Abstract
Purpose
To investigate the time-period characteristics associated with morphologic changes in central serous chorioretinopathy (CSC) using fundus autofluorescence (FAF).
Patients and methods
Retrospective, cross-sectional observational case series. Patients were classified into three groups: acute and chronic according to the onset of subjective symptoms of 6 weeks and sequelae patients who have history and symptoms but no serous retinal detachment (SRD). We compared FAF images to obtain characteristic findings according to the chronicity.
Results
A total of 52 eyes were included in this study. Acute CSC eyes were characterized by decreased FAF intensity at the leakage point in 13/22 eyes (56.5%) and staining patterns with various levels of fluorescence signal (hyperautofluorescent (10 eyes, 43.5%), hypoautofluorescent (1 eye, 4.3%), and minimal changes (12 eyes, 52.2%)) in the area of SRD. In chronic CSC eyes, hyperautofluorescent (14 eyes, 63.6%) or minimal changes (8 eyes, 36.4%) were observed in the area of SRD. Discrete dots with increased FAF intensity were observed in chronic CSC eyes (P<0.001). Eyes with sequelae of CSC had mixed FAF patterns over areas of retinal pigment epithelium (RPE) atrophy in seven eyes (100%, P<0.001)) and descending tracts which showed various FAF intensities according to the RPE and photoreceptor status (P<0.001).
Conclusion
FAF imaging patterns in CSC eyes differ according to the course of the disease, reflecting RPE and outer retinal changes. Detailed investigation using FAF could help to estimate the duration of CSC and determine the proper treatment modality.
Introduction
Idiopathic central serous chorioretinopathy (CSC) typically affects young and middle-aged adults, and is characterized by a serous detachment of the neurosensory retina at the posterior pole caused by choroidal vascular hyperpermeability that can be visualized on indocyanine green angiography (IA) and by focal leakage at the level of the retinal pigment epithelium (RPE) seen on fluorescein angiography (FA).1, 2, 3, 4 Recently, fundus autofluorescence (FAF) imaging has been used to study various retinal disorders. FAF is related to lipofuscin changes within the RPE and outer retina and subretinal space alterations.5, 6, 7, 8
A number of studies have reported on detailed FAF changes in eyes with CSC.7, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19 These changes include increased or decreased FAF intensity at the site of the leakage, and retinal detachment in acute CSC, and irregular and increased FAF intensity in chronic-recurrent CSC.10, 11, 17, 20 Confluent, granular hypoautofluorescence and descending tracts were reported as a characteristic pattern of CSC.7, 12
When evaluating CSC, an understanding of the chronicity of the disease is important in determining the treatment plan. Acute CSC usually has a self-limited natural course, whereas chronic CSC with sustained subretinal detachment may cause permanent visual disturbance if not treated. Therefore, determination of the chronicity of the disease is important both for treatment choice and for predicting prognosis.
To date, the chronicity of CSC has usually been determined by the subjective recall of the patient because until now there has been no definite objective method to estimate it. In this study, we classified CSC according to the onset of subjective symptoms and investigated the relationship between our classification and FAF changes.
Subjects and methods
Patients
From October 2009 to May 2011, we retrospectively reviewed the medical records of patients with idiopathic CSC who were examined at Hanyang University Hospital. All patients underwent comprehensive ophthalmic examinations, including spectral domain-optical coherence tomography (SD-OCT), FA, and IA. SD-OCT was performed using a 3D OCT-1000 or -2000 (Topcon, Tokyo, Japan) cube volume scan. The FA, IA, and FAF were obtained using an F-10 confocal scanning laser ophthalmoscope (cSLO; Nidek, Gmagori, Japan).
Idiopathic CSC was diagnosed based on the presence of a serous detachment of the neurosensory retina involving the macula, as demonstrated by SD-OCT, leakage at the level of RPE on FA and hyperpermeability on IA. Patients with steroid-induced CSC, organ transplant–associated CSC or a history of previous treatment such as laser photocoagulation or photodynamic therapy (PDT) were also excluded. We excluded severe media opacity such as cataract, which can degrade image quality causing unreadable FAF.
