Pyroptosis is an inflammatory, caspase-dependent (caspase-1 or caspase-4/-5/-11) mode of cell death, initiated by formation of the inflammasome (or pyroptosome) in response to infection or danger signals. This form of death is characterised by swelling and ultimately, lysis of the cell. The molecular mechanisms by which cell swelling, and hence pyroptosis, occur were unknown until recently. Gasdermin D (GSDMD), a member of the gasdermin family of proteins, was identified in 2015 as the death effector protein in caspase-11 and caspase-1-mediated cell death. There is a caspase cleavage site in GSDMD which releases the N-terminus of the protein, which then causes cell death.1, 2 The molecular mechanism underlying cell lysis has now been revealed by a number of groups.3, 4, 5, 6 Upon caspase-dependent cleavage, the GSDMD N-terminus (GSDMD-NT) can bind to lipids such as cardiolipin (CL) and phosphatidylethanolamine (PE) leading to pore formation in membranes. These GSDMD pores are 10–20 nM in size, and formation of these pores in cells drives pyroptosis.3, 4, 5, 6
The evidence to support GSDMD-NT pore formation in eukaryotic cells driving pyroptosis is compelling. An intriguing possibility suggested by Liu et al5 is that GSDMD-NT is also bactericidal and is able to kill different species of bacteria. The authors reasoned that because the GSDMD-NT can bind to CL, a key constituent of many bacterial membranes, then this protein might also kill bacteria. Treatment with GSDMD-NT does indeed reduce viability of Escherichia coli and Staphylococcus aureus. 5 Ding et al4 also showed that overexpression of the GSDMD-NT in E. coli is bactericidal, and that this peptide induces lysis of Bacillus megaterium protoplasts. Gasdermin D also reduces intracellular numbers of Listeria monocytogenes, although it is not clear whether it is able to kill intracellular bacteria directly, or whether this is a consequence of host cell death.5
These data raise a number of interesting questions. The major membrane lipid components in E. coli are phosphatidylglycerol (PG), PE and CL.7 In E. coli, CL can be synthesised from two molecules of PG by the CL synthase ClsA or from PG and PE by ClsC.8 A third enzyme, ClsB, has CL synthase activity in vitro, but in vivo activity has not been demonstrated.9 E. coli ΔclsABC strains lacking all three enzymes produce no CL,8 suggesting that this mutant may not be lysed by GSDMD-NT. There is considerable variation in membrane composition between different bacteria, with some species able to synthesise numerous other phospholipids to either supplement, or sometimes replace, PG, PE and CL. These include methylated PE derivatives, phosphatidylcholine (PC), phosphatidylinositol (PI) and phosphatidylserine (PS). 7 Certain pathogenic bacteria such as Campylobacter jejuni, Porphyromonas gingivalis, Clostridium perfringens and Borrelia burgdorferi do not make CL (reviewed in ref. 7), so it would seem reasonable to predict resistance to GSDMD killing in these species. CL concentrates at the poles and septa of E. coli cells, where it colocalises with the osmosensory transporter ProP.10 GSDMD may, therefore, preferentially target and form pores at the bacterial poles and septa, potentially colocalising with ProP. GSDMD also binds to PS, an intermediate in PE biosynthesis, that forms a minor lipid component of many bacterial species.7, 11 PS is a major lipid component of Clostridium botulinum and several other anaerobic bacteria,11, 12, 13 and could serve as an alternative or additional target for GSDMD in these species.
GSDMD shares several features with antimicrobial peptides (AMPs), some of which are also expressed by macrophages, and also interact with and penetrate membranes.14 It is difficult to directly compare the microbicidal activity of GSDMD with AMPs because of the wide range of assay conditions, bacterial strains and protocols used to assess the potency of these molecules. The GSDMD effective dose 50 (ED50) (the dose of compound that inhibits bacterial growth in 50% of the population), however, appears to be approximately 100 nM for E. coli and S. aureus,5 which is comparable to the AMPs LL-37 and HBD-3, and is substantially lower than HBD-1 and HBD-2.15
In conclusion, the N-terminus of GSDMD is an effective killer of eukaryotic and, potentially, prokaryotic cells (Figure 1). Determining the functional roles of GSDMD and the other gasdermin family members in greater detail may lead to the identification of novel classes of cell and bacterial death molecules. Given that GSDMD does not kill host cells from the outside, due to selective targeting of the inner leaflet of the plasma membrane,5 gasdermin derived peptides could be potentially developed as antimicrobial agents to treat extracellular bacterial infections.
