Abstract
Background
In recent years, antibiotic resistance has been indicated as a paramount threat to public health. The use of bacteriophages appears to be a safer alternative for the control of bacterial infections.
Objectives
The present study aims to explore sewage water for the presence of indigenous bacteriophages, and to investigate their antibacterial potential against Methicillin-resistant Staphylococcus aureus (MRSA).
Methods
Bacterial isolates were first collected and identified from pus samples taken from the surgical and burn units using standard microbiological procedures. A cefoxitin disk screen test was then used and interpreted according to the clinical laboratory standards institute (CLSI) guidelines for the detection of MRSA. The sewage samples were processed and the phages enriched using S. aureus as a host organism. Turbid and clear plaques of different sizes were isolated using an overlay method, purified, and then enumerated by means of a dilution method.
Results
The phages exhibited good lytic activity against MRSA when tested in-vitro, and the highest activity was attained within three to six hours of phage infection. The isolated phage pq/48 was also found efficient in decreasing the bacterial count during an in-vivo trial in rabbits. A protein analysis using SDS-PAGE revealed 10 proteins of between 20 kDa and 155 kDa in size.
Conclusions
The overall results indicated that bacteriophages isolated from sewage exhibited excellent lytic activity against MRSA strains. In conclusion, bacteriophages can be further characterized and appear to be a promising candidate for phage therapy against MRSA in the future.
Keywords: MRSA, Bacteriophage, SDS-PAGE, Clear Plaques, In-Vitro Efficacy, In-Vivo Model
1. Background
The extensive use of antibiotics for nutritive and therapeutic purposes is linked to the progression of antibiotic resistance, which has been declared one of the most pressing threats to public health. In effect, the rate of development of new antibiotic drugs is not fast enough to replace those drugs that have become less effective over the years. Every year, around 80,000 hospital patients are infected with resistant strains of Staphylococcus aureus (1). Therefore, the threat of S. aureus is not just its ability to induce a diverse range of life-threatening infections, but also its exceptional behavior in terms of mounting antimicrobial resistance (2).
Staphylococcus aureus is gradually acquiring resistance to previously effective antimicrobial agents. Methicillin-resistant S. aureus (MRSA) is more difficult to arrest due to its resistance to the antibiotics that are usually recommended. Not only is it resistant to beta-lactam antibiotics, it also shows resistance to fluoroquinoles, aminoglycosides, and glycopeptides (3). This remarkable potential of S. aureus to adopt resistance to currently available antimicrobial agents presents an even greater threat than before. The problem is worsening exponentially, since MRSA’s resistance to antibiotics is extending from hospitals out into the community (2). In Pakistan, its prevalence has grown dramatically from 5% in 1989 to 51% in 2003 (4).
Bacteriophages, or phages, are viruses that infect bacteria. They work by perfusing bacterial cells, and in the case of lytic phages, they impact on bacterial metabolic rates, leading to bacterial lysis (5). Bacteriophage abundance is thought to be ten times higher than that of bacteria, making bacteriophages the most expanded life form on earth. Conventional medicine can gain advantages from the lytic skill of phages, as they offer significant therapeutic capability due to their potency in inducing lethal effects in the host bacterium (6). More than 200 S. aureus phages have been characterized during the last 10 years (7). Bacteriophage therapy has a number of advantages over conventional antibiotic therapies. Unlike antibiotics, which may be bacteriostatic, phages are bactericidal in nature. Thus, bacteria are incapable of recovering their viability following a phage infection (8). Furthermore, the high specificity of phages for their target bacteria helps to prevent the establishment of secondary infections due to microbial imbalance (9). The development of the phage system is relatively economic. Moreover, phage therapy exhibits a high degree of versatility in terms of phage formulation and applications; for example, phages can be used in combination with several antibiotics, or a mixture of diverse phages administered as a cocktail may be useful in boosting their antibacterial spectrum (1, 10).
2. Objectives
The aim of the present study was to isolate and characterize the bacteriophages specific to S. aureus from indigenous sewage water sources. Further, their lytic activity in-vitro was evaluated, in addition to bacteriophage therapy in rabbits in-vivo. The selected phages were further characterized using the SDS-PAGE technique.