Classification of the disease according to its chronicity
We classified CSC patients into three categories: acute, chronic, and sequelae.21 CSC patients with onset of subjective symptoms such as visual loss, metamorphopsia, chromatopsia, or micropsia within the past 6 weeks were defined as acute and those whose symptoms began in the past 6 weeks or more were classified as chronic. We enrolled patients with a first episode of CSC as acute CSC only if they satisfied the following conditions: there was no recurrent episode on history taking or in the patient's medical record and patients did not have any background fundus changes that would suggest recurrent or chronic CSC such as atrophic retinal and RPE changes, descending tract or atypical dye leakage on FA at initial exam. Sequelae of CSC were defined based on symptoms exhibited by the group of the patients with apparent previous history of the disease in their medical records and with remnant symptoms but for whom SD-OCT revealed no serous retinal detachment (SRD). Recurrent CSC was defined if patients with previous apparent resolution of CSC in history taking or medical records had a reappearance of the CSC-related symptoms and SRD revealed by SD-OCT. The patients with recurrent CSC were excluded in this study.
Analysis of FAF images
FAF was excited by blue light (490 nm) used originally for FA. A barrier filter with a cutoff at 513 nm was used to block excitation. The confocal detection unit uses a pinhole aperture to suppress light from above or below the confocal plane. The size of the scanning field was wide-angle 60°. The digital images were saved for processing, and an average image was composited from the 20 original images to reduce noise and to obtain more detail. All images were obtained by the same well-trained technician, who applied the same protocol to all patients. To avoid contaminating the fluorescence with residual dye from previous FA and IA examinations, FAF examination was performed before FA and IA.
FAF images were evaluated retrospectively and compared with FA and SD-OCT findings by two retina specialists in a masked fashion. To determine the area of SRD on FAF, the FAF image was superimposed onto the fundus image provided by Topcon SD-OCT, which was used to register the location of the lesion on the B-scan image.
For the standardization and objectification of image analysis, we used a commercially available image analysis program (Adobe Photoshop 7.0, Adobe Systems Inc., San Jose, CA, USA) and measured the average value of histogram of SRD lesions seen on FAF images.22, 23 The degree of FAF intensity change was determined by comparing with background intensity (SRD-free extramacular area confirmed in SD-OCT). Then the differences of background intensity and FAF were classified into 0–20 unit for minimal, more than 20 unit compared with background intensity for hyperautofluorescent (hyper-FAF: higher than background intensity) or hypoautofluorescent (hypo-FAF: lower than background intensity).
We investigated the specific FAF patterns according to the each disease chronicity. We analyzed the FAF characteristics and FAF intensities of the foveal center as well as the SRD area. In addition, other FAF findings of specific lesions including leaking point, discrete spot, and the descending tract were checked carefully.
Statistical analyses were performed using SPSS software for Windows version 17.0 (SPSS Inc, Chicago, IL, USA). Pearson's χ2 test or Fisher's exact test was used to compare FAF patterns between CSC groups.
This study followed the ethical standards enumerated in the Declaration of Helsinki and was approved by the Institutional Review Board of the Hanyang University Hospital.
Results
A total of 52 eyes of 50 patients were included in this study; these included 23 eyes with acute CSC, 22 eyes with chronic CSC and 7 eyes with sequelae of CSC. The mean age of the patients in the study was 49.52±8.04 years (range, 32–68 years). Males represented 40 of the patients (42 eyes) and females represented 10 patients (10 eyes). Detailed clinical profiles of the eyes studied are presented in Supplementary Digital Content (Table) and Table 1.
Table 1. Demographics and clinical characteristics of acute, chronic, and sequelae of CSC groups.
| Acute (n=23) | Chronic (n=22) | Sequelae (n=7) | P-value | |
|---|---|---|---|---|
| Age | 47.22±6.28 | 51.27±8.63 | 51.57±10.41 | 0.202 |
| Symptom duration | 12.83±12.09 | 365.00±541.99 | 1251.43±28.18 | <0.001 |
| SRD height | 308.73±164.02 | 208.14±137.12 | – | <0.001 |
Abbreviation: SRD, serous retinal detachment.
