Abstract
Background
Campylobacter jejuni is one of the major causes of infectious diarrhea worldwide. The distending cytolethal toxin (CDT) of Campylobacter spp. interferes with normal cell cycle progression. This toxic effect is considered a result of DNase activity that produces chromosomal DNA damage. To perform this event, the toxin must be endocytosed and translocated to the nucleus.
Objectives
The aim of this study was to evaluate the role of the cytoskeleton in the translocation of CDT to the nucleus.
Methods
Campylobacter jejuni ATCC 33291 and seven isolates donated from Instituto de Biotecnologia were used in this study. The presence of CDT genes in C. jejuni strains was determined by PCR. To evaluate the effect of CDT, HeLa cells were treated with bacterial lysate, and the damage and morphological changes were analyzed by microscopy, immunofluorescence staining, and flow cytometry. To evaluate the role of the cytoskeleton, HeLa cells were treated with either latrunculin A or by nocodazole and analyzed by microscopy, flow cytometry, and immunoquantification (ELISA).
Results
The results obtained showed that the eight strains of C. jejuni, including the reference strain, had the ability to produce the toxin. Usage of latrunculin A and nocodazole, two cytoskeletal inhibitors, blocked the toxic effect in cells treated with the toxin. This phenomenon was evident in flow cytometry analysis and immunoquantification of Cdc2-phosphorylated.
Conclusions
This work showed that the cytotoxic activity of the C. jejuni CDT is dependent on its endocytosis. The alteration in the microtubules and actin filaments caused a blockage transit of the toxin, preventing it from reaching the nucleus of the cell, as well as preventing DNA fragmentation and alteration of the cell cycle. The CDT toxin appears to be an important element for the pathogenesis of campylobacteriosis, since all clinical isolates showed the presence of cdtA, cdtB and cdtC genes.
Keywords: CDC2 Protein Kinase, Cytolethal Distending Toxin, Cytoskeleton, Latrunculin A, Nocodazole, Campylobacter jejuni
1. Background
Campylobacter jejuni is one of the most common causative agents of foodborne infectious illness in humans. A bacterial membrane-associated protein, this cytolethal distending toxin (CDT) has been identified as one of the virulence factors required for the pathogenesis of C. jejuni (1, 2). Cytolethal distending toxin is a tripartite protein toxin composed of three subunits, CdtA, CdtB, and CdtC, encoded by an operon comprising cdtA, cdtB, and cdtC (3). Several bacterial species have been identified that contain CDT, including Aggregatibacter actinomycetemcomitans (4), Campylobacter sp. (5), Escherichia coli (6), Haemophilus ducreyi (7), Helicobacter sp. (8), Shigella dysenteriae (9), and Salmonella enterica (10).
Cytolethal distending toxin holotoxin functions as an “AB2” toxin, in which CdtB is the active toxic unit “A” of the AB2 toxin. CdtA and CdtC make up the “B2” units required for CDT binding to target cells and for the delivery of CdtB into the cell interior (3, 11, 12). The nature of the surface receptor is still poorly characterized. However, the binding of CDT requires intact lipid rafts, where CdtA and CdtC can interact with the cell membrane and enable the translocation of the holotoxin across the cell membrane (13-15). The toxin is retrograde transported into the nuclear compartment, where the CdtB subunit exhibits type I DNase activity. Cellular intoxication induces DNA damage and activation of the DNA damage response, which results in arrest of the target cells in the G1 and/or G2 phases of the cell cycle and activation of DNA repair mechanisms, cellular distention and nuclear enlargement, and Cdc2 and ataxia-telangiectasia-mutated protein (ATM) phosphorylation. Cells that fail to repair the damage will senesce or undergo apoptosis (11, 16-20).
Some bacterial protein toxin internalization had typically been observed to involve rearrangement of the host cytoskeletal structure, resulting in endocytosis (21-23). This internalization can occur through multiple routes, including clathrin-dependent endocytosis, caveolae, phagocytosis, macropinocytosis, and several other clathrin-independent pathways (24). Actin and tubulin have particularly well-characterized roles during internalization: actin, which is composed of microfilaments (MFs), has a clear role in generating force to assist uptake, while tubulin microtubules (MTs) are involved in the transport of endocytic vesicles (25). However, some questions remain regarding the internalization pathway and the role of microfilaments and microtubules in the intracellular trafficking of CDT. Latrunculin A, a disruptor of actin microfilament organization, and nocodazole, a disruptor of microtubules, both have been widely used in assays of vesicular traffic (26).
2. Objectives
The aim of this study was to evaluate the role of the cytoskeleton in the translocation of CDT to the nucleus.
3. Methods
3.1. Bacterial Culture
Campylobacter jejuni ATCC 33291 and seven isolates donated from Instituto de Biotecnología, UNAM were used in this study. All strains were grown on Campylobacter blood-free selective agar base (Oxoid, USA), with 5% sheep’s whole blood, under microaerophilic conditions (5% O2, and 10% CO2) at 37°C for 24 - 48 hours.
3.2. Detection of cdt Genes From Campylobacter jejuni
The presence of CDT genes in Campylobacter strains was determined by PCR. The sequences of all cdt gene primers (IDT, USA), sizes of PCR amplicons, and PCR conditions used in this study are presented in Table 1.
Table 1. List of Primers, Size of Amplicons, and PCR Conditions Used in this Studya.
| Primers | Sequences | Amplicon Size | PCR Conditions |
|---|---|---|---|
| cdt A gene | cytA1: 5’-TGCAAAAAATTATAGTTTTTATTTTATGTTG-3’; cytA2: 5’-TCGTACCTCTCCTTGGCG-3’ | 807 pb | 94°C 1 minute; 62°C 1 minute;72°C 1 min; (30 cycles) |
| cdt B gene | cytB1: 5’-ATGAAAAAAATTATATGTTTATTTTTATC-3’; cytB2: 5’-TTTTCTAAAATTTACTGGAAAATG -3’ | 781pb | 94°C 1 minute; 55°C 1 minute; 72°C 1 minute; (30 cycles) |
| cdt C gene | cyt C1: 5’-ATGAAAAAAATTATTACTTTGTTTTTTATG-3’; cytC2: 5’-TTCTAAAGGGGTAGCAGCTG-3’ | 570 pb | 94°C 1 minute; 57°C 1 minute; 72°C 1 minute; (30 cycles) |
aAll primers were designed for this study.
