al. A biologic definition of Burkitt`s lymphoma from transcriptional and genomic profilling. N Engl J Med. 2006;354:2419–30. doi: 10.1056/NEJMoa055351. [DOI] [PubMed] [Google Scholar]
- 9.Barrans SL, Fenton JAL, Banham A, Roger G, et al. Strong expression of FOXP1 identifies a distinct subset of diffuse large B-cell lymphoma) DLBCL) patients with poor outcome. Blood. 2006;104:2933–3935. doi: 10.1182/blood-2004-03-1209. [DOI] [PubMed] [Google Scholar]
- 10.Choi WW, Weisenburger DD, Greiner TC, Piris MA, Banham AH, Delabie J, et al. A now immunostain algorithm classifies diffuse large B cell lymphoma into molecular subtipes with high accuracy. Clin Cancer Res. 2009;115(17):5494–502. doi: 10.1158/1078-0432.CCR-09-0113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bai M, Skyrlas A, Agnantis NJ, Kamina S, Tsanou E, Grepi C, Galani V, Kanavaros P. Diffuse large B-cell lymphomas with germinal centre B-cell ike differentiation immunophenotypic profile are associated with high apoptotic index, high expression of the proapoptotic proteins bax, bak and bid and low expression of the aniapoptotic protein bclxl. Modern Pathology. 2004 Jull;17(7):847–56. doi: 10.1038/modpathol.3800130. [DOI] [PubMed] [Google Scholar]
- 12.Von Imhoff GW, et al. Prognostic impact of germinal center-associated proteins and chromosomal breakpoints in poor-risk diffuse large B-cell lymphoma. J Clin Oncol. 2006 Sep 1;24(25):4135–42. doi: 10.1200/JCO.2006.05.5897. [DOI] [PubMed] [Google Scholar]
- 13.Bai M, Skyrlas A, Agnantis NJ, Kamina S, Kitsoulis P, Kanavaros P. Clustetr analysis of apoptosis-associated bcl2 family proteins in diffuse large B-cell lymphomas. Relations with the apoptotic index the proliferation profile and the B-cell differentiation immunophenotypes. Anticancer Res. 2004 Sep-Oct;24(5A):3081–8. [PubMed] [Google Scholar]
- 14.Oh YH, Park CK. Prognostic evaluation of nodal diffuse large B cell lymphoma by immunohistochemical profiles with emphasis on CD138 expression as a poor prognostic factor. J Korean Med Sci. 2006;21:397–405. doi: 10.3346/jkms.2006.21.3.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Paepe P, Achten R, Verhoef G, Wlodarska I, Stul M, Vanhentenrijk V, Praet M, De Wolf-Peeters C. Large cleaved and immunoblastic lymphoma may represent two distinct clinicopahologic entities the group of diffuse large B-cell lymphomas. J Clin Oncol. 2005 Oct 1;23(28):7060–8. doi: 10.1200/JCO.2005.15.503. [DOI] [PubMed] [Google Scholar]
- 16.Sofo HA, et al. Prognostic and predictive significance of CD10 expression in diffuse large B cell lymphoma. Leukemia Research-clinical and laboratory studies. 2007;131:S89. [Google Scholar]
- 17.Coiffier B, et al. J Clin Oncol. 2007;25:185. doi: 10.1200/JCO.2006.10.5957. [DOI] [PubMed] [Google Scholar]
- 18.Berglund M, Thunberg U, Amini RM, Book M, Roos G, Erlanson M, Linderoth J, Dictor M, Jerkeman M, Cavallin-Stahl E, Sundstrom C, Rehn-Eriksson S, Backlin C, Hagberg H, Rosenquist R, Enblad G. Evaluation of immunophenothype in diffuse large B-cell lymphoma and its impact on prognosis. Mod Pathol. 2005 Aug;18(8):1113–20. doi: 10.1038/modpathol.3800396. [DOI] [PubMed] [Google Scholar]
- 19.Winter JN, et al. Prognostic significance of Bcl-6 protein expression in DLBCL treated with CHOP or R-CHOP: a prospective correlative study. Blood. 2006;107:4207–13. doi: 10.1182/blood-2005-10-4222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bonavida B. Rituximab-induced inhibition of antiapoptotic cell survival pathways: implications in chemo/immunoresistance, rituximab unresponsiveness, prognostic and novel therapeutic interventions. Oncogene. 2007;26:3629–36. doi: 10.1038/sj.onc.1210365. [DOI] [PubMed] [Google Scholar]
- 21.Jazirehi AR, et al. Rituximab (chimeric anti-CD20 monoclonal antibody) inhibits the constitutive Nuclear factor-κB signaling pathway in non-Hodgkin’s lymphoma B-cell lines: role in sensitization to chemotherapeutic drug-induced apoptosis. Cancer Res. 2005;65:264–76. [PubMed] [Google Scholar]
- 22.Mounier N, et al. Rituximab plus CHOP (R-CHOP) overcomes bcl-2-associated resistance to chemotherapy in elderly patients with diffuse large B-cell lymphoma (DLBCL) Blood. 2003;101:4279–84. doi: 10.1182/blood-2002-11-3442. [DOI] [PubMed] [Google Scholar]
- 23.Davis RE, et al. Constitutive nuclear factor kappaB activity is required for survival of activated B cell-like diffuse large B cell lymphoma cells. J Exp Med. 2001;194:1861–74. doi: 10.1084/jem.194.12.1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Muris JJ, Meijer CJ, Vos W, van Krieken JH, Jiwa NM, Ossenkoppele GJ, Oudejans JJ. Immunohistochemical profilling based on Bcl-2, CD10 and MUM1 expression improves risk stratification in patients with primary nodal diffuse large B cell lymphoma. J Pathol. 2006 Apr;208(5):714–23. doi: 10.1002/path.1924. [DOI] [PubMed] [Google Scholar]


