Abstract
HEN1-dependent methylation of the 3′-terminal nucleotide is a crucial step in plant microRNA (miRNA) biogenesis. Here we report that several viral RNA silencing suppressors (P1/HC-Pro, p21 and p19) inhibit miRNA methylation. These suppressors have distinct effects on different miRNAs. We also show that miRNA* is methylated in vivo in a suppressor-sensitive manner, suggesting that the viral proteins interfere with miRNA/miRNA* duplexes. p19 and p21 bind both methylated and unmethylated miRNA/miRNA* duplexes in vivo. These findings suggest miRNA/miRNA* as the in vivo substrates for the HEN1 miRNA methyltransferase and raise intriguing possibilities regarding the cellular location of miRNA methylation.
Keywords: Viral RNA silencing suppressor, Methylation, microRNA/microRNA*, HEN1
1. Introduction
MicroRNAs (miRNAs) are 20- to 24-nucleotide (nt) RNAs that function as sequence-specific regulators of gene expression through translational repression and/or transcript cleavage [1]. miRNA/miRNA* duplex intermediates are formed by DICER or DICER-like enzymes, which generate products with 2-nt 3′ overhangs containing 5′ monophosphate and 3′-OH groups. The miRNA strand is incorporated into the RNA-induced silencing complex, while the passenger miRNA* strand is ejected and degraded [1]. We and others recently demonstrated that the 2′-OH of the 3′-terminal nucleotide of miRNAs and siRNAs is methylated in Arabidopsis by a methyltransferase, HUA ENHANCER1 (HEN1) [2–5]. The reduced accumulation and heterogeneity in size of miRNAs, as well as loss of miRNA function, in hen1 mutants [6–9] reflect the importance of miRNA methylation in plants. The size increase of small RNAs in the hen1-1 mutant is due to the addition of one to five U residues to the 3′ ends of the small RNAs by a novel uridylation activity targeting the 3′ ends of unmethylated miRNAs and siRNAs [3]. Therefore, one role of small RNA methylation is likely protecting 3′ ends of the small RNAs from uridylation activity. HEN1 uses miRNA/miRNA* duplexes as substrates in vitro [2,4], but it is not known whether it also acts on miRNA/miRNA* duplexes in vivo. It is also unknown where in the cell miRNA methylation occurs.
Most plant viruses encode RNA silencing suppressors, which condition susceptibility by interfering with the host antiviral silencing response. Many viral RNA silencing suppressors are also pathogenicity factors that cause developmental defects in host plants [10]. The miRNA pathway is inhibited by several viral silencing suppressors [11–13], such as the Beet yellows virus 21 kDa protein (p21) [14], the Tomato bushy stunt virus 19 kDa protein (p19) [15,16] and the Turnip mosaic virus silencing suppressor, P1/HC-Pro [17–19]. Each of these suppressors stabilizes miRNA/miRNA* duplexes [12,13]. Although P1/HC-Pro was initially thought to stabilize duplexes by an indirect mechanism [12], recent studies using in vitro and in vivo assays revealed that all three suppressors interact directly with small RNA duplexes [20]. Since P1/HC-Pro, p21 and p19 act on small RNA duplexes, they may affect miRNA methylation if miRNA/miRNA* duplexes are the in vivo substrates of HEN1. Recently it was shown that the expression of P1/HC-Pro weakly affects the modification of miRNAs in tobacco [5].
Here we report that viral RNA silencing suppressors p21, p19 and P1/HC-Pro interfere with the methylation of miRNAs.
2. Materials and methods
2.1. Transgenic plants
Arabidopsis thaliana Col-0 transgenic plants expressing influenza HA epitope-tagged P1/HC-Pro,p21, p19 or the empty expression vector were constructed as described [12]. Seeds were grown under selection for phosphoinothricin resistance and pools of primary transformants were analyzed.
2.2. Detection of small RNAs by RNA filter hybridization
RNA isolation, gel electrophoresis, blotting, hybridization and washes for miRNAs and 5S RNAs were performed as described [6]. 5′ end-labeled (32P) antisense DNA or LNA oligonucleotides were used as probes. Radioactive signals were detected and quantified with a phosphorimager.