3.3. CDT Preparations
Cytolethal distending toxin preparations were obtained as described previously by Whitehouse (27). The preparations applied in all assays were evaluated by SDS-PAGE (28) and quantified by Bradford (29).
3.4. Cell Culture and CDT Treatment
HeLa cells were grown in Eagle’s minimal essential medium with 10% fetal calf serum, 2 mM L-glutamine, 1% Na-bicarbonate, and 0.1 mM sodium pyruvate (all reagents from Gibco, USA). For CDT treatment, 5 × 104 cells were seeded in tissue culture plates (Nunc, Denmark) with MEM medium and 150 µg/mL of toxin. Cells were examined under an inverted microscope (Velab, Mex) every 24 hours for observation of morphologic changes or damage. As a DNA damage positive control, we used 50 mM Etoposide (VP-16-Lemery, Mex) (30, 31). For the inhibition of translocation of toxin, HeLa cells were treated with either 1 µM latrunculin A (Sigma-Aldrich, USA) or by addition of 30 µM of nocodazole (Sigma-Aldrich, USA). The drugs were present for 30 minutes prior to the CDT infection assay.
3.5. Fluorescence
Standard immunofluorescence staining and visualization under a confocal epifluorescence microscope (E800, Nikon, Japan) was performed to assess morphologic patterns of cells. Cells were cultured on chamber slides (Nalge, USA) and incubated with the CDT preparation for 48 hours at 37°C, in an atmosphere of 5% CO2. They were then washed with PBS and fixed for 15 minutes with 4% paraformaldehyde in PBS at room temperature. Fluorescent phalloidin (200 U/mL; Alexa 488, Molecular Probes, USA) was then added to detect actin microfilaments. After 30 minutes of incubation with phalloidin, cells were washed in PBS before propidium iodide (Sigma, USA) staining and microscopic examination (32).
3.6. Measurement of DNA Fragmentation
HeLa cells treated with CDT preparation, as described above, were processed for evaluation of DNA fragmentation, as described (32, 33). Extracted DNA from lysed cells was dissolved in 10 µL distilled water and treated with RNase A (Thermo Scientific, USA) 10 µg/mL at 37°C for 45 minutes and electrophoresed on a 1.2% agarose gel containing 0.5 µg of ethidium bromide per mL. DNA fragments were visualized under UV transillumination (Gel Doc XR, BioRad, USA). Cells exposed to 50mM of etoposide were used as a positive control.
3.7. Immunoquantification of Cdc2-Phosphorylated
HeLa cells with CDT treatment, with or without latrunculin A and nocodazole were washed twice with PBS. Cells were resuspended and lysed for five minutes at 4°C in RIPA buffer (1% [v/v] Triton X-100, 1% [v/v] sodium deoxycholate, 0.1% [v/v] sodium dodecyl sulfate [SDS], 20 mM Tris-HCl, pH 7.4, 150 mM NaCl, and 1 mM EDTA in distilled water) (Thermo Scientific, USA) and supplemented with protease inhibitor and phosphatase inhibitors (GE Healthcare, USA). After that, the samples were centrifuged at 14,000 g for 15 minutes at 4°C, and the protein in the resulting supernatants was quantified by Bradford assay (29). The determination of Cdc2-phosphorylated (P-Cdc2) was tested using an enzyme-linked immunosorbent assay (ELISA-Sandwich), using rabbit monoclonal antibody against P-Cdc2 and Cdc2 (Cell Signaling, USA). The absorbance was read at 450 nm in a microplate reader (Model 680, BioRad, USA).
3.8. Cell Cycle Analysis
In order to establish the proportion of cells in each phase of the cell cycle, cells were trypsinized for three minutes, centrifuged and washed once with PBS. The cell pellet was suspended and fixed on ice for 15 minutes with 1 mL of cold 70% ethanol. The cells were subsequently centrifuged and the cell pellet suspended in 1 mL of propidium iodide (PI) solution (0.05 mg/mL PI, 0.02 mg/mL RNase, 0.3% NP40 [Roche, USA] 1 mg/mL sodium citrate) for one hour at 4°C (34, 35). Flow cytometry analysis was performed using a FAC Sort flow cytometer (Becton and Dickinson, USA). Data from 104 cells was collected and analyzed using the Cell Quest software (Becton and Dickinson, USA).
3.9. Statistical Analysis
Results were entered and analyzed using ANOVA. P values less than 0.05 were taken to indicate statistical significance.
4. Results
4.1. Detection of the cdt Gene in Campylobacter jejuni
In order to determine whether the eight strains of C. jejuni, including the reference strain (ATCC 33291), had the ability to produce the toxin, a PCR test was performed to detect the genes encoding the three subunits of the CDT toxin. All strains tested showed amplifications of 807 bp fragments for cdtA, 781 bp for cdtB, and 570 bp for cdtC, confirming the existence of the genes for this toxin. From one strain (the reference strain), the nucleotide sequence of the amplified product was obtained and analyzed to identify important regions described in another CDT toxin family.
4.2. CDT Preparations
To obtain the CDT toxin, a reference strain of C. jejuni was used (ATCC 33291). The results obtained in the SDS page showed the presence of approximately six bands, with molecular weights ranging from 60 to 90 kDa (data no shown). This preparation was used in all infection assays to evaluate the cytopathic effect of CDT and DNA damage in HeLa cells.