Acute CSC
Of 23 eyes with acute CSC with angiographic evidence of a focal leakage accompanying SRD, FAF intensity was decreased at the leakage point (13 eyes, 56.5%). In acute CSC, FAF showed staining patterns with various levels of fluorescence signal in the area of SRD (hyper-FAF, 10 eyes, 43.5% hypo-FAF, 1 eye, 4.3% and minimal changes, 12 eyes, 52.2% Table 2 and Figure 1a–c).
Table 2. Comparison of fundus autofluorescent patterns among acute, chronic and sequelae of CSC groups.
| Acute (n=23)(%) | Chronic (n=22)(%) | Sequelae (n=7)(%) | P-valuea | |
|---|---|---|---|---|
| SRD | ||||
| Hyper-FAF | 10 (43.5) | 14 (63.6) | – | 0.296 |
| Hypo-FAF | 1 (4.3) | 0 (0) | – | – |
| Minimal changes | 12 (52.2) | 8 (36.4) | – | – |
| Leaking on FA—hypo-FAF | 13 (56.5) | 7 (31.8) | – | 0.085 |
| Discrete dots with hyper-FAF | 0 (0) | 10 (45.5) | 0 (0) | <0.001 |
| Irregular FAF with RPE atrophy | 0 (0) | 0 (0) | 7 (100) | <0.001 |
| Descending tract | ||||
| Hyper-FAF | 0 (0) | 3 (13.6) | 2 (28.6) | <0.001 |
| Hypo-FAF | 0 (0) | 0 (0) | 2 (28.6) | – |
| Hyper+hypo | 0 (0) | 0 (0) | 2 (28.6) | – |
Abbreviations: FA, fluorescein angiography; FAF, fundus autoflurescent; RPE, retinal pigment epithelium; SRD, serous retinal detachment.
Fisher's exact test.
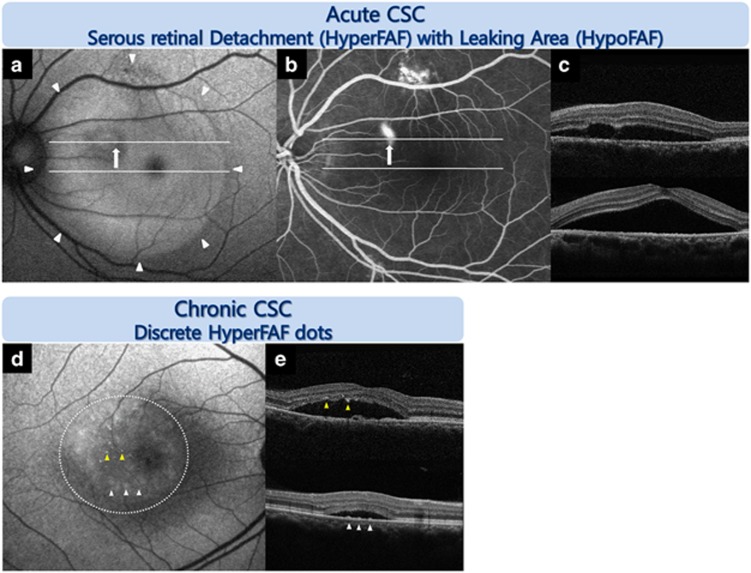
Figure 1.
Representative FAF images from an eye with idiopathic CSC according to the chronicity of the disease. Acute CSC: FAF image (a) showing hyper-FAF in the area of SRD (white arrowhead) obtained by SD-OCT image (c) and hypo-FAF at the leaking area (white arrow) obtained by fluorescein angiography (FA) (b). Chronic CSC: discrete small dots with increased FAF intensity were observed (d). One pattern showed a greater number of tiny, discrete dots with intense hyper-FAF that corresponded to hyper-reflective dots attached to the posterior surface of the detached retina on SD-OCT (yellow arrowhead) (e). Another pattern showed more frequent, larger, diffuse, and vague hyper-FAF spots that corresponded to the accumulation on the bottom of the SRD, which appeared to be the RPE protrusion on SD-OCT (white arrow; e).