2.3. Analysis of the methylation status of small RNAs
Sodium periodate treatments were done as described [2,21,22]. RNA was dissolved in borax/boric acid buffer (0.06 M, pH 8.6) and sodium periodate (200 mM in water) was added to a final concentration of 25 mM. The RNA was then incubated in darkness at room temperature. After 1 h of incubation, 1/10 volume of glycerol was added to the RNA and the incubation was continued for an additional 30 min. The RNA was then precipitated in the presence of ethanol. For β elimination, the sodium periodate-treated RNA was dissolved in NaOH/borax/boric acid buffer (0.055 M, pH 9.5), incubated at 45 °C for 90 min, and precipitated with ethanol.
2.4. Co-immunoprecipitation
The immunoprecipitation of HA-tagged viral RNA silencing suppressors and RNA isolation from precipitates were performed as described [12]. Briefly, tissues from transgenic Arabidopsis were ground in liquid nitrogen and homogenized in 5 ml/g lysis buffer (50 mM Tris–HCl at pH 7.4, 100 mM KCl, 2.5 mM MgCl2, 0.1% NP-40, and 2× complete protease inhibitor cocktail; Roche), and centrifuged for 15 min at 9500 × g. Clarified lysates were pre-cleared with protein A-agarose, reacted with anti-HA (Roche) for 1 h at 4 °C, and then incubated with protein A-agarose beads for 3 h at 4 °C. Precipitates were washed three times in lysis buffer and divided for protein and RNA analysis. Nucleic acid was recovered by treatment with 3 volumes of proteinase K solution (100 mM Tris–HCl, pH 7.4, 10 mM EDTA, 150 mM NaCl, 2% SDS, and 0.2 μg/μl proteinase K) for 15 min at 65 °C followed by extraction with saturated phenol and phenol:chloroform and ethanol precipitation.
3. Results
3.1. Viral silencing suppressors inhibit miRNA methylation
To determine if p19, p21 and P1/HCPro affect the methylation of the 3′-terminal nucleotide of miRNAs, total RNA from Arabidopsis transgenic lines that constitutively expressed these viral silencing suppressors [12] was treated with sodium periodate followed by β elimination. Specific miRNAs were analyzed by RNA filter hybridization. The reactions eliminate a 3′ nucleotide containing both 2′ and 3′ OH groups on the ribose, resulting in an RNA product that is one nucleotide shorter than the substrate RNA and that contains a 3′ phosphate group [21]. Therefore, miRNAs that contain a 3′ terminal ribose with both 2′ and 3′ OH groups will migrate faster during electrophoresis by between one and two nucleotides after the treatment. Methylated miRNAs whose 3′ terminal ribose contains a 2′-O-methyl group do not participate in the reactions and remain unchanged in mobility. This method was previously used to study miRNA 3′ end ribose methylation [2,22]. Although this method does not distinguish a 2′-O-methyl modification from other potential modifications on the 3′ terminal ribose, previous mass spectrometry studies demonstrated that methylation is the only modification of an Arabidopsis miRNA [2]. We used this method to evaluate 3′ terminal methylation of miRNAs in the RNA silencing suppressor-expressing plants. The methylation status of miR159, miR167, miR171 and miR172 was analyzed in pools of primary transformants containing each of the three viral silencing suppressors or the vector DNA alone [12]. As shown in Fig. 1A, a portion of the miRNAs from the suppressor-expressing plants migrated more quickly after β elimination reactions, except for miR171 from p21 transgenic lines. No mobility change was detected in the vector-transformed plants, indicating that the miRNA pools in control plants were fully or near fully methylated. The proportion of unmethylated miRNAs ranged from 17.5% (miR171) to 42.7% (miR159) in p19-expressing plants, 19.5% (miR159) to 68% (miR172) in P1/HC-Pro-expressing plants and from undetectable (miR171) to 88.5% (miR172) in p21-expressing plants (Fig. 1B). Therefore, the viral silencing suppressors partially affected miRNA methylation, but to different levels depending on the miRNA. The previously observed truncated forms of miRNAs in p19 transgenic plants [12,13,23] were detected here, and all truncated molecules were shifted after β elimination (Fig. 1A). This indicates that the truncated miRNAs are not methylated.