3. Methods
3.1. Bacterial Isolates
The study subject was approved by the ethical review committee (ERC) of government college University Faisalabad (Vide No. ERC/GCUF/35). Samples were taken aseptically from surgical and burn wound patients attending allied hospital Faisalabad in Pakistan. A total of 65 samples were collected from the patients using sterile swabs. The swabs were homogenized in normal saline and were immediately transferred to the postgraduate research laboratory of the department of microbiology at government college University Faisalabad (Pakistan), as described (11). Mannitol salt agar (Difco, USA) was used as a selective and differential medium for the isolation of S. aureus. Swab samples were streaked directly on mannitol salt agar plates. The Kirby Bauer disk diffusion method was applied for the detection of MRSA using a cefoxitin disk (30 µg) (Difco, USA) (12, 13).
3.2. Isolation of Bacteriophages
The bacteriophages for use against S. aureus were isolated from sewage water collected in screw-capped bottles from different areas of Faisalabad in Pakistan. The sewage water samples were then processed for phage isolation as described previously (14). Briefly, the samples were first centrifuged at 4,000 rpm for 20 minutes, and a supernatant was filtered using a 0.22 µm syringe filter. The filtrate was incubated for 15 minutes at 37°C after the addition of 150 µL of chloroform. Subsequently, 10 mL of fresh bacterial culture and 25 mL of broth were mixed with 15 mL of the filtrate. The mixture was incubated overnight at 37°C in a shaking incubator at 160 rpm. The centrifugation and filtration steps were repeated, and an active bacterial culture was added to the filtrate once more. The mixture was incubated again. For phage enrichment, the whole process was repeated two to three times. The final enriched filtrate was assessed for lytic activity by means of a spot assay.
3.3. Assessment of Filtrate Efficacy by Spot Assay
The spot assay was carried out as described previously (15, 16). Positive samples were selected, from which the most susceptible host strains and the most active sewage filtrates were processed further.
3.4. Agar Overlay Assay for Plaque Morphology
For the identification of plaques, an agar overlay method was used, as described (17). The morphology of each plaque in terms of clarity and size was noted.
3.5. Purification and Count of Individual Plaques
Well isolated plaques were chosen for the purification and phage count process. Individual plaques were picked using a sterile micro-pipette tip, as follows: the tip was first inserted into the center of a well isolated plaque and was swirled to obtain a single plaque. The plaque was then suspended in saline magnesium (SM) buffer, and an overlay method was used to obtain purified plaques of each type on the lawn of the individual host bacterium, as described previously (14). For the phage count (plaque forming unit (PFU) determination), a SM buffer supplemented with gelatin was prepared, and the phages were counted, as described previously.
3.6. Effect of Salts
For effective attachment to or intracellular progression into host cells, phages require certain divalent ions. In this assay, the effect of two divalent salts (MgSO4 and CaCl2) was assessed on phage action, as described previously, using 5 mM working solutions of divalent salts, namely, MgSO4 and CaCl2 (18).
3.7. In-Vitro Assessment of the Lytic Activity of Bacteriophages
The in-vitro lytic activity of the isolated phage was assessed on a solid medium. Following infection by the phage lysate, the bacterial host strain was analyzed for bacterial count at different time intervals (15). Ten test tubes were labelled clearly, and about 3 mL of fresh broth was poured into each test tube. Then, 50 µL of bacterial host strain, with turbidity adjusted to 0.5 McFarland standards, was inoculated into each tube except for the last, which served as a bacterial control. About 100 µL of the phage sample (PFU = 108) was added to all the test tubes. The first tube served as a phage control. All the tubes were then incubated at 37°C. The samples were removed at regular intervals (15 minutes, 30 minutes, and 1, 2, 3, 6, 12, and 24 hours), and 100 µL of the sample was plated and incubated for bacterial count purposes.
3.8. In-Vivo Evaluation of the Bacteriophages
The efficacy of the bacteriophages was assessed using an infection model (19). Healthy, disease-free six to seven month old rabbits were grouped into four sets of three rabbits each. Exponentially growing MRSA was pelleted by means of centrifugation at 3,000 rpm. A washing pellet was then suspended in phosphate buffer saline to make a suspension that could be used as a bacterial inoculum. A wound was created aseptically on the shaved flanks of the rabbits. The four sets of rabbits were categorized as follows: first set: negative control; second set: bacterial control; third set: treatment group; fourth set: prevention group. As the negative control, the first set received only normal saline. The bacterial control set was treated with 100 µL of bacterial challenge. For the treatment group, bacteriophages (PFU 2 × 108) were sprayed over the wound after its infection with bacterial challenge. The bacteriophage application was followed by the same dose of bacterial challenge in the prevention group. Wound-healing ability was assessed by observing the wound contraction rate, and by determining the bacterial count after 48 hours.
