Abstract
The induction, formation and maintenance of memory represent dynamic processes modulated by multiple factors including the circadian clock and sleep. Chronic sleep restriction has become common in modern society due to occupational and social demands. Given the impact of cognitive impairments associated with sleep deprivation, there is a vital need for a simple animal model in which to study the interactions between chronic sleep deprivation and memory. We used the marine mollusk Aplysia californica, with its simple nervous system, nocturnal sleep pattern and well-characterized learning paradigms, to assess the effects of two chronic sleep restriction paradigms on short-term (STM) and long-term (LTM) associative memory. The effects of sleep deprivation on memory were evaluated using the operant learning paradigm, learning that food is inedible, in which the animal associates a specific netted seaweed with failed swallowing attempts. We found that two nights of 6 h sleep deprivation occurring during the first or last half of the night inhibited both STM and LTM. Moreover, the impairment in STM persisted for more than 24 hours. A milder, prolonged sleep deprivation paradigm consisting of 3 consecutive nights of 4 h sleep deprivation also blocked STM, but had no effect on LTM. These experiments highlight differences in the sensitivity of STM and LTM to chronic sleep deprivation. Moreover, these results establish Aplysia as a valid model for studying the interactions between chronic sleep deprivation and associative memory paving the way for future studies delineating the mechanisms through which sleep restriction affects memory formation.
Keywords: Aplysia, sleep deprivation, associative memory, learning
1. Introduction
Increasingly, individuals are working longer hours with approximately 19% of adults working more than 48 hours per week and 7% working more than 60 hours per week (Alterman et al., 2013). Consequently, chronic sleep restriction has become prevalent in modern society as a result of occupational and social demands (Akerstedt and Wright, 2009; Costa, 2015; Liu et al., 2016). Approximately 35% of American adults and an increasing number of children and adolescents report insufficient sleep at night (Centers for Disease Control and Prevention, 2011, 2012; Liu et al., 2016). In national surveys conducted in 2005 and 2010, one-third of adult workers reported an average of less than 6 hours sleep per night (Centers for Disease Control and Prevention, 2011, 2012; Luckhaupt et al., 2010). Insufficient sleep and sleep disorders not only represent a public health problem in the United States and western countries, but are also arising as a significant health issue in countries across Africa and Asia (Stranges et al., 2012). Chronic sleep restriction results in poor performance in tasks measuring sustained attention such as the psychomotor vigilance test (Mollicone et al., 2010), working memory (Jiang et al., 2011; Drummond et al., 2012) and long-term memory (Lo et al., 2016a; Lo et al., 2016b). Both mild (~3 h/day) and harsh (~7 h/day) sleep restriction for 1-2 weeks result in cumulative adverse effects on attention and cognition (Dinges et al., 1997; Belenky et al., 2003; Van Dongen et al., 2003).
In light of the accumulating evidence regarding the adverse effects of even short periods of sleep restriction on performance and memory, relatively little is known about the mechanisms through which restricted sleep affects performance and cognitive function. In rodent models, chronic sleep restriction paradigms involve extended sleep deprivation protocols frequently continuing for multiple weeks or months (Alzoubi et al., 2012; Rothman et al., 2013; Zielinski et al., 2013; Alzoubi et al., 2016) making it difficult to isolate the time points at which to resolve the molecular consequences of sleep deprivation. As the first step in establishing a simple model system in which the interactions between chronic sleep restriction and memory could be investigated at the molecular and cellular levels, we investigated the effects of chronic sleep deprivation on the induction of short-term (STM) and long-term (LTM) associative memory using a relatively simple invertebrate model system, Aplysia californica. Aplysia sleep almost solely during the night exhibiting decreased responsiveness to appetitive and aversive stimuli during sleep (Vorster et al., 2014). Following a single night of sleep deprivation, Aplysia exhibit rebound sleep demonstrating homeostatic as well as circadian regulation of sleep (Vorster et al., 2014). Recently, Aplysia also has been used as a model to investigate the effects of acute sleep deprivation on memory in which it was found that a single night of 9 h sleep deprivation inhibited the induction of short and long-term memory (Krishnan et al., in press).
Using the operant learning paradigm, learning that food is inedible (LFI), in which the animal associates a specific netted seaweed with the inability to swallow the seaweed (Susswein et al., 1986), we compared the effects of two different patterns of repeated sleep deprivation (6 h/day for 2 consecutive nights or 4 h/day for 3 consecutive nights) on STM and LTM. We found that two consecutive nights of 6 h/day sleep deprivation during either the first half (ZT 12-ZT 18) or last half (ZT 18-ZT 24) of the night blocked the induction of both STM and LTM. Moreover, the impairment in STM following two nights of restricted sleep persisted for 24 h. In contrast, there were no persistent effects of repeated sleep deprivation on LTM. As the effects of chronic sleep restriction on cognitive impairments is proportional to the degree of restricted sleep in humans (Belenky et al., 2003; Van Dongen et al., 2003), we also investigated the effects of a milder form of chronic sleep restriction, in which the animals were sleep deprived for 4 h/night for 3 consecutive nights, on short and long-term LFI memory. We found that three nights of mild sleep restriction inhibited STM, although no adverse effects were observed on the induction of 24 h LTM. Thus, short-term memory appears to be more sensitive to the detrimental effects of chronic sleep restriction. These behavioral studies established Aplysia as a suitable model system for investigating the interactions between repeated sleep restriction and memory formation.
2. Materials and Methods
2.1. Animal Maintenance
Wild-caught Aplysia californica (100-200g; South Coast Bio-Marine, San Pedro, CA) were maintained within individual boxes in chilled 110 gallon tanks containing artificial seawater (Instant Ocean) at 15°C on a 12h light/12h dark (LD) cycle. Animals were fed romaine lettuce every two – three days with feeding times varied across the day.
2.2. Sleep Deprivation
Sleep deprivation was performed as previously described (Vorster et al, 2014) with slight modifications. Animals were transferred in the dark to individual, chilled and aerated plastic open containers (25 cm × 30 cm) filled with artificial seawater and varying substrates (large smooth stones, small pebbles mixed with coral sand, aquarium filter, terrarium liner). Animals were sleep deprived through context changes every 30 min via transfer to a different container and tactile stimulation. Each animal was observed once per minute to assess mobility and the animal was handled if the animal remained immobile with a resting body posture (Vorster et al., 2014) for 3 consecutive observations. Typically, animals were handled 2 – 5 times per half hour to achieve sleep deprivation. Sleep deprivation procedures were performed in the dark under dim red light.
2.3. Behavioral training and testing
All animals, including experimental, control and naïve animals, were fed to satiation with laver seaweed as in previous studies of non-associative and associative memory in Aplysia (Fernandez et al., 2003; Lyons et al., 2005; Michel et al., 2012; Michel et al., 2013) and then removed from appetitive stimuli for 6 days prior to LFI training or testing. All training was performed at Zeitgeber Time 1 (ZT 1) with ZT 0 defined as the time of lights on and ZT 12 referencing lights off. LFI training was performed as previously described using a single 25 min training protocol (Michel et al., 2012; Michel et al., 2013). During LFI training, animals were presented with laver seaweed that provides strong chemosensory cues in a tulle-mesh bag that could not be swallowed. Animals responded with head-waving, orienting toward the seaweed and biting responses. Although all animals were trained using a 25 min training protocol, small individual variation in the duration of training occurs between animals due to the time necessary to gently extract the seaweed bag from the mouth during cycles of protraction and retraction of the radula. Testing occurred using similar procedures as training, either 30 min later for short-term memory (STM) or 24 h later for long-term memory (LTM) and proceeded until 3 min elapsed without the animal taking the netted seaweed into the mouth after egestion. Two parameters were measured during testing: total response time and the cumulative time the netted seaweed was retained in the mouth. Response times were measured individually for each animal using digital waterproof stopwatches (VWR). Memory is represented by a decrease in response times when trained animals are compared to naïve animals. Naïve animals were tested for behavioral responses either at the time of training (similar Zeitgeber time and time elapsed since feeding to satiation) for control and sleep-deprived animals or at the time of LTM testing. As no significant differences were observed in the responses between the two groups of naïve animals, the data was pooled for each set of experiments. For all behavior experiments including sleep deprivation, LFI training and LFI testing, a single experiment involved multiple individuals so that an experimenter at any one step was frequently blind to the history of the animal or subsequent experimental plans for the animal.