Fig. 1.
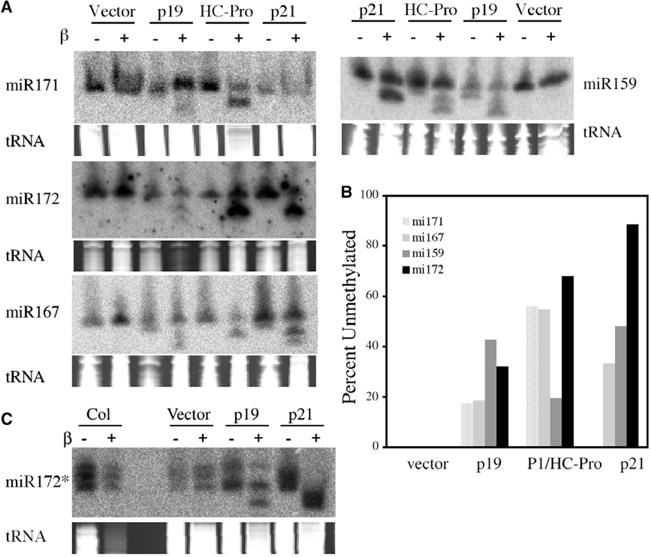
The viral silencing suppressors inhibit miRNA methylation. (A) The methylation status of miRNAs in various transgenic lines. Total RNAs from vector-transformed or viral silencing suppressor-transformed plants were either subjected (+) or not (−) to the chemical modification reactions and probed for various miRNAs by filter hybridization. The region of the stained gels corresponding to where tRNAs migrate is shown below the hybridization images to indicate the amount of total RNAs used. (B) Percentage of unmethylated miRNAs in various transgenic lines. Radioactive signals from (A) were quantified with a phosphorimager. The percentage was calculated as: {unmethylated miRNA/(unmethylated miRNA + methylated miRNA)} * 100%. The truncated miRNAs in p19 and HC-Pro lines were not included. (C) The methylation of miR172b* in various transgenic lines. Although the identities of the three species are unknown, they are all methylated in the vector-transformed plants.
3.2. miRNA* is methylated in vivo
Although steady state levels of most miRNA* species are generally not detected using standard blotting conditions, miR172b* is an exception [24]. To determine if miRNA* species are methylated in vivo, the methylation status of miR172b* in Col-0 control plants and plants expressing p19 or p21 was tested. miR172b* was detected only in the methylated form in the control plants. Unmethylated miR172* was detected in plants expressing p19 and p21 (Fig. 1C).
3.3. Co-immunoprecipitation (co-IP) of methylated and unmethylated miRNAs with viral silencing suppressors
p21, p19 and HC-Pro bind miRNA/miRNA* duplexes in vivo. The interaction between duplexes and HC-Pro may be dependent on cellular factors, and detection of HC-Pro-duplex complexes in vivo is sensitive to experimental conditions [12,13,20]. To determine if these viral silencing suppressors have a preference for unmethylated or methylated miRNAs, the methylation status of miRNAs associated with co-immunoprecipitated (co-IP) suppressor complexes was analyzed. Modified suppressors were immunoprecipitated using an anti-hemagglutinin (HA) monoclonal antibody, which recognizes an influenza HA epitope tag fused to the C terminus of each suppressor [12].
miR167, miR171 and miR172 co-immunoprecipitated differentially with the three suppressors. Each miRNA co-immunoprecipitated with p21, whereas only miR167 and miR171 were detected in co-IP fractions with p19 (Fig. 2). Only miR172 co-immunoprecipitated with HA-tagged HC-Pro (Fig. 2). However, in all cases of miRNAs that co-immunoprecipitated with suppressor, the methylation status was similar to that detected in the input samples (Fig. 2), indicating that each suppressor binds both methylated and unmethylated miRNAs.
Fig. 2.