Chronic CSC
Of the 22 eyes with chronic CSC, 14 showed hyper-FAF (63.6%), and 8 showed minimal FAF changes (36.4%) in the area of the SRD. In the chronic CSC patients, 10 eyes showed discrete dots with increased FAF intensity (45.5%) that corresponded to subretinal precipitates in SD-OCT images; this was not observed in acute CSC (Table 2, Figures 1 and 3). Among the chronic CSC patients, three eyes showed an intense hyper-FAF area just inferiorly adjacent to the SRD (Supplementary Figure 1A).
Sequelae of CSC
In six eyes with sequelae of CSC, descending tracts were found; these tracts showed various FAF patterns corresponding to their RPE status (Figure 2a–c). Two eyes had descending tracts with increased FAF, corresponding to hypofluorescent areas without window defects on FA and the area of an intact RPE with damaged outer photoreceptor layer on SD-OCT. In contrast, the other two eyes had descending tracts with decreased FAF, which corresponded to hyperfluorescent areas with window defects on FA and the areas of both the RPE and outer photoreceptor layer atrophy on SD-OCT (Table 2).
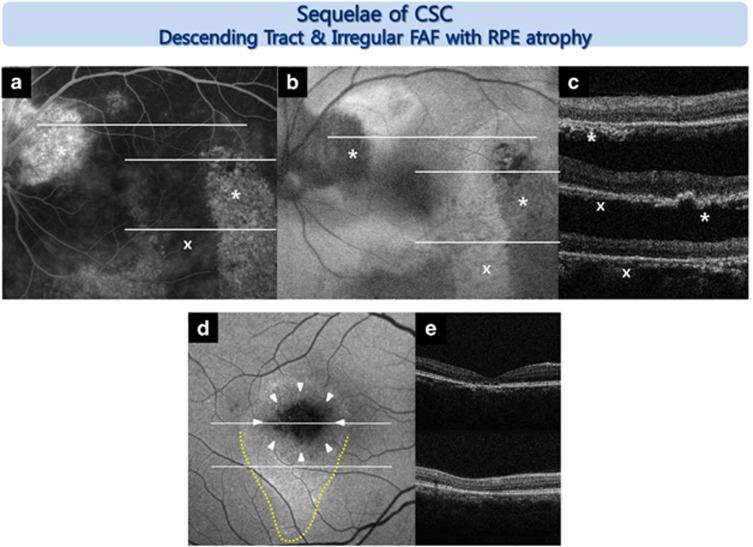
Figure 2.
Representative FAF images from an eye with sequelae of CSC. Descending tract: various FAF changes were observed in the descending tract according to the chronicity of the disease (a–c, d yellow dotted line). The old descending tract (*) showed a window defect in the RPE (area of hyperfluorescence in fluorescein angiography (FA)) and hypo-FAF, corresponding to both RPE and photoreceptor damage as determined by SD-OCT. A recent descending tract (x) showed no window defect in the RPE on the FA image and hyper-FAF, which corresponded to an intact RPE on the SD-OCT image. Irregular FAF with RPE atrophy: focal absolute hypo-FAF and heterogeneous FAF patterns mixing hyper- and hypointensity (d, white arrowhead) over areas of RPE atrophic thinning (e) were observed.
All seven eyes with sequelae of CSC revealed focal absolute hypo-FAF and heterogeneous FAF patterns mixing hyper- and hypointensity. The area of focal absolute hypo-FAF corresponded to the area with RPE atrophic thinning on SD-OCT. This pattern was only observed in eyes with sequelae of CSC (Figure 2d and e).