2.4. Statistical Analysis
Statistical analysis of the data was performed using one-way ANOVA with Bonferroni's post-hoc analysis for comparisons between groups. P values less than 0.05 were considered significant.
3. Results
3.1. Repeated 6 hour sleep deprivation inhibits long-term memory
In humans, chronic sleep restriction leads to cumulative cognitive impairments (Belenky et al., 2003; Van Dongen et al., 2003). To determine whether chronic sleep restriction affected memory in Aplysia, we investigated the effect of two nights of 6 h sleep deprivation on the induction of long-term memory. Aplysia sleep approximately 7.5 hours per night with sleep onset occurring 1 – 2 hours after lights off and anticipatory activity beginning approximately 1.5 h prior to dawn (Vorster et al., 2014). Previously, we found that Aplysia sleep in longer bouts during the first half of the night with shorter resting bouts and more frequent awakenings observed during the last half of the night (Vorster et al., 2014). Accordingly, we characterized the effects of chronic sleep restriction during both the first and last half of the night on memory formation. Animals were sleep deprived using periodic contextual changes and gentle handling for 6 h for 2 consecutive nights during the first half of the night (ZT 12 - ZT 18; Figure 1A and 1B) or during the last half of the night (ZT 18 - ZT 24; Figure 1C and 1D). As predicted, on night 1 the animals required almost no handling during the first hour after lights off from ZT 12 – ZT 13 (mean handling per animal 1.36 ± 0.17) and only slightly more handling during the second hour after lights off from ZT 13 – ZT 14 (mean handling per animal 2.99 ± 0.26) reflecting the natural activity pattern of the animal. However, when sleep deprivation occurred during the last half of the night starting at ZT 18, more handling was necessary during the entire time period as the normal pre-dawn anticipatory activity was not observed, presumably due to the prior sleep deprivation (mean handling per animal from ZT 22- ZT 23 = 6.85 ±0.41; mean handling per animal from ZT 23 – lights on = 6.35 ± 0.40). The effects of sleep deprivation were cumulative as additional handling was required for both phases of sleep deprivation on night 2 (data not shown).
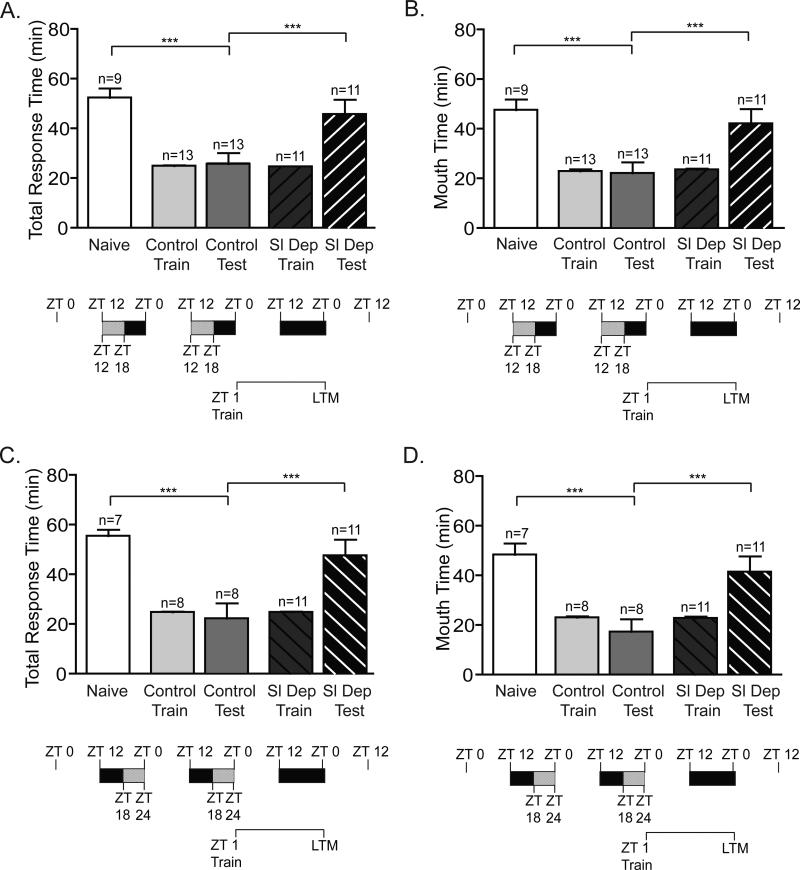
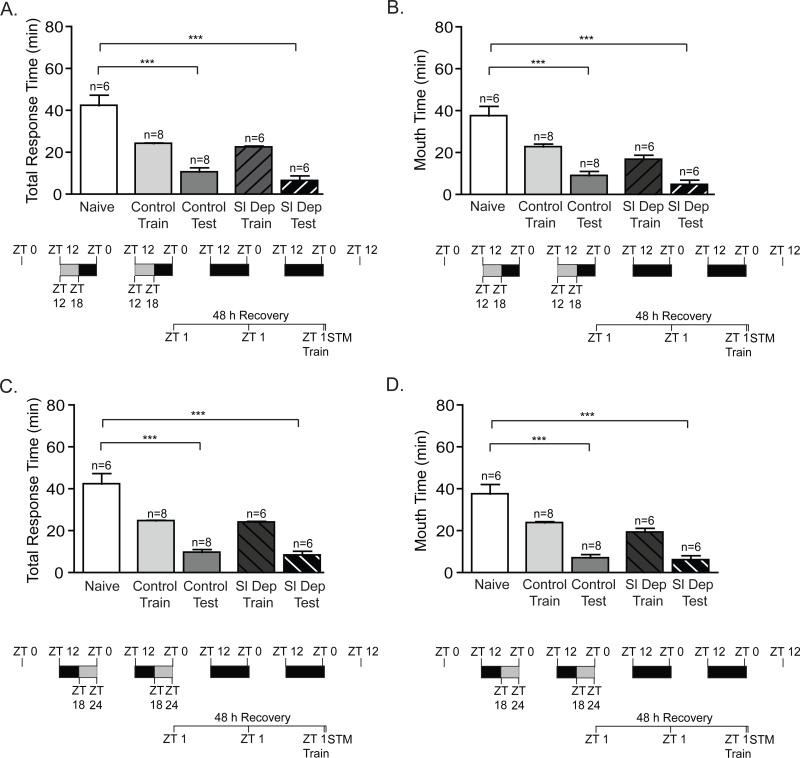
Figure 1. Two nights of repeated sleep deprivation impairs long-term memory.
To investigate the effect of repeated sleep restriction on long-term memory, animals were sleep deprived for the first 6 hours of the night (ZT 12-ZT 18) or during last 6 hours of the night (ZT 18-24) for two consecutive nights. Animals were trained at ZT 1 on the following day and tested for LTM 24 h after training. No significant differences were observed between the training times for non-sleep deprived animals (Control Train) and sleep-deprived animals (Sl Dep Train) irrespective of the timing of sleep deprivation. A) Animals sleep deprived (Sl Dep Test) during the first 6 hours of the night did not exhibit LTM with significantly longer total response time compared to trained non-sleep deprived animals (Control Test) (one-way ANOVA F(4,52) = 10.14, P < 0.0001). Asterisks represent Bonferroni's post-hoc analyses ***P < 0.001. (B) Sleep deprived trained animals retained the seaweed in the mouth during testing, a second parameter to assess memory, for significantly longer than non-sleep deprived trained animals with times similar to naïve animals (one-way ANOVA F(4,52) = 12.19, P < 0.0001). Asterisks denote significant differences with ***P < 0.001. C) Animals sleep deprived during the last 6 hours of the night had deficits in LTM with significantly longer total response time compared to trained non-sleep deprived animals (one-way ANOVA F(4,40) = 11.35, P < 0.0001). D) Animals sleep deprived in the last half of the night retained the seaweed in the mouth during testing for significantly longer than non-sleep deprived trained animals with times similar to naïve animals (one-way ANOVA F(4,40) = 9.069, P < 0.0001). Asterisks denote significant differences with ***P < 0.001.