4.3. Effect of CDT Treatment on HeLa Cells
To evaluate the effect of the CDT toxin, HeLa cells were treated with 150 mg/mL of CDT preparations for 48 hours. Every 24 hours, cells were viewed on an inverted microscope to determine damage and morphological changes caused by the toxin. Staining of cells with fluorescent dyes, including phalloidin and propidium iodide, was used in evaluating the actin microfilaments and nuclear morphology of the treated cells. Cells stained green represented actin, whereas reddish or orange staining represented nuclei. The CDT preparation from C. jejuni induced cytopathic damage after 24 hours of incubation. Treated cells presented enlargement (completely round cells with a diameter greater than that of untreated cells) and fragmentation of the nucleus, with an abnormal distribution of chromatin (Figure 1). The toxic effect of CDT on DNA was evaluated using agarose gel electrophoresis. The results of this analysis show DNA fragmentation similar to that observed in apoptosis (Figure 2).
Figure 1. CDT and Etoposide Produce Similar Effects in HeLa Cells.
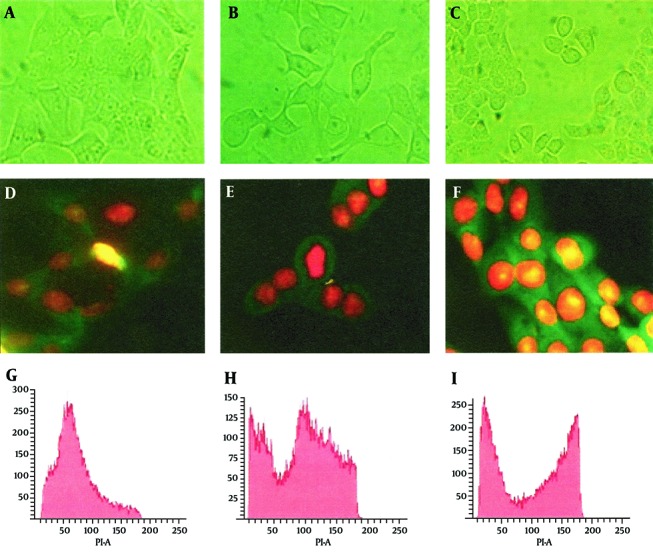
Morphological studies by inverted microscope at actual magnification 40 × and G2 arrest in HeLa cells by CDT and etoposide. Panel A, D and G cells without treatment; Panel B, E and H cells treated with etoposide; Panel C, F and I cells treated with CDT. Panels D, E, and F, cells stained with phalloidin/propidium iodide; Panels G, H, and I, flow cytometry analysis (percentage of cells ± SE, in three independent experiments).
Figure 2. CDT Induces DNA Fragmentation.
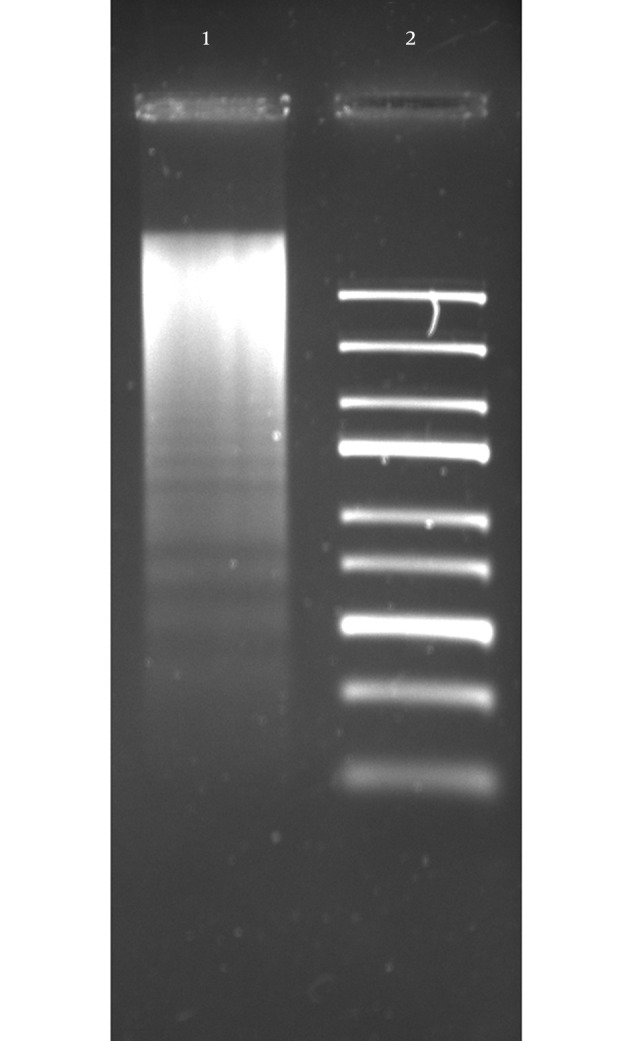
HeLa cells were subjected to 150 µg/mL of CDT preparation for 24 hours, after DNA was obtained and visualized by electrophoresis, in 1.2% agarose gel stained with ethidium bromide. Lane 1, DNA obtained from HeLa cells with CDT treatment; Lane 2, Gene Ruler Express DNA Ladder (Fermentas, USA).
The effect of the toxin on the cell cycle was also assessed by flow cytometry (FACS). In this experiment, the DNA content of untreated cells and cells treated with the toxin was measured. As a positive control, cells treated with etoposide were included. The results in this section show that 69% of cells treated with CDT toxin underwent a block in the G2/M phase, with minimal accumulation of DNA in the S phase, and 76% of those treated with etoposide were also blocked in the G2/M phase of the cell cycle, while only 18% of untreated cells showed a blockage at the G2/M and a high proportion of cells in the S phase. Having established that the CDT toxin from C. jejuni was able to block the cell cycle in the G2/M phase, the amount of phosphorylated Cdc2 in these cells was evaluated, because this cyclin is essential to the regulation of the cell cycle. Quantitation was performed in an immunoassay (ELISA) using monoclonal antibodies and protein extract from cells treated with toxin and etoposide. The results of this analysis show that the amount of phosphorylated Cdc-2 in toxin-treated cells was similar to that of cells treated with etoposide (Figure 3).