3.9. Molecular Characterization Using the SDS-PAGE Technique
The proteins of the isolated phages were analyzed by means of a sodium dodecyl sulphate polyacrylamide gel electrophoresis (SDS-PAGE) technique carried out using the Hoefer SE300 mini VE integrated vertical electrophoresis system (Bio-Rad, USA) (20). Then, 10 mL of resolving gel was prepared by mixing 2.5 mL of 1.5 M tris HCl, 4 mL of 10% SDS, 0.1 mL of 10% ammonium persulphate, and 0.004 ml of TEMED. A 3 mL preparation of stacking gel was prepared by mixing 0.5 mL of the 30% acrylamide solution, 0.38 mL of the 1.5 M tris HCl, 0.03 ml of the 10% SDS, 0.03 mL of the 10% ammonium persulphate (APS), and 0.003 ml of the TEMED. A sample buffer was prepared for the solubilization of the sample to be processed for protein analysis. This buffer was composed of a 1.20% Trizma base, 4% SDS, 3% dithiothreitol, 0.20% bromophenol blue, and 20% glycerol. A 1X running buffer was prepared from a Trizma base of 0.3%, glycine 1.87%, and sodium dodecyl sulfate 0.1%. The pH was adjusted to 8.3. The staining solution was composed of Coomassie brilliant blue 0.25%, glacial acetic acid 10% (v/v), and methanol 45% (v/v). Previously prepared and refrigerated phage lysates were pelleted by centrifugation at 21,000 rpm at 4°C (20). The pellet obtained as a result of centrifugation was dissolved in 2X sample buffers. By inserting a comb into the stacking gel, wells were created for sample loading. Following sample loading, electrophoresis was carried out at 2 mA/cm2 for 60 to 80 minutes. The running of the samples was monitored through observation of the position of the bromophenol blue dye, and the process was struck off when the dye had completely left the gel front. Coomassie brilliant blue staining solution was used to stain the processed gel for 30 minutes. After removal of the excess stain, the gel was visualized using the ImageJ software (Bio-Rad, USA), and the results were documented.
4. Results
4.1. Percentage of Positive Samples
The processing of 65 samples, swabbed from burn and surgical wounds, resulted in the isolation of 48 (73%) S. aureus; in other words, the testing of burn and surgical wounds was 81% (39) and 19% (9) positive for S. aureus, respectively. Of all the isolates tested for cefoxitin, 19 (40%) were found to be MRSA, while 29 (60%) were Methicillin-sensitive Staphylococcus aureus (MSSA). The instance of MRSA from the surgical and burn samples was 84% and 16%, respectively (Table 1).
Table 1. Distribution of Methicillin-Resistant and -Sensitive S. aureus Isolates from Wound Samples.
| Origin of Isolates | Samples Collected | S. aureus Isolates |
|---|---|---|
| Surgical unit | 50 | MSSA 23 |
| MRSA 16 | ||
| Total 39 | ||
| Burn unit | 15 | MSSA 6 |
| MRSA 3 | ||
| Total 9 | ||
| Total | 65 | 48 |
4.2. Potential of Sewage Water for Isolating Bacteriophages
A total of 15 sewage water samples, collected from different areas located in and around Faisalabad, were processed for phage isolation. The final filtrate obtained after the processing and enrichment of each sample was separately assessed against twelve MRSA and three MSSA isolates. Except for three, all of the samples were found to be positive.
4.3. Spot Assay
The purpose of this assay was to evaluate the efficacy of enriched filtrates obtained from 15 sewage water samples. The majority of the samples were positive for the presence of bacteriophages against the selected host range. The highest activity of about 93% was achieved from three filtrates obtained from SWC, SWF, and SWK, and these were collected from Douglas Pura, Mureed Wala, and Satyana Road, respectively. Except for three samples that were found to be negative, the rest all showed good activity. The highest susceptibility was 80%, which was observed in four bacterial isolates, whereas the lowest was 20% and was observed in SA42 (Table 2).