Animals were trained during the early day (ZT 1) using the LFI paradigm and tested for LTM 24 h after training. Although previous research has shown that a single night of 9 h sleep deprivation does not affect the baseline responses of animals to the seaweed stimulus used in training (Krishnan et al., in press), potentially, the repeated sleep deprivation protocol could have affected the responses of the animals during training through either decreased sensory awareness of the presented stimuli or a fatigue induced decrease in motor responses. However, no significant differences were observed between the training times for non-sleep deprived animals and sleep-deprived animals (Figure 1). Sleep deprived animals, irrespective of the phase in which sleep deprivation occurred, showed no memory with response times similar to naïve animals and significantly greater than non-sleep deprived animals (Figure 1). Non-sleep deprived trained animals demonstrated robust LTM with significantly decreased total response time and the time the seaweed was retained in the mouth compared to naïve animals. In comparison, a single night of acute sleep deprivation for 6 h during the first half of the night (ZT 12-ZT 18) blocked the induction of LTM in half of the animals while sleep deprivation during the last half of the night (ZT 18-ZT 24) blocked LTM in all animals (Supplemental Figure 1). Overall, these experiments demonstrate the cumulative adverse effects of repeated sleep deprivation on long-term LFI memory.
3.2. Two nights of 6 hour sleep deprivation blocks short-term memory
The mechanisms underlying the formation of LTM and STM are different, with LTM requiring gene transcription and subsequent protein synthesis (Sweatt, 2010; Michel et al., 2012). Consequently, the effect of chronic sleep restriction on STM may differ from the adverse impact observed for LTM. In rats, chronic sleep deprivation for 8 h/day for 6 weeks impaired both short-term and long-term spatial memory (Alzoubi et al., 2012; Alzoubi et al., 2016). To investigate whether repeated sleep deprivation inhibited STM, animals were sleep deprived for 6 h during either the first half of the night (ZT 12-ZT 18; Figure 2A and 2B) or the last half of the night (ZT 18-ZT 24; Figure 2C and 2D) for 2 days, trained at ZT 1 using the LFI paradigm and tested for STM 30 minutes after training. As previously, no differences were observed in the training responses between non-sleep deprived and sleep deprived animals (Figure 2). Similar to the results in the LTM experiments, sleep deprived animals demonstrated no memory with both the total response time and the time the seaweed was retained in the mouth similar to naïve animals. No differences were observed based upon the timing of sleep deprivation. Non-sleep deprived trained animals exhibited robust STM (Figure 2). In comparison, a single night of acute sleep deprivation for 6 h during the first or last half of the night did not inhibit STM (Supplemental Figure 2). Thus, repeated sleep deprivation results in cumulative effects that adversely impact STM.
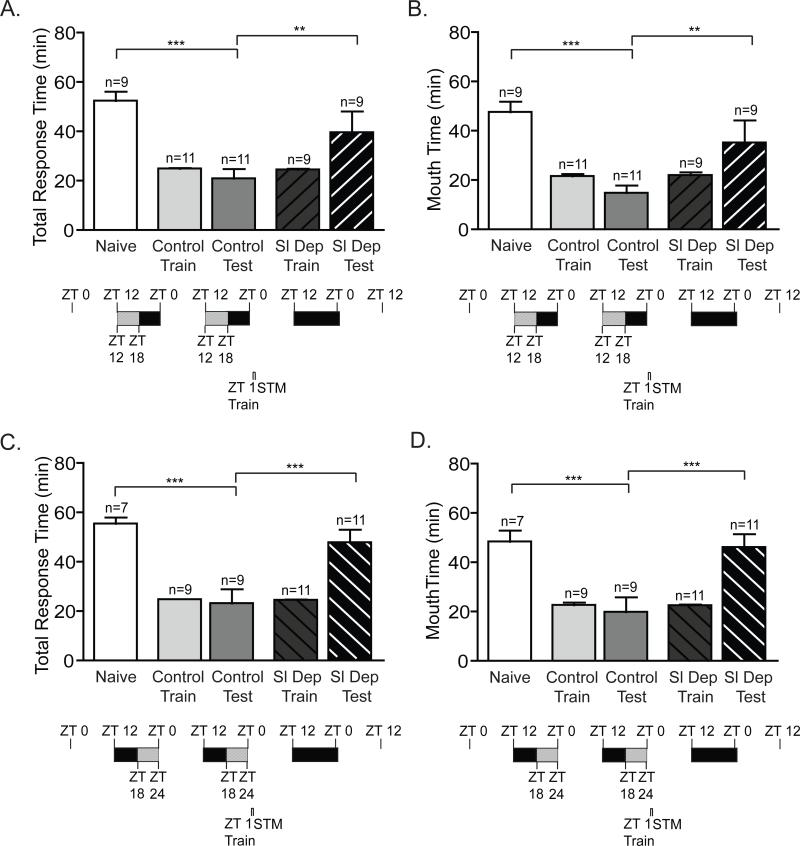
Figure 2. Two nights of sleep deprivation blocks short-term memory.
To assess the effect of two nights of sleep deprivation on STM, animals were sleep deprived for the first 6 hours of the night (ZT 12-ZT 18) or during last 6 hours of the night (ZT 18-24), trained at ZT 1 and tested for STM 30 min after training. No differences existed in training duration between groups (Control Train compared to Sl Dep Train). (A) Animals sleep deprived (Sl Dep Test) during the first 6 hours of the night did not exhibit STM with significantly longer total response time compared to trained non-sleep deprived animals (Control Test) (one-way ANOVA F(4,44) = 9.372, P < 0.0001). Asterisks represent Bonferroni's post-hoc analyses ***P < 0.001 and **P<0.01. (B) Sleep deprived trained animals retained the seaweed in the mouth with times similar to naïve animals and significantly greater times than non-sleep deprived trained animals (one-way ANOVA F(4,44) = 8.648, P < 0.0001). C) Animals sleep deprived during the last 6 hours of the night had impaired STM with significantly longer total response time compared to trained non-sleep deprived animals (one-way ANOVA F(4,42) = 14.98, P < 0.0001). Asterisks represent Bonferroni's post-hoc analyses ***P < 0.001. D) Sleep deprived trained animals retained the seaweed in the mouth significantly longer than non-sleep deprived trained animals (one-way ANOVA F(4,42) = 11.25, P < 0.0001). Asterisks denote significant differences with ***P < 0.001.
3.3. Chronic sleep restriction persistently affects short-term but not long-term memory
The time necessary for an individual to recover from multiple days of sleep restriction depends upon the extent of sleep deprivation (Dinges et al., 1997; Belenky et al., 2003) suggesting that chronic sleep restriction induces long-lasting molecular and cellular changes. To assess whether the inhibitory effects of two days of sleep deprivation on the induction of LTM were persistent in Aplysia, animals were sleep-deprived for 2 consecutive nights and allowed to recover for an uninterrupted day and night prior to LFI training. Animals were trained at ZT 1 the following day (either 31 hours after the end of sleep deprivation that occurred during the first half of the night or 25 hours after the end of sleep deprivation that occurred at the end of the night) and then tested for LTM 24 h after training. Sleep deprived trained animals demonstrated robust LTM regardless of whether sleep deprivation occurred during the early or late night (Figure 3). Moreover, when sleep deprivation occurred during the last 6 hours of the night, sleep-deprived trained animals actually demonstrated more robust memory than non-sleep deprived animals (Figure 3C and 3D). Thus, the impairment of 24 h LTM induced by two nights of sleep deprivation does not persist and could be alleviated by a 24 h period following sleep deprivation.