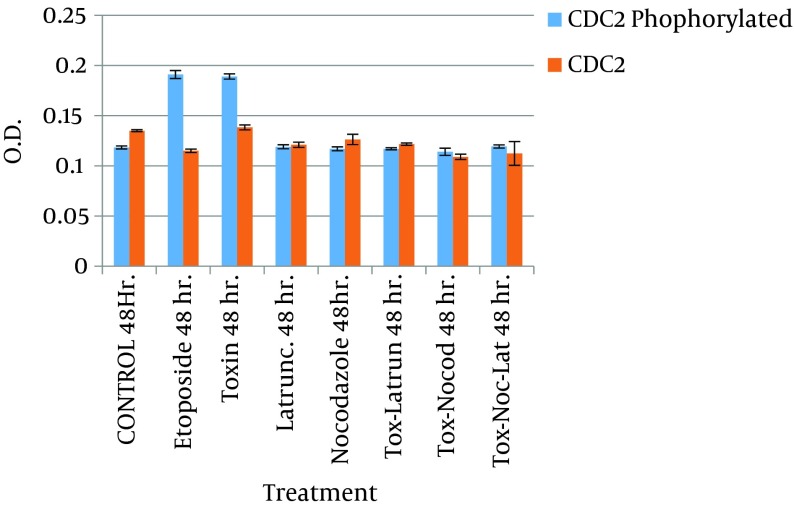
Figure 3. Determination of Cdc2-P by Quantitative Enzyme-Linked Immunosorbent Assay (ELISA).
The graphics show variations in the concentration of Cdc2-P measured in samples with different treatments. Values are given as the percentage of Cdc2-P ± SE, where n = 3 independent experiments.
4.4. Effect of Nocodazole and Latrunculin A on the Toxic Activity of CDT
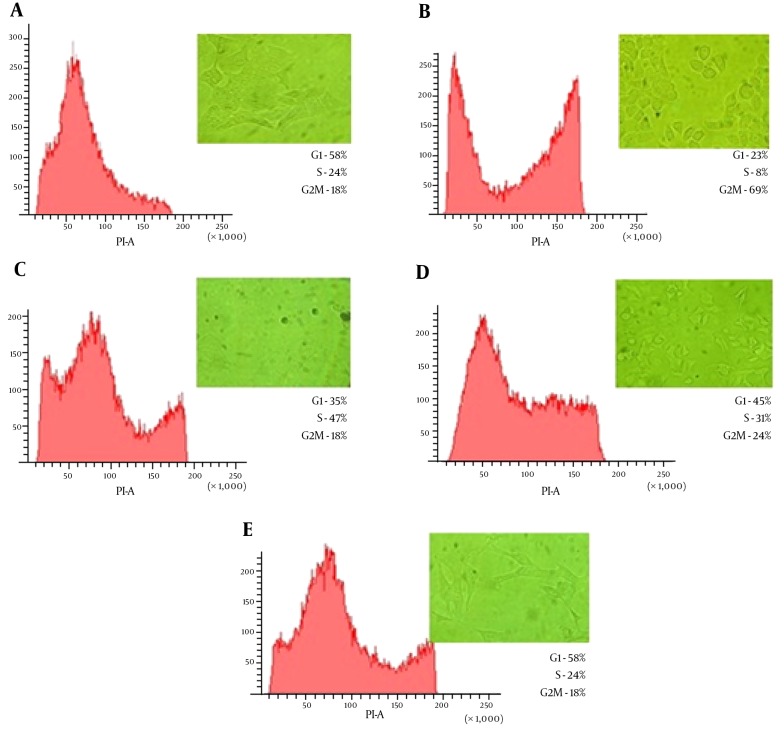
Having evaluated the cytotoxic effects of CDT toxin in HeLa cells, we determined whether actin microfilaments and microtubules were involved in the transit of the toxin. To do this, HeLa cells were treated with 1 µM latrunculin A or 30 µM nocodazole, 30 minutes before CDT treatment, for 48 hours. As in the previous experiment, HeLa cells were viewed on an inverted microscope every 24 hours. In the first 24 hours (Figure 4), cells that were treated with the CDT toxin and latrunculin showed minimal cytopathic damage, based on the elongation of the cell cytoplasm. In cell cycle analysis, latrunculin was able to decrease 45% of blocked cells in the G2/M phase, compared with toxin treated cells only. Similarly, cells treated with CDT and nocodazole showed no apparent morphological damage. This was also confirmed by the results of FACS analysis, with only 18% of cells in the G2/M phase, similar to the untreated cells (Figure 4).
Figure 4. Latrunculin A and Nocodazole Affect CDT Activity in HeLa Cells.
Flow cytometry analysis and morphological alterations in HeLa cells treatment with cytoskeletal inhibitors and CDT (10,000 nuclei were analyzed by sample). Panel A, cells without treatment; Panel B, cells treated with CDT; Panel C, cells treated with CDT + nocodazole; Panel D, cells treated with CDT + latrunculin A; Panel E, cells treated with CDT + nocodazole + latrunculin A.
When latrunculin and nocodazole were used in tandem, the morphological changes observed were very similar to those seen with latrunculin alone. However, an appreciable S phase of the cell cycle was noted in FACS analysis (Figure 4). This suggests that the cells were able to maintain adequate replication. With this experiment, we were able to show that both latrunculin and nocodazole interfere with intracellular transit of CDT toxin. Besides, the ELISA results showed that the amount of phosphorylated Cdc2 in HeLa cells treated with CDT toxin and with cytoskeleton inhibitors (i.e., latrunculin and nocodazole) were lower than in cells treated with CDT and etoposide (Figure 3).
5. Discussion
Many investigations have clearly established that bacterial toxins function as virulence factors. These toxins have precise effects on different processes in eukaryotic cells; for example, some interfere with intracellular signaling by interacting with specific proteins in different signaling cascades and others, such as CDT, interfere in the cell cycle (36-38). For these activities, toxins have to reach specific places in the cell, using the normal routes of intracellular transit. This paper explores the role of the cytoskeleton in the biological activity of CDT through different assays in which pharmacological inhibitors were added to cell cultures treated with the CDT of C. jejuni.