Discussion
No appropriate classification system for the time stage of CSC has been developed. In general, CSC is arbitrarily classified as acute or chronic according to its duration (3 or 6 months).21, 24, 25, 26 To investigate the time-periodic characteristics of CSC in more detail, we divided CSC patients according to the chronicity of their symptoms.
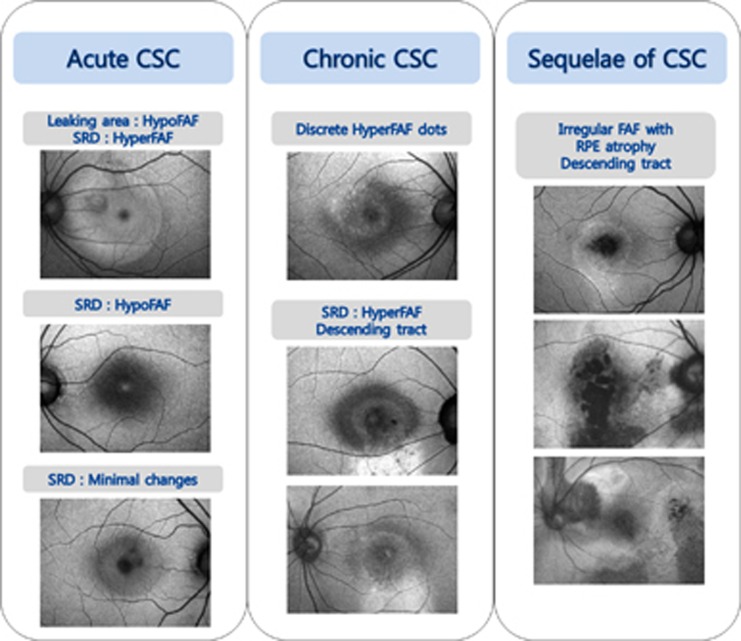
Representative FAF patterns according to the chronicity of disease are summarized in Figure 3 at a glance view and presented as a schematic diagram in Supplementary Figure 2.
Figure 3.
Representative fundus autofluorescence (FAF) images from an eye with idiopathic central serous chorioretinopathy (CSC) according to the chronicity at-a-glance-view. CSC, central serous chorioretinopathy; FAF, fundus autofluorescence; RPE, retinal pigment epithelium; SRD, serous retinal detachment.
Interpretation of FAF findings in CSC
Autofluorescence of serous retinal detachment
Our finding that the majority of CSC cases in all stages were associated with hyper-FAF or minimal changes in the area of SRD, and that only a minority of acute CSC cases revealed hypo-FAF contradicts findings presented in a previous report.11 This hypo-FAF may have been caused by the presence of dense macular pigment rather than by the blocking effect of SRD. Individual or racial differences in the presence of macular pigments may also cause confusion in the interpretation of FAF.
Autofluorescence in the leakage point on fluorescein angiography
In our study, focally decreased FAF intensity observed at the leakage point on FA corresponded to the presence of a focal RPE defect above PED on SD-OCT. Mechanical defects or the complete absence of RPE at the acute leakage point may indicate decreased levels or the absence of lipofuscin, resulting in decreased FAF in this area.5, 11, 25, 27
Some patients with acute CSC showed a broad decrease in FAF intensity that corresponded to the area of the PED on SD-OCT. Serous fluid below the PED may dilute the autofluorescence; alternatively or in addition, changed metabolic activity of RPE at the PED may influence the FAF, resulting in a pattern of decreased FAF on PED that extends beyond the focal leakage point. However, the exact mechanism of hypofluorescent in the area of PED remains unclear, and further studies are needed.
Autofluorescence of discrete dots
Among the 52 eyes with CSC, 10 eyes had small discrete dots of increased FAF intensity. These small discrete dots of increased FAF corresponded to pinpoint hyper-reflective dots in SD-OCT images. Discrete spots on the fundus appeared as two different FAF patterns (Figure 1d and e). One pattern showed more discrete, tiny dots with intense hyper-FAF; these corresponded to hyper-reflective dots attached to the posterior surface of the detached retina on SD-OCT. These dots were thought to be macrophages that had accumulated lipofuscin by phagocytosis of the outer segments of photoreceptors.28 Another pattern showed a greater number of larger, diffuse, and vague hyper-FAF spots that corresponded to the accumulation on the bottom of the SRD area, which seemed to be the RPE protrusion on SD-OCT. We first discriminate these two patterns of precipitate with FAF modality.