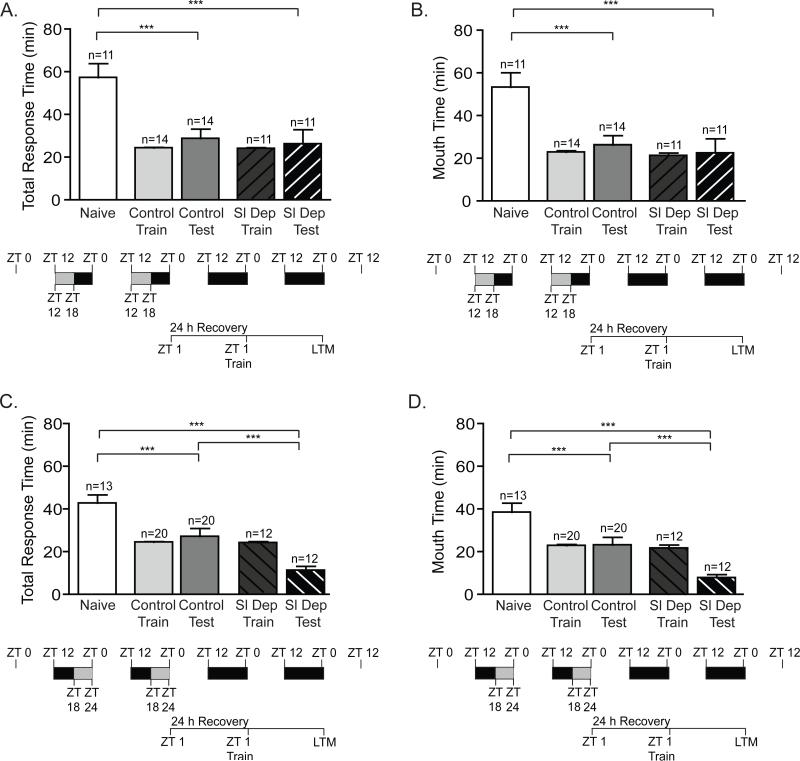
Figure 3. Chronic sleep deprivation had no persistent effects on long-term memory.
To assess whether sleep deprivation had persistent effects on LTM, animals were sleep deprived for the first 6 hours of the night (ZT 12-ZT 18) or during last 6 hours of the night (ZT 18-24) for two consecutive nights, allowed to recover for 24 h (full day and night), trained at ZT 1 and then tested for LTM. No significant differences were observed between the training times for non-sleep deprived animals (Control Train) and sleep-deprived animals (Sl Dep Train). A) Animals sleep deprived (Sl Dep Test) during the first 6 hours of the night and trained at ZT 1 (~31 h after SD) exhibited robust LTM as measured by total response time (one-way ANOVA F(4,56) = 9.733, P < 0.0001). Asterisks represent Bonferroni's post-hoc analyses ***P < 0.001 for testing between naïve and non-sleep deprived animals and between naives and sleep deprived animals. (B) Sleep deprived trained animals also demonstrated robust LTM when the time the seaweed was retained in the mouth was measured (one-way ANOVA F(4,56) = 8.437, P < 0.0001). Asterisks denote significant differences with ***P < 0.001. C and D) Animals sleep deprived during the last 6 hours of the night and trained at ZT 1 (~24 h after SD) also showed robust LTM in C) Total response time (one-way ANOVA F(4,72) = 13.85, P < 0.0001). Asterisks represent Bonferroni's post-hoc analyses ***P < 0.001. D) Time the seaweed was retained in the mouth (one-way ANOVA F(4,72) = 12.64, P < 0.0001). Asterisks denote significant differences with ***P < 0.001.
Given the mechanistic differences between STM and LTM, we also investigated whether STM was persistently affected by two nights of restricted sleep using a similar protocol. Surprisingly, we found that two nights of 6 h sleep deprivation blocked STM for at least 24 hours after the end of sleep deprivation. Sleep deprived animals exhibited total response times similar to naïve animals and significantly different than trained non-sleep deprived animals that demonstrated robust STM (Figure 4). The phase of the sleep deprivation did not affect the responses. As 24 h recovery time was insufficient to relieve the impairment in STM, additional experiments were performed with a minimum 48 hour period of recovery between the end of sleep deprivation and training. Animals were sleep-deprived for 6 h for 2 d during the first (ZT 12- ZT 18) or last half (ZT 18-ZT 24) of the night and allowed to recover for two full days and nights prior to LFI training. Animals were trained at ZT 1 and tested for STM. The extended recovery time was sufficient to ameliorate the effects of sleep deprivation as sleep deprived animals demonstrated robust STM 30 min after training (Figure 5) with observed response times similar to non-sleep deprived trained control animals. Thus, STM appears more susceptible to disruption by repeated nights of sleep deprivation requiring increased recovery time for effective training and memory formation.
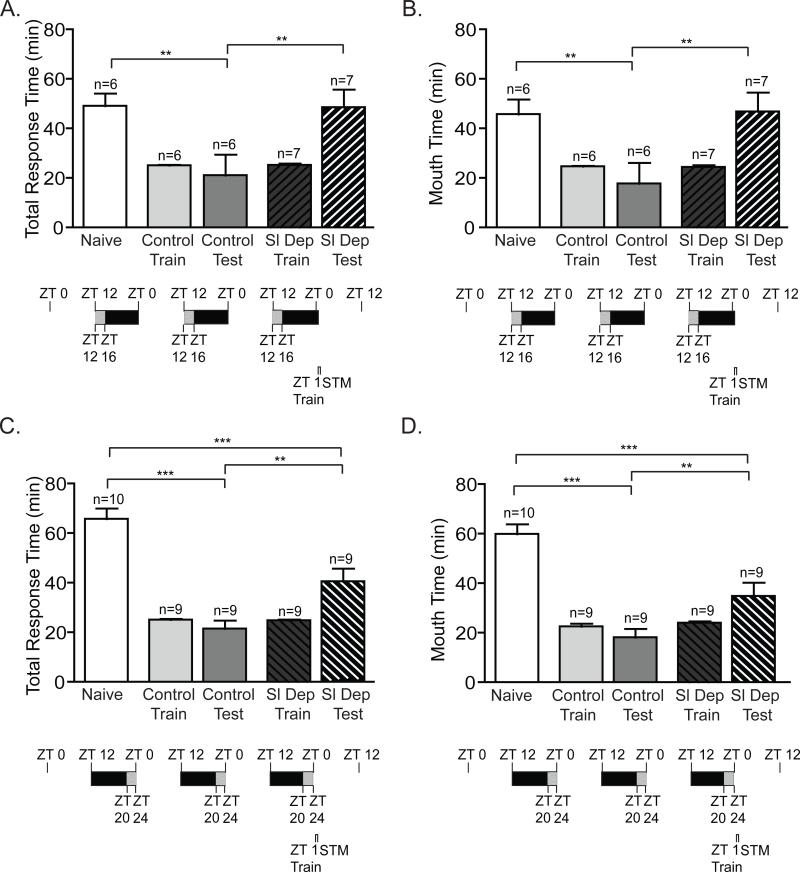
Figure 4. Two consecutive nights of chronic sleep deprivation persistently inhibited short-term memory.