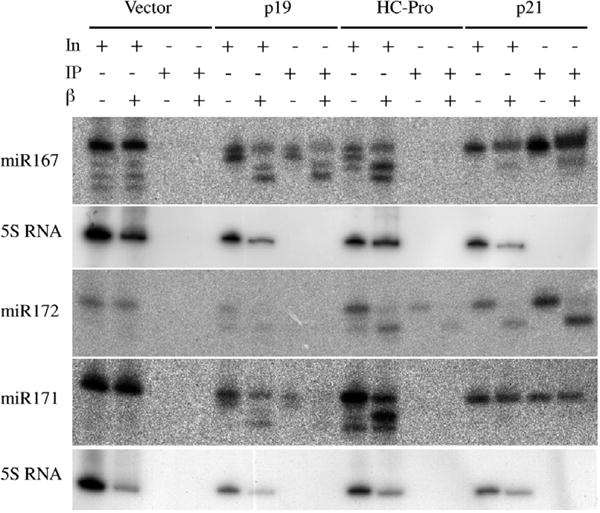
Co-immunoprecipitation (co-IP) of methylated and unmethylated miRNAs with the viral silencing suppressors. RNAs from the IP input (IN) sample or from the immunoprecipitates were either subject (+) or not (−) to the chemical modification reactions and probed for various miRNAs. The miR172 and miR171 panels were from the same blot and share the 5S rRNA control (bottom panel).
4. Discussion
We conclude that miRNA/miRNA* is most likely the in vivo substrate for HEN1-mediated methylation, which is consistent with the in vitro substrate specificity of the enzyme [2,4]. This conclusion is supported by the following observations: (1) miR172b* is methylated in vivo; and (2) viral silencing suppressors that sequester miRNA/miRNA* duplexes interfere with methylation of both strands. Furthermore, the fact that methylated miRNAs are indentified in complexes with silencing suppressors that bind miRNA/miRNA* duplexes prior to unwinding suggests that the products of the HEN1-mediated methylation reaction exist in the form of RNA duplexes. Although this does not preclude duplex formation after the HEN1-mediated methylation reaction, the most likely scenario is that the substrates of HEN1 are small RNA duplexes.
How do viral silencing suppressors affect miRNA methylation? First, they may compete with HEN1 for substrate miRNA/miRNA* duplexes. Sequestration by the suppressors could exclude HEN1 from interacting with duplexes or prevent HEN1 access to the 2′ OH of the 3′-terminal nucleotide. Second, the viral silencing suppressors may bind directly to HEN1 and inhibit its activity. However, we were unable to detect HEN1 as a co-IP component with viral silencing suppressor complexes using the anti-HA serum (data are not shown), arguing against this hypothesis. It is still possible that these suppressors interact with other factors required for HEN1 function. Third, viral suppressors may affect the subcellular localization of HEN1. It was previously reported that p19 relocates the ALY protein from the nucleus to the cytoplasm [25], indicating the potential of suppressors to affect subcellular protein trafficking. Whether or not these suppressors affect HEN1 localization remains to be determined. It should be noted that our studies were done with transgenic Arabidopsis expressing the viral RNA silencing suppressors with the strong 35 S promoter. It remains to be determined whether or not these proteins inhibit miRNA methylation during viral infections, although it is clear that miRNA functions are inhibited in TuMV-infected plants [11].
We showed that protecting the 3′ termini of small RNAs from an unknown uridylation activity is a function of small RNA methylation [3]. Unlike in hen1 mutants, we did not detect any size increase of miRNAs lacking methylation in plants expressing viral RNA silencing suppressors (Figs. 1 and 2). This result is not contradictory to the proposed protection function of small RNA methylation. Rather, unwinding of miRNA/miRNA* may be crucial for the uridylation activity targeting unmethylated miRNAs [3]. It was recently shown that infection of Arabidopsis with Oilseed rape mosaic tobamovirus (ORMV) leads to reduced methylation of endogenous miRNAs and siRNAs, suggesting that ORMV also inhibits HEN1 [26]. Intriguingly, the unmethylated small RNAs in ORMV infected plants become larger and heterogeneous in size as in hen1 mutants [26], indicating that the ORMV suppressor does not inhibit the uridylation activity.