Table 2. Spot Assay of the Activity of Bacteriophages Isolated From Sewage Samples Against S. aureus.
| No. | Sewage Sample | SA6 | SA11 | SA15 | SA17 | SA18 | SA22 | SA26 | SA27 | SA33 | SA42 | SA45 | SA48 | SA1 | SA12 | SA40 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | SWA | + | + | + | - | - | + | + | + | - | - | - | + | + | + | + |
| 2 | SWB | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 3 | SWC | + | + | + | + | + | + | + | + | + | - | + | + | + | + | + |
| 4 | SWD | + | - | + | - | + | - | + | + | - | - | - | + | + | + | - |
| 5 | SWE | - | + | + | - | - | + | + | + | - | - | - | + | + | + | + |
| 6 | SWF | + | + | + | + | + | - | + | + | + | + | + | + | + | + | + |
| 7 | SWG | + | - | - | - | - | - | + | + | - | - | - | + | + | + | + |
| 8 | SWH | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 9 | SWI | + | - | + | + | + | - | + | + | - | - | + | + | - | + | + |
| 10 | SWJ | + | + | + | + | + | - | + | + | - | + | - | + | + | + | + |
| 11 | SWK | + | + | + | + | + | + | + | + | + | - | + | + | + | + | + |
| 12 | SWL | - | - | + | - | - | - | + | + | + | - | - | + | + | + | + |
| 13 | SWM | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 14 | SWN | + | - | + | + | + | + | + | + | + | + | - | + | + | + | + |
| 15 | SWO | - | - | + | - | + | - | + | + | + | - | + | + | + | + | + |
4.4. Plaque Morphology and Cell Imaging
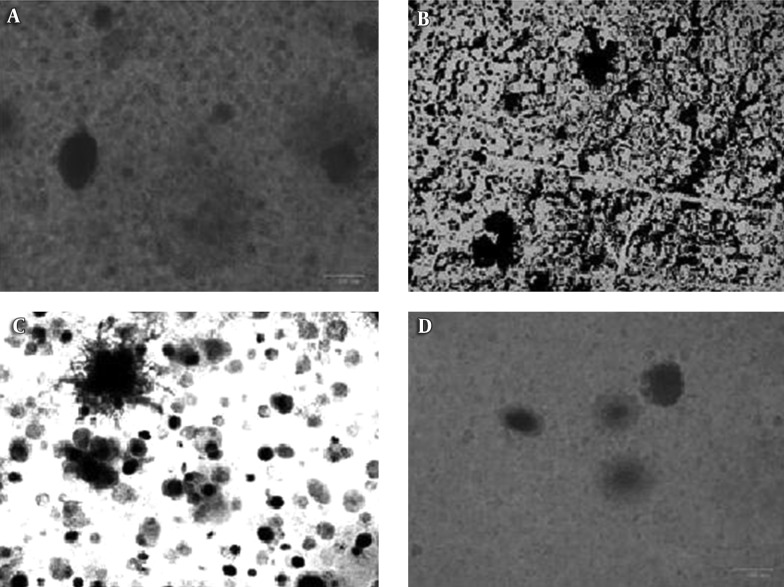
The most effective filtrates (SWC, SWF, and SWK) and the most susceptible S. aureus isolates were processed for the isolation of bacteriophages. Small- to medium-sized plaques, as well as large plaques, were obtained using the agar overlay method. The plaques were clear and turbid, with sharp and undefined boundaries. The presence of plaques and their morphology was confirmed using the ZOETM cell imager (Bio-Rad, USA) (Figure 1).
Figure 1. Plaques of Different Types and Sizes Viewed Through a Cell Imager (Bio Rad).
A and B, numerous pinheads and a few large plaques; C, Clear plaques with sharp edges; D, Turbid plaques.
4.5. Phage Purification, Enumeration, and Selection
Except for pinheads, each type of plaque was picked and purified separately using two host bacterial strains, namely, SA27 and SA48. The purified phage plates were processed further for phage enumeration using a lysate preparation followed by a tenfold dilution procedure. Lastly, the PFU was calculated. The lysate prepared from the plate of medium-sized clear plaques was selected randomly for further assays and was termed as pq/48. Its activity was assessed against 15 host strains and was found to be effective against 80% of those host strains. SA22 and SA42 also showed resistance.
4.6. Effect of Salts
CaCl2 displayed effects on the morphology as well as the PFU of the plaques. No significant effect was observed in the case of MgSO4.