A) Animals sleep deprived (Sl Dep Test) during the first 6 hours of the night for two nights and trained 31 h after the end of sleep deprivation at ZT 1 did not exhibit STM with significantly longer total response time compared to trained non-sleep deprived animals (Control Test) and similar to naïve animals (one-way ANOVA F(4,43) = 8.751, P < 0.0001). Asterisks represent Bonferroni's post-hoc analyses ***P < 0.001 and **P<0.01. (B) Sleep deprived trained animals retained the seaweed in the mouth with times significantly longer than non-sleep deprived control animals (one-way ANOVA F(4,43) = 7.347, P < 0.0001). Asterisks denote significant differences with ***P < 0.001 and *P<0.01. C and D) Animals sleep deprived during the last 6 hours of the night for two nights and trained 24 h after the end of sleep deprivation at ZT 1 displayed no short term memory as seen for C) Total response time (one-way ANOVA F(4,43) = 8.071, P < 0.0001). Asterisks represent Bonferroni's post-hoc analyses ***P < 0.001 and **P<0.01. D) Time the seaweed was retained in the mouth (one-way ANOVA F(4,43) = 6.230, P < 0.0001). Asterisks denote significant differences with ***P < 0.001 and **P<0.01.
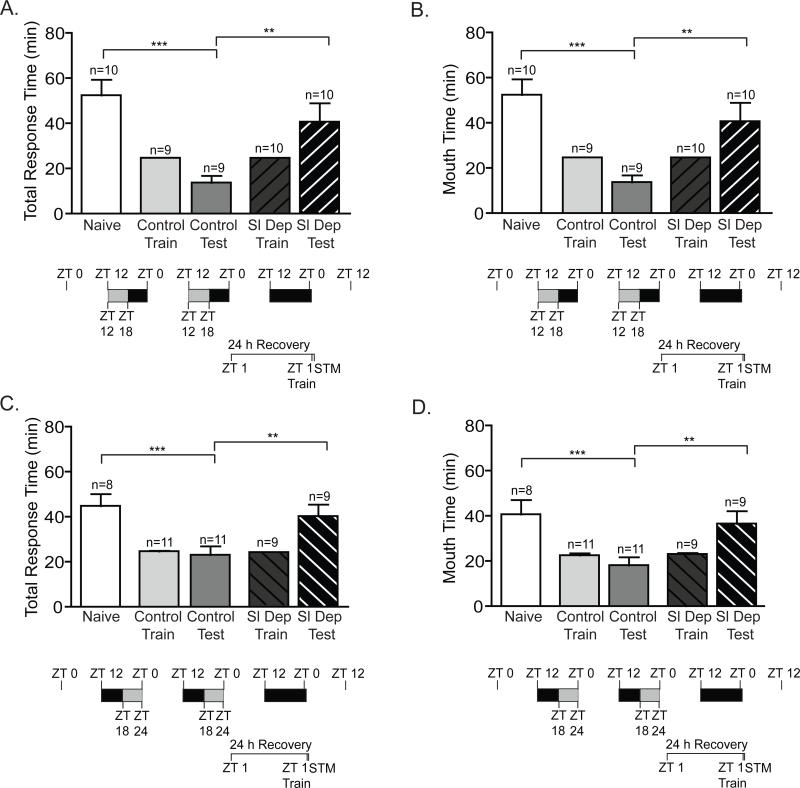
Figure 5. Impairment in STM induced by 2 consecutive nights of chronic sleep deprivation is alleviated after 48 hours.
A and B) Animals sleep deprived (Sl Dep Test) during the first 6 hours of the night for two nights and allowed to recover for 55 hours prior to training exhibited STM with significantly shorter total response time compared to naïves and similar to trained non-sleep deprived animals (Control Test): A) Total response time (one-way ANOVA F(4,29) = 32.20, P < 0.0001). Asterisks represent Bonferroni's post-hoc analyses ***P < 0.001. B) Time the seaweed was retained in the mouth (one-way ANOVA F(4,29) = 25.30, P < 0.0001). Asterisks denote significant differences with ***P < 0.001. C and D) Animals sleep deprived for two nights during the last 6 hours of the night and trained approximately 49 hours after sleep deprivation at ZT 1 demonstrated robust STM: A) Total response time (one-way ANOVA F(4,29) = 36.92, P < 0.0001). Asterisks represent Bonferroni's post-hoc analyses ***P < 0.001. D) Time the seaweed was retained in the mouth (one-way ANOVA F(4,29) = 32.32, P < 0.0001). Asterisks denote significant differences with ***P < 0.001.
3.4. Prolonged, but milder sleep restriction impacts STM to a greater extent than LTM
Given the unexpected difference in the persistent effects of 2 nights of 6 h sleep deprivation on STM and LTM, we hypothesized that a milder sleep restriction protocol extending over a longer time period may also differentially impact STM and LTM. Animals were sleep deprived for a shorter period of time (4 h) for 3 consecutive nights during the early night (ZT 12-ZT 16) or during late night (ZT 20-ZT 24). As with the 2 day sleep restriction paradigm, additional handling was required to prevent the animals from sleeping on nights 2 and 3 during both phases compared to night 1 (data not shown). Animals were then trained at ZT 1 and tested either 30 min later for STM or 24 h later for LTM. Three days of sleep restriction had no effect on the animals’ responses during training as no significant differences were observed in training times between the groups (Figure 6). We found that sleep deprivation (4 h) for 3 consecutive nights inhibited STM independent of when the sleep deprivation occurred. Animals sleep deprived during the first 4 h of the night displayed no memory with response times similar to naïve animals whereas non-sleep deprived animals exhibited robust STM (Figure 6A and 6B). Sleep deprivation during the last 4 hours of the night reduced STM with sleep-deprived animals exhibiting significantly greater response times than non-sleep deprived trained animals, (Figure 6C and 6D). In contrast, a 4 h period of chronic sleep restriction for 3 consecutive nights either during the first or the last part of the night had no detrimental effects on 24 h LTM. Sleep deprived animals showed robust memory with response times comparable to non-sleep deprived trained animals (Figure 7). These results suggest that STM is more sensitive to the ongoing effects of chronic sleep restriction than LTM.
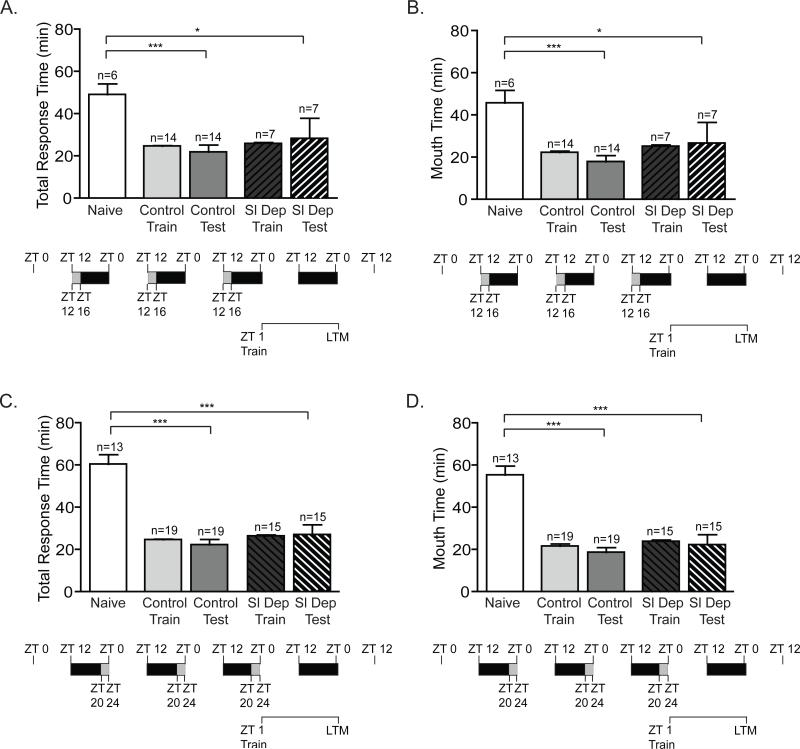
Figure 6. Milder, prolonged sleep restriction inhibits short-term memory.