Given the fact that many viral silencing suppressors do not affect miRNA/miRNA* duplex formation [12,13] and that they interfere with the methylation of miRNA in Arabidopsis, they could be excellent tools to study when and where the methylation process occurs. For instance, p19 and p21 probably function in the cytoplasm [14,21,25] after miRNA/miRNA* duplexes are generated by DCL1 in the nucleus and subsequently exported to the cytoplasm [12,27,28]. This would mean that at least a portion of miRNA/miRNA* duplexes is methylated in the cytoplasm.
Acknowledgments
This work was supported by NSF (MCB-0612958 to X.C. and MCB-0209836 to J.C.).
Footnotes
Edited by Shou-Wei Ding
References
- 1.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 2.Yu B, Yang Z, Li J, Minakhina S, Yang M, Padgett RW, Steward R, Chen X. Methylation as a crucial step in plant microRNA biogenesis. Science. 2005;307:932–935. doi: 10.1126/science.1107130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li J, Yang Z, Yu B, Liu J, Chen X. Methylation protects miRNAs and siRNAs from a 3′-end uridylation activity in Arabidopsis. Curr Biol. 2005;15:1501–1507. doi: 10.1016/j.cub.2005.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang Z, Ebright YW, Yu B, Chen X. HEN1 recognizes 21–24 nt small RNA duplexes and deposits a methyl group onto the 2′OH of the 3′ terminal nucleotide. Nucleic Acid Res. 2006;34:667–675. doi: 10.1093/nar/gkj474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ebhardt HA, Thi EP, Wang MB, Unrau PH. Extensive 3′ modification of plant small RNAs is modulated by helper component-proteinase expression. Proc Natl Acad Sci USA. 2005;102:13398–13403. doi: 10.1073/pnas.0506597102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Park W, Li J, Song R, Messing J, Chen X. CARPEL FACTORY, a Dicer homolog, and HEN1, a novel protein, act in microRNA metabolism in Arabidopsis thaliana. Curr Biol. 2002;12:1484–1495. doi: 10.1016/s0960-9822(02)01017-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boutet S, Vazquez F, Liu J, Beclin C, Fagard M, Gratias A, Morel JB, Crete P, Chen X, Vaucheret H. Arabidopsis HEN1: a genetic link between endogenous miRNA controlling development and siRNA controlling transgene silencing and virus resistance. Curr Biol. 2003;13:843–848. doi: 10.1016/s0960-9822(03)00293-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Han MH, Goud S, Song L, Fedoroff N. The Arabidopsis double-stranded RNA-binding protein HYL1 plays a role in microRNA-mediated gene regulation. Proc Natl Acad Sci USA. 2004;101:1093–1098. doi: 10.1073/pnas.0307969100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xie Z, Johansen LK, Gustafson AM, Kasschau KD, Lellis AD, Zilberman D, Jacobsen SE, Carrington JC. Genetic and functional diversification of small RNA pathways in plants. PLoS Biol. 2004;2:E104. doi: 10.1371/journal.pbio.0020104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Voinnet O. Induction and suppression of RNA silencing: insights from viral infections. Nat Rev Genet. 2005;6:206–220. doi: 10.1038/nrg1555. [DOI] [PubMed] [Google Scholar]
- 11.Kasschau KD, Xie Z, Allen E, Llave C, Chapman EJ, Krizan KA, Carrington JC. P1/HC-Pro, a viral suppressor of RNA silencing, interferes with Arabidopsis development and miRNA function. Dev Cell. 2003;4:205–217. doi: 10.1016/s1534-5807(03)00025-x. [DOI] [PubMed] [Google Scholar]
- 12.Chapman EJ, Prokhnevsky AI, Gopinath K, Dolja VV, Carrington JC. Viral RNA silencing suppressors inhibit the microRNA pathway at an intermediate step. Genes Dev. 2004;18:1179–1186. doi: 10.1101/gad.1201204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dunoyer P, Lecellier CH, Parizotto EA, Himber C, Voinnet O. Probing the microRNA and small interfering RNA pathways with virus-encoded suppressors of RNA silencing. Plant Cell. 2004;16:1235–1250. doi: 10.1105/tpc.020719. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 14.Reed JC, Kasschau KD, Prokhnevsky AI, Gopinath K, Pogue GP, Carrington JC, Dolja VV. Suppressor of RNA silencing encoded by Beet yellows virus. Virology. 2003;306:203–209. doi: 10.1016/s0042-6822(02)00051-x. [DOI] [PubMed] [Google Scholar]