4.7. In-Vitro Evaluation of Phage Lytic Activity
The MRSA host strain (108 CFU/mL) was infected with phage lysate at 108 PFU/mL, and 100 µL of this suspension was plated after incubation periods of 15 minutes, 30 minutes, and 1, 2, 3, 6, 12, and 24 hours. The bacterial count began to reduce after an incubation period of 30 minutes, but a significant reduction was seen three to six hours after phage infection.
4.8. In-Vivo Efficacy of Bacteriophages
The antibacterial ability of bacteriophages was determined in-vivo using a rabbit wound model. The MRSA dose was maintained at 1.5 × 108 CFU/mL, while the phage dose was approximately 108 PFU/mL. The rabbits were challenged with 100 µL from each dose. Four groups of rabbits were divided into negative control, positive control, treatment, and prevention groups. The wound healing-ability of the phages was assessed 24 to 48 hours after phage application. Wound healing exhibited a positive trend in all of the groups except for the bacterial control group, where inflammatory signs, poor healing, and abscess formation were observed (Table 3). The wound contraction rate was also higher in the phage-treated group. The wound swabs collected after 48 hours displayed a higher bacterial count in the bacterial control group compared to the phage-treated groups.
Table 3. In-vivo Phage Activity in a Rabbit Wound Modela.
| Experimental Group | Healing Effect | Inflammatory Signs | Abscesses |
|---|---|---|---|
| Negative control | + | - | - |
| Bacterial control | - | + | + |
| Treatment group | + | - | - |
| Prevention group | + | - | - |
aNegative control, normal saline treated group; bacterial control, Treated with bacterial challenge; treatment group, treated with bacteriophage 30 to 45 minutes after bacterial challenge; prevention group, bacteriophage application following the bacterial challenge.
4.9. Proteome Analysis Using SDS-PAGE
The total phage proteins of pq/27 and pq/48 were analyzed by means of Sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) using a 12% concentration of resolving gel. Staining with Coomassie blue exposed 10 distinct bands in different sample rows. The molecular weights showed variation, and the overall results revealed three different kinds of phages against S. aureus from the collected sewage samples on the basis of the number and size of proteins present (Figure 2).
Figure 2. SDS-PAGE Analysis of Selected Bacteriophages.
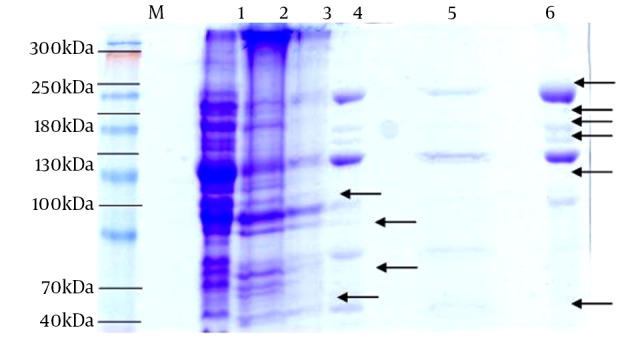
Lanes 1, 2, and 3 for phage pq/27; 4, 5, and 6 for phage pq/48; lane M, marker; bands in lane 6, 20 kDa, 185 kDa, 155 kDa, 140 kDa, 115 kDa, and 55 kDa; bands in lane 4 not present in lane 6, 90 kDa and 75 kDa; bands in lane 3 not present in lanes 6 and 4, 105 kDa and 65 kDa.
5. Discussion
Today, there is an increasing trend toward antibiotic resistance due to over use and unnecessary use of antibiotics. In essence, the root of the problem is the recent progression of resistance into the community. MRSA poses a real threat, and the day-to-day management of the complications associated with this organism is becoming ever more difficult. Bacteriophages are a potential substitute for antibiotics in the treatment of bacterial infections, and current studies aim to investigate sewage water sources for the isolation and characterization of indigenous bacteriophages to assess their lytic activity against MRSA.
A total of 48 (73%) S. aureus samples were isolated from 65 swabs collected from wound patients admitted to the burn and surgical units of allied hospital Faisalabad. Other researchers have also investigated wound sites for S. aureus, and a high prevalence (70%) of S. aureus in clinical samples like blood and surgical wounds has been reported (12). One study documented the colonization of S. aureus in burn patients within less than 48 hours of injury (21). In another study, it was found that the prevalence of MRSA in burn patients in Karachi was 24% (22).