To determine whether chronic sleep deprivation for 4 hours per night inhibited STM,animals were sleep deprived for either the first 4 hours of the night (ZT 12-ZT 16) or the last 4 hours of the night (ZT 20-24) for three consecutive nights, trained at ZT 1 and tested for STM. No significant differences were observed between the training times for non-sleep deprived animals (Control Train) and sleep-deprived animals (Sl Dep Train) regardless of the phase of sleep deprivation. A) Animals sleep deprived (Sl Dep Test) during the first 4 hours of the night failed to exhibit STM with significantly longer total response time compared to trained non-sleep deprived animals (Control Test; one-way ANOVA F(4,27) = 6.457, P < 0.0001). Asterisks represent Bonferroni's post-hoc analyses **P < 0.001. (B) Sleep deprived trained animals also retained the seaweed in the mouth with times longer than non-sleep deprived control animals and similar to naives (one-way ANOVA F(4,27) = 5.372, P < 0.001). Asterisks denote significant differences with **P < 0.001. C) Animals sleep deprived (SD Test) during the last 4 hours of the night and trained at ZT 1 showed deficits in short-term memory with significantly longer total response time compared to non-sleep deprived control animals (one-way ANOVA F(4,41) = 30.41, P < 0.0001). Asterisks represent Bonferroni's post-hoc analyses ***P < 0.001 and **P < 0.001. D) Sleep deprived trained animals retained the seaweed in the mouth with significantly longer times than non-sleep deprived animals (one-way ANOVA F(4,41) = 24.74, P < 0.0001). Asterisks denote significant differences with ***P < 0.001 and **P<0.01.
Figure 7. Milder, prolonged sleep deprivation does not impair long-term memory.
Animals were sleep deprived for the first 4 hours of the night (ZT 12-ZT 16) or during last 4 hours of the night (ZT 20-24) for three consecutive nights, trained at ZT 1 and tested for LTM 24 h after training. As previously, no significant differences were observed between the training times for non-sleep deprived animals (Control Train) and sleep-deprived animals (Sl Dep Train). A) Animals sleep deprived (Sl Dep Test) during the first 4 hours of the night and trained at ZT 1 exhibited robust LTM with significantly shorter total response time compared to naïves and similar to trained non-sleep deprived animals (Control Test) (one-way ANOVA F(4,43) = 5.383, P < 0.001). Asterisks represent Bonferroni's post-hoc analyses ***P < 0.001 and *P<0.05. (B) Sleep deprived trained animals retained the seaweed in the mouth with times similar to non-sleep deprived control animals and significantly different than naives (one-way ANOVA F(4,43) = 8.437, P < 0.001). Asterisks denote significant differences with ***P < 0.001 and *P<0.05 for testing between naives and sleep deprived animals. C) Animals sleep deprived during the last 4 hours of the night showed robust LTM with significantly shorter total response time compared to naïve animals. (one-way ANOVA F(4,76) = 25.57, P < 0.0001). Asterisks represent Bonferroni's post-hoc analyses ***P < 0.001. D) Sleep deprived trained animals retained the seaweed in the mouth with significantly shorter times than naïve animals (one-way ANOVA F(4,76) = 24.47, P < 0.0001). Asterisks denote significant differences with ***P < 0.001.
4. Discussion
Across the globe, sleep or rather the lack thereof, has arisen as a major health and public safety issue. Insufficient sleep contributes to an increased incidence of cardiovascular, metabolic and neurodegenerative diseases (Banks and Dinges, 2007; Mullington et al., 2009; Aho et al., 2016). Chronic restricted sleep also results in decrements in memory and performance (Alhola and Polo-Kantola, 2007; Goel et al., 2009). As the underlying mechanisms through which insufficient sleep lead to the impairment in cognitive function are not well understood, our objective was to identify a simple model system in which the interactions between repeated nights of sleep restriction and memory formation could be studied. The marine mollusk Aplysia californica, with a central nervous system composed of 20,000 neurons organized into discrete ganglia, provides an ideal model for studying the interactions between sleep and memory formation. Aplysia also has proven an excellent model system for differentiating cellular signaling pathways and synaptic mechanisms underlying memory formation (Hawkins et al., 2006; Mayford et al., 2012; Kandel et al., 2014). Due to the high degree of conservation in cellular signaling pathways, the identification of molecular mechanisms underlying memory formation in Aplysia has been broadly extrapolated across phylogeny (Hunter, 2008; Kandel, 2012; Kandel et al., 2014).
We found that two nights of 6 h sleep deprivation inhibited both short and long-term memory whereas a milder 4 h sleep deprivation paradigm for three consecutive nights only affected short-term memory. Interestingly, the block on memory formation was independent of whether the sleep deprivation occurred during the first or the last part of the night. As Aplysia remain mostly awake and intermittently active during the first two hours after lights off, the sleep deprivation protocols were milder on the first night for animals that received sleep deprivation during the early portion of the night compared to animals that had sleep deprivation during the last half of the night. This difference in sleep deprivation between the first and last half of the night was apparent in the single night sleep deprivation experiments as sleep deprivation during the last half of the night inhibited LTM in a significantly greater percentage of the animals compared to sleep deprivation during the first half of the night. However, the cumulative effects of sleep deprivation were noticeable during subsequent nights as more handling was required in all phases to maintain sleep deprivation. Sleep deprivation during the last part of the night for both the 4 and 6 h sleep deprivation protocols blocked the pre-dawn anticipatory activity normally observed presumably due to fatigue in the animals. Thus, the phylogenetically conserved homeostatic sleep drive overshadows the circadian regulation of locomotor activity.
Understanding the temporal window necessary for recovery of cognitive function following sleep deprivation is an essential factor prior to identification of the molecular aspects through which sleep deprivation adversely affects memory. Children and adults frequently sleep longer on the weekends to compensate for the adverse health effects and fatigue induced by shorter sleep durations during the week though this extended recovery sleep may induce circadian rhythm anomalies (Wittmann et al., 2006; Roenneberg, 2013). The amount of recovery sleep needed following sleep deprivation has traditionally been correlated with the degree of sleep loss (Lamond et al., 2007; Banks et al., 2010); however, recent research suggests that the recovery time needed to ameliorate the adverse effects of sleep deprivation may be underestimated (Banks et al., 2010). We found that 24 h was insufficient recovery time from 2 nights of 6 h sleep deprivation to allow the induction of short-term memory, even though this recovery period was adequate to permit the formation of LTM observed 24 h after training. Nevertheless, it remains possible that a period of 24 hours of recovery after sleep deprivation may not be sufficient for all forms of LTM as longer forms of LTM, e.g. 48 h or 72 h memory, were not examined.
Mechanistically, memory may be partitioned into multiple steps including the encoding of sensory information, acquisition of the behavior, memory formation and the recall of memory (Abel and Lattal, 2001; Sweatt, 2010). Although temporal or mechanistic overlap may exist between these steps, molecular processes often are specific to individual stages permitting independent analysis of the processes in memory. In STM, the formation of memory relies upon kinase activation and phosphorylation of downstream target molecules. For LTM, memory formation may be further separated into the early induction steps of memory involving receptor activation and kinase signaling pathways, and the molecular consolidation of memory necessitating protein synthesis and gene expression (Michel et al., 2011a; Kandel, 2012). How does chronic sleep restriction affect these processes? Behaviorally, many human studies of chronic sleep restriction have focused on sensory perception or the encoding of sensory stimuli employing repeated performance assays during the sleep restriction period (Dinges et al., 1997). Chronic sleep restriction has been found to target attention and stimulus valuation resulting in attention deficits and alterations in stimulus perception (Van Dongen et al., 2003; Durmer and Dinges, 2005). For example, 3 hours of restricted sleep per night for a week increased reaction times in the psychomotor vigilance test (Belenky et al., 2003). Similarly, we also observed changes in response to sensory stimuli following repeated sleep restriction with a significant increase in latency of the response to the seaweed in sleep deprived animals compared to non-sleep deprived animals for most of the sleep deprivation protocols we used with the exception for the 3 day paradigm when sleep deprivation occurred during the early portion of the night (data not shown).
