Abstract
Eosinophils are frequently elevated in pathological conditions and can cause tissue damage and disease exacerbation. The number of eosinophils in the blood is largely regulated by factors controlling their production in the bone marrow. While several exogenous factors, such as interleukin-5, have been described to promote eosinophil differentiation, comparatively little is known about eosinophil-intrinsic factors that control their de novo generation. Here, we report that the small atypical GTPase RhoH is induced during human eosinophil differentiation, highly expressed in mature blood eosinophils and further upregulated in patients suffering from a hypereosinophilic syndrome. Overexpression of RhoH increases, in a Rho-associated protein kinase-dependent manner, the expression of GATA-2, a transcription factor involved in regulating eosinophil differentiation. In RhoH−/− mice, we observed reduced GATA-2 expression as well as accelerated eosinophil differentiation both in vitro and in vivo. Conversely, RhoH overexpression in bone marrow progenitors reduces eosinophil development in mixed bone marrow chimeras. These results highlight a novel negative regulatory role for RhoH in eosinophil differentiation, most likely in consequence of altered GATA-2 levels.
Eosinophils are short-lived effector and regulatory cells that are increasingly recognized as having key roles in the immune system, including maintenance of long-term antibody responses and defence against bacteria and viruses.1, 2 On the other hand, their potentially destructive nature becomes apparent in a number of eosinophilic diseases such as hypereosinophilic syndromes (HES), diseases characterized by eosinophil-mediated tissue damage and organ dysfunction.3, 4 Eosinophils contribute to disease pathology directly and indirectly by releasing cytotoxic molecules and by supporting an ongoing inflammation.1 Besides HES, eosinophilia can be observed in a wide range of infectious, allergic and autoimmune diseases, as well as in cancer.3, 5 Given the increasing incidence of such diseases and the often unsatisfactory treatment options available, it is of considerable importance to better understand factors governing eosinophil biology under both normal and inflammatory conditions.
Eosinophil numbers are largely regulated by their production in the bone marrow where they differentiate from myeloid precursors.6 Lineage commitment is controlled by GATA-1, GATA-2, PU.1 and c/EBP transcription factors. For instance, mice lacking GATA-1 or c/EBP expression are devoid of eosinophils, and transduction of hematopoietic progenitor cells (HPC) with either of these genes promotes differentiation into eosinophils.7 However, the situation is not always straightforward. Lineage specification and maturation strongly depend on the relative levels of these transcription factors and their interaction partners or competitors. For example, while GATA-2 expression is required for the proper differentiation of several haematopoietic lineages, including eosinophils,8 high GATA-2 expression inhibits HPC proliferation.9, 10 Similarly, overexpression of GATA-2 in progenitor cells in the presence of C/EBPα drives eosinophil differentiation, while it promotes mast cell/basophil generation in the absence of C/EBPα.11 Furthermore, while GATA-2 induces the expression of eosinophil-derived neurotoxin, it also antagonizes GATA-1-mediated expression of other eosinophil-related genes, including major basic protein.12, 13, 14 These apparently discordant findings highlight the need for a better understanding of the molecular mechanisms controlling eosinophil differentiation.
The best studied cytokines promoting eosinophil development are interleukin (IL)-3, IL-5, and granulocyte/macrophage colony-stimulating factor (GM-CSF).6 In addition to their role in eosinophilopoiesis, they also enhance eosinophil survival.15 Of these eosinophil hematopoietins, IL-5 is the most specific for the eosinophil lineage. Transgenic overexpression of IL-5 in mice results in eosinophilia, whereas deletion of the IL-5 gene strongly reduces eosinophil counts in blood and lungs following allergen challenge.6, 16 Furthermore, many eosinophilic diseases are associated with increased IL-5 levels.3, 17 These findings together with the knowledge of the critical role of IL-5 for eosinophil generation and survival have prompted the development of therapeutic anti-IL-5 and anti-IL-5 receptor antibodies, which are currently being investigated in eosinophilic disorders.1, 18
RhoH belongs to the family of small GTPases that are involved in diverse cellular processes, including signalling, vesicle transport, migration, proliferation and differentiation. In contrast to most family members, RhoH is GTPase deficient and, therefore, its activity is assumed to be regulated at the level of protein expression rather than by switching between active (GTP-bound) and inactive (GDP-bound) forms.19 RhoH is exclusively expressed in the hematopoietic system and is mutated in a number of lymphomas and leukemias, but the mechanisms by which dysregulated RhoH contributes to malignancy remain unclear.
With respect to its physiological functions, RhoH is involved in T-cell receptor (TCR) signalling, where it regulates the subcellular localization of ZAP70 and LCK.20, 21, 22 Following TCR stimulation, RhoH is degraded.23 RhoH−/− mice have reduced T-cell numbers due to a failure of positive selection, and impaired peripheral T-cell responses.21, 24 This phenotype is similar to that of two recently described natural RhoH-deficient siblings, in which impaired T-cell function resulted in susceptibility to certain viral infections.25 In mast cells, RhoH is critical for FcɛRI-dependent signal transduction.26 RhoH has also been found to negatively regulate several processes, including T-cell adhesion and migration, stem cell proliferation and homing,27 IL-3 signalling in a B cell line28 and leukotriene production in neutrophils,29 but its function in eosinophils is unknown.
In this study, we report that RhoH deficiency promotes, and RhoH overexpression reduces, eosinophil differentiation in vitro and in vivo. Moreover, RhoH induces the developmentally important transcription factor GATA-2 through a Rho-associated protein kinase (ROCK)-dependent mechanism. In addition, RhoH appears to promote cellular quiescence in a manner similar to what has been reported for GATA-2.10 Furthermore, our data suggest that IL-5 induces RhoH in immature eosinophils, a pathway that also remains functional following maturation. Taken together, our data suggest that IL-5 not only drives eosinophil differentiation, but also induces an inhibitory pathway limiting this process in which RhoH has a major role.