For the investigation of indigenous bacteriophages that are effective against MRSA, waste water samples were collected from different areas located in and around Faisalabad city. In our study, 80% of the samples were found to be positive for the presence of bacteriophages, which is in accordance with previous research reporting the presence of bacteriophages in sewage water (12, 19, 23). All hospital effluents were found to be positive for the presence of bacteriophages. This might be due to the fact that hospital waste water is rich in bacterial contaminants from the hospital environment, which provides an excellent host range for all types of bacteriophages. The above explains why most of the researchers in this area were able to isolate phages from hospital waste water (14).
Individual enriched filtrates were assessed for the selection of the best filtrate for phage isolation by spotting the enriched filtrate over the plates of host strains. Enriched filtrates from sewage sources SWC, SWK, and SWF were found to be more efficient, with 93% efficacy, followed by sewage sources SWN and WSJ, with an 87% and 80% efficacy level, respectively. SWG was found to be the least efficient of all the positive samples, with an efficacy level of only 47%. Three of the samples were found to be negative for bacteriophages over the whole host range, and all of these were of domestic origin. All of the samples were assessed against 12 MRSA and three MSSA samples selected randomly. SA26, SA27, SA48, and SA12 were found to be the most susceptible, with an 80% level of susceptibility to all the filtrates. Samples SA15, SA1, and SA40 followed, with a 73% susceptibility level. SA42 was found to be the least efficient, with a 20% susceptibility level. This difference in susceptibility to bacteriophages might be due to the fact that each bacterial strain exhibits varying potential in terms of phage attacks. Again, more than one type of phage can infect a single host strain, making some of the host strains most susceptible and others less susceptible.
Enriched filtrates with an efficacy level higher than 80% and host strains exhibiting a susceptibility level between 70% and 80% were chosen for the agar overlay assays. In addition, phages were characterized by the presence of clear zones. The plaques thus obtained were confirmed using a cell imager (Bio-Rad, USA). Plaques were of two types, namely, clear and turbid. A similar morphology of plaques has been reported previously (23).
The turbid plaques were of medium size, while the clear plaques were pinheads, medium sized and, in some cases, large. The medium-sized turbid and clear plaques were chosen for further characterization because of their abundance and consistency over a wide host range. Adsorption to the host cell surface is the first step in phage replication. Divalent ions may play some role in phage adsorption in the course of bacterial infection. During the salt induction assay, a significant increase in PFU from 1.11 × 108 to 1.88 × 108 was noted after the incorporation of CaCl2 into the soft agar in SA48. Conversely, in host strain SA27, a PFU of 1.62 × 108 compared to control 1.11 × 108 without salt incorporation was observed. MgSO4 neither enhanced the plaque size nor lessened the PFU, showing neither a positive nor a negative effect on phage adsorption. These results are in accordance with previous findings indicating that the effectiveness of different salt solutions with a different concentration of Ca ions played a role in phage adsorption and enhanced phage titration, while other salts like Mg, Fe, and Zinc played no significant role (18).
In-vitro efficacy was evaluated by means of a bacterial count at different time intervals after infection by the phage lysate at a PFU of 108. A decline in the bacterial cell population was observed within three hours of the phage infection, highlighting its lytic ability against MRSA (15). In-vivo lytic activity was assessed in a rabbit wound model; wound contraction was greater in the treatment and prevention groups than in the non-treatment and bacterial control groups. In the phage-treated group, the wound had almost healed within six days compared to the wounds in the non-treatment groups. The bacterial count also started to decrease after 48 hours. These results are in line with those of a previous study that investigated the wound-healing properties in a rat model, and found evidence of an increased bacterial count and abscess formation in the non-treated group compared to phage-treated group (19).
Phage pq/27 and pq/48 were further characterized by their proteome analysis. Using SDS-PAGE, about ten distinct proteins were pictured in the gel. The protein size ranged between 20 kDa and 185 kDa. Lane three represented two distinct proteins of 105 kDa and 65 kDa. A protein band of 65 kDa in phage MSA6 was reported as a major tail sheath protein on the basis of sequence homology with staphylococcal phage K, which is a member of the Myoviridae family (24). Another study reported the same findings of a tail protein about 78 kDa in size (23). On this basis, it can be suggested that pq/27 may resemble the phages of the Myoviridae family, but this requires further investigation and characterization. Lanes 4, 5, and 6 showed the protein bands linked to phage pq/48. The major proteins in lanes 4 and 5 were 90, 75, 20, 185, 155, 140, 115, and 55 kDa in size. A phage protein of about 100 kDa was also reported (14).