Autofluorescence of descending tract
The descending tract showed various FAF changes correlated with the chronicity of the disease. In chronic CSC, intense hyper-FAF areas just inferiorly adjacent to the SRD were present. The descending tracts were short and broad compared with the narrower, longer old descending tracts observed in sequelae of CSC (Supplementary Figure 1).
In sequelae of CSC, descending tracts with both increased and decreased FAF were observed. The corresponding FA and OCT findings suggest that the descending tracts with increased FAF intensity may have been formed relatively recently. These FAF changes are thought to be caused by the accumulation of damaged photoreceptor cell debris in intact RPE cells. As CSC progresses, damage of the RPE follows damage of the photoreceptor cells, suggesting that the descending tracts with decreased FAF could represent relatively old lesions. Because the chronicity of descending tracts is easily distinguishable using FAF imaging, FAF imaging could potentially be used to localize recent lesions and inform treatment strategies.
Characteristic FAF findings according to the chronicity of CSC
In eyes with acute CSC, the majority of cases presented relatively homogenous hyper-FAF or minimal changes in the area of SRD. Some eyes with acute CSC had focally decreased FAF intensity at the leakage point on FA and a focal RPE defect on SD-OCT.
In eyes with chronic CSC, hyper-FAF or minimal changes in the area of the SRD were frequently observed. Most patients with chronic CSC showed more heterogeneous patterns of hyper-FAF in the area of the SRD than those with acute CSC. This pattern might appear due to uneven distribution of fluorophores within the subretinal fluid by multifocal shedding of the outer photoreceptor layer and phagocytosis by macrophages. Small discrete dots with increased FAF intensity were observed in chronic CSC eyes (P<0.001), indicating that when these findings were detected very early stage CSC could be excluded. These precipitates characterized in chronic CSC.
Patients with sequelae of CSC revealed focal absolute hypo-FAF and heterogeneous FAF patterns with mixed hyper- and hypointensity over areas of RPE atrophic thinning. Resolution of the SRD, absorption of the accumulated subretinal materials and a damaged and thinned RPE may lead to the mixed FAF patterns that characterize this stage of the disease. Characteristic descending tracts with various FAF patterns similar to those described above were found at this stage.
Clinical applications and limitations of our study
The strength of our study, compared with the previous studies, is that we analyzed the FAF patterns in great detail ‘according to the chronicity'. We especially focused on the association between the chronicity of the CSC and special patterns of FAF and we found some chronicity-specific FAF findings.
The results of our study can be applied to various clinical situations. First, our findings may be useful for determining the chronicity of CSC at the initial visit in some patients for whom the time of onset of symptoms is unclear. Our previous study suggested that detailed interpretation of SD-OCT findings may be useful in determining the chronicity of CSC in most cases.21 Thus, the simultaneous use of FAF and SD-OCT may be helpful in understanding the natural course of CSC and determining the chronicity of CSC without depending on subjective recall of the patients, leading to a more accurate assessment of the status and prognosis of the disease.
A second clinical application of our study is demonstrated by the fact that we applied the results obtained in the study when selecting the proper treatment modality to use in the clinic. Spontaneous resolution of CSC could be expected without treatment in patients who had unclear onset of subjective symptoms but FAF patterns suggesting acute CSC. In contrast, PDT seemed appropriate in patients who had unclear onset of subjective symptoms but FAF patterns suggesting chronic CSC.
The current study is limited by its retrospective cross-sectional design and by the small sample size of each group. The stage of CSC was determined only by the onset of subjective symptoms; therefore, there may be controversy over the exact duration of symptoms. To better study the natural history of CSC, it would be necessary to perform a longitudinal study from acute to chronic stages of the illness with the same patient. Furthermore, because FAF is not an absolute quantitative imaging modality and image findings may vary with circumstances such as macular pigments and image enhancement, it is difficult to generalize the FAF findings related to CSC disease.