Results
RhoH is expressed in human eosinophils and upregulated upon IL-5 stimulation
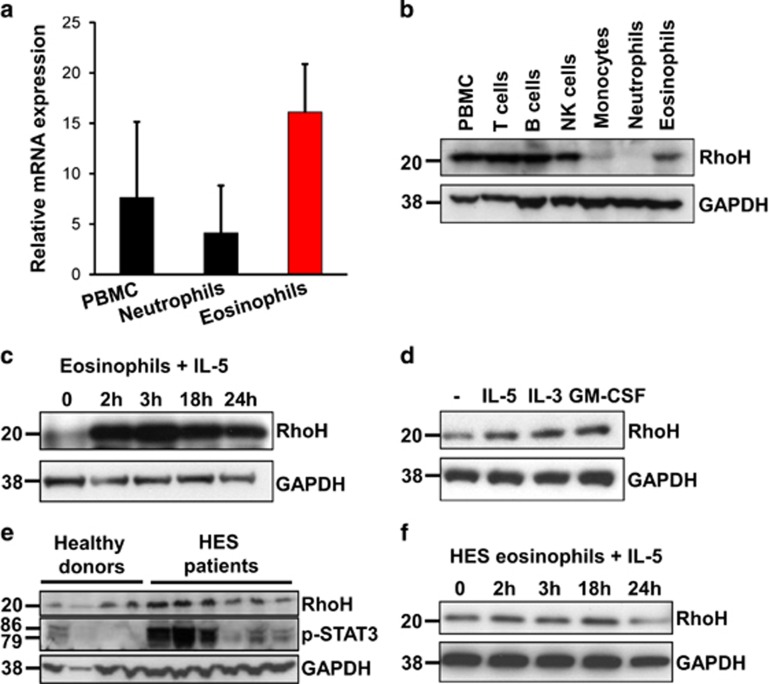
To investigate whether RhoH is expressed in eosinophils under physiological conditions, we isolated eosinophils from peripheral blood of healthy donors and found that eosinophils expressed higher levels of RhoH mRNA than peripheral blood mononuclear cells (PBMC) or neutrophils from the same individuals (Figure 1a). To examine RhoH protein expression in more detail, we isolated different leukocyte subsets for immunoblot analysis. We observed that eosinophils and NK cells express RhoH. In addition, in agreement with the previous observations,23 RhoH protein was present in T and B cells, but was hardly detectable in monocytes and neutrophils (Figure 1b).
Figure 1.
RhoH expression in peripheral blood eosinophils. Leukocyte subsets were isolated from peripheral blood and RhoH expression was measured by qPCR (a) or immunoblotting (b). Representative immunoblots of eosinophils from healthy donors stimulated with 10 ng/ml IL-5 (c) or 10 ng/ml of IL-5, IL-3 or GM-CSF for 3 h (d), freshly isolated eosinophils from healthy donors or HES patients (e), or IL-5 stimulated eosinophils from HES patients (f), are presented. Values in panel (a) represent means +/− S.D. Data in panels (b–f) are representative for at least three independent experiments
We next investigated the regulation of RhoH by eosinophil hematopoietins. RhoH was rapidly upregulated in eosinophils in response to IL-5 (Figure 1c), IL-3 and GM-CSF (Figure 1d). Of note, the magnitude of the response differed considerably between donors 3 h after stimulation (Figures 1c and d), ranging from 1.5-fold to more than 10-fold induction, probably reflecting differences in intrinsic responsiveness and/or degrees of pre-activation. The reduction of RhoH signal at later time points is mirrored by a reduction in GAPDH signal and is likely the result of a loss of cell viability.
To address the in vivo relevance of these findings, we assessed RhoH expression in eosinophils from healthy donors and patients with HES, a condition frequently characterized by overproduction of IL-5 or IL-3 by T cells or other cells.3, 17 We found that in eosinophils from some HES patients, RhoH was upregulated (Figure 1e). Notably, increased RhoH expression was accompanied by phosphorylation of signal transducer and activator of transcription 3 (STAT3). Why STAT3 phosphorylation varied considerably among HES patients is unclear, as the eosinophilia in all cases was likely driven by at least one of the eosinophil hematopoietins IL-3, IL-5 or GM-CSF and none of these patients had evidence for the presence of an eosinophilic leukemia. Therefore, we speculate that STAT3 phosphorylation is a dynamic process and depends not only on extracellular cytokine concentrations at a given time point. Nevertheless, the increased STAT3 phosphorylation levels point to the possibility that increased RhoH levels in HES patients might have been caused by in vivo exposure to at least one eosinophil hematopoietin. This notion was further substantiated by the finding that eosinophils derived from HES patients failed to further upregulate RhoH in response to in vitro stimulation with IL-5 (Figure 1f).