Therefore, the isolated phages were found to have close relationships with previously reported phages on the basis of a molecular analysis, and they also exhibited good therapeutic potential when tested in-vitro and in-vivo. An abundance of bacteriophages in the natural environment, in addition to the ease with which they can be isolated, make them a good contender for phage therapy. Isolated phages need to be further characterized; in particular, the protein segments of isolated phages need to be assessed for their antibacterial ability against MRSA. If found effective, they may be used in the future for commercial lysate preparations. The next research step will be to focus on the efficacy of isolated phages against VISA and MDR.
On the basis of this study, it was concluded that the characterized bacteriophages exhibited good lytic activity against indigenous strains of S. aureus, and that the phages can be isolated easily from indigenous sources like waste water etc. The isolated phage pq/48 holds good antibacterial efficacy and can serve as a bio-control agent against infections induced by S. aureus. The study also provides a base line for the investigation of other sources of phage isolation. Additionally, the isolated phages themselves have considerably more potential for further characterization, especially on the molecular side. Generally, they may be a good candidate for phage therapy, not just against MRSA but also against other multidrug-resistant strains as well.
Footnotes
Authors’ Contribution:Study concept and design: Muhammad Hidayat Rasool; literature search and practical research work: Rukhsana Yousaf and Muhammad Saqalein; manuscript write-up: Rukhsana Yousaf and Mohsin Khurshid; intellectual input and critical review: Muhammad Hidayat Rasool and Abu Baker Siddique.
Funding/Support:This research was supported by the department of microbiology, government college University Faisalabad, Pakistan.
References
- 1.Kutateladze M, Adamia R. Bacteriophages as potential new therapeutics to replace or supplement antibiotics. Trends Biotechnol. 2010;28(12):591–5. doi: 10.1016/j.tibtech.2010.08.001. [DOI] [PubMed] [Google Scholar]
- 2.Schito GC. The importance of the development of antibiotic resistance in Staphylococcus aureus. Clin Microbiol Infect. 2006;12 Suppl 1:3–8. doi: 10.1111/j.1469-0691.2006.01343.x. [DOI] [PubMed] [Google Scholar]
- 3.Bishop EJ, Howden BP. Treatment of Staphylococcus aureus infections: new issues, emerging therapies and future directions. Expert Opin Emerg Drugs. 2007;12(1):1–22. doi: 10.1517/14728214.12.1.23. [DOI] [PubMed] [Google Scholar]
- 4.Khan S, Rasheed F, Zahra R. Genetic Polymorphism of agr Locus and Antibiotic Resistance of Staphylococcus aureus at two hospitals in Pakistan. Pak J Med Sci. 2014;30(1):172–6. doi: 10.12669/pjms.301.4124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mattey M, Spencer J. Bacteriophage therapy--cooked goose or phoenix rising? Curr Opin Biotechnol. 2008;19(6):608–12. doi: 10.1016/j.copbio.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 6.Deurenberg RH, Vink C, Kalenic S, Friedrich AW, Bruggeman CA, Stobberingh EE. The molecular evolution of methicillin-resistant Staphylococcus aureus. Clin Microbiol Infect. 2007;13(3):222–35. doi: 10.1111/j.1469-0691.2006.01573.x. [DOI] [PubMed] [Google Scholar]
- 7.Ackermann HW. Bacteriophage observations and evolution. Res Microbiol. 2003;154(4):245–51. doi: 10.1016/S0923-2508(03)00067-6. [DOI] [PubMed] [Google Scholar]
- 8.Stratton CW. Dead bugs don't mutate: susceptibility issues in the emergence of bacterial resistance. Emerg Infect Dis. 2003;9(1):10–6. doi: 10.3201/eid0901.020172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Skurnik M, Pajunen M, Kiljunen S. Biotechnological challenges of phage therapy. Biotechnol Lett. 2007;29(7):995–1003. doi: 10.1007/s10529-007-9346-1. [DOI] [PubMed] [Google Scholar]