This study is the first report that compares FAF findings from patients whose CSC has been classified into different time stages. A detailed investigation of the FAF patterns of such patients could help to estimate the duration of CSC and determine the proper treatment modality for the disease.
Footnotes
Supplementary Information accompanies this paper on Eye website (http://www.nature.com/eye)
Author contributions
BRL had full access to the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Dr Byung Ro Lee, MD is a consultant for Nidek, Gamagori, Japan. The remaining authors declare no conflict of interest.
Supplementary Material
References
- Aggio FB, Roisman L, Melo GB, Lavinsky D, Cardillo JA, Farah ME. Clinical factors related to visual outcome in central serous chorioretinopathy. Retina 2010; 30(7): 1128–1134. [DOI] [PubMed] [Google Scholar]
- Chen SN, Hwang JF, Tseng LF, Lin CJ. Subthreshold diode micropulse photocoagulation for the treatment of chronic central serous chorioretinopathy with juxtafoveal leakage. Ophthalmology 2008; 115(12): 2229–2234. [DOI] [PubMed] [Google Scholar]
- Spaide RF, Campeas L, Haas A, Yannuzzi LA, Fisher YL, Guyer DR et al. Central serous chorioretinopathy in younger and older adults. Ophthalmology 1996; 103(12): 2070–2079. [DOI] [PubMed] [Google Scholar]
- Wang M, Munch IC, Hasler PW, Prünte C, Larsen M. Central serous chorioretinopathy. Acta Ophthalmol 2008; 86(2): 126–145. [DOI] [PubMed] [Google Scholar]
- Schmitz-Valckenberg S, Holz FG, Bird AC, Spaide RF. Fundus autofluorescence imaging: review and perspectives. Retina 2008; 28(3): 385–409. [DOI] [PubMed] [Google Scholar]
- Spaide R. Autofluorescence from the outer retina and subretinal space: hypothesis and review. Retina 2008; 28(1): 5–35. [DOI] [PubMed] [Google Scholar]
- Spaide RF, Klancnik JM Jr. Fundus autofluorescence and central serous chorioretinopathy. Ophthalmology 2005; 112(5): 825–833. [DOI] [PubMed] [Google Scholar]
- Sparrow JR, Yoon KD, Wu Y, Yamamoto K. Interpretations of fundus autofluorescence from studies of the bisretinoids of the retina. Invest Ophthalmol Vis Sci 2010; 51(9): 4351–4357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayata A, Tatlipinar S, Kar T, Unal M, Ersanli D, Bilge AH. Near-infrared and short-wavelength autofluorescence imaging in central serous chorioretinopathy. Br J Ophthalmol 2009; 93(1): 79–82. [DOI] [PubMed] [Google Scholar]
- Eandi CM, Ober M, Iranmanesh R, Peiretti E, Yannuzzi LA. Acute central serous chorioretinopathy and fundus autofluorescence. Retina 2005; 25(8): 989–993. [DOI] [PubMed] [Google Scholar]
- Framme C, Walter A, Gabler B, Roider J, Sachs HG, Gabel VP. Fundus autofluorescence in acute and chronic-recurrent central serous chorioretinopathy. Acta Ophthalmol Scand 2005; 83(2): 161–167. [DOI] [PubMed] [Google Scholar]
- Imamura Y, Fujiwara T, Spaide RF. Fundus autofluorescence and visual acuity in central serous chorioretinopathy. Ophthalmology 2011; 118(4): 700–705. [DOI] [PubMed] [Google Scholar]
- Matsumoto H, Kishi S, Sato T, Mukai R. Fundus autofluorescence of elongated photoreceptor outer segments in central serous chorioretinopathy. Am J Ophthalmol 2011; 151(4): 617–623. [DOI] [PubMed] [Google Scholar]