Mature eosinophils from RhoH knockout mice exhibit no abnormalities regarding life span, surface phenotype or migration
To determine the functional role of RhoH in resting and activated eosinophils, we attempted to overexpress RhoH in primary human eosinophils. However, primary granulocytes are notoriously difficult to transfect. As we have previously observed that the use of lentiviruses is not suitable for overexpressing proteins in granulocytes,30 we tested the nucleofection approach reported by Xu et al. 31 for mouse neutrophils. Using a GFP control construct, we observed GFP expression in up to 40% of eosinophils (Supplementary Figure S1a). However, we found that the vast majority of eosinophils died during the procedure and surviving eosinophils showed strong signs of nonspecific activation, such as downregulation of the IL-5/IL-3/GM-CSF common β-chain ((CD131); Supplementary Figure S1b). This is in agreement with a recent publication using human neutrophils and an identical protocol.32
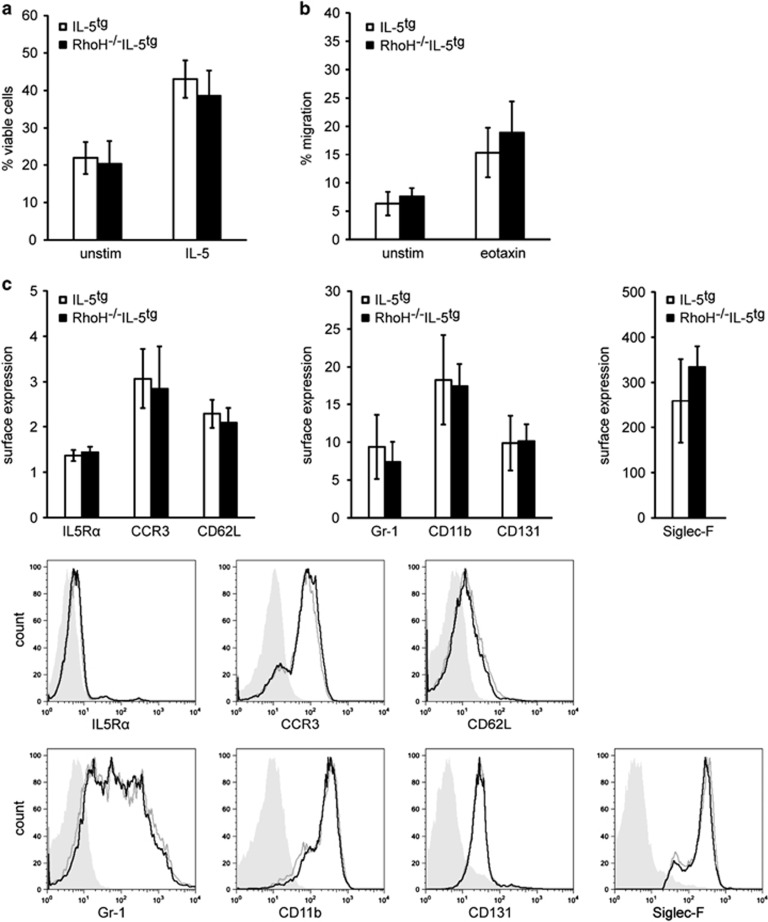
To circumvent the difficulties experienced with manipulating RhoH levels in human eosinophils, we crossed RhoH−/− mice to mice carrying the IL-5 transgene. IL-5tg mice have increased levels of eosinophils, allowing their isolation in larger numbers.16 Eosinophils from RhoH−/−IL-5tg mice did not show any obvious alterations in cellular viability, migratory behaviour (spontaneous or eotaxin-mediated), surface immunophenotype (IL-5Rα, CCR3, CD62L, Gr-1, CD11b, CD131) or spontaneous and induced cytokine (IL-4, IL-6, IL-13) production compared with eosinophils from IL-5tg mice (Figure 2 and data not shown).
Figure 2.
Absence of RhoH had no effect on viability, migration and immunophenotype of eosinophils obtained from IL-5 overexpressing mice. Eosinophils were isolated from the spleens of IL5tg mice or RhoH−/−IL5tg mice and analyzed in vitro. (a) Viability after 48 h in culture. (b) Migration in the absence of a stimulus or after 100 ng/ml eotaxin treatment. (c) Expression of the indicated surface molecules of freshly isolated eosinophils. Values represent means +/− S.D. (n≥4). Representative FACS plots are shown. Filled gray: isotype control, black line: RhoH−/−IL5tg, gray line: IL5tg eosinophils
RhoH induces GATA-2 expression
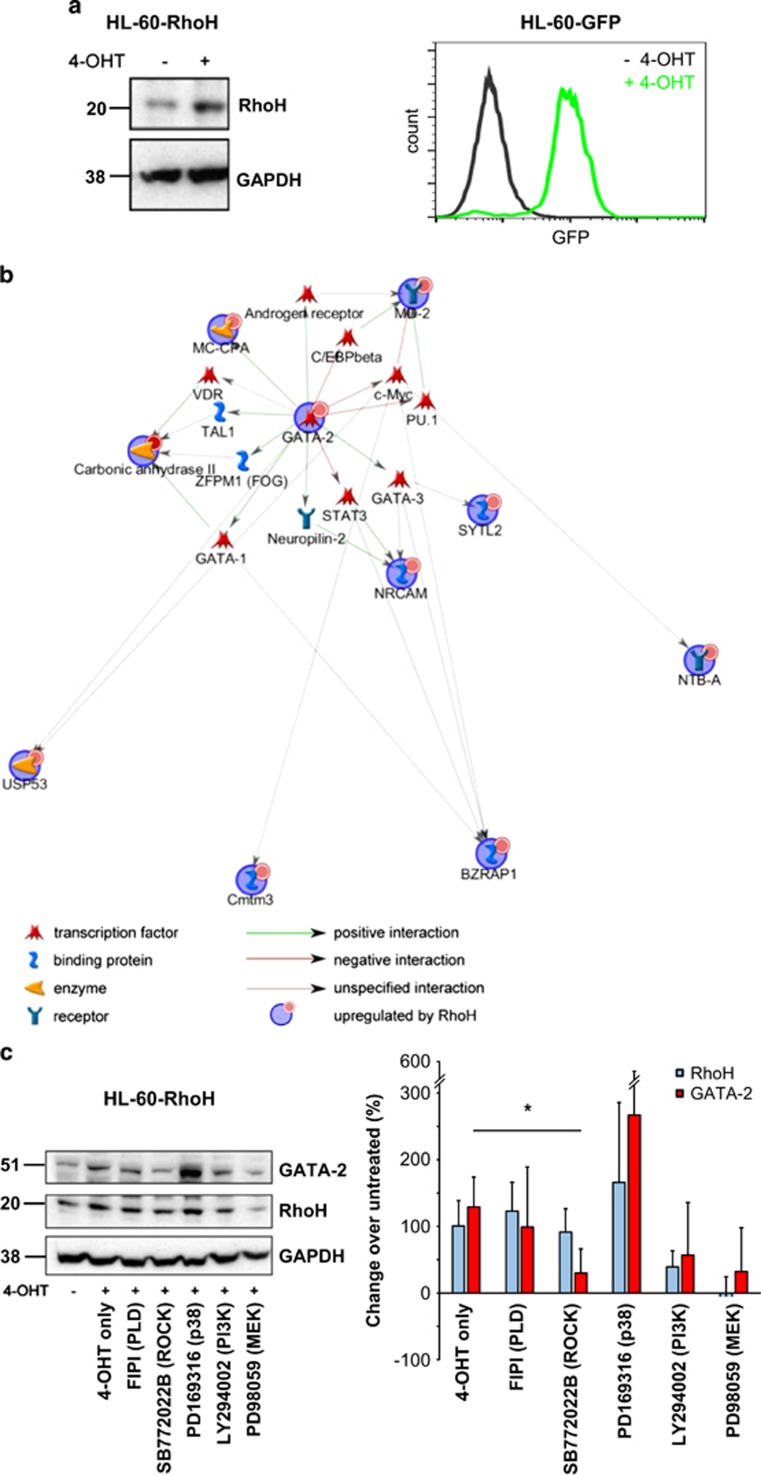
As genetic modification of human eosinophils was problematic and functional screening with mouse eosinophils did not uncover a role for RhoH in mature eosinophils, we lentivirally transduced the eosinophilic HL-60c15 line with 4-hydroxytamoxifen (4-OHT)-inducible constructs coding for RhoH (HL-60-RhoH) or GFP (HL-60-GFP)33 as a control. Induction of RhoH or GFP by 4-OHT was confirmed by western blot or fluorescence-activated cell sorting (FACS) analysis (Figure 3a). Using microarrays, gene expression of untreated HL-60-RhoH cells was compared with that of HL-60-RhoH cells in which RhoH had been induced. To exclude genes that were induced by 