- 10.Kutter E, De Vos D, Gvasalia G, Alavidze Z, Gogokhia L, Kuhl S, et al. Phage therapy in clinical practice: treatment of human infections. Curr Pharm Biotechnol. 2010;11(1):69–86. doi: 10.2174/138920110790725401. [DOI] [PubMed] [Google Scholar]
- 11.de Macedo JL, Santos JB. Bacterial and fungal colonization of burn wounds. Mem Inst Oswaldo Cruz. 2005;100(5):535–9. doi: 10.1590/s0074-02762005000500014. [DOI] [PubMed] [Google Scholar]
- 12.Gupta R, Prasad Y. Efficacy of polyvalent bacteriophage P-27/HP to control multidrug resistant Staphylococcus aureus associated with human infections. Curr Microbiol. 2011;62(1):255–60. doi: 10.1007/s00284-010-9699-x. [DOI] [PubMed] [Google Scholar]
- 13.Mathews AA, Thomas M, Appalaraju B, Jayalakshmi J. Evaluation and comparison of tests to detect methicillin resistant S. aureus. Indian J Pathol Microbiol. 2010;53(1):79–82. doi: 10.4103/0377-4929.59189. [DOI] [PubMed] [Google Scholar]
- 14.Sangha KK, Kumar BV, Agrawal RK, Deka D, Verma R. Proteomic Characterization of Lytic Bacteriophages of Staphylococcus aureus Isolated from Sewage Affluent of India. Int Sch Res Notices. 2014;2014:265298. doi: 10.1155/2014/265298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.O'Flaherty S, Ross RP, Meaney W, Fitzgerald GF, Elbreki MF, Coffey A. Potential of the polyvalent anti-Staphylococcus bacteriophage K for control of antibiotic-resistant staphylococci from hospitals. Appl Environ Microbiol. 2005;71(4):1836–42. doi: 10.1128/AEM.71.4.1836-1842.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sunagar R, Patil SA, Chandrakanth RK. Bacteriophage therapy for Staphylococcus aureus bacteremia in streptozotocin-induced diabetic mice. Res Microbiol. 2010;161(10):854–60. doi: 10.1016/j.resmic.2010.09.011. [DOI] [PubMed] [Google Scholar]
- 17.Kaur S, Harjai K, Chhibber S. Methicillin-resistant Staphylococcus aureus phage plaque size enhancement using sublethal concentrations of antibiotics. Appl Environ Microbiol. 2012;78(23):8227–33. doi: 10.1128/AEM.02371-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chhibber S, Kaur T, Kaur S. Essential role of calcium in the infection process of broad-spectrum methicillin-resistant Staphylococcus aureus bacteriophage. J Basic Microbiol. 2014;54(8):775–80. doi: 10.1002/jobm.201300051. [DOI] [PubMed] [Google Scholar]
- 19.VinodKumar CS, Srinivasa H, Basavarajappa KG, Patil U, Bandekar N, Patil R. Abrogation of Staphylococcus aureus wound infection by bacteriophage in diabetic rats. Int J Pharm Sci Drug Res. 2011;3(3):202–7. [Google Scholar]
- 20.Zimecki M, Artym J, Kocieba M, Weber-Dabrowska B, Borysowski J, Gorski A. Effects of prophylactic administration of bacteriophages to immunosuppressed mice infected with Staphylococcus aureus. BMC Microbiol. 2009;9:169. doi: 10.1186/1471-2180-9-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alghalibi S, Humaid A, Alshaibani E, Alhamzy E. Microorganisms associated with burn wound infection in Sana’a, Yemen. Egypt. Acad. Egypt Acad J Biol Sci. 2011;3(1):19–25. [Google Scholar]
- 22.Naqvi ZA, Hashmi K, Kharal SA. Methicillin resistant Staphylococcus aureus (MRSA) in burn patients. Pak J Pharmacol. 2007;24(2):7–11. [Google Scholar]
- 23.Han JE, Kim JH, Hwang SY, Choresca CJ, Shin SP, Jun JW, et al. Isolation and characterization of a Myoviridae bacteriophage against Staphylococcus aureus isolated from dairy cows with mastitis. Res Vet Sci. 2013;95(2):758–63. doi: 10.1016/j.rvsc.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 24.Kwiatek M, Parasion S, Mizak L, Gryko R, Bartoszcze M, Kocik J. Characterization of a bacteriophage, isolated from a cow with mastitis, that is lytic against Staphylococcus aureus strains. Arch Virol. 2012;157(2):225–34. doi: 10.1007/s00705-011-1160-3. [DOI] [PubMed] [Google Scholar]