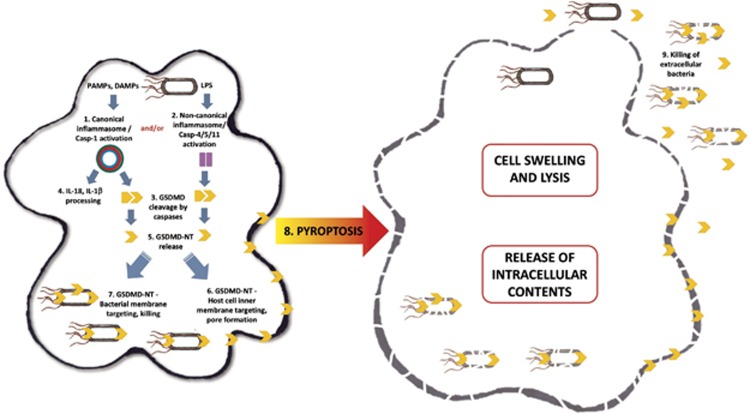
Figure 1.
Triggering of the canonical inflammasome (1) by bacterial ligands such as flagellin leads to caspase-1 activation and subsequent cleavage of gasdermin D (GSDMD) (3), and of pro-IL-1β and pro-IL-18 (4). Lipopolysaccharide (LPS) driven initiation of the non-canonical inflammasome (2) activates caspase-4/caspase-5/caspase-11 which also cleave GSDMD (4). The cytotoxic N-terminal fragment of gasdermin D (GSDMD-NT) is then released (5) and targets phospholipids in the host cell membrane and assembles into pores (6). Processed GSDMD-NT, via its affinity for cardiolipin and phosphatidylserine, may also target and kill intracellular bacteria (7), possibly colocalising with cardiolipin rich regions such as the bacterial poles and septa. GSDMD-NT pore formation in the host cell leads to pyroptosis (8) and release of intracellular contents. It is possible GSDMD-NT released during cell lysis may also target and kill extracellular bacteria (9)
The authors declare no conflict of interest.
References
- Kayagaki N et al. Nature 2015; 526: 666–671. [DOI] [PubMed]
- Shi J et al. Nature 2015; 526: 660–665. [DOI] [PubMed]
- Aglietti RA et al. Proc Natl Acad Sci USA 2016; 113: 7858–7863. [DOI] [PMC free article] [PubMed]
- Ding J et al. Nature 2016; 535: 111–116. [DOI] [PubMed]
- Liu X et al. Nature 2016; 535: 153–158. [DOI] [PMC free article] [PubMed]
- Sborgi L et al. EMBO J 2016: e201694696.
- Sohlenkamp C, Geiger O. FEMS Micro Rev 2016; 40: 133–159. [DOI] [PubMed]
- Tan BK et al. Proc Natl Acad Sci USA 2012; 109: 16504–16509. [DOI] [PMC free article] [PubMed]
- Guo D, Tropp BE. Biochim Biophys Acta 2000; 1483: 263–274. [DOI] [PubMed]
- Romantsov T et al. Mol Microbiol 2007; 64: 1455–1465. [DOI] [PubMed]
- Van Golde LM et al. FEBS Lett 1975; 53: 57–60. [DOI] [PubMed]
- Evans RI et al. Int J Food Microbiol 1998; 40: 159–167. [DOI] [PubMed]
- Lata P et al. Int J Syst Evol Microbiol 2012; 62: 2674–2679. [DOI] [PubMed]
- Mansour SC, Pena OM, Hancock REW. Trends Immunol 2014; 35: 443–450. [DOI] [PubMed]
- Chen X et al. J Dermatol Sci 2005; 40: 123–132. [DOI] [PubMed]