The CDT was first described in one strain of Escherichia coli, isolated from a pediatric case of gastroenteritis with encephalopathy. After identification in E. coli, CDT production was also reported in other pathogen microorganisms, such as S. dysenteriae, H. hepaticus, H. ducreyi, A. actynomycetemcomitans, and C. jejuni (11). In this work, we evaluated the presence of the cdt gene in eight strains: one reference and seven isolated. All strains showed by PCR test the presence of the cdtB gene, which is the toxic subunit.
Other authors, such as Johnson et al. (5), have also reported the presence of the cdtB gene, working with more than 700 strains of Campylobacter, including 583 of C. jejuni, 109 of C. coli, 16 of C. lari, and 7 of C. fetus. None of these strains was correlated by serotype, biotype, or country of origin (5). These results suggest that the cdt operon is conserved in microorganisms of the Campylobacter genus, at least in the C. jejuni species. This conservation does not occur in the Salmonella enterica serovar Typhi, which does not encode apparent homologs of CdtA or CdtC, only expressing cdtB (10).
The distending cytolethal toxins have unusual mechanisms of action: the interference with normal cell cycle progression. This toxic effect is considered to be exclusive to this toxin, and it is a result of DNase activity that produces chromosomal DNA damage (2, 12). No other components of the Campylobacter bacteria cell have this activity. For this reason, CDT preparations obtained from bacterial cell lysates have been used in many other investigations (27). In this research, CDT preparations were used to treat HeLa cells. The results observed were distention, nuclear fragmentation, abnormal chromatin condensation, cell cycle arrest in the G2 phase, and apoptosis.
There are a number of ways in which CDT might cause HeLa cells to become blocked in G2. For example, CDT might act directly on the CDC2 kinase or on proteins that interact directly with CDC2, such as the CDC25 phosphatase, which carries out the reaction that activates CDC2 for entry into mitosis (37). The possible analogy between CDT and a prototype DNA-damaging agent, etoposide, whose early effects on HeLa cells had been studied before (39), led us to comparatively analyze in detail the properties of these agents. We showed that both agents induce arrest of cell proliferation associated with a G2 block and the progressive swelling of blocked cells. Other investigators had also used CDT preparations and obtained the same results, in concordance with this investigation (40).
However, recent new evidence of other activities has been reported in other CDT toxins, specifically in A. actinomycetemcomitans, where analysis of the toxin suggests that blocking of the cell cycle occurs not only as a result of DNA damage, but also by phosphatidylinositol-3, 4, 5-triphosphate (PIP3) phosphatase activity (41). In Campylobacter, this data has not been investigated, and sequence analysis does not show evidence of this activity. However, more data is required to demonstrate this.
Cytolethal distending toxin affects a variety of epithelial cells, including HeLa, CHO, Vero, and Hep-2. This activity appears to be more effective in younger cell cultures at 60% confluence than in mature cells at 100% of confluence (42-44). This suggests that the toxic activity of CDT is closely linked to the cell cycle. For this reason, in this investigation, cells in culture were deprived of sera, previous to treatment with CDT lysate, in order to synchronize the cell cycle and quantify the effect. However, in FACS, the cells were seen in other phases. This phenomenon may be due to a continuous accumulation of some signals that can stimulate the beginning of the S-phase. This signal may have accumulated along the cell division cycle and, therefore, may be independent of the cell cycle, rather than cell-cycle specific. According to this hypothesis, in each phase of the cell cycle, there are a number of S-phase triggering signals, characteristic of that particular phase.
Starvation prevents the accumulation of these triggering signals, and cells are arrested in the G1 phase. But, some of these cells may not be arrested at this time or specific phase in the cell cycle, because they have different levels of activation signals (45). In assays where the effect of the toxin was evident, an arrest in the G2 phase was observed. However, other phases were also present, due to the situation described above (40). Nevertheless, the effect could still be measured, and cell distension and apoptosis were present. We can assume that each cell type has its own scenario in which the effect of the toxin is expressed; the detailed study of this scenario can help in the understanding of toxic activity, which is one of the objectives of this research.
In C. jejuni CDT, the B subunit has enzymatic activity (DNase), and the other subunits are involved in the recognition of a receptor on the cell surface, which is a ganglioside (2). Numerous proteins and lipids on the cell surface display this type of galactoside, which would explain the large capacity of this toxin to bind to different cell lines (46). Once bound to the cell surface, the toxin by itself is capable of directing its traffic to the nucleus (2, 43, 47). In this respect, it has been suggested that the toxin could enter the cell through endocytosis, and once inside endoplasmic reticulum, diffuses into the nucleus through the nuclear pore.
However, in the case of C. jejuni CDT, several issues remain unresolved. One of them is that, if this toxin is endocytosed, it would require the involvement of the cytoskeleton. This work has shown that both actin and tubulin filaments are important and required for the transit of the toxin by the use of two inhibitors of the formation of the cytoskeleton. As seen in the results of ELISA and FACS, nocodazole-treated cells showed less apparent effect on the activity of the toxin. Nocodazole is a pharmacological agent that exerts its effect in cells by interfering with the polymerization of microtubules.
Microtubules are one constituent of the cytoskeleton, and the dynamic microtubule network has several important roles in the cell, including vesicular transport. Some studies have also reported that nocodazole can arrest cells in the G2 phase. However, the amount used is critical for this effect to be seen (48). The analysis of cells treated with nocodazole alone showed a basal level of arrest in the G2 phase, compared to the toxin-nocodazole treated cells, in which the effect disappeared. The same results were observed in assays with latrunculin A.
Latrunculins are a family of toxins that binds actin monomers near the nucleotide binding site and prevents them from polymerizing. This effect results in the disruption of actin filaments of the cytoskeleton (49). In our toxin assays, the cells treated with latrunculin A showed a reduced CDT effect. Therefore, the amount of Cdc2 and cells in the G2 phase showed a significant statistical difference between cells treated or not treated with nocodazole and latrunculin A. These results suggest an important participation of the cytoskeleton in the biological activity of this toxin, specifically in its intracellular transit.