- 15.Voinnet O, Pinto VM, Baulcombe DC. Suppression of gene silencing: a general strategy used by diverse DNA and RNA viruses of plants. Proc Natl Acad Sci USA. 1999;96:14147–14152. doi: 10.1073/pnas.96.24.14147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Silhavy D, Molnár A, Lucioli A, Szittya G, Hornyik C, Tavazza M, Burgyán J. A viral protein suppresses RNA silencing and binds silencing-generated, 21- to 25-nucleotide double-stranded RNAs. EMBO J. 2002;21:3070–3080. doi: 10.1093/emboj/cdf312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Anandalakshmi R, Pruss GJ, Ge X, Marathe R, Smith TH, Vance VB. A viral suppressor of gene silencing in plants. Proc Natl Acad Sci USA. 1998;95:13079–13084. doi: 10.1073/pnas.95.22.13079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brigneti G, Voinnet O, Wan-Xiang L, Ding SW, Baulcombe DC. Viral pathogenicity determinants are suppressors of transgene silencing. EMBO J. 1998;17:6739–6746. doi: 10.1093/emboj/17.22.6739. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 19.Kasschau KD, Carrington JC. A counter-defensive strategy of plant viruses: suppression of posttranscriptional gene silencing. Cell. 1998;95:461–470. doi: 10.1016/s0092-8674(00)81614-1. [DOI] [PubMed] [Google Scholar]
- 20.Lakatos L, Csorba T, Pantaleo V, Chapman EJ, Carrington JC, Liu YP, Dolja VV, Calvino LF, Lopez-Moya JJ, Burgyan J. Small RNA binding is a common strategy to suppress RNA silencing by several viral suppressors. EMBO J. doi: 10.1038/sj.emboj.7601164. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alefelder S, Patel BK, Eckstein F. Incorporation of terminal phosphorothioates into oligonucleotides. Nucleic Acids Res. 1998;26:4983–4988. doi: 10.1093/nar/26.21.4983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hutvagner G, McLachlan J, Pasquinelli AE, Balint E, Tuschl T, Zamore PD. A cellular function for the RNA-interference enzyme Dicer in the maturation of the let-7 small temporal RNA. Science. 2001;293:834–838. doi: 10.1126/science.1062961. [DOI] [PubMed] [Google Scholar]
- 23.Papp I, Mette MF, Aufsatz W, Daxinger L, Schauer SE, Ray A, van der Winden J, Matzke J, Matzke AJ. Evidence for nuclear processing of plant micro RNA and short interfering RNA precursors. Plant Physiol. 2003;132:1382–1390. doi: 10.1104/pp.103.021980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang XJ, Reyes JL, Chua NH, Gaasterland T. Arabidopsis thaliana microRNAs and their mRNA targets. Genome Biol. 2004;5:R65. doi: 10.1186/gb-2004-5-9-r65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Uhrig JF, Canto T, Marshall D, MacFarlane SA. Relocalization of nuclear ALY proteins to the cytoplasm by the tomato bushy stunt virus P19 pathogenicity protein. Plant Physiol. 2004;135:2411–2423. doi: 10.1104/pp.104.046086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Akbergenov R, Si-Ammour A, Blevins T, Amin I, Kutter C, Vanderschuren H, Zhang P, Gruissem W, Meins F, Hohn JT, Pooggin MM. Molecular characterization of geminivirus-derived small RNAs in different plant species. Nucleic Acids Res. 2006;34:436–444. doi: 10.1093/nar/gkj447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Park MY, Wu G, Gonzalez-Sulser A, Vaucheret H, Poethig RS. Nuclear processing and export of microRNAs in Arabidopsis. Proc Natl Acad Sci USA. 2005;102:3691–3696. doi: 10.1073/pnas.0405570102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zamore PD. Plant RNAi: how a viral silencing suppressor inactivates siRNA. Curr Biol. 2004;14:R198–R200. doi: 10.1016/j.cub.2004.02.021. [DOI] [PubMed] [Google Scholar]