However, changes in the response to the sensory stimulus do not appear to be the mechanism through which chronic sleep deprivation inhibits associative memory in Aplysia as the animals that failed to demonstrate memory did interact with the seaweed stimulus during training. Moreover, in both of our repeated sleep restriction paradigms, STM appeared more susceptible to inhibition than LTM in contrast to what we observed following a single night of 6 h sleep deprivation in which LTM was most greatly affected. As the same training paradigm induces short and long-term LFI memory, this suggests that neither acute nor chronic sleep restriction induced memory deficits via changes in sensory perception or the encoding of sensory stimuli as this should have affected short and long-term memory equally. Since one night of 6 h sleep deprivation affected LTM, but not STM, one can speculate that acute sleep deprivation targets molecular consolidation, thus affecting long-term but not short-term memory. Likewise, the immediate effects of two nights of six hour sleep restriction may also impact the molecular consolidation of long-term memory.
Conversely, the persistent effects of repeated six hour sleep restriction and the milder four hour sleep restriction paradigm inhibited short-term but not long-term LFI memory. What enables long-term memory to be more resilient against the effects of repeated sleep restriction? The induction of long-term LFI memory involves the activation of multiple kinase signaling pathways including protein kinase A, protein kinase C, protein kinase G and multiple waves of MAPK activation whereas for short-term LFI memory, only immediate MAPK signaling has been identified as necessary for memory (Michel et al., 2011a; Michel et al., 2011b). Although disruption of any of these pathways through pharmacological inhibition blocks LTM, potentially these pathways may work synergistically during LTM formation providing a compensatory mechanism to buffer the effects of sleep deprivation. It has been hypothesized based on neuroimaging studies in humans that repeated sleep deprivation may induce compensatory mechanisms including the recruitment of additional neuronal groups to counteract the adverse effects on cognitive performance (Drummond and Brown, 2001; Drummond et al., 2005). Recently, researchers found increased serum BDNF levels in individuals with 24 h total wakefulness raising the possibility that molecular mechanisms modulating neural circuit activity may also adapt to sleep deprivation to maintain cognitive performance (Giacobbo et al., 2016). Thus, it is possible in our studies that LTM was more resistant than STM to the effects of sleep deprivation as a result of the greater potential for adaptation during long-term memory formation.
The current study highlights the phylogenetically conserved relationship between sleep deprivation and adverse impacts on short and long-term memory. These behavioral studies establish Aplysia as a valid model for investigating the effects of repeated sleep restriction on memory paving the way for future molecular studies delineating the mechanisms through which sleep affects memory formation.
Supplementary Material
Highlights.
In Aplysia, 2 nights of 6 h sleep deprivation blocks short and long-term memory
The effects of sleep deprivation on induction of STM, but not LTM, persist for 24 h
Short, but not long-term memory is blocked by 3 nights of 4 h sleep deprivation
STM appears to be more sensitive to chronic sleep restriction than LTM
Aplysia represents a good model for studying the interactions of sleep and memory
Acknowledgements
We would like to thank the following undergraduate students for their assistance with sleep deprivation experiments: Grant Talkington, Mandi Abdelahad, Jamie Anne Mortel, Alexa Ramirez, Alyssa Allem and Valentina Saracino. This work was supported by grant R21NS088835 from the National Institute on Neurological Disorders and Stroke.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Interest: The authors have no conflicts of interest.
Appendix A. Supplementary Material
Supplemental Figures 1 and 2 accompany this manuscript.
References
- Abel T, Lattal KM. Molecular mechanisms of memory acquisition, consolidation and retrieval. Curr Opin Neurobiol. 2001;11:180–187. doi: 10.1016/s0959-4388(00)00194-x. [DOI] [PubMed] [Google Scholar]
- Aho V, Ollila HM, Kronholm E, Bondia-Pons I, Soininen P, Kangas AJ, Hilvo M, Seppälä I, Kettunen J, Oikonen M, et al. Prolonged sleep restriction induces changes in pathways involved in cholesterol metabolism and inflammatory responses. Sci Rep. 2016;6:24828. doi: 10.1038/srep24828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akerstedt T, Wright KP. Sleep Loss and Fatigue in Shift Work and Shift Work Disorder. Sleep Med Clin. 2009;4:257–271. doi: 10.1016/j.jsmc.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alhola P, Polo-Kantola P. Sleep deprivation: Impact on cognitive performance. Neuropsychiatr Dis Treat. 2007;3:553–567. [PMC free article] [PubMed] [Google Scholar]
- Alterman T, Luckhaupt SE, Dahlhamer JM, Ward BW, Calvert GM. Prevalence rates of work organization characteristics among workers in the U.S.: data from the 2010 National Health Interview Survey. Am J Ind Med. 2013;56:647–659. doi: 10.1002/ajim.22108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alzoubi KH, Khabour OF, Albawaana AS, Alhashimi FH, Athamneh RY. Tempol prevents chronic sleep-deprivation induced memory impairment. Brain Res Bull. 2016;120:144–150. doi: 10.1016/j.brainresbull.2015.11.017. [DOI] [PubMed] [Google Scholar]
- Alzoubi KH, Khabour OF, Rashid BA, Damaj IM, Salah HA. The neuroprotective effect of vitamin E on chronic sleep deprivation-induced memory impairment: the role of oxidative stress. Behav Brain Res. 2012;226:205–210. doi: 10.1016/j.bbr.2011.09.017. [DOI] [PubMed] [Google Scholar]
- Banks S, Dinges DF. Behavioral and physiological consequences of sleep restriction. J Clin Sleep Med. 2007;3:519–528. [PMC free article] [PubMed] [Google Scholar]
- Banks S, Van Dongen HP, Maislin G, Dinges DF. Neurobehavioral dynamics following chronic sleep restriction: dose-response effects of one night for recovery. Sleep. 2010;33:1013–1026. doi: 10.1093/sleep/33.8.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belenky G, Wesensten NJ, Thorne DR, Thomas ML, Sing HC, Redmond DP, Russo MB, Balkin TJ. Patterns of performance degradation and restoration during sleep restriction and subsequent recovery: a sleep dose-response study. J Sleep Res. 2003;12:1–12. doi: 10.1046/j.1365-2869.2003.00337.x. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention Effect of short sleep duration on daily activities--United States, 2005-2008. Morb Mortal Wkly Rep. 2011;60:239–242. [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention Short Sleep duration among workers—United Sates, 2010. In Morb Mortal Wkly Rep. 2012;61(16):281–285. [PubMed] [Google Scholar]
- Costa G. Sleep deprivation due to shift work. Handb Clin Neurol. 2015;131:437–446. doi: 10.1016/B978-0-444-62627-1.00023-8. [DOI] [PubMed] [Google Scholar]
- Dinges DF, Pack F, Williams K, Gillen KA, Powell JW, Ott GE, Aptowicz C, Pack AI. Cumulative sleepiness, mood disturbance, and psychomotor vigilance performance decrements during a week of sleep restricted to 4-5 hours per night. Sleep. 1997;20:267–277. [PubMed] [Google Scholar]
- Drummond SP, Anderson DE, Straus LD, Vogel EK, Perez VB. The effects of two types of sleep deprivation on visual working memory capacity and filtering efficiency. PLoS One. 2012;7:e35653. doi: 10.1371/journal.pone.0035653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond SP, Bischoff-Grethe A, Dinges DF, Ayalon L, Mednick SC, Meloy MJ. The neural basis of the psychomotor vigilance task. Sleep. 2005;28:1059–1068. [PubMed] [Google Scholar]