Eash and Atwood (50) used latrunculin A and nocodazole to determine the role of microfilaments and microtubules during early viral infection of BK virus in Vero cells. Their results showed that the disassembly of the microtubule network caused by nocodazole was crucial for the BKV infectious entry. In contrast, disassembly of the actin filaments with latrunculin A did not impede BKV infection. This phenomenon is very similar to the results we obtained, when cells were treated with nocodazole and latrunculin. Our results suggest that the toxin needs in a large degree of the microtubules in a lower percentage the actin filaments to follow the retrograde transport route from the plasma membrane to the nucleus.
Finally, considering that endocytosis is important in a great number of cellular functions (24), it is not rare that bacteria use this cellular process to introduce toxins into cells, in order to infect the host. The regulation of endocytotic pathways is closely coupled with the ability of cells to recognize, respond, and adapt to external stimuli. Different endocytic mechanisms, including clathrin-mediated endocytosis, caveolae-mediated endocytosis/rafts, macropinocytosis, and transitions between endosomes, are regulated by signaling molecules (24, 46). While clathrin-mediated endocytosis is the main route of endocytosis for extracellular ligands and the plasma membrane, another alternate route that is actively involved in endocytosis of extracellular particles, associated with regions known as lipid rafts, is the process called endocytosis mediated by caveolae, which is involved in cholesterol homeostasis, recycling of glycosylphosphatidylinositol-anchored proteins, and transcytotic transport of glycosphingolipid and serum components (46). Some viruses or toxins use this route to reach the endoplasmic reticulum, such as the SV40 virus and cholera toxin. Given the structural similarity of the CDT and cholera toxin, this pathway may be involved in the entry of the C. jejuni CDT (50, 51).
In conclusion, this work showed that C. jejuni CDT uses the cytosketon to move into the cell in order to reach the cell nucleus. Polymerization of microtubules might be important in the retrograde transport route of CDT, with a lower participation of actin filaments. However, other molecules might be involved in this route, as SNARES and COPI-II proteins. Considering the important role that toxins have in the pathogenesis of campylobacteriosis and other infections, all knowledge generated in this area will serve to propose and develop new strategies for the control of pathogens.
Acknowledgments
We are grateful to Doctorado en Ciencias Biológicas y de la Salud, UAM-Xochimilco throughout this process and to Dr. Edith Cortés Barberena for her advice on flow cytometry.
Footnotes
Authors’ Contribution:Estela T. Méndez-Olvera contributed to the study plan, design, acquisition, analysis, interpretation, critical revision, and drafting of the manuscript. Daniel Martínez-Gómez contributed to the analysis, interpretation, critical revision of the manuscript, study supervision, administrative, technical, and material support. Jaime A. Bustos-Martínez, Yolanda López-Vidal, and Antonio Verdugo-Rodríguez contributed to critical revision of the manuscript, study supervision, administrative, technical, and material support. All authors read and approved the final manuscript.
Funding/Support:This work was supported by Universidad Autonoma Metropolitana, Unidad Xochimilco-Acuerdos del Rector 2007 and by Consejo Nacional de Ciencia y Tecnología, México (CONACYT-96960).
References
- 1.Young KT, Davis LM, Dirita VJ. Campylobacter jejuni: molecular biology and pathogenesis. Nat Rev Microbiol. 2007;5(9):665–79. doi: 10.1038/nrmicro1718. [DOI] [PubMed] [Google Scholar]
- 2.Pickett CL, Pesci EC, Cottle DL, Russell G, Erdem AN, Zeytin H. Prevalence of cytolethal distending toxin production in Campylobacter jejuni and relatedness of Campylobacter sp. cdtB gene. Infect Immun. 1996;64(6):2070–8. doi: 10.1128/iai.64.6.2070-2078.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lara-Tejero M, Galan JE. CdtA, CdtB, and CdtC form a tripartite complex that is required for cytolethal distending toxin activity. Infect Immun. 2001;69(7):4358–65. doi: 10.1128/IAI.69.7.4358-4365.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sugai M, Kawamoto T, Peres SY, Ueno Y, Komatsuzawa H, Fujiwara T, et al. The cell cycle-specific growth-inhibitory factor produced by Actinobacillus actinomycetemcomitans is a cytolethal distending toxin. Infect Immun. 1998;66(10):5008–19. doi: 10.1128/iai.66.10.5008-5019.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Johnson WM, Lior H. A new heat-labile cytolethal distending toxin (CLDT) produced by Campylobacter spp. Microb Pathog. 1988;4(2):115–26. doi: 10.1016/0882-4010(88)90053-8. [DOI] [PubMed] [Google Scholar]
- 6.Peres SY, Marches O, Daigle F, Nougayrede JP, Herault F, Tasca C, et al. A new cytolethal distending toxin (CDT) from Escherichia coli producing CNF2 blocks HeLa cell division in G2/M phase. Mol Microbiol. 1997;24(5):1095–107. doi: 10.1046/j.1365-2958.1997.4181785.x. [DOI] [PubMed] [Google Scholar]