4-OHT alone, 4-OHT-treated HL-60-GFP cells were also included in the analysis. Among the genes found to be specifically upregulated by RhoH, most had no known functions in eosinophil physiology (Supplementary Table S1). To further explore the patterns underlying these data, we performed network and Gene Ontology (GO) analysis using Metacore (Figure 3b and Supplementary Figure S2). The resulting interaction network centred on GATA-2, a transcription factor involved in eosinophil differentiation. Similarly, the GO processes associated with the data included ‘eosinophil differentiation' and ‘eosinophil fate commitment', and a prominent component of these GO processes was again GATA-2, suggesting that RhoH might influence eosinophil differentiation by altering GATA-2 expression.
Figure 3.
GATA-2 induction in RhoH overexpressing cells. (a) Induction of RhoH (immunoblot) or GFP expression (FACS) by 4-OHT in transduced cells. (b) Network analysis of genes selectively regulated by RhoH as determined by microarray analysis. Input genes (i.e., those regulated by RhoH) are marked with blue circles, interactions with arrows (green: positive regulatory interactions, red: negative regulatory interactions, gray: unspecified interactions). Small red circles indicate upregulation. Microarray analysis was performed with three biological replicates per condition. (c) Regulation of GATA-2 protein by RhoH and ROCK. HL-60-RhoH cells were induced to overexpress RhoH with 4-OHT in the presence or absence of different signalling inhibitors. The molecular target of each inhibitor is given in brackets. A representative immunoblot and quantification of the results are shown (n=3). Expression of RhoH and GATA-2 were normalized to that of GAPDH, and then to that of the untreated sample. The quantification graph shows the change in expression relative to the untreated control. Values represent means +/− S.D. *P<0.05 for GATA-2 expression compared with the 4-OHT-treated sample
A link between RhoH and GATA-2 has not been described previously. To investigate which molecular pathways might be involved in RhoH-induced GATA-2 upregulation, we treated HL-60-RhoH cells with inhibitors for different signalling pathways before induction (Figure 3c). RhoH induction led to upregulation of GATA-2 at the protein level, confirming the microarray data. P38 inhibition resulted in GATA-2 upregulation, but this was not statistically significant. MEK and PI3K inhibition lead to reduced GATA-2 induction, but RhoH also failed to be upregulated under those conditions, suggesting that the inhibitors act upstream of RhoH in this system and that lack of GATA-2 upregulation is likely a consequence of lack of RhoH induction. Interestingly, of the pharmacological inhibitors tested, only the ROCK inhibitor SB772022B prevented GATA-2 upregulation without affecting RhoH induction (Figure 3c), indicating that ROCK is a downstream effector of RhoH and mediates RhoH-induced GATA-2 upregulation.