- Ojima A, Iida T, Sekiryu T, Maruko I, Sugano Y. Photopigments in central serous chorioretinopathy. Am J Ophthalmol 2011; 151(6): 940–952.e1. [DOI] [PubMed] [Google Scholar]
- Ozmert E, Batioglu F. Fundus autofluorescence before and after photodynamic therapy for chronic central serous chorioretinopathy. Ophthalmologica 2009; 223(4): 263–268. [DOI] [PubMed] [Google Scholar]
- Sekiryu T, Iida T, Maruko I, Saito K, Kondo T. Infrared fundus autofluorescence and central serous chorioretinopathy. Invest Ophthalmol Vis Sci 2010; 51(10): 4956–4962. [DOI] [PubMed] [Google Scholar]
- von Ruckmann A, Fitzke FW, Fan J, Halfyard A, Bird AC. Abnormalities of fundus autofluorescence in central serous retinopathy. Am J Ophthalmol 2002; 133(6): 780–786. [DOI] [PubMed] [Google Scholar]
- von Ruckmann A, Schmidt KG, Fitzke FW, Bird AC, Jacobi KW. [Serous central chorioretinopathy. Acute autofluorescence of the pigment epithelium of the eye]. Ophthalmologe 1999; 96(1): 6–10. [DOI] [PubMed] [Google Scholar]
- Weinberger AW, Lappas A, Kirschkamp T, Mazinani BA, Huth JK, Mohammadi B et al. Fundus near infrared fluorescence correlates with fundus near infrared reflectance. Invest Ophthalmol Vis Sci 2006; 47(7): 3098–3108. [DOI] [PubMed] [Google Scholar]
- Smith RT, Chan JK, Nagasaki T, Sparrow JR, Barbazetto I. A method of drusen measurement based on reconstruction of fundus background reflectance. Br J Ophthalmol 2005; 89(1): 87–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song IS, Yu Shin, Lee BR. Time-periodic characteristics in the morphology of idiopathic central serous chorioretinopathy evaluated by volume scan using spectral-domain optical coherence tomography. Am J Ophthalmol 2012; 154(2):366–375. [DOI] [PubMed] [Google Scholar]
- Park YM, Lee MH, Lee JE, Oum BS. Fundus autofluorescence in acute and chronic-recurrent central serous chorioretinopathy. J Korean Ophthalmol Soc 2009; 50(10):1353–1358. [Google Scholar]
- Spaide RF. Fundus autofluorescence and age-related macular degeneration. Ophthalmology 2003; 110(2): 392–399. [DOI] [PubMed] [Google Scholar]
- Fujimoto H, Gomi F, Wakabayashi T, Sawa M, Tsujikawa M, Tano Y. Morphologic changes in acute central serous chorioretinopathy evaluated by fourier-domain optical coherence tomography. Ophthalmology 2008; 115(9): 1494–1500, 500 e1-2. [DOI] [PubMed] [Google Scholar]
- Hirami Y, Tsujikawa A, Sasahara M, Gotoh N, Tamura H, Otani A et al. Alterations of retinal pigment epithelium in central serous chorioretinopathy. Clin Experiment Ophthalmol 2007; 35(3): 225–230. [DOI] [PubMed] [Google Scholar]
- Shinojima A, Hirose T, Mori R, Kawamura A, Yuzawa M. Morphologic findings in acute central serous chorioretinopathy using spectral domain-optical coherence tomography with simultaneous angiography. Retina 2010; 30(2): 193–202. [DOI] [PubMed] [Google Scholar]
- Gupta V, Gupta P, Dogra MR, Gupta A. Spontaneous closure of retinal pigment epithelium microrip in the natural course of central serous chorioretinopathy. Eye (Lond) 2010; 24(4): 595–599. [DOI] [PubMed] [Google Scholar]
- Maruko I, Iida T, Ojima A, Sekiryu T. Subretinal dot-like precipitates and yellow material in central serous chorioretinopathy. Retina 2011; 31(4): 759–765. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.