- 7.Cope LD, Lumbley S, Latimer JL, Klesney-Tait J, Stevens MK, Johnson LS, et al. A diffusible cytotoxin of Haemophilus ducreyi. Proc Natl Acad Sci U S A. 1997;94(8):4056–61. doi: 10.1073/pnas.94.8.4056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Young VB, Knox KA, Schauer DB. Cytolethal distending toxin sequence and activity in the enterohepatic pathogen Helicobacter hepaticus. Infect Immun. 2000;68(1):184–91. doi: 10.1128/iai.68.1.184-191.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Okuda J, Kurazono H, Takeda Y. Distribution of the cytolethal distending toxin A gene (cdtA) among species of Shigella and Vibrio, and cloning and sequencing of the cdt gene from Shigella dysenteriae. Microb Pathog. 1995;18(3):167–72. doi: 10.1016/s0882-4010(95)90022-5. [DOI] [PubMed] [Google Scholar]
- 10.Haghjoo E, Galan JE. Salmonella typhi encodes a functional cytolethal distending toxin that is delivered into host cells by a bacterial-internalization pathway. Proc Natl Acad Sci U S A. 2004;101(13):4614–9. doi: 10.1073/pnas.0400932101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guerra L, Cortes-Bratti X, Guidi R, Frisan T. The biology of the cytolethal distending toxins. Toxins (Basel). 2011;3(3):172–90. doi: 10.3390/toxins3030172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lara-Tejero M, Galan JE. Cytolethal distending toxin: limited damage as a strategy to modulate cellular functions. Trends Microbiol. 2002;10(3):147–52. doi: 10.1016/s0966-842x(02)02316-8. [DOI] [PubMed] [Google Scholar]
- 13.Hu X, Nesic D, Stebbins CE. Comparative structure-function analysis of cytolethal distending toxins. Proteins. 2006;62(2):421–34. doi: 10.1002/prot.20767. [DOI] [PubMed] [Google Scholar]
- 14.Cortes-Bratti X, Chaves-Olarte E, Lagergard T, Thelestam M. Cellular internalization of cytolethal distending toxin from Haemophilus ducreyi. Infect Immun. 2000;68(12):6903–11. doi: 10.1128/iai.68.12.6903-6911.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nesic D, Hsu Y, Stebbins CE. Assembly and function of a bacterial genotoxin. Nature. 2004;429(6990):429–33. doi: 10.1038/nature02532. [DOI] [PubMed] [Google Scholar]
- 16.Lindmark B, Rompikuntal PK, Vaitkevicius K, Song T, Mizunoe Y, Uhlin BE, et al. Outer membrane vesicle-mediated release of cytolethal distending toxin (CDT) from Campylobacter jejuni. BMC Microbiol. 2009;9:220. doi: 10.1186/1471-2180-9-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jinadasa RN, Bloom SE, Weiss RS, Duhamel GE. Cytolethal distending toxin: a conserved bacterial genotoxin that blocks cell cycle progression, leading to apoptosis of a broad range of mammalian cell lineages. Microbiology. 2011;157(Pt 7):1851–75. doi: 10.1099/mic.0.049536-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eshraghi A, Maldonado-Arocho FJ, Gargi A, Cardwell MM, Prouty MG, Blanke SR, et al. Cytolethal distending toxin family members are differentially affected by alterations in host glycans and membrane cholesterol. J Biol Chem. 2010;285(24):18199–207. doi: 10.1074/jbc.M110.112912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lin CD, Lai CK, Lin YH, Hsieh JT, Sing YT, Chang YC, et al. Cholesterol depletion reduces entry of Campylobacter jejuni cytolethal distending toxin and attenuates intoxication of host cells. Infect Immun. 2011;79(9):3563–75. doi: 10.1128/IAI.05175-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guerra L, Teter K, Lilley BN, Stenerlow B, Holmes RK, Ploegh HL, et al. Cellular internalization of cytolethal distending toxin: a new end to a known pathway. Cell Microbiol. 2005;7(7):921–34. doi: 10.1111/j.1462-5822.2005.00520.x. [DOI] [PubMed] [Google Scholar]
- 21.Lord JM, Smith DC, Roberts LM. Toxin entry: how bacterial proteins get into mammalian cells. Cell Microbiol. 1999;1(2):85–91. doi: 10.1046/j.1462-5822.1999.00015.x. [DOI] [PubMed] [Google Scholar]
- 22.Sandvig K, van Deurs B. Transport of protein toxins into cells: pathways used by ricin, cholera toxin and Shiga toxin. FEBS Lett. 2002;529(1):49–53. doi: 10.1016/s0014-5793(02)03182-4. [DOI] [PubMed] [Google Scholar]
- 23.Bischofberger M, van der Goot FG. Exotoxin secretion: getting out to find the way in. Cell Host Microbe. 2008;3(1):7–8. doi: 10.1016/j.chom.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 24.Doherty GJ, McMahon HT. Mechanisms of endocytosis. Annu Rev Biochem. 2009;78:857–902. doi: 10.1146/annurev.biochem.78.081307.110540. [DOI] [PubMed] [Google Scholar]
- 25.Granger E, McNee G, Allan V, Woodman P. The role of the cytoskeleton and molecular motors in endosomal dynamics. Semin Cell Dev Biol. 2014;31:20–9. doi: 10.1016/j.semcdb.2014.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Day CA, Baetz NW, Copeland CA, Kraft LJ, Han B, Tiwari A, et al. Microtubule motors power plasma membrane tubulation in clathrin-independent endocytosis. Traffic. 2015;16(6):572–90. doi: 10.1111/tra.12269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Whitehouse CA, Balbo PB, Pesci EC, Cottle DL, Mirabito PM, Pickett CL. Campylobacter jejuni cytolethal distending toxin causes a G2-phase cell cycle block. Infect Immun. 1998;66(5):1934–40. doi: 10.1128/iai.66.5.1934-1940.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brunelle JL, Green R. One-dimensional SDS-polyacrylamide gel electrophoresis (1D SDS-PAGE). Methods Enzymol. 2014;541:151–9. doi: 10.1016/B978-0-12-420119-4.00012-4. [DOI] [PubMed] [Google Scholar]
- 29.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–54. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 30.Meresse P, Dechaux E, Monneret C, Bertounesque E. Etoposide: discovery and medicinal chemistry. Curr Med Chem. 2004;11(18):2443–66. doi: 10.2174/0929867043364531. [DOI] [PubMed] [Google Scholar]
- 31.Rello-Varona S, Gamez A, Moreno V, Stockert JC, Cristobal J, Pacheco M, et al. Metaphase arrest and cell death induced by etoposide on HeLa cells. Int J Biochem Cell Biol. 2006;38(12):2183–95. doi: 10.1016/j.biocel.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 32.Gavrieli Y, Sherman Y, Ben-Sasson SA. Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J Cell Biol. 1992;119(3):493–501. doi: 10.1083/jcb.119.3.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Matassov D, Kagan T, Leblanc J, Sikorska M, Zakeri Z. Measurement of apoptosis by DNA fragmentation. Methods Mol Biol. 2004;282:1–17. doi: 10.1385/1-59259-812-9:001. [DOI] [PubMed] [Google Scholar]
- 34.Vindelov LL, Christensen IJ, Jensen G, Nissen NI. Limits of detection of nuclear DNA abnormalities by flow cytometric DNA analysis. Results obtained by a set of methods for sample-storage, staining and internal standardization. Cytometry. 1983;3(5):332–9. doi: 10.1002/cyto.990030505. [DOI] [PubMed] [Google Scholar]
- 35.Pozarowski P, Darzynkiewicz Z. Analysis of cell cycle by flow cytometry. Methods Mol Biol. 2004;281:301–11. doi: 10.1385/1-59259-811-0:301. [DOI] [PubMed] [Google Scholar]
- 36.Oswald E, Nougayrede JP, Taieb F, Sugai M. Bacterial toxins that modulate host cell-cycle progression. Curr Opin Microbiol. 2005;8(1):83–91. doi: 10.1016/j.mib.2004.12.011. [DOI] [PubMed] [Google Scholar]
- 37.Lara-Tejero M, Galan JE. A bacterial toxin that controls cell cycle progression as a deoxyribonuclease I-like protein. Science. 2000;290(5490):354–7. doi: 10.1126/science.290.5490.354. [DOI] [PubMed] [Google Scholar]
- 38.Alouf JE. Bacterial protein toxins. An overview. Methods Mol Biol. 2000;145:1–26. doi: 10.1385/1-59259-052-7:1. [DOI] [PubMed] [Google Scholar]
- 39.Lock RB, Galperina OV, Feldhoff RC, Rhodes LJ. Concentration-dependent differences in the mechanisms by which caffeine potentiates etoposide cytotoxicity in HeLa cells. Cancer Res. 1994;54(18):4933–9. [PubMed] [Google Scholar]
- 40.De Rycke J, Oswald E. Cytolethal distending toxin (CDT): a bacterial weapon to control host cell proliferation? FEMS Microbiol Lett. 2001;203(2):141–8. doi: 10.1111/j.1574-6968.2001.tb10832.x. [DOI] [PubMed] [Google Scholar]
- 41.Shenker BJ, Walker LP, Zekavat A, Dlakic M, Boesze-Battaglia K. Blockade of the PI-3K signalling pathway by the Aggregatibacter actinomycetemcomitans cytolethal distending toxin induces macrophages to synthesize and secrete pro-inflammatory cytokines. Cell Microbiol. 2014;16(9):1391–404. doi: 10.1111/cmi.12299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bezine E, Vignard J, Mirey G. The cytolethal distending toxin effects on Mammalian cells: a DNA damage perspective. Cells. 2014;3(2):592–615. doi: 10.3390/cells3020592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Thelestam M, Frisan T. Cytolethal distending toxins. Rev Physiol Biochem Pharmacol. 2004;152:111–33. doi: 10.1007/s10254-004-0030-8. [DOI] [PubMed] [Google Scholar]
- 44.Nakajima T, Hirayama J, Tazumi A, Hayashi K, Tasaki E, Asakura M, et al. Comparative analysis of Campylobacter lari cytolethal distending toxin (CDT) effect on HeLa cells. J Basic Microbiol. 2012;52(5):559–65. doi: 10.1002/jobm.201100297. [DOI] [PubMed] [Google Scholar]
- 45.Buder-Hoffmann S, Palmer C, Vacek P, Taatjes D, Mossman B. Different accumulation of activated extracellular signal-regulated kinases (ERK 1/2) and role in cell-cycle alterations by epidermal growth factor, hydrogen peroxide, or asbestos in pulmonary epithelial cells. Am J Respir Cell Mol Biol. 2001;24(4):405–13. doi: 10.1165/ajrcmb.24.4.4290. [DOI] [PubMed] [Google Scholar]
- 46.Quest AF, Leyton L, Parraga M. Caveolins, caveolae, and lipid rafts in cellular transport, signaling, and disease. Biochem Cell Biol. 2004;82(1):129–44. doi: 10.1139/o03-071. [DOI] [PubMed] [Google Scholar]
- 47.Smith JL, Bayles DO. The contribution of cytolethal distending toxin to bacterial pathogenesis. Crit Rev Microbiol. 2006;32(4):227–48. doi: 10.1080/10408410601023557. [DOI] [PubMed] [Google Scholar]
- 48.Ho YS, Duh JS, Jeng JH, Wang YJ, Liang YC, Lin CH, et al. Griseofulvin potentiates antitumorigenesis effects of nocodazole through induction of apoptosis and G2/M cell cycle arrest in human colorectal cancer cells. Int J Cancer. 2001;91(3):393–401. doi: 10.1002/1097-0215(200002)9999:9999<::aid-ijc1070>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 49.Braet F, De Zanger R, Jans D, Spector I, Wisse E. Microfilament-disrupting agent latrunculin A induces and increased number of fenestrae in rat liver sinusoidal endothelial cells: comparison with cytochalasin B. Hepatology. 1996;24(3):627–35. doi: 10.1053/jhep.1996.v24.pm0008781335. [DOI] [PubMed] [Google Scholar]
- 50.Eash S, Atwood WJ. Involvement of cytoskeletal components in BK virus infectious entry. J Virol. 2005;79(18):11734–41. doi: 10.1128/JVI.79.18.11734-11741.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Saslowsky DE, te Welscher YM, Chinnapen DJ, Wagner JS, Wan J, Kern E, et al. Ganglioside GM1-mediated transcytosis of cholera toxin bypasses the retrograde pathway and depends on the structure of the ceramide domain. J Biol Chem. 2013;288(36):25804–9. doi: 10.1074/jbc.M113.474957. [DOI] [PMC free article] [PubMed] [Google Scholar]