RhoH is upregulated during differentiation of human eosinophils
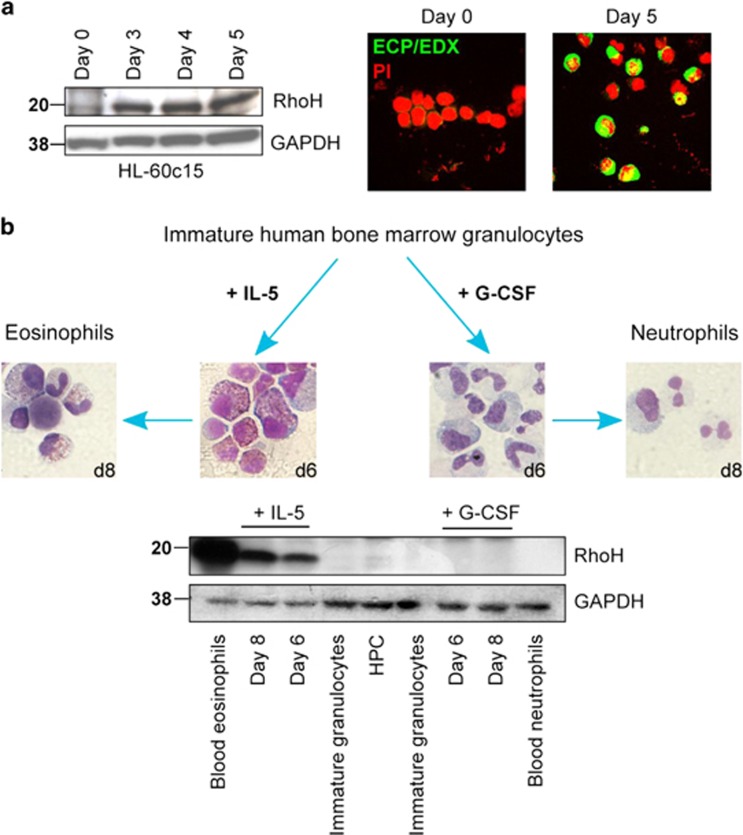
As the microarray data suggested a role of RhoH in eosinophilopoiesis, we investigated whether RhoH would regulate this process. We found that RhoH expression was low in undifferentiated HL-60c15 cells, but was upregulated during differentiation into eosinophil-like cells (Figure 4a). To further substantiate these data in a more physiological system, we isolated HPC and immature myeloid cells from human bone marrow. Immature myeloid cells were differentiated into mature neutrophils by addition of G-CSF, or into eosinophils by addition of IL-5, and analyzed by immunoblot (Figure 4b). RhoH was barely detectable in HPC and was undetectable in immature myeloid cells, differentiating and mature neutrophils. Differentiation into eosinophils, on the other hand, resulted in strong upregulation of RhoH, explaining the relatively high RhoH levels in mature peripheral blood eosinophils.
Figure 4.
RhoH expression in differentiating eosinophils. (a) HL-60c15 cells were differentiated into eosinophil-like cells for the indicated number of days. Left: Immunoblot. RhoH is induced during differentiation. Right: Immunofluorescence. The expression of eosinophil cationic protein/eosinophil-derived neurotoxin ECP/EDN is induced (original magnification × 630), confirming eosinophil differentiation. (b) Immature granulocytes isolated from human bone marrow were differentiated into mature neutrophils by addition of G-CSF, or eosinophils by addition of IL-5 for 8 days. Differentiation and maturation were confirmed by morphological assessment of Diff-Quik-stained cytospins (original magnification × 630 for all panels). RhoH expression was determined in mature blood eosinophils and neutrophils, hematopoietic progenitor cells (HPC) and immature granulocytes isolated from human bone marrow, as well as for in vitro differentiated eosinophils or neutrophils
RhoH −/− mice have increased eosinophil numbers in peripheral blood and bone marrow
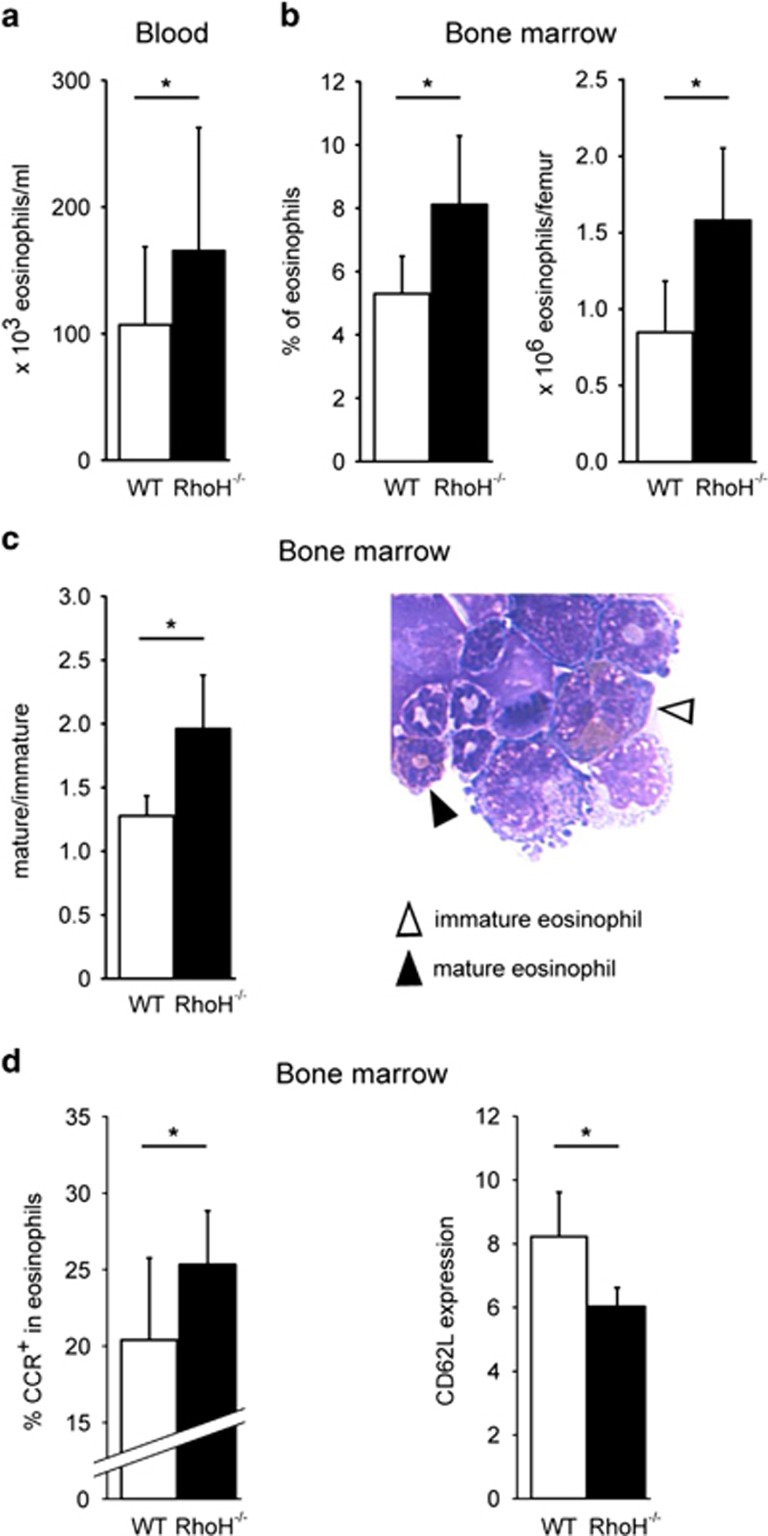
To investigate a potential role of RhoH in eosinophil differentiation in vivo, we analyzed the peripheral blood and bone marrow of RhoH−/− and wild-type (WT) mice, and found that absolute eosinophil counts were clearly increased in the peripheral blood of knockout mice (Figure 5a). Similarly, eosinophils were more abundant in the bone marrow of RhoH−/− mice (Figure 5b), suggesting that the elevated number of eosinophils in peripheral blood was due to increased de novo production. Unexpectedly, we also found that eosinophils in the bone marrow of RhoH−/− mice were more mature than the WT as assessed by morphological criteria (Figure 5c). Mature eosinophils differ in their surface phenotype from their immature counterparts, having upregulated the eotaxin receptor CCR3 and downregulated CD62L.34 In agreement with the morphological data, we observed increased CCR3 and decreased CD62L expression in the absence of RhoH, confirming a more mature phenotype (Figure 5d).
Figure 5.
Increased eosinophils and eosinophil maturity in the absence of RhoH in vivo. Eosinophil numbers in blood (a) and bone marrow (b) of WT and RhoH−/− mice. Cell differentials were determined by Diff-Quik staining and microscopy. (c) Ratio mature/immature eosinophils in freshly isolated bone marrow of WT and knockout mice. Filled triangle: mature eosinophil, open triangle: immature eosinophil (original magnification × 630). (d) Flow cytometry. Percentage of CCR3+ and level of CD62L expression on eosinophils in freshly isolated bone marrow. (n≥4). *P<0.05
RhoH overexpression leads to reduced eosinophil differentiation in mixed bone marrow chimera
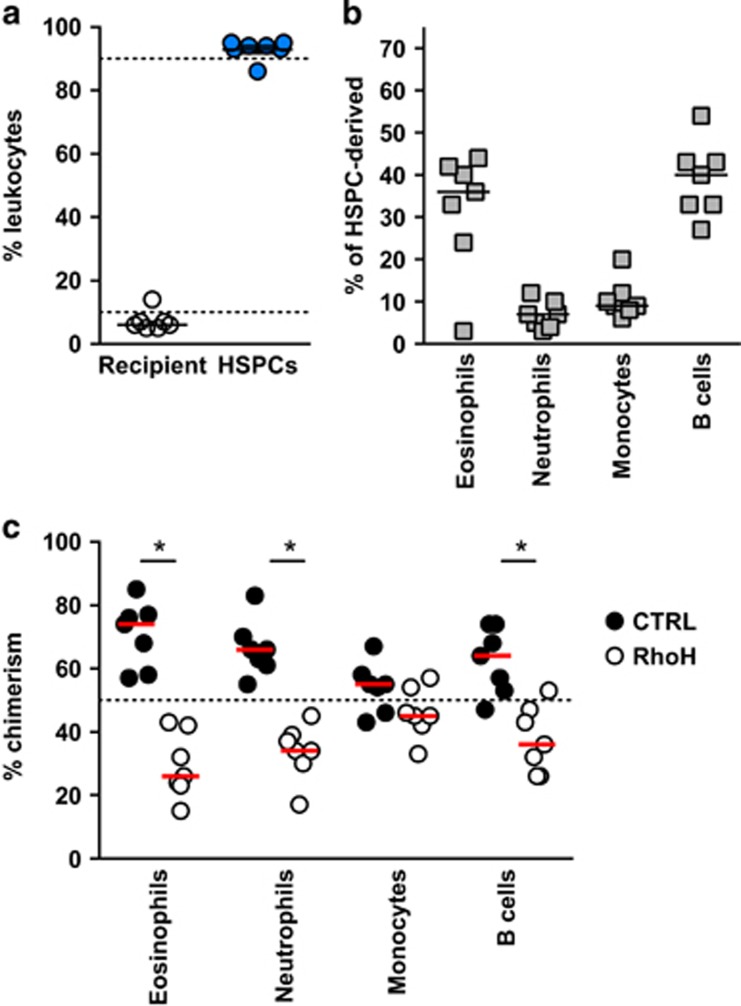
To further substantiate the effects of RhoH on eosinophil differentiation in vivo, we reconstituted the hematopoietic compartment of lethally irradiated IL-5tg mice with a 1 : 1 mixture of hematopoietic stem and progenitor cells (HSPCs) transduced with either a RhoH overexpressing or control lentivirus vector (Supplementary Figure S3a, b). Four weeks post transfer, blood leukocyte were typically 95% of donor origin (Figure 6a; Supplementary Figure S3c and d). The remaining 5% cells from the recipient mice were principally T cells, which sustained high levels of IL-5 as indicated by a substantial blood eosinophilia in most mice (Figure 6b). The relative chimerism of donor-derived leukocytes revealed a highly significant skewing in favor of eosinophils originating from CD45.2 progenitors transduced with control lentivirus at the expense of eosinophils from RhoH overexpressing progenitors (CD45.1). Of note, a similar, although less intense skewing, was observed in neutrophils and B cells, but not monocytes (Figure 6c; Supplementary Figure S4). At 4 weeks post transfer, most circulating cells are derived from progenitors rather than stem cells. A similar analysis performed at 8 weeks post transfer showed that RhoH overexpression affected all leukocyte subsets in blood and bone marrow (Supplementary Figure S3e and S5). In our competitive system, the early (4 weeks) skewing of eosinophils and neutrophils, but not monocytes, suggests that RhoH has a strong negative effect on some, but not all lineage-committed progenitors, including eosinophils. The results at 8 weeks are consistent with previous reports showing that RhoH is a broad negative regulator of hematopoietic stem and/or uncommitted progenitor cell engraftment.27 As a cautionary note, the latter results may be due in part to allelic differences in the CD45 locus, which was reported to give a competitive advantage to CD45.2 HSPCs in long-term engraftment studies (>12 weeks).35, 36, 37 However, the extent of skewing observed in eosinophils compared with B cells and neutrophils 4 weeks after transfer more strongly supports an effect of RhoH overexpression in eosinophil progenitors than a general effect of the CD45 allele on HSPCs.
Figure 6.
RhoH overexpression in HSPCs of mixed bone marrow chimeras. (a) Percentage of donor-derived and HSPC-derived blood leukocytes 4 weeks post irradiation and HSPC transfer. (b) Percentage of blood leukocyte subsets from HSPC-derived cells. (c) Relative percentage of RhoH overexpressing and control HSPC-derived cells for each leukocyte subset. *P<0.01
RhoH does not affect IL-5R expression in primary eosinophils
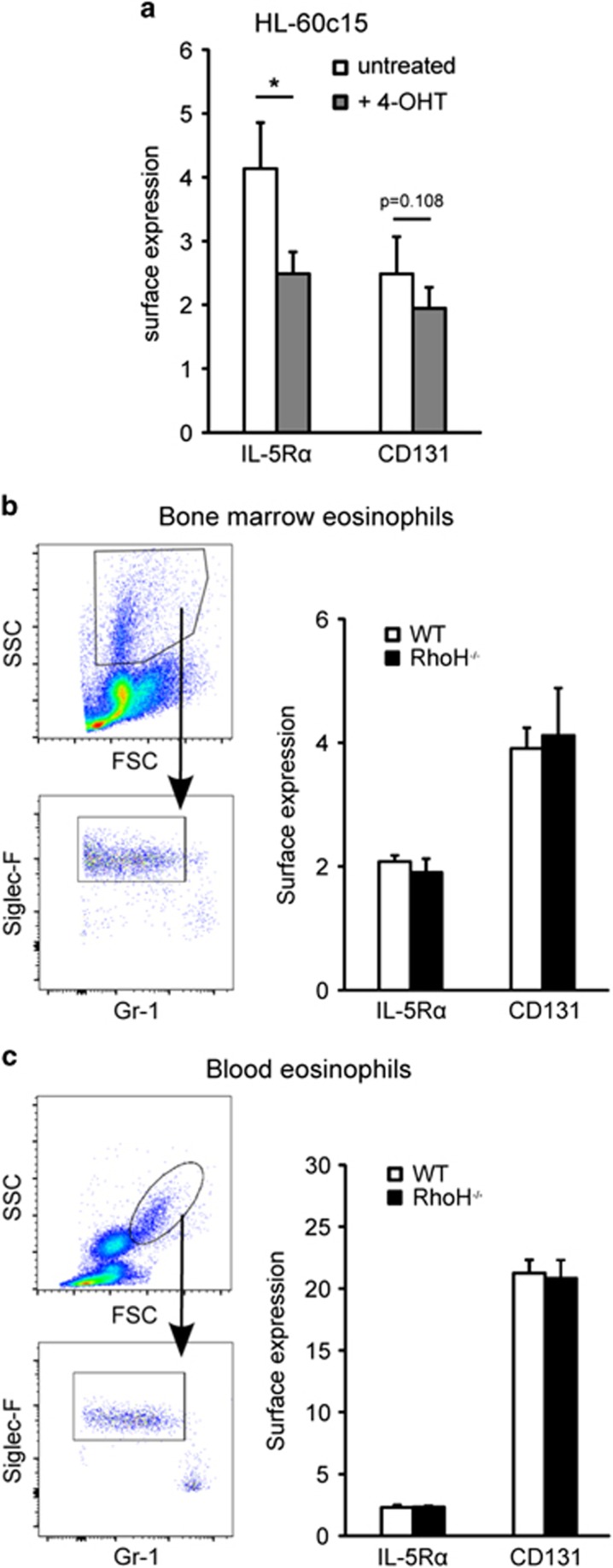
RhoH has been reported to downregulate the alpha chain of the IL-3 receptor (CD123) in a B cell line,28 diminishing IL-3 signalling. As eosinophil levels are mainly regulated through the closely related IL-5, and we had observed upregulation of RhoH in response to IL-5 in eosinophils (Figure 1), we determined if RhoH can downregulate the IL-5 receptor in eosinophils. Although surface expression of the IL-5Rα (CD125) and β-chain (CD131) were reduced following RhoH induction in HL-60c15 cells (Figure 7a), we observed no difference in IL-5R expression on bone marrow or peripheral blood eosinophils between WT and RhoH−/− mice (Figures 7b and c), similar to the findings with transgenic animals (Figure 2). This suggests that elevated eosinophil numbers in the absence of RhoH are not due to an altered IL-5 receptor expression in vivo.
Figure 7.
RhoH does not affect IL-5 receptor expression on primary mouse eosinophils. (a) Expression of IL-5Rα and IL-5Rβ (CD131) in HL-60-RhoH cells induced to overexpress RhoH with 4-OHT. (b) Gating strategy and expression of IL-5 receptor chains on freshly isolated mouse bone marrow eosinophils of WT and RhoH−/− mice. (c) Gating strategy and expression of IL-5 receptor chains on freshly isolated mouse blood eosinophils of WT and RhoH−/− mice. Values represent means +/− S.D. (n=4). *P<0.05
Lack of RhoH results in increased eosinophil differentiation in vitro and reduced GATA-2 expression in bone marrow
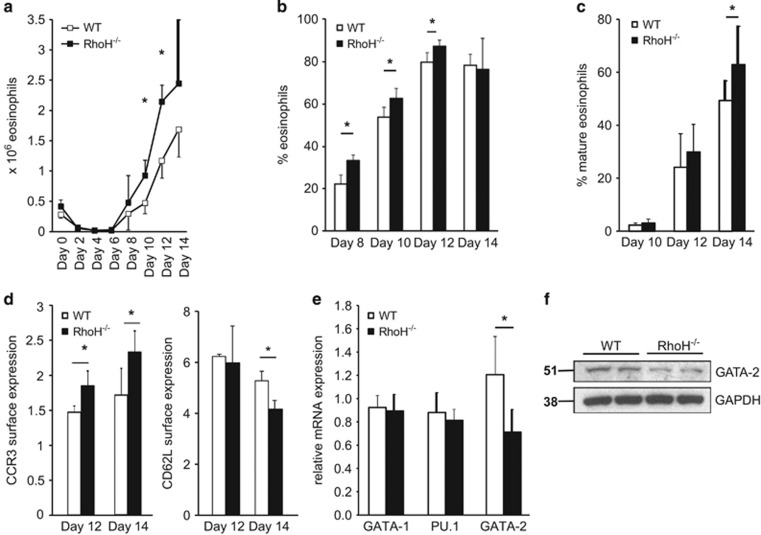
To corroborate our findings that RhoH deficiency promotes de novo eosinophil differentiation, we employed a cell culture model to differentiate eosinophils from mouse bone marrow.38 Using this model, we observed that eosinophil differentiation is indeed enhanced in the absence of RhoH (Figure 8). Importantly, although the percentage of eosinophils was higher in the bone marrow of RhoH−/− mice at the beginning of the culture, at day 6 virtually no eosinophils could be detected in either knockout or WT cultures, excluding the possibility that the differences observed were due to eosinophils surviving through the culture period. Newly differentiating eosinophils were discernible from day 8 onwards and subsequently developed into mature cells. This process was accelerated in the absence of RhoH as shown by increased eosinophil numbers (Figures 8a and b) and faster maturation as assessed by morphology and CCR3/CD62L expression (Figures 8c and d). Again, no differences in IL-5R expression were detectable (Supplementary Figures S6a and b).
Figure 8.
Enhanced eosinophil differentiation in the absence of RhoH. Bone marrow was cultured in the presence of cytokines, and cell numbers and differentials determined every second day by counting and microscopic analysis of cytospins stained with Diff-Quik. Total number (a) and percentage (b) of eosinophils from bone marrow of WT and RhoH−/− mice. (c) Mature eosinophils as percentage of total eosinophils in culture, demonstrating faster maturation of RhoH−/− eosinophils. Results are representative of three independent experiments with 3–4 mice per group. (d) CCR3 and CD62L expression on in vitro differentiated eosinophils on days 12 and 14 as determined by flow cytometry. (e) GATA-1, PU.1, and GATA-2 mRNA expression in freshly isolated bone marrow (n=4). (f) Immunoblot analysis of GATA-2 protein expression in freshly isolated bone marrow. *P<0.05
To address a possible role of GATA-2 in the observed phenotype, we assessed the expression of GATA-2, as well as that of GATA-1 and PU.1 in the bone marrow of WT and RhoH−/− mice. While GATA-1 and PU.1 levels were unchanged, GATA-2 mRNA and protein expression were reduced in the absence of RhoH (Figures 8e and f), suggesting that RhoH also regulates GATA-2 levels in vivo.
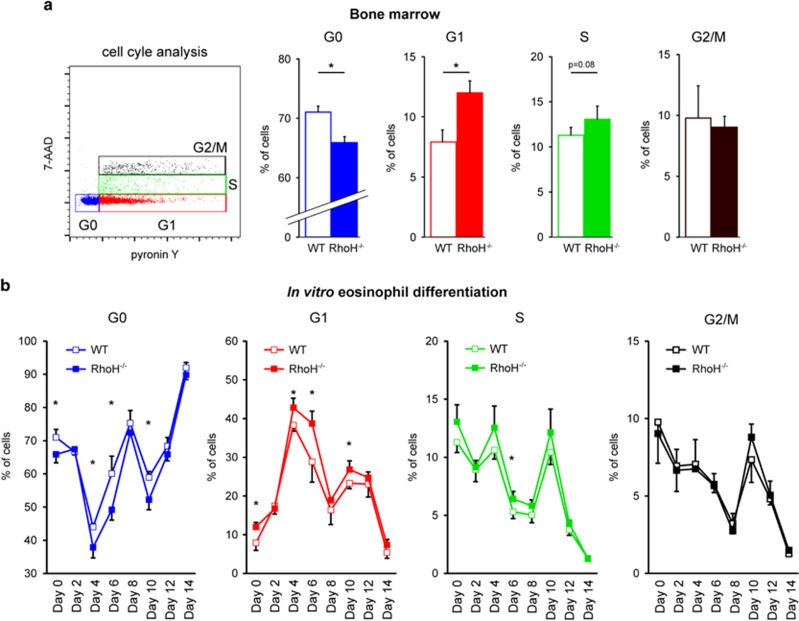
Lack of RhoH is associated with increased numbers of differentiating eosinophils entering the cell cycle
An increased number of eosinophils in the absence of RhoH could be the result of decreased cell death during differentiation or increased proliferation. As induction of GATA-2 has been reported to affect cell cycling and proliferation of HPC by increasing their quiescence (G0) residency,10 we examined cell cycle phase distribution and viability of freshly isolated bone marrow cells and during eosinophil differentiation in vitro. While we did not observe any differences in viability (Supplementary Figure S6c), we found that RhoH deficiency did have an effect on the cell cycle (Figure 9). Using a staining protocol with 7-AAD and pyronin Y, which allows the discrimination of cells in G1 from cells in G0, as well as S and G2/M phases, we found that in the absence of RhoH, the percentage of quiescent cells having withