Abstract
Delineating the factors that affect behavioral and neurological responses to alcohol is critical to facilitate measures for preventing or treating alcohol abuse. The high degree of conserved molecular and physiological processes make Drosophila melanogaster a valuable model for investigating circadian interactions with alcohol-induced behaviors and examining sex-specific differences in alcohol sensitivity. We found that wild-type Drosophila exhibit rhythms in alcohol-induced sedation under light-dark and constant dark conditions with considerably greater alcohol exposure necessary to induce sedation during the late (subjective) day and peak sensitivity to alcohol occurring during the late (subjective) night. The circadian clock also modulated the recovery from alcohol-induced sedation with flies regaining motor control significantly faster during the late (subjective) day. As predicted, the circadian rhythms in sedation and recovery were absent in flies with a mutation in the circadian gene period or arrhythmic flies housed in constant light conditions. Flies lacking a functional circadian clock were more sensitive to the effects of alcohol with significantly longer recovery times. Similar to other animals and humans, Drosophila exhibit sex-specific differences in alcohol sensitivity. We investigated whether the circadian clock modulated the rhythms in the Loss-of-Righting Reflex, alcohol-induced sedation, and recovery differently in males and females. We found that both sexes demonstrate circadian rhythms in the Loss-of-Righting Reflex and sedation with the differences in alcohol sensitivity between males and females most pronounced during the late subjective day. Recovery of motor reflexes following alcohol sedation also exhibited circadian modulation in male and female flies, although the circadian clock did not modulate the difference in recovery times between the sexes. These studies provide a framework outlining how the circadian clock modulates alcohol-induced behaviors in Drosophila and identifies sexual dimorphisms in the circadian modulation of alcohol behaviors.
Keywords: Ethanol, biological rhythms, circadian, sexual dimorphism, sedation
Introduction
Alcohol is the most commonly abused substance in the U.S. resulting in severe individual health consequences and enormous economic impacts to businesses and society. Acute binge drinking represents a critical issue with 40.6% of adults between ages 18–25 engaging in binge drinking (Substance Abuse and Mental Health Services Administration, 2014). Moreover, the Center for Disease Control and Prevention reports that alcohol poisoning is the cause of 2,200 deaths per year in the United States (Center for Disease Control and Prevention, 2015). Understanding the factors underlying alcohol responses is necessary to ameliorate the individual and societal costs resulting from acute alcohol abuse.
The circadian clock broadly regulates behavioral and physiological activities of the body including the rest-activity cycle, immune responses, cardiovascular activity, metabolism, gene expression and enzymatic activity (Ko and Takahashi, 2006; Kyriacou and Hastings, 2010; Gamble et al., 2014). The role of the circadian clock in regulating physiological responses to alcohol initially was identified more than 50 years ago with early toxicity studies demonstrating a time of day specificity in alcohol lethality (Haus and Halberg, 1959). Recent behavioral research has shown a daily rhythm in the effects of alcohol on postural control in mice (Perreau-Lenz et al., 2009) and circadian regulation of alcohol-induced decrements in motor reflexes in Drosophila (van der Linde and Lyons, 2011). Furthermore, the absence of a functional circadian clock exacerbates alcohol-induced tissue damage, such as seen for alcohol-induced intestinal hyperpermeability (Forsyth et al., 2015). However, the mechanism through which the circadian clock interacts with alcohol responsiveness at the behavioral and cellular levels is not well understood creating the need for a model system appropriate to conduct joint research in alcohol neurobiology and the circadian clock. Additionally, the consequences of alcohol abuse and excessive consumption affect males and females differently. Men are more prone to binge drinking than women, but women are more vulnerable to alcohol-induced liver and brain damage (Hommer et al., 2001; Pfefferbaum et al., 2001). Thus, any model system for alcohol research should be suitable to accommodate studies on both males and females.
The fruit fly, Drosophila melanogaster with its well-characterized circadian clock (Allada and Chung, 2010), represents an excellent model for investigating circadian modulation of alcohol neurobiology. With clear parallels to mammalian systems at the molecular and behavioral levels, Drosophila is a suitable model for dissecting molecular and neural pathways of alcohol regulation (Wolf et al., 2002; Devineni and Heberlein, 2009; Devineni and Heberlein, 2010; Grotewiel and Bettinger, 2015). Upon initial exposure to alcohol, flies exhibit hyperactivity and behavioral disinhibition (Wolf et al., 2002; Lee et al., 2008), followed by loss of motor coordination with prolonged alcohol exposure. Further increases in alcohol exposure induce sedation with continued alcohol exposure leading to death (Scholz et al., 2000; Guarnieri and Heberlein, 2003; Scholz, 2009; Rodan and Rothenfluh, 2010). Previous research found that the circadian clock modulates alcohol sensitivity in Drosophila as observed through the Loss of Righting Reflex (LoRR) (van der Linde and Lyons, 2011). However, not all alcohol induced behaviors and alcohol processes are regulated by the circadian clock. Similar to humans, Drosophila develop rapid and chronic functional tolerance with repeated occurrences of alcohol exposure resulting in decreased sensitivity to subsequent alcohol exposure (Wolf and Heberlein, 2003). Rapid tolerance following a single alcohol exposure does not appear to be modulated by the circadian clock (van der Linde and Lyons, 2011). Moreover, alcohol absorbance in Drosophila is not circadianly regulated.
We used Drosophila melanogaster to characterize the circadian modulation of sedation and recovery following a single alcohol exposure. We also investigated whether the circadian clock differentially modulates alcohol-induced behaviors in males and females. Alcohol differentially affects male and female Drosophila with females exhibiting greater sensitivity to alcohol-induced sedation, although males display higher mortality in response to high alcohol doses (Devineni and Heberlein, 2012). Thus, Drosophila presents an excellent model for investigating circadian modulation of alcohol behaviors and analyzing differences between male and females. We found that the circadian clock modulates alcohol-induced sedation such that flies were most sensitive to alcohol during the mid-to-late (subjective) night under light-dark (LD) or constant dark conditions. Surprisingly, despite longer alcohol exposures necessary during the late day (ZT or CT 9) to achieve 100% sedation, flies regained control of motor reflexes significantly faster during the late subjective day compared to the mid to late subjective night. Both males and females exhibit a circadian rhythm in their sedative and recovery responses to alcohol with males sedating slower at all time points and recovering faster than females. However, we found phase specificity in the degree of difference in sensitivity between males and females for both LoRR and sedation with the greatest difference observed between males and females during the late subjective day (CT 9). These results demonstrate that alcohol sedation and recovery varies with circadian time and the circadian clock modulates the difference in acute alcohol sensitivity between males and females.
Materials and Methods
Fly Maintenance
Wild-type Canton-S (CS) flies and per01 flies in a CS background were maintained at 25 °C in 12h–12h light-dark cycles and reared on molasses-cornmeal medium. Circadian experiments were performed on the 2nd day of constant darkness (DD). Zeitgeber Time (ZT) 0 refers to dawn or “lights on” while ZT 12 represents dusk and the time when lights were turned off. For experiments conducted in DD, the Circadian Time (CT) refers to the subjective free-running time of the animal with respect to the previous LD entrainment cycle. In Drosophila, circadian rhythms under constant dark conditions (DD) approximate 24 hours so that the Circadian Time reflects the previous Zeitgeber Time. All experiments were performed in an environmentally controlled room at 25 °C and 60–70% relative humidity. All experiments were performed in the dark using dim red light.
Alcohol Exposure
Exposure to alcohol vapor was performed as described previously (van der Linde and Lyons, 2011; van der Linde et al., 2014). Four tubes of Drosophila (3–5 days old; ~30 flies per tube) were exposed to ethanol vapor by mixing proportional fractions of an airstream bubbled through deionized water with an airstream bubbled through 95% ethanol (Koptec) to achieve the pre-determined percentage of ethanol vapor. Flow rates for airstreams were monitored during the experiment to maintain predetermined flow rates and ensure a steady concentration of ethanol vapor.
Behavioral Assay for the Loss-of-Righting Reflex (LoRR)
The loss-of-righting reflex assay was performed as previously described in Drosophila (van der Linde and Lyons, 2011; van der Linde et al., 2014). Prior to the start of alcohol exposure, flies were habituated to dark room conditions for one hour (25° Celsius, 60 – 70 % humidity). During continuous exposure to 30% ethanol vapor, flies received a gentle tap to the tube every 5 minutes over the course of an hour. At each interval, flies were scored to determine the number of flies that could not right themselves, with the LoRR representing the fraction of flies that lost the ability to right themselves. For all experiments, flies of two circadian time points (an LD time point and a reversed cycle-DL time point) were tested simultaneously. The 50% LoRR was estimated for each experiment by linear extrapolation. The difference in male-female responses was determined from experiments in which one tube of male flies and one tube of female flies from each time point were exposed to alcohol. The difference in 50% LoRR between males and females was calculated for each experiment.
Sedation
On the second day of DD, flies were exposed to 50% alcohol vapor for one hour and visually assayed for sedation at 5-minute intervals. A gentle tap was administered to each vial and flies were scored as sedated if lying immobile with no coordinated leg movement, particularly the two front legs as spontaneous twitching may be observed in the middle and hind legs (Cowmeadow et al., 2005). For all experiments, flies of two circadian time points were tested simultaneously. The time at which 50% of the flies reached sedation (ST50) was determined by linear extrapolation. For all experiments performed during light time points, flies were acclimated to the dark for one hour prior to the experiment to avoid potential acute effects of light on alcohol sensitivity.
Recovery
Flies were transferred to new vials containing food after 100% of the flies reached sedation (the sedation end-point was different for each circadian time and between males and females). Vials were tapped every five minutes to assess recovery of postural control and the number of flies that were standing upright or walking were counted. For each circadian time point, the time at which 50% of the flies recovered from sedation (RT50) was determined by linear extrapolation.
Statistics
Statistics were performed using Graph Pad PRISM version 6.0. Differences between circadian time points were assessed using an analysis of variance (ANOVA), with 50% LoRR, 50% ST or 50% RT as the dependent variable and circadian time as the categorical independent variable. Post-hoc analyses were performed using the Bonferroni correction for multiple comparisons.
Results
Circadian Clock Modulates Alcohol-Induced Sedation
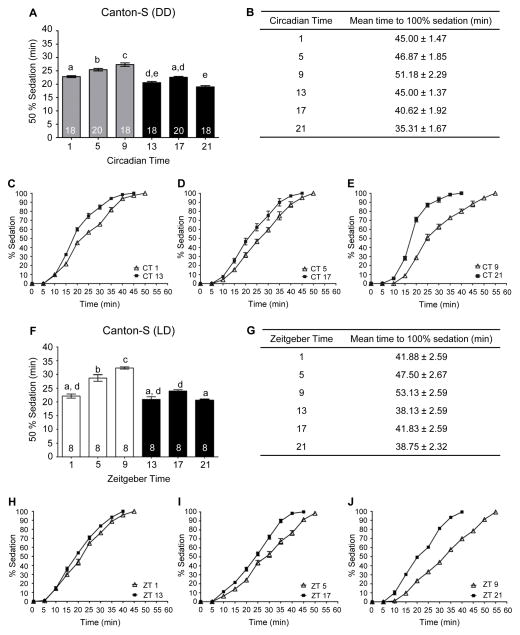
After prolonged exposure to alcohol, flies lose coordination of motor reflexes affecting postural control, specifically the ability to right themselves (Moore et al., 1998; Scholz et al., 2000; van der Linde and Lyons, 2011). Previously, we found that the circadian clock modulates the LoRR in flies with the greatest sensitivity to alcohol observed during the mid-to-late subjective night (van der Linde and Lyons, 2011). To better understand circadian modulation of alcohol neurobiology, we investigated whether the circadian clock modulated additional alcohol-induced behaviors potentially controlled by other neuronal circuits. Sedation in response to alcohol is a sleep like state characterized by immobility and represents a behavior that may be separated from the initial loss of postural control as assessed by the LoRR. Low to moderate levels of alcohol exposure can induce a loss of motor coordination in the majority of flies without inducing sedation, as flies may continue exhibiting active and seemingly coordinated leg movement in attempts at righting themselves (van der Linde et al., 2014; data not shown). In humans, alcohol sedation is induced during the descending limb of the blood alcohol concentration curve and consists of motor impairment and inactivity (Hendler et al., 2013) and requires higher alcohol concentrations relative to motor and balance impairments alone (Dubowski, 2006). The increased alcohol exposure necessary for sedation may reflect the effect of alcohol on an increased number of neuronal circuits or brain regions affected by alcohol or may represent the recruitment of additional cell signaling pathways in response to alcohol exposure. Although previous research suggested that sedation also may be modulated by the circadian clock (van der Linde et al., 2014), no comprehensive study has been performed examining the effects of the circadian clock on sedation or the recovery from sedation in Drosophila. To investigate if the circadian clock modulated alcohol-induced sedation, flies were exposed to 50% alcohol vapor with behavioral state assessed every five minutes. We found a significant circadian rhythm in alcohol-induced sedation with a phase similar to the rhythm in LoRR (Figure 1A and 1B). Flies sedated significantly faster during the mid-to-late subjective night (CT 17 & CT 21) indicating greater alcohol sensitivity at these times. In addition, circadian differences in alcohol sedation were apparent throughout the length of alcohol exposure (Figure 1C – 1E).
Figure 1. Circadian rhythms in alcohol-induced sedation.
(A) The time necessary for 50% of wild-type flies to become sedated is circadianly modulated. Sedation using 50% alcohol vapor was measured at six time points during the 2nd day of DD in age-matched mixed populations of male and female CS flies (ANOVA: F5, 106 = 44.01, p < 0.0001). Post-hoc analyses performed using Bonferroni corrections for multiple comparisons. Means and standard error of the mean plotted for all experiments, n (number of vials of flies) on bars for each group. Different letters above columns denote statistically different responses at time points (p < 0.05), while shared letters between groups indicate no difference. (B) The time for 100% of the flies to become sedated was significantly higher at CT 5 and 9 (ANOVA: F5, 106 = 39.13, p < 0.001). (C) Complete time courses for CT 1 and 13; (D) 5 and 17 and (E) 9 and 21. (F) The time necessary for 50% of wild-type flies to become sedated during alcohol exposure is rhythmically regulated under LD conditions. Sedation using 50% alcohol vapor was measured at six time points for flies housed in LD cycles in age-matched mixed populations of male and female CS flies (ANOVA: F5, 42 = 38.66, p < 0.0001). For all light time points, flies were habituated to the dark for one hour prior to the experiment with all experiments conducted in the dark using dim red light. (G) The time for flies to reach 100% sedation was significantly regulated under LD conditions ((ANOVA: F5, 42 = 40.41, p < 0.0001). (H) Complete time courses for ZT 1 and 13; (I) 5 and 17 and (J) 9 and 21.
While experiments performed under constant conditions reveal circadian regulation and prevent potential confounds such as the masking effects by light, we were interested in whether modulation of alcohol sensitivity also occurred in light-dark conditions. Age-matched flies were tested for alcohol-induced sedation at six time points of the light-dark cycle. We found a significant rhythm in alcohol-induced sedation for flies maintained in LD cycles with a similar phase to the observed circadian rhythm (Figure 1F – 1J). Significantly longer alcohol exposure was necessary for sedation during the late day compared to flies exposed to alcohol during the night.
Functional Circadian Clock is Necessary for the Rhythm in Alcohol Sedation
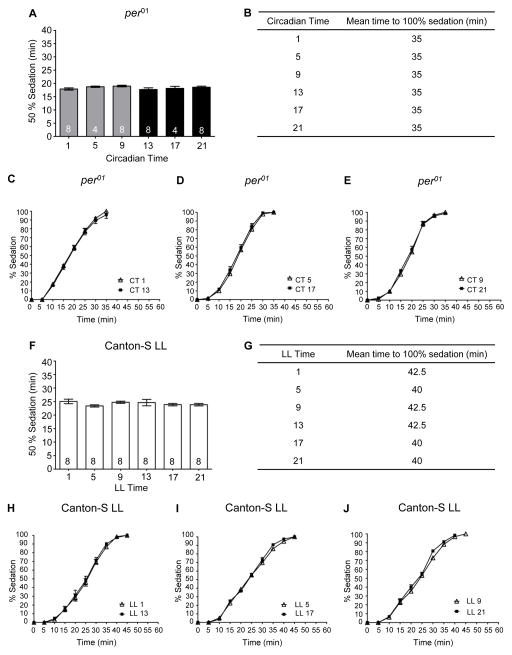
To verify that the observed rhythm in sedation was dependent upon the circadian clock, we performed similar experiments using the circadian per01 mutant. Sedation was assessed at six time points on the second day of DD. No time of day differences were observed in alcohol-induced sedation for the per01 mutant flies (Figure 2A). In comparison to wild-type CS flies, the duration of alcohol exposure necessary for 50% alcohol-induced sedation in per01 mutant flies was similar to the most sensitive phase of the wild-type flies at CT 21 and significantly different than the length of alcohol exposure required for 50% sedation of wild-type flies at CT 9 (ANOVA: F2, 73 = 154.2, p < 0.0001, per0;, n = 40, vs CS CT 9, n = 18, post-hoc test p < 0.01). The increased sensitivity of the per01 mutants also can be observed in the slope of the response curve during the period of alcohol exposure (Figure 2A–2E).
Figure 2. Rhythm in alcohol-induced sedation is abolished in flies lacking a functional circadian clock.
(A) There was no significant time of day difference in the time necessary for 50% of per01 flies to become sedated during alcohol exposure (ANOVA: F5, 34 = 1.16, p = 0.35). (B) The time for 100% of the per01 flies to become sedated was the same at all time points for all experiments as measured using 5 min bins. Complete time courses shown for alcohol-induced sedation in per01 flies for (C) CT 1 and CT 13, (D) CT 5 and 17 and (E) CT 9 and 21. F) There was no significant time of day difference in the time necessary for 50% flies housed in LL conditions to become sedated during alcohol exposure (ANOVA: F5, 42 = 0.87, p = 0.51). (G) The time for 100% of CS flies housed in LL to become sedated. Complete time courses shown for alcohol-induced sedation in CS-LL flies for (H) 1 and 13, (I) 5 and 17 and (J) 9 and 21.
In addition to genetic manipulations, the circadian clock can be rendered non-functional by environmental manipulations. In Drosophila, constant light exposure is sufficient to dampen molecular circadian oscillations and abolish circadian rhythms in locomotor activity, memory formation, and the rhythm in alcohol-induced LoRR (Konopka et al., 1989; Ewer et al., 1992; Power et al., 1995; Price et al., 1995; Yoshii et al., 2005; Lyons and Roman, 2009; van der Linde and Lyons, 2011). To corroborate the necessity of the circadian oscillator for modulation of alcohol-induced sedation, we transferred newly eclosed flies to LL conditions and tested them for alcohol sensitivity on the 4th day of LL. We found that LL conditions abolished the rhythm in alcohol-induced sedation with no significant differences observed in sedation between any of the time points (Figure 2F–J). The length of alcohol exposure necessary for sedation was at mid-trough levels compared to CS flies in DD with flies in LL significantly more sensitive to the sedative effects of alcohol than CS flies at CT 9 and significantly less sensitive than CS flies at CT 21(ANOVA: F2, 81 = 72.60, p < 0.0001, LL flies, n = 48, vs CS CT 9, n = 18, post-hoc test p < 0.001; vs CS CT 21, n = 18, post-hoc test p < 0.001). These results confirm that an intact circadian oscillator is necessary for the circadian rhythm in alcohol-induced sedation and suggest that a non-functional circadian oscillator may result in phase-specific changes in alcohol sensitivity.
Circadian Rhythm in the Recovery of Motor Control
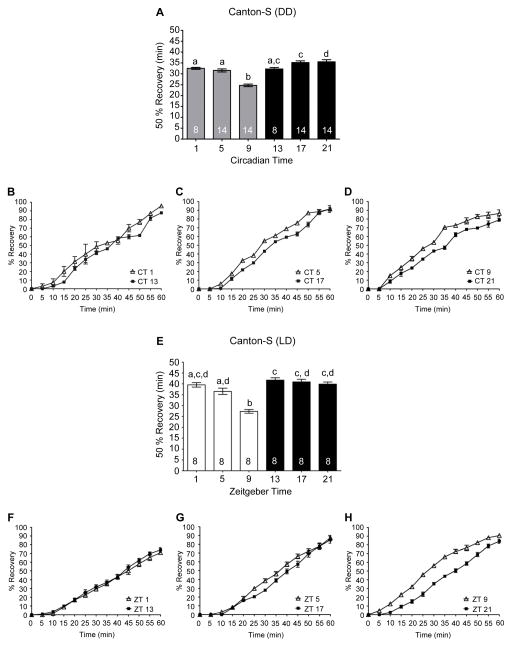
The duration of recovery may be linked to the effects of alcohol on neuronal function, underlying alcohol toxicity at the cellular and tissue levels, or differences in ethanol metabolism. In Drosophila, alcohol absorbance does not appear to be regulated by the circadian clock (van der Linde and Lyons, 2011). Moreover, recovery may not be tightly correlated with alcohol absorbance and metabolism as recovery of the righting reflex following alcohol-induced sedation in mice takes longer in females than males, even with lower blood alcohol levels (Gamsby et al., 2013). Similarly in Drosophila, recovery does not appear correlated with ethanol metabolism as ethanol-induced sedation and recovery can be genetically dissociated (Singh and Heberlein, 2000). Thus, recovery from the effects of alcohol-induced sedation is likely dependent upon the effects of alcohol on neuronal function or alcohol toxicity. To determine whether the circadian clock modulated the rate at which flies recovered from the sedative effects of alcohol, flies were exposed to 50% alcohol vapor until 100% of the flies were sedated. As expected, this resulted in significantly longer alcohol exposure at CT or ZT 9 than at 17 or 21 (Figure 1B and 1G). For our experiments, recovery was defined as the restoration of the righting reflex in which flies regained coordinated motor control to resume an upright position after a gentle tap. Although sedation recovery can be evaluated in Drosophila as the re-emergence of a standing posture, the recovery of the righting reflex represents a more complete recovery and is similar to assays in mice in which the LoRR duration is measured after ethanol injections (Perreau-Lenz et al., 2009). Given that flies at CT 9 received a significantly longer duration of alcohol exposure, we predicted that if the circadian clock did not modulate recovery, flies at CT 9 would take longer to recover the righting reflex. If the circadian clock modulated recovery, we hypothesized that the time to regain motor control would be comparable across time points since flies at CT 9 received a greater alcohol exposure. Surprisingly, we found a circadian rhythm in alcohol recovery with flies recovering significantly faster at CT 9 compared to CT 13, 17 and 21 even with the longer alcohol exposures at CT 9 (Figure 3A – 3D). We confirmed these results with analysis of recovery alcohol-induced sedation using flies in LD cycles. We found that flies exposed to alcohol late in the day at ZT 9 recovered significantly faster than flies exposed to alcohol during the night (Figure 3E – 3H).
Figure 3. Circadian clock modulates recovery from the sedative effects of alcohol.
(A) The time necessary for 50% of the flies to exhibit the righting reflex following sedation in DD, means and SEMs plotted (ANOVA: F5, 66 = 29.67, p < 0.001). Columns with common letters indicate no significant differences between time points based upon Bonferroni post hoc analyses. (B, C, D) The complete recovery curves for CT 1 and 13, 5 and 7, and 9 and 21 are shown respectively. (E) The time necessary for 50% of the flies to regain the righting reflex following alcohol-induced sedation was significantly affected by time of day in LD cycles with flies at ZT 9 exhibiting significantly shorter recovery times (ANOVA: F5, 42 = 23.25, p < 0.0001). (F, G, H) The complete recovery curves for ZT 1 and 13, 5 and 7, and 9 and 21 are shown respectively.
Rhythm in Recovery from Alcohol-induced Sedation Requires the Circadian Clock
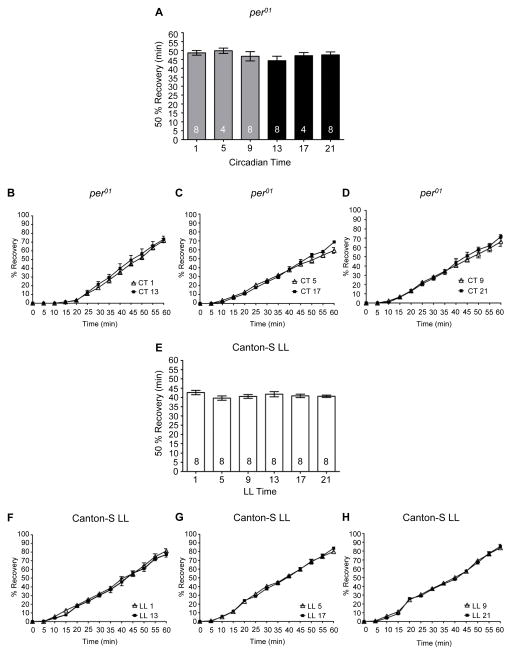
To verify that a functional circadian clock was necessary for the observed rhythms in recovery from alcohol-induced sedation we analyzed recovery in per01 mutants and flies housed in LL conditions. As expected, recovery from alcohol-induced sedation in per01 flies did not show any time of day differences (Figure 2E – 2G). Due to the increased sensitivity of per01 flies, the duration of alcohol exposure for 100% of the flies to reach sedation averaged 35.0 min, a significantly shorter length of alcohol exposure compared to CS flies at CT 1, 5, 9, 13, and 17 (ANOVA: F6, 125 = 42.85, p < 0.0001; Bonferroni post-hoc analyses p < 0.05). per01 flies took significantly longer to recover than CS flies at either CT 9 or CT 21(ANOVA: F2, 65 = 133.8, p < 0.0001; post-hoc comparisons per01 vs. CT 9 and per01 vs. CT 21 p < 0.001). These results confirm that the rhythms in recovery are dependent upon a functional circadian oscillator and demonstrate the robustness of recovery from alcohol-induced sedation in wild-type CS flies compared to per01 flies. Similarly, flies in LL conditions did not exhibit any time of day differences in the recovery from alcohol-induced sedation (Figure 4E – 4H). LL flies took significantly longer to recover than CS flies at either CT 9 or at CT 21 (ANOVA: F2, 73 = 163.60, p < 0.0001, Bonferroni post-hoc analyses for LL vs. CT 9 and LL vs. CT 21 p < 0.001).
Figure 4. A functional circadian clock is necessary for rhythms in alcohol-induced recovery.
(A) There was no significant time of day difference in the time necessary for 50% of per01 flies to recover from alcohol-induced sedation as assessed by recovery of the righting reflex (ANOVA: F5, 34 = 0.76, p = 0.59). Complete time courses shown for recovery of the righting reflex following alcohol-induced sedation in per01 flies for (B) CT 1 and 13, (C) CT 5 and 17 and (D) CT 9 and 21. (E) There was no significant time of day difference in the time necessary for 50% of CS flies housed under LL conditions to recover from alcohol-induced sedation as assessed by recovery of the righting reflex (ANOVA: F5, 42 = 0.95, p = 0.46). Complete time courses shown for recovery of the righting reflex following alcohol-induced sedation in CS flies in LL for (F) 1 and 13, (G) 5 and 17 and (H) 9 and 21.
Circadian Modulation of Alcohol-induced Behaviors in Males and Females
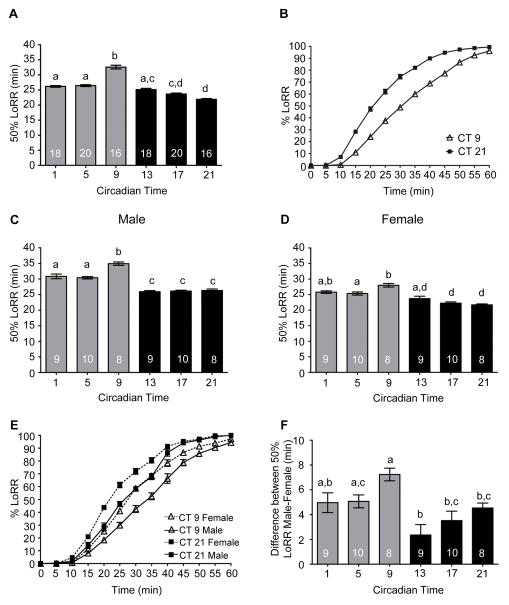
As differences in alcohol sensitivity between males and females are observed from Drosophila to rodents to humans, and differences in circadian locomotor activity are also observed in Drosophila (Fujii et al., 2007), we extended our investigations to determine whether the circadian clock differentially regulated alcohol-induced behaviors between males and females. For comparison, we replicated our previous studies with mixed populations of age-matched flies using a lower concentration of alcohol vapor (30%) to achieve a slow steady curve for better assessment of differences in alcohol sensitivity (Figure 5A and 5B). We found a robust circadian rhythm in the LoRR with flies significantly more sensitive to alcohol in the subjective night (CT 21). We then measured the LoRR with alcohol exposure in separated male and female wild-type flies in concurrent experiments. Males and females were separated approximately 30 hours after eclosion and maintained separately. We found a significant circadian rhythm in LoRR with analogous phases in males and females (Figure 5C and 5D). Interestingly, the amplitude of the circadian rhythm was higher in males than in females. At every time point, females reached 50% LoRR faster than male flies with the difference in response to alcohol exposure between male and female flies apparent throughout the entirety of the alcohol exposure (Figure 5E). Analysis of sex and circadian effects with a two way ANOVA determined that a significant interaction effect occurred between sex and circadian time (Interaction F(5, 96) = 4.60, p = .0008). Using analysis of the magnitude of the difference in sensitivity between males and females in paired experiments, a significantly greater difference in the sensitivity between male and female flies was observed in the late subjective day at CT 9 compared to the night (Figure 5F). Thus, although the circadian clock modulates alcohol sensitivity in male and female flies, phase specific differences exist in the relative sensitivity between the sexes.
Figure 5. Circadian modulation of the alcohol-induced Loss-of-Righting Reflex in males and females.
LoRR induced by 30% alcohol vapor was measured at six time points during the 2nd day of DD. (A) Mixed populations of male and female CS flies exhibited significant circadian rhythms in the LoRR (ANOVA: F5, 102 = 86.52, p < 0.001). (B) Complete course of LoRR during 1 h alcohol exposure shown for CT 9 and CT 21. (C) Circadian rhythm in 50% LoRR for male CS flies (ANOVA: F5, 48 = 53.55, p < 0.001) and (D) female CS flies (ANOVA: F5, 48 = 19.64, p < 0.001). (E) Comparison of time course of LoRR during 1 h alcohol exposure shown for males and females, CT 9 and CT 21. (F) Magnitude of the difference in LoRR between males and females in yoked experiments is phase-specific with the greatest difference between the sexes observed at CT 9 (ANOVA: F5, 48 = 6.23, p < 0.002).
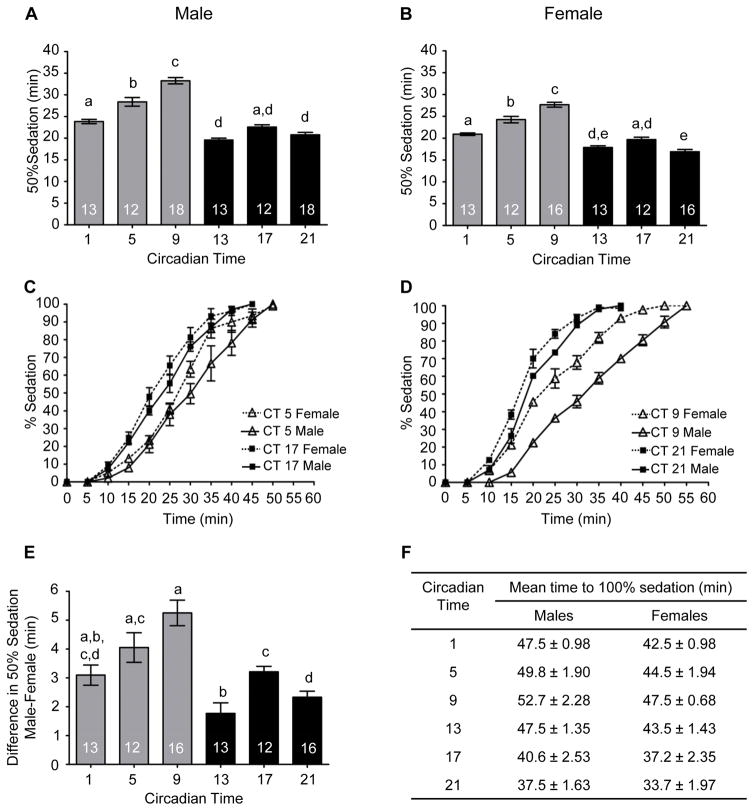
We also investigated circadian modulation of sedation separately in males and females. (Figure 6A and 6B). Both males and females demonstrated significant circadian rhythms in alcohol-induced sedation with the greatest sensitivity to alcohol sedation occurring during the late subjective night. Similar to previous research (Devineni and Heberlein, 2012); females reached 50% sedation faster than males, indicating a greater sensitivity to alcohol sedation in females (Figure 6C and 6D). Although females were more sensitive to alcohol sedation than males at all the circadian time points tested, significant interaction effects were observed between sex and circadian time (Two way ANOVA Interaction F(5, 156) = 2.58, p = .028). Greater differences in alcohol sedation between males and females were observed during the mid to late subjective day compared to the night (Figure 6E and 6F).
Figure 6. The circadian clock differentially affects sensitivity to alcohol-induced sedation in males and females.
(A) The time to 50% sedation for males was circadianly regulated (ANOVA: F5, 80 = 71.25, p < 0.0001). (B) Circadian rhythm in sedation for females (ANOVA: F5, 76 = 66.20, p < 0.0001). (C) The complete sedation time courses for males and females at CT 5 and 17 and (D) CT 9 and 21. (E) The difference in sensitivity of males and females to alcohol sedation was regulated by the circadian clock observed (ANOVA: F5, 75 = 12.52, p < 0.0001). (F) Mean time for 100% of the males (ANOVA: F5, 80 = 61.95, p < 0.0001) and females (ANOVA: F5, 76 = 58.19, p < 0.0001) to reach sedation was circadianly regulated.
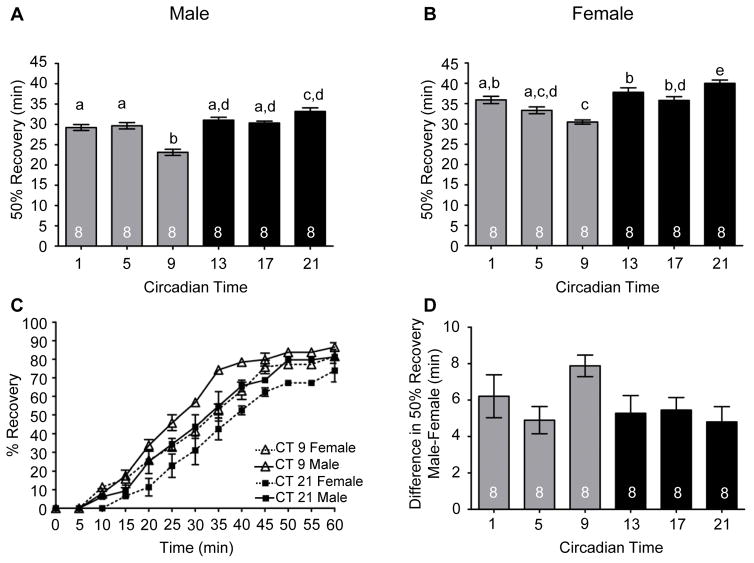
We extended these studies to determine whether differences occurred in circadian modulation of alcohol recovery between males and females. Males and females were exposed to 50% alcohol vapor until 100% of the flies were sedated and recovery assessed as described above. We found that the circadian clock modulated the recovery from alcohol-induced sedation in males and females (Figure 7A and 7B). Males and females exhibited the fastest recovery from alcohol sedation during the late subjective day (CT 9) and the slowest recovery during the late subjective night (CT 21; Figure 7A – 7C). Compared to females, males took longer to reach 100% sedation (Figure 6F) and recovered significantly faster than females from alcohol sedation at all circadian times. No time of day variance or phase specificity was seen in the difference in recovery between males and females from alcohol-induced sedation as determined by two way ANOVA (Interaction F(5, 84) = 1.49, p = .229) and by one way ANOVA analysis of the magnitude of the difference between males and females at each time point (Figure 7D).
Figure 7. Circadian modulation of alcohol recovery in males and females.
(A) Males (ANOVA: F5, 42 = 21.58, p < 0.001) and (B) females (ANOVA: F5, 42 = 14.72, p < 0.001) display a circadian rhythm in alcohol recovery. (C) The complete recovery time course for males and females at CT 9 and 21. (D) No phase specific differences were observed between males and females for recovery from alcohol-induced sedation (ANOVA: F5, 42 = 2.020, p < 0.098).
Sensitivity to Sedation is Different during the Late Subjective Night in Mated vs Virgin flies
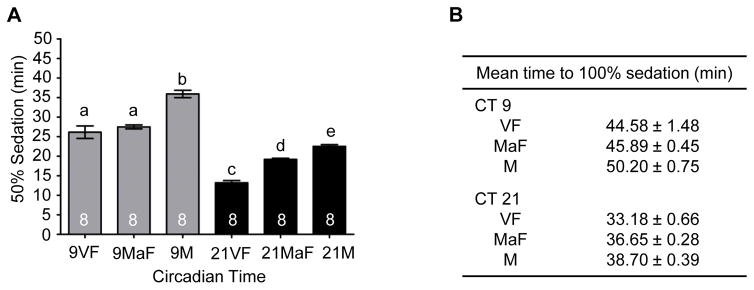
As a first step in understanding the difference in alcohol responses between male and female flies at CT 9 compared to CT 21, we tested whether the reproductive status of the females influenced sensitivity to the ethanol exposure at different times of the day. Research has shown that mating and reproduction significantly decrease the immune defense capabilities of female Drosophila (Peng et al., 2005; Short et al., 2012; Short and Lazzaro, 2013). Potentially, reproductive status also may affect the response to stress induced by the re-allocation of resources to egg-production. To investigate potential differences in alcohol sensitivity, virgin females were collected within 4 hours of eclosion. Remaining newly eclosed females and males were collected and housed together in vials for approximately 30 hours prior to separation and placement into DD. Drosophila females become receptive to courtship between 12 and 14 hours after eclosion (Ashburner et al., 2005). We performed control experiments to validate mating within the 30 h length of incubation of males and females. We found that 30 h of male female group housing resulted in 86.2% of females mated as determined by progeny production when females were separated into individual tubes following mating period (n = 13 vials of mixed males and females, 65 females assessed). Alcohol-induced sedation was assessed on the second day of DD at two time points (CT9 and CT 21) with concurrent comparisons of virgin females, mated females and male flies. We found no significant difference in the sensitivity to alcohol-induced sedation between virgin females and mated females during the late subjective day (CT 9; Figure 8). However, virgin females were more sensitive than mated females to the sedative effects of alcohol during the late subjective night. As this was a time point at which the difference in alcohol sensitivity between males and females was minimized in our previous experiments (Figure 6E), presumably most of the females were mated in those experiments. These results show that reproductive status does not account for the difference observed between males and females in alcohol sensitivity during the late subjective day (CT 9).
Figure 8. Circadian modulation of sedation in virgin and mated females.
(A) The mean time to 50% sedation for virgin females (VF), mated females (MaF) and males (M) for the late subjective day (CT 9) and night (CT 21) (ANOVA: F5, 42 = 82.87, p < 0.001). Post-hoc analyses revealed no significant differences in alcohol sedation between virgin and mated females at CT 9 while virgin females demonstrated significantly increased sensitivity at CT 21. Virgin and mated females sedated significantly faster than males at CT 9 and 21. (B) Mean time to 100% sedation in virgin females, mated females and males.
Discussion
Identification of the factors that modulate the effects of alcohol on behavior and the processes through which alcohol impedes neuronal function or induces cellular damage is necessary to understand alcohol pathologies and develop future therapies. Alcohol bidirectionally interacts with the circadian clock. Acute and chronic alcohol abuse shift core circadian oscillator function and disrupt circadian rhythms, and the circadian clock modulates alcohol consumption and the physiological effects of alcohol (Brick et al., 1984; Rosenwasser, 2015). Alcohol-induced tissue damage is exacerbated with circadian dysfunction potentially acting as a lynchpin in the severity of alcohol-induced liver damage (Forsyth et al., 2015). Well-established as a model system for circadian biology (Allada and Chung, 2010), Drosophila has become a powerful model for identifying genes involved in alcohol use disorders (Grotewiel and Bettinger, 2015).
Previously we found that the circadian clock modulated the acute sensitivity to alcohol by assessing the LoRR in Drosophila; whereas rapid tolerance to alcohol did not appear to be independently modulated by the circadian clock (van der Linde and Lyons, 2011). This dichotomy suggests that the circadian influence on alcohol-induced behaviors varies between behaviors. Potentially, anatomical, molecular or functional differences between alcohol-induced behaviors could underlie the differential circadian modulation. With exposure to low alcohol concentrations, flies exhibit hyperactivity, followed by decreased postural control and sedation with increased alcohol exposure. Although LoRR has been used as a marker for alcohol-induced sedation when flies or mice are exposed to high alcohol concentrations (Rothenfluh et al., 2006; McClure and Heberlein, 2013), impairment of motor control and complete sedation represent distinct behaviors that can be temporally separated in flies using moderate alcohol exposures (van der Linde et al., 2014). To determine the range of circadian interactions with alcohol-induced behaviors, we investigated whether the circadian clock modulated alcohol-induced sedation and the recovery from sedation. We found robust circadian rhythms in alcohol-induced sedation with increased sensitivity to sedation observed during the mid-late subjective night (CT 17 – CT 21). The phase of modulation was similar to what was previously observed for LoRR, although as expected higher alcohol concentrations were necessary to induce sedation relative to LoRR. In mice, the circadian clock modulates alcohol-induced sedation with an opposite phase as expected for a nocturnal animal. Alcohol-induced sedation is rhythmically regulated with the longest duration at ZT 11 during the late day in wild-type mice with circadian clock mutant Per2 mice arrhythmic (Perreau-Lenz et al., 2009). Thus, it appears that circadian modulation of alcohol-induced behaviors is coordinated with the animal’s activity period.
Multiple neurons and neural circuits are implicated in alcohol-induced LoRR and sedation in Drosophila. In adult flies, the central complex is important for locomotion and ethanol-induced changes in locomotion (Scholz et al., 2000; Scholz, 2009; Rodan and Rothenfluh, 2010) and is comprised of four synaptic neuropil domains: the fan shaped body, the ellipsoid body, paired noduli and photocerebral bridge. The central complex also has been implicated in the development of functional tolerance to ethanol (Rodan et al., 2002; Scholz, 2009; Rodan and Rothenfluh, 2010) with a subset of neurons within the ellipsoid body mediating the effects of alcohol in sedation and the development of rapid tolerance to alcohol (Nässel, 2002; Urizar et al., 2007). Alcohol-induced sedation also involves the neuroendocrine system through neurons in the pars intercerebralis and the pars lateralis (McClure and Heberlein, 2013; Sha et al., 2014), neurosecretory clusters analogous to the mammalian hypothalamus (de Velasco et al., 2007). Peripheral axons of these neurosecretory cells project to a neuroendocrine gland complex, the ring gland, comparable to the hypothalamic-pituitary axis in mammals (reviewed in (Nässel, 2002). This axis functions in energy metabolism, water balance, growth and reproduction (Nässel, 2002). Additionally, neurons within the pars intercerebralis and pars lateralis serve in regulating the amount of sleep and rest/activity rhythms in Drosophila (Cavanaugh et al., 2014; Afonso et al., 2015). A subset of neurons within the pars lateralis secrete the neuropeptide Corazonin (Crz), the insect ortholog of the gonadotropin-releasing hormone, which functions in metabolism, energy expenditure, homeostatic control and stress responses (Choi et al., 2005; Zhao et al., 2010) and have been recently shown to mediate alcohol-induced sedation (McClure and Heberlein, 2013; Sha et al., 2014). However, the neurons within these brain regions do not contain circadian oscillators indicating that temporal information must be relayed from circadian oscillatory cells. In the Drosophila brain, approximately 110 – 150 circadian pacemaker neurons are organized into seven primary clusters: the large (l-LNvs) and small ventral lateral neurons (s-LNvs), the dorsal lateral neurons (LNds), the lateral posterior neurons (LPNs) and three groups of dorsal neurons (DN1, DN2, DN3; (Shafer et al., 2006; Allada and Chung, 2010; Helfrich-Förster, 2014).
The s-LNvs function as the primary drivers for circadian activity as functional clocks within these neurons are both necessary and sufficient for circadian locomotor activity rhythms in constant conditions (Frisch et al., 1994; Renn et al., 1999; Grima et al., 2004; Stoleru et al., 2004; Allada and Chung, 2010) Synchronization between circadian oscillatory neurons and temporal relay of information to other neurons for circadian rhythms occurs through the neuropeptide Pigment Dispersing Factor (PDF) released by the s-LNvs (Renn et al., 1999; Grima et al., 2004; Stoleru et al., 2004; Allada and Chung, 2010). The s-LNvs send widespread projections throughout the Drosophila brain and terminals of these neurons lie close to CRZ neuron cell bodies in the pars lateralis (Kaneko and Hall, 2000; Choi et al., 2005) suggesting a possible functional connection through which the circadian clock can modulate ethanol-induced sedation. Dorsal terminals of the circadian PDF neurons also arborize near the pars intercebralis soma suggestive of circadian modulation of arousal and sleep promoting neurons within the pars intercerebralis (Gorostiza et al., 2014). Thus, the anatomical interactions from circadian pacemaker cells to the pars intercerebralis and the pars lateralis provide a mechanism for circadian modulation of alcohol sedation.
Additional neurons in other brain regions also may be involved or responsive to ethanol-induced sedation and potentially functioning in circadian interactions with alcohol behaviors. Although the mushroom bodies are not necessary for alcohol-induced sedation (Rodan et al., 2002; Rodan and Rothenfluh, 2010), alcohol locomotor responsiveness and alcohol-induced behaviors may involve mushroom body neurons (King et al., 2014). The mushroom body functions in olfactory associative memory (Davis, 2011; Guven-Ozkan and Davis, 2014) and has been shown to be involved in acquired ethanol preference (Xu et al., 2012; Ojelade et al., 2015), ethanol-induced hyperactivity (King et al., 2011), the habituation to ethanol-induced startle (Cho et al., 2004), and the conditioned preference or aversion to alcohol (Kaun et al., 2011). As the circadian clock also has been shown to modulate olfactory conditioning in both short and long-term memory (Lyons and Roman, 2009; Fropf et al., 2014), interactions between the circadian pacemaker cells and the mushroom body have been hypothesized. Anatomically, circadian PDF neurons project to the mushroom bodies with circadian changes in the synaptic contacts (Gorostiza et al., 2014) providing a mechanism for conveyance of temporal information necessary for circadian regulation of behavior and physiological processes. Thus, circadian input through mushroom body neurons may contribute to circadian modulation of alcohol-induced behaviors.
The time course of recovery from the sedative effects of alcohol may be an important parameter affecting alcohol abuse and addiction. As recovery time differences may influence alcohol consumption preferences, we examined whether the circadian clock mediated the recovery from alcohol –induced sedation using the recovery of postural control as the criterion. Despite greater alcohol exposure necessary for sedation during the late subjective day, flies recovered faster during this phase compared to the subjective night. Thus, at the same phase, circadian modulation results in decreased sensitivity to the sedative properties of alcohol and speeds the recovery process from those sedative effects. In mice, the circadian clock affects recovery from alcohol hangover symptoms including motor coordination and anxiety with constant darkness shortening recovery periods and circadian desynchronization inhibiting the recovery (Karadayian et al., 2014). We found that the absence of a functional circadian clock in per01 mutants significantly increased the time necessary to recover from the sedative effects of alcohol. Circadian differences in recovery from the deleterious effects of alcohol abuse may influence time-of-day drinking preferences or binge drinking. Time-of-day drinking preferences for alcohol have been long established in rodent models (Geller, 1971; Geller and Purdy, 1979; Wasielewski and Holloway, 2001; Trujillo et al., 2011) and humans (Arfken, 1988; Danel et al., 2003). The evening preference for alcohol consumption is exacerbated by an evening chronotype as individuals categorized as evening-types display greater alcohol consumption and increased risks of alcohol abuse and binge drinking (Prat and Adan, 2011; Urbán et al., 2011; Watson et al., 2013).
Alcohol consumption preferences and the deleterious effects of alcohol vary between males and females across species. Similarly, in Drosophila the sensitivity to alcohol differs between males and females with females more acutely sensitive to the sedative effects of alcohol while males are more sensitive to alcohol-induced hyperactivity and alcohol-induced mortality (Devineni and Heberlein, 2012). We investigated whether the circadian clock modulated alcohol sensitivity differently in males and females. Whereas the phase responses in LoRR and sedation were similar between males and females, the amplitude of the circadian rhythm was significantly reduced in females compared to males. Interestingly, the difference in male-female sensitivity to alcohol was most pronounced during the late subjective day with differences minimized during the night. Sleep and alcohol-induced sedation may involve common or overlapping neural circuitry and signaling pathways so potentially sleep differences between the sexes may partially account for the difference in alcohol-induced sedation between the sexes. In Drosophila, male and female sleep varies significantly during the day as opposed to sleep during the night with males showing greater daytime sleep (Huber et al., 2004; Andretic and Shaw, 2005). However, differences in daytime sleep patterns appear unlikely to explain the difference in alcohol sedation as daytime sleep is greatly reduced in mated females during the day compared to virgin female or male flies (Isaac et al., 2010). We found no difference in alcohol-induced sedation between mated and virgin female flies during the subjective day and both were significantly different than male flies (Figure 8) suggesting that the timing of sleep and the sensitivity to alcohol-induced sedation are distinctly regulated. In the wild, fruit flies locate food sources through the attraction to ethanol vapor. Potentially, the damped amplitude of the circadian rhythm in females with comparatively increased sensitivity during the late subjective day is coordinated with the predicted higher alcohol vapor concentrations due to higher afternoon temperatures that may be encountered by flies in the wild. This increased sensitivity may counterbalance the attraction of females for egg-laying in ethanol rich food sources (Mckenzie and Parsons, 1972; McKenzie, 1972; Azanchi et al., 2013) to prevent egg-laying under conditions in which the alcohol levels may be toxic to development (McClure and Heberlein, 2013).
Anatomically, sexual dimorphism exists in multiple brain regions in Drosophila with male-enlarged regions including the olfactory neurons, pars intercerbralis and the gamma lobes of mushroom body (Cachero et al., 2010). Neuroendocrine regulation mediated by a subset of pars intercerebralis neurons regulates sexual dimorphism in locomotor activity (Belgacem and Martin, 2002). Potentially, the pars intercerebralis acting as a junction for circadian modulation of multiple output pathways results in amplitude differences in circadian modulation of alcohol-induced behaviors between males and females. Sexual dimorphism in neuropeptidergic signaling such as observed for Neuropeptide F (NPF) (Lee et al., 2006), the Drosophila ortholog of Neuropeptide Y, also may partially account for some of the differences in circadian mediated alcohol sensitivity. NPF is expressed in subsets of circadian pacemaker cells, functions in circadian regulation of evening locomotor activity, and has been hypothesized to couple the core oscillator with output signals and behaviors (Hermann et al., 2012; He et al., 2013a; He et al., 2013b). The involvement of the NPF (NPY) pathway in alcohol-induced behavioral responses and intake is conserved from Drosophila to mammals with NPF in Drosophila acting as a key signaling pathway in mediating alcohol sedation (Wen et al., 2005; Chen et al., 2008). NPF also functions through reward pathways in voluntary alcohol intake (Shohat-Ophir et al., 2012). Thus, while sex-specific gene expression may underlie the basic difference in alcohol sensitivity between males and females, sexual dimorphism in neuroendocrine signaling may generate sex-specific circadian modulation of alcohol sensitivity.
Understanding circadian interactions with alcohol provides a foundation for future therapeutic treatments for alcohol use disorders and addiction. Recent research using rodent models has shown that inhibition of Casein-Kinase-1 –epsilon/delta, a key component of the circadian oscillator, decreases relapse drinking after alcohol withdrawal (Perreau-Lenz et al., 2012). Similarly, phase-specific treatment with melatonin or the melatonin receptor agonist agomelatine also reduces relapse-like alcohol consumption after withdrawal in rats (Vengeliene et al., 2015). Given the impact of the circadian clock with alcohol consumption and alcohol toxicity and the bidirectional impact of alcohol use on circadian rhythms, there is a continuing need to understand the mechanisms of these interactions. Using Drosophila, a genetically tractable model, the current study lays the groundwork for future research investigating the neural circuitry and molecular signaling pathways through which the circadian clock modulates alcohol-induced behaviors.
Acknowledgments
The authors would like to thank Dr. Gregg Roman for helpful discussions and Harini C. Krishnan for assistance with preliminary sedation experiments and manuscript preparation. This work was supported by the National Institutes of Health, National Institute on Alcohol Abuse and Alcoholism grant R21AA021233.
References
- Afonso DJ, Liu D, Machado DR, Pan H, Jepson JE, Rogulja D, Koh K. TARANIS Functions with Cyclin A and Cdk1 in a Novel Arousal Center to Control Sleep in Drosophila. Curr Biol. 2015;25:1717–1726. doi: 10.1016/j.cub.2015.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allada R, Chung BY. Circadian organization of behavior and physiology in Drosophila. Annu Rev Physiol. 2010;72:605–624. doi: 10.1146/annurev-physiol-021909-135815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andretic R, Shaw PJ. Essentials of sleep recordings in Drosophila: moving beyond sleep time. Methods Enzymol. 2005;393:759–772. doi: 10.1016/S0076-6879(05)93040-1. [DOI] [PubMed] [Google Scholar]
- Arfken CL. Temporal pattern of alcohol consumption in the United States. Alcohol Clin Exp Res. 1988;12:137–142. doi: 10.1111/j.1530-0277.1988.tb00147.x. [DOI] [PubMed] [Google Scholar]
- Ashburner M, Golic KG, Hawley RS. Drosophila: A Laboratory Handbook. Cold Spring Harbor Laboratory, Cold Spring Harbor; New York: 2005. p. 1331. [Google Scholar]
- Azanchi R, Kaun KR, Heberlein U. Competing dopamine neurons drive oviposition choice for ethanol in Drosophila. Proc Natl Acad Sci U S A. 2013;110:21153–21158. doi: 10.1073/pnas.1320208110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belgacem YH, Martin JR. Neuroendocrine control of a sexually dimorphic behavior by a few neurons of the pars intercerebralis in Drosophila. Proc Natl Acad Sci U S A. 2002;99:15154–15158. doi: 10.1073/pnas.232244199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brick J, Pohorecky LA, Faulkner W, Adams MN. Circadian variations in behavioral and biological sensitivity to ethanol. Alcohol Clin Exp Res. 1984;8:204–211. doi: 10.1111/j.1530-0277.1984.tb05840.x. [DOI] [PubMed] [Google Scholar]
- Cachero S, Ostrovsky AD, Yu JY, Dickson BJ, Jefferis GS. Sexual dimorphism in the fly brain. Curr Biol. 2010;20:1589–1601. doi: 10.1016/j.cub.2010.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanaugh DJ, Geratowski JD, Wooltorton JR, Spaethling JM, Hector CE, Zheng X, Johnson EC, Eberwine JH, Sehgal A. Identification of a circadian output circuit for rest:activity rhythms in Drosophila. Cell. 2014;157:689–701. doi: 10.1016/j.cell.2014.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Center for Disease Control and Prevention. [Accessed July 30, 2015];Alcohol Poisoning Deaths, CDC Vital Signs. 2015 Jan; www.cdc.gov/vitalsigns/alcohol-poisoning-deaths.
- Chen J, Zhang Y, Shen P. A protein kinase C activity localized to neuropeptide Y-like neurons mediates ethanol intoxication in Drosophila melanogaster. Neuroscience. 2008;156:42–47. doi: 10.1016/j.neuroscience.2008.07.008. [DOI] [PubMed] [Google Scholar]
- Cho W, Heberlein U, Wolf FW. Habituation of an odorant-induced startle response in Drosophila. Genes Brain Behav. 2004;3:127–137. doi: 10.1111/j.1601-183x.2004.00061.x. [DOI] [PubMed] [Google Scholar]
- Choi YJ, Lee G, Hall JC, Park JH. Comparative analysis of Corazonin-encoding genes (Crz’s) in Drosophila species and functional insights into Crz-expressing neurons. J Comp Neurol. 2005;482:372–385. doi: 10.1002/cne.20419. [DOI] [PubMed] [Google Scholar]
- Cowmeadow RB, Krishnan HR, Atkinson NS. The slowpoke gene is necessary for rapid ethanol tolerance in Drosophila. Alcohol Clin Exp Res. 2005;29:1777–1786. doi: 10.1097/01.alc.0000183232.56788.62. [DOI] [PubMed] [Google Scholar]
- Danel T, Jeanson R, Touitou Y. Temporal pattern in consumption of the first drink of the day in alcohol-dependent persons. Chronobiol Int. 2003;20:1093–1102. doi: 10.1081/cbi-120025533. [DOI] [PubMed] [Google Scholar]
- Davis RL. Traces of Drosophila memory. Neuron. 2011;70:8–19. doi: 10.1016/j.neuron.2011.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Velasco B, Erclik T, Shy D, Sclafani J, Lipshitz H, McInnes R, Hartenstein V. Specification and development of the pars intercerebralis and pars lateralis, neuroendocrine command centers in the Drosophila brain. Dev Biol. 2007;302:309–323. doi: 10.1016/j.ydbio.2006.09.035. [DOI] [PubMed] [Google Scholar]
- Devineni AV, Heberlein U. Preferential ethanol consumption in Drosophila models features of addiction. Curr Biol. 2009;19:2126–2132. doi: 10.1016/j.cub.2009.10.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devineni AV, Heberlein U. Addiction-like behavior in Drosophila. Commun Integr Biol. 2010;3:357–359. doi: 10.4161/cib.3.4.11885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devineni AV, Heberlein U. Acute ethanol responses in Drosophila are sexually dimorphic. Proc Natl Acad Sci U S A. 2012;109:21087–21092. doi: 10.1073/pnas.1218850110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubowski K. Stages of Acute Influence/Intoxication. Nutritional Sciences:From Fundamentals to Food. In: McGuire M, Beerman K, editors. Wadsworth, Cengage Learning. 3. Belmont, California: 2006. p. 305. [Google Scholar]
- Ewer J, Frisch B, Hamblen-Coyle MJ, Rosbash M, Hall JC. Expression of the period clock gene within different cell types in the brain of Drosophila adults and mosaic analysis of these cells’ influence on circadian behavioral rhythms. J Neurosci. 1992;12:3321–3349. doi: 10.1523/JNEUROSCI.12-09-03321.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsyth CB, Voigt RM, Burgess HJ, Swanson GR, Keshavarzian A. Circadian rhythms, alcohol and gut interactions. Alcohol. 2015;49:389–398. doi: 10.1016/j.alcohol.2014.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisch B, Hardin PE, Hamblen-Coyle MJ, Rosbash M, Hall JC. A promoterless period gene mediates behavioral rhythmicity and cyclical per expression in a restricted subset of the Drosophila nervous system. Neuron. 1994;12:555–570. doi: 10.1016/0896-6273(94)90212-7. [DOI] [PubMed] [Google Scholar]
- Fropf R, Zhang J, Tanenhaus AK, Fropf WJ, Siefkes E, Yin JC. Time of day influences memory formation and dCREB2 proteins in Drosophila. Front Syst Neurosci. 2014;8:43. doi: 10.3389/fnsys.2014.00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii S, Krishnan P, Hardin P, Amrein H. Nocturnal male sex drive in Drosophila. Curr Biol. 2007;17:244–251. doi: 10.1016/j.cub.2006.11.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamble KL, Berry R, Frank SJ, Young ME. Circadian clock control of endocrine factors. Nat Rev Endocrinol. 2014;10:466–475. doi: 10.1038/nrendo.2014.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamsby JJ, Templeton EL, Bonvini LA, Wang W, Loros JJ, Dunlap JC, Green AI, Gulick D. The circadian Per1 and Per2 genes influence alcohol intake, reinforcement, and blood alcohol levels. Behav Brain Res. 2013;249:15–21. doi: 10.1016/j.bbr.2013.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geller I. Ethanol preference in the rat as a function of photoperiod. Science. 1971;173:456–459. doi: 10.1126/science.173.3995.456. [DOI] [PubMed] [Google Scholar]
- Geller I, Purdy R. Biochemistry and Pharmacology of Ethanol. Plenum Press; New York: 1979. Interrelationships between ethanol consumption and circadian rhythm. [Google Scholar]
- Gorostiza EA, Depetris-Chauvin A, Frenkel L, Pírez N, Ceriani MF. Circadian pacemaker neurons change synaptic contacts across the day. Curr Biol. 2014;24:2161–2167. doi: 10.1016/j.cub.2014.07.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grima B, Chélot E, Xia R, Rouyer F. Morning and evening peaks of activity rely on different clock neurons of the Drosophila brain. Nature. 2004;431:869–873. doi: 10.1038/nature02935. [DOI] [PubMed] [Google Scholar]
- Grotewiel M, Bettinger JC. Drosophila and Caenorhabditis elegans as Discovery Platforms for Genes Involved in Human Alcohol Use Disorder. Alcohol Clin Exp Res. 2015;39:1292–1311. doi: 10.1111/acer.12785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guarnieri DJ, Heberlein U. Drosophila melanogaster, a genetic model system for alcohol research. Int Rev Neurobiol. 2003;54:199–228. doi: 10.1016/s0074-7742(03)54006-5. [DOI] [PubMed] [Google Scholar]
- Guven-Ozkan T, Davis RL. Functional neuroanatomy of Drosophila olfactory memory formation. Learn Mem. 2014;21:519–526. doi: 10.1101/lm.034363.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haus E, Halberg F. 24-Hour rhythm in susceptibility of C mice to a toxic dose of ethanol. J Appl Physiol. 1959;14:878–880. doi: 10.1152/jappl.1959.14.6.878. [DOI] [PubMed] [Google Scholar]
- He C, Cong X, Zhang R, Wu D, An C, Zhao Z. Regulation of circadian locomotor rhythm by neuropeptide Y-like system in Drosophila melanogaster. Insect Mol Biol. 2013a;22:376–388. doi: 10.1111/imb.12027. [DOI] [PubMed] [Google Scholar]
- He C, Yang Y, Zhang M, Price JL, Zhao Z. Regulation of sleep by neuropeptide Y-like system in Drosophila melanogaster. PLoS One. 2013b;8:e74237. doi: 10.1371/journal.pone.0074237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helfrich-Förster C. From neurogenetic studies in the fly brain to a concept in circadian biology. J Neurogenet. 2014;28:329–347. doi: 10.3109/01677063.2014.905556. [DOI] [PubMed] [Google Scholar]
- Hendler RA, Ramchandani VA, Gilman J, Hommer DW. Stimulant and sedative effects of alcohol. Curr Top Behav Neurosci. 2013;13:489–509. doi: 10.1007/7854_2011_135. [DOI] [PubMed] [Google Scholar]
- Hermann C, Yoshii T, Dusik V, Helfrich-Förster C. Neuropeptide F immunoreactive clock neurons modify evening locomotor activity and free-running period in Drosophila melanogaster. J Comp Neurol. 2012;520:970–987. doi: 10.1002/cne.22742. [DOI] [PubMed] [Google Scholar]
- Hommer D, Momenan R, Kaiser E, Rawlings R. Evidence for a gender-related effect of alcoholism on brain volumes. Am J Psychiatry. 2001;158:198–204. doi: 10.1176/appi.ajp.158.2.198. [DOI] [PubMed] [Google Scholar]
- Huber R, Hill SL, Holladay C, Biesiadecki M, Tononi G, Cirelli C. Sleep homeostasis in Drosophila melanogaster. Sleep. 2004;27:628–639. doi: 10.1093/sleep/27.4.628. [DOI] [PubMed] [Google Scholar]
- Isaac RE, Li C, Leedale AE, Shirras AD. Drosophila male sex peptide inhibits siesta sleep and promotes locomotor activity in the post-mated female. Proc Biol Sci. 2010;277:65–70. doi: 10.1098/rspb.2009.1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko M, Hall JC. Neuroanatomy of cells expressing clock genes in Drosophila: transgenic manipulation of the period and timeless genes to mark the perikarya of circadian pacemaker neurons and their projections. J Comp Neurol. 2000;422:66–94. doi: 10.1002/(sici)1096-9861(20000619)422:1<66::aid-cne5>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Karadayian AG, Lores-Arnaiz S, Cutrera RA. The effect of constant darkness and circadian resynchronization on the recovery of alcohol hangover. Behav Brain Res. 2014;268:94–103. doi: 10.1016/j.bbr.2014.03.048. [DOI] [PubMed] [Google Scholar]
- Kaun KR, Azanchi R, Maung Z, Hirsh J, Heberlein U. A Drosophila model for alcohol reward. Nat Neurosci. 2011;14:612–619. doi: 10.1038/nn.2805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King I, Tsai LT, Pflanz R, Voigt A, Lee S, Jäckle H, Lu B, Heberlein U. Drosophila tao controls mushroom body development and ethanol-stimulated behavior through par-1. J Neurosci. 2011;31:1139–1148. doi: 10.1523/JNEUROSCI.4416-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King IF, Eddison M, Kaun KR, Heberlein U. EGFR and FGFR pathways have distinct roles in Drosophila mushroom body development and ethanol-induced behavior. PLoS One. 2014;9:e87714. doi: 10.1371/journal.pone.0087714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko CH, Takahashi JS. Molecular components of the mammalian circadian clock. Hum Mol Genet. 2006;15(Spec No 2):R271–277. doi: 10.1093/hmg/ddl207. [DOI] [PubMed] [Google Scholar]
- Konopka RJ, Pittendrigh C, Orr D. Reciprocal behaviour associated with altered homeostasis and photosensitivity of Drosophila clock mutants. J Neurogenet. 1989;6:1–10. doi: 10.3109/01677068909107096. [DOI] [PubMed] [Google Scholar]
- Kyriacou CP, Hastings MH. Circadian clocks: genes, sleep, and cognition. Trends Cogn Sci. 2010;14:259–267. doi: 10.1016/j.tics.2010.03.007. [DOI] [PubMed] [Google Scholar]
- Lee G, Bahn JH, Park JH. Sex- and clock-controlled expression of the neuropeptide F gene in Drosophila. Proc Natl Acad Sci U S A. 2006;103:12580–12585. doi: 10.1073/pnas.0601171103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HG, Kim YC, Dunning JS, Han KA. Recurring ethanol exposure induces disinhibited courtship in Drosophila. PLoS One. 2008;3:e1391. doi: 10.1371/journal.pone.0001391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons LC, Roman G. Circadian modulation of short-term memory in Drosophila. Learn Mem. 2009;16:19–27. doi: 10.1101/lm.1146009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure KD, Heberlein U. A small group of neurosecretory cells expressing the transcriptional regulator apontic and the neuropeptide corazonin mediate ethanol sedation in Drosophila. J Neurosci. 2013;33:4044–4054. doi: 10.1523/JNEUROSCI.3413-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mckenzie JA, Parsons PA. Alcohol Tolerance: An Ecological parameter in the relative success of Drosophila melanogaster and Drosophila simulans. Oecologia. 1972;10:373–388. doi: 10.1007/BF00345738. [DOI] [PubMed] [Google Scholar]
- McKenzie JC. Social implications of alcohol consumption. Proc Nutr Soc. 1972;31:99–106. doi: 10.1079/pns19720022. [DOI] [PubMed] [Google Scholar]
- Moore MS, DeZazzo J, Luk AY, Tully T, Singh CM, Heberlein U. Ethanol intoxication in Drosophila: Genetic and pharmacological evidence for regulation by the cAMP signaling pathway. Cell. 1998;93:997–1007. doi: 10.1016/s0092-8674(00)81205-2. [DOI] [PubMed] [Google Scholar]
- Nässel DR. Neuropeptides in the nervous system of Drosophila and other insects: multiple roles as neuromodulators and neurohormones. Prog Neurobiol. 2002;68:1–84. doi: 10.1016/s0301-0082(02)00057-6. [DOI] [PubMed] [Google Scholar]
- Ojelade SA, Jia T, Rodan AR, Chenyang T, Kadrmas JL, Cattrell A, Ruggeri B, Charoen P, Lemaitre H, Banaschewski T, Büchel C, Bokde AL, Carvalho F, Conrod PJ, Flor H, Frouin V, Gallinat J, Garavan H, Gowland PA, Heinz A, Ittermann B, Lathrop M, Lubbe S, Martinot JL, Paus T, Smolka MN, Spanagel R, O’Reilly PF, Laitinen J, Veijola JM, Feng J, Desrivières S, Jarvelin MR, Schumann G, Rothenfluh A Consortium I. Rsu1 regulates ethanol consumption in Drosophila and humans. Proc Natl Acad Sci U S A. 2015 doi: 10.1073/pnas.1417222112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng J, Zipperlen P, Kubli E. Drosophila sex-peptide stimulates female innate immune system after mating via the Toll and Imd pathways. Curr Biol. 2005;15:1690–1694. doi: 10.1016/j.cub.2005.08.048. [DOI] [PubMed] [Google Scholar]
- Perreau-Lenz S, Vengeliene V, Noori HR, Merlo-Pich EV, Corsi MA, Corti C, Spanagel R. Inhibition of the casein-kinase-1-ε/δ/prevents relapse-like alcohol drinking. Neuropsychopharmacology. 2012;37:2121–2131. doi: 10.1038/npp.2012.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perreau-Lenz S, Zghoul T, de Fonseca FR, Spanagel R, Bilbao A. Circadian regulation of central ethanol sensitivity by the mPer2 gene. Addict Biol. 2009;14:253–259. doi: 10.1111/j.1369-1600.2009.00165.x. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Rosenbloom M, Deshmukh A, Sullivan E. Sex differences in the effects of alcohol on brain structure. Am J Psychiatry. 2001;158:188–197. doi: 10.1176/appi.ajp.158.2.188. [DOI] [PubMed] [Google Scholar]
- Power J, Ringo J, Dowse H. The role of light in the initiation of circadian activity rhythms of adult Drosophila melanogaster. J Neurogenet. 1995;9:227–238. doi: 10.3109/01677069509084159. [DOI] [PubMed] [Google Scholar]
- Prat G, Adan A. Influence of circadian typology on drug consumption, hazardous alcohol use, and hangover symptoms. Chronobiol Int. 2011;28:248–257. doi: 10.3109/07420528.2011.553018. [DOI] [PubMed] [Google Scholar]
- Price JL, Dembinska ME, Young MW, Rosbash M. Suppression of PERIOD protein abundance and circadian cycling by the Drosophila clock mutation timeless. EMBO J. 1995;14:4044–4049. doi: 10.1002/j.1460-2075.1995.tb00075.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renn SC, Park JH, Rosbash M, Hall JC, Taghert PH. A pdf neuropeptide gene mutation and ablation of PDF neurons each cause severe abnormalities of behavioral circadian rhythms in Drosophila. Cell. 1999;99:791–802. doi: 10.1016/s0092-8674(00)81676-1. [DOI] [PubMed] [Google Scholar]
- Rodan AR, Kiger JA, Heberlein U. Functional dissection of neuroanatomical loci regulating ethanol sensitivity in Drosophila. J Neurosci. 2002;22:9490–9501. doi: 10.1523/JNEUROSCI.22-21-09490.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodan AR, Rothenfluh A. The genetics of behavioral alcohol responses in Drosophila. Int Rev Neurobiol. 2010;91:25–51. doi: 10.1016/S0074-7742(10)91002-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenwasser AM. Sleep, circadian rhythms and alcohol: introduction and overview. Alcohol. 2015;49:297. doi: 10.1016/j.alcohol.2015.04.002. [DOI] [PubMed] [Google Scholar]
- Rothenfluh A, Threlkeld RJ, Bainton RJ, Tsai LT, Lasek AW, Heberlein U. Distinct behavioral responses to ethanol are regulated by alternate RhoGAP18B isoforms. Cell. 2006;127:199–211. doi: 10.1016/j.cell.2006.09.010. [DOI] [PubMed] [Google Scholar]
- Scholz H. Intoxicated fly brains: neurons mediating ethanol-induced behaviors. J Neurogenet. 2009;23:111–119. doi: 10.1080/01677060802471676. [DOI] [PubMed] [Google Scholar]
- Scholz H, Ramond J, Singh CM, Heberlein U. Functional ethanol tolerance in Drosophila. Neuron. 2000;28:261–271. doi: 10.1016/s0896-6273(00)00101-x. [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration. Summary of National Findings. Rockville, MD: 2014. Results from the 2013 National Survey on Drug Use and Health; pp. 14–4863. NSDUH Series H-48, HHS Publication No. (SMA) [Google Scholar]
- Sha K, Choi SH, Im J, Lee GG, Loeffler F, Park JH. Regulation of ethanol-related behavior and ethanol metabolism by the Corazonin neurons and Corazonin receptor in Drosophila melanogaster. PLoS One. 2014;9:e87062. doi: 10.1371/journal.pone.0087062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafer OT, Helfrich-Förster C, Renn SC, Taghert PH. Reevaluation of Drosophila melanogaster’s neuronal circadian pacemakers reveals new neuronal classes. J Comp Neurol. 2006;498:180–193. doi: 10.1002/cne.21021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shohat-Ophir G, Kaun KR, Azanchi R, Mohammed H, Heberlein U. Sexual deprivation increases ethanol intake in Drosophila. Science. 2012;335:1351–1355. doi: 10.1126/science.1215932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Short SM, Lazzaro BP. Reproductive status alters transcriptomic response to infection in female Drosophila melanogaster. G3 (Bethesda) 2013;3:827–840. doi: 10.1534/g3.112.005306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Short SM, Wolfner MF, Lazzaro BP. Female Drosophila melanogaster suffer reduced defense against infection due to seminal fluid components. J Insect Physiol. 2012;58:1192–1201. doi: 10.1016/j.jinsphys.2012.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh CM, Heberlein U. Genetic control of acute ethanol-induced behaviors in Drosophila. Alcohol Clin Exp Res. 2000;24:1127–1136. [PubMed] [Google Scholar]
- Stoleru D, Peng Y, Agosto J, Rosbash M. Coupled oscillators control morning and evening locomotor behaviour of Drosophila. Nature. 2004;431:862–868. doi: 10.1038/nature02926. [DOI] [PubMed] [Google Scholar]
- Trujillo JL, Do DT, Grahame NJ, Roberts AJ, Gorman MR. Ethanol consumption in mice: relationships with circadian period and entrainment. Alcohol. 2011;45:147–159. doi: 10.1016/j.alcohol.2010.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbán R, Magyaródi T, Rigó A. Morningness-eveningness, chronotypes and health-impairing behaviors in adolescents. Chronobiol Int. 2011;28:238–247. doi: 10.3109/07420528.2010.549599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urizar NL, Yang Z, Edenberg HJ, Davis RL. Drosophila homer is required in a small set of neurons including the ellipsoid body for normal ethanol sensitivity and tolerance. J Neurosci. 2007;27:4541–4551. doi: 10.1523/JNEUROSCI.0305-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Linde K, Fumagalli E, Roman G, Lyons LC. The FlyBar: administering alcohol to flies. J Vis Exp. 2014 doi: 10.3791/50442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Linde K, Lyons LC. Circadian modulation of acute alcohol sensitivity but not acute tolerance in Drosophila. Chronobiol Int. 2011;28:397–406. doi: 10.3109/07420528.2011.577921. [DOI] [PubMed] [Google Scholar]
- Vengeliene V, Noori HR, Spanagel R. Activation of Melatonin Receptors Reduces Relapse-Like Alcohol Consumption. Neuropsychopharmacology. 2015 doi: 10.1038/npp.2015.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasielewski JA, Holloway FA. Alcohol’s interactions with circadian rhythms. A focus on body temperature. Alcohol Res Health. 2001;25:94–100. [PMC free article] [PubMed] [Google Scholar]
- Watson NF, Buchwald D, Harden KP. A twin study of genetic influences on diurnal preference and risk for alcohol use outcomes. J Clin Sleep Med. 2013;9:1333–1339. doi: 10.5664/jcsm.3282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen T, Parrish CA, Xu D, Wu Q, Shen P. Drosophila neuropeptide F and its receptor, NPFR1, define a signaling pathway that acutely modulates alcohol sensitivity. Proc Natl Acad Sci U S A. 2005;102:2141–2146. doi: 10.1073/pnas.0406814102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf FW, Heberlein U. Invertebrate models of drug abuse. J Neurobiol. 2003;54:161–178. doi: 10.1002/neu.10166. [DOI] [PubMed] [Google Scholar]
- Wolf FW, Rodan AR, Tsai LT, Heberlein U. High-resolution analysis of ethanol-induced locomotor stimulation in Drosophila. J Neurosci. 2002;22:11035–11044. doi: 10.1523/JNEUROSCI.22-24-11035.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu S, Chan T, Shah V, Zhang S, Pletcher SD, Roman G. The propensity for consuming ethanol in Drosophila requires rutabaga adenylyl cyclase expression within mushroom body neurons. Genes Brain Behav. 2012;11:727–739. doi: 10.1111/j.1601-183X.2012.00810.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshii T, Heshiki Y, Ibuki-Ishibashi T, Matsumoto A, Tanimura T, Tomioka K. Temperature cycles drive Drosophila circadian oscillation in constant light that otherwise induces behavioural arrhythmicity. Eur J Neurosci. 2005;22:1176–1184. doi: 10.1111/j.1460-9568.2005.04295.x. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Bretz CA, Hawksworth SA, Hirsh J, Johnson EC. Corazonin neurons function in sexually dimorphic circuitry that shape behavioral responses to stress in Drosophila. PLoS One. 2010;5:e9141. doi: 10.1371/journal.pone.0009141. [DOI] [PMC free article] [PubMed] [Google Scholar]