- Drummond SP, Brown GG. The effects of total sleep deprivation on cerebral responses to cognitive performance. Neuropsychopharmacology. 2001;25:S68–73. doi: 10.1016/S0893-133X(01)00325-6. [DOI] [PubMed] [Google Scholar]
- Durmer JS, Dinges DF. Neurocognitive consequences of sleep deprivation. Semin Neurol. 2005;25:117–129. doi: 10.1055/s-2005-867080. [DOI] [PubMed] [Google Scholar]
- Fernandez RI, Lyons LC, Levenson J, Khabour O, Eskin A. Circadian modulation of long-term sensitization in Aplysia. Proc Natl Acad Sci U S A. 2003;100:14415–14420. doi: 10.1073/pnas.2336172100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giacobbo BL, Corrêa MS, Vedovelli K, de Souza CE, Spitza LM, Gonçalves L, Paludo N, Molina RD, da Rosa ED, Argimon, et al. Could BDNF be involved in compensatory mechanisms to maintain cognitive performance despite acute sleep deprivation? An exploratory study. Int J Psychophysiol. 2016;99:96–102. doi: 10.1016/j.ijpsycho.2015.11.008. [DOI] [PubMed] [Google Scholar]
- Goel N, Rao H, Durmer JS, Dinges DF. Neurocognitive consequences of sleep deprivation. Semin Neurol. 2009;29:320–339. doi: 10.1055/s-0029-1237117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins RD, Kandel ER, Bailey CH. Molecular mechanisms of memory storage in Aplysia. Biol Bull. 2006;210:174–191. doi: 10.2307/4134556. [DOI] [PubMed] [Google Scholar]
- Hunter P. Ancient rules of memory. The molecules and mechanisms of memory evolved long before their 'modern' use in the brain. EMBO Rep. 2008;9:124–126. doi: 10.1038/sj.embor.2008.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang F, VanDyke RD, Zhang J, Li F, Gozal D, Shen X. Effect of chronic sleep restriction on sleepiness and working memory in adolescents and young adults. J Clin Exp Neuropsychol. 2011;33:892–900. doi: 10.1080/13803395.2011.570252. [DOI] [PubMed] [Google Scholar]
- Kandel ER. The molecular biology of memory: cAMP, PKA, CRE, CREB-1, CREB-2, and CPEB. Mol Brain. 2012;5:14. doi: 10.1186/1756-6606-5-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandel ER, Dudai Y, Mayford MR. The molecular and systems biology of memory. Cell. 2014;157:163–186. doi: 10.1016/j.cell.2014.03.001. [DOI] [PubMed] [Google Scholar]
- Krishnan HC, Gandour CE, Ramos JL, Wrinkle MC, Sanchez-Pacheco JJ, Lyons LC. Acute Sleep Deprivation Blocks Short and Long-Term Operant Memory in Aplysia. Sleep. doi: 10.5665/sleep.6320. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamond N, Jay SM, Dorrian J, Ferguson SA, Jones C, Dawson D. The dynamics of neurobehavioural recovery following sleep loss. J Sleep Res. 2007;16:33–41. doi: 10.1111/j.1365-2869.2007.00574.x. [DOI] [PubMed] [Google Scholar]
- Liu Y, Wheaton AG, Chapman DP, Cunningham TJ, Lu H, Croft JB. Prevalence of Healthy Sleep Duration among Adults--United States, 2014. MMWR Morb Mortal Wkly Rep. 2016;65:137–141. doi: 10.15585/mmwr.mm6506a1. [DOI] [PubMed] [Google Scholar]
- Lo JC, Bennion KA, Chee MW. Sleep restriction can attenuate prioritization benefits on declarative memory consolidation. J Sleep Res. 2016a doi: 10.1111/jsr.12424. 10.1111/jsr.12424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo JC, Ong JL, Leong RL, Gooley JJ, Chee MW. Cognitive Performance, Sleepiness, and Mood in Partially Sleep Deprived Adolescents: The Need for Sleep Study. Sleep. 2016b;39:687–698. doi: 10.5665/sleep.5552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luckhaupt SE, Tak S, Calvert GM. The prevalence of short sleep duration by industry and occupation in the National Health Interview Survey. Sleep. 2010;33:149–159. doi: 10.1093/sleep/33.2.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons LC, Rawashdeh O, Katzoff A, Susswein AJ, Eskin A. Circadian modulation of complex learning in diurnal and nocturnal Aplysia. Proc Natl Acad Sci U S A. 2005;102:12589–12594. doi: 10.1073/pnas.0503847102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayford M, Siegelbaum SA, Kandel ER. Synapses and memory storage. Cold Spring Harb Perspect Biol. 2012;4:a005751. doi: 10.1101/cshperspect.a005751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel M, Gardner JS, Green CL, Organ CL, Lyons LC. Protein phosphatase-dependent circadian regulation of intermediate-term associative memory. J Neurosci. 2013;33:4605–4613. doi: 10.1523/JNEUROSCI.4534-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel M, Green CL, Eskin A, Lyons LC. PKG-mediated MAPK signaling is necessary for long-term operant memory in Aplysia. Learn Mem. 2011a;18:108–117. doi: 10.1101/lm.2063611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel M, Green CL, Gardner JS, Organ CL, Lyons LC. Massed training-induced intermediate-term operant memory in Aplysia requires protein synthesis and multiple persistent kinase cascades. J Neurosci. 2012;32:4581–4591. doi: 10.1523/JNEUROSCI.6264-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel M, Green CL, Lyons LC. PKA and PKC are required for long-term but not short-term in vivo operant memory in Aplysia. Learn Mem. 2011b;18:19–23. doi: 10.1101/lm.2026311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mollicone DJ, Van Dongen HP, Rogers NL, Banks S, Dinges DF. Time of day effects on neurobehavioral performance during chronic sleep restriction. Aviat Space Environ Med. 2010;81:735–744. doi: 10.3357/asem.2756.2010. [DOI] [PubMed] [Google Scholar]
- Mullington JM, Haack M, Toth M, Serrador JM, Meier-Ewert HK. Cardiovascular, inflammatory, and metabolic consequences of sleep deprivation. Prog Cardiovasc Dis. 2009;51:294–302. doi: 10.1016/j.pcad.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roenneberg T. Chronobiology: the human sleep project. Nature. 2013;498:427–428. doi: 10.1038/498427a. [DOI] [PubMed] [Google Scholar]
- Rothman SM, Herdener N, Frankola KA, Mughal MR, Mattson MP. Chronic mild sleep restriction accentuates contextual memory impairments, and accumulations of cortical Aβ and pTau in a mouse model of Alzheimer's disease. Brain Res. 2013;1529:200–208. doi: 10.1016/j.brainres.2013.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stranges S, Tigbe W, Gómez-Olivé FX, Thorogood M, Kandala NB. Sleep problems: an emerging global epidemic? Findings from the INDEPTH WHO-SAGE study among more than 40,000 older adults from 8 countries across Africa and Asia. Sleep. 2012;35:1173–1181. doi: 10.5665/sleep.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Susswein AJ, Schwarz M, Feldman E. Learned changes of feeding behavior in Aplysia in response to edible and inedible foods. J Neurosci. 1986;6:1513–1527. doi: 10.1523/JNEUROSCI.06-05-01513.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweatt JD. Mechanisms of Memory. 2nd ed Academic Press; Elsevier; 2010. [Google Scholar]
- Van Dongen HP, Maislin G, Mullington JM, Dinges DF. The cumulative cost of additional wakefulness: dose-response effects on neurobehavioral functions and sleep physiology from chronic sleep restriction and total sleep deprivation. Sleep. 2003;26:117–126. doi: 10.1093/sleep/26.2.117. [DOI] [PubMed] [Google Scholar]
- Vorster AP, Krishnan HC, Cirelli C, Lyons LC. Characterization of sleep in Aplysia californica. Sleep. 2014;37:1453–1463. doi: 10.5665/sleep.3992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittmann M, Dinich J, Merrow M, Roenneberg T. Social jetlag: misalignment of biological and social time. Chronobiol Int. 2006;23:497–509. doi: 10.1080/07420520500545979. [DOI] [PubMed] [Google Scholar]
- Zielinski MR, Davis JM, Fadel JR, Youngstedt SD. Influence of chronic moderate sleep restriction and exercise training on anxiety, spatial memory, and associated neurobiological measures in mice. Behav Brain Res. 2013;250:74–80. doi: 10.1016/j.bbr.2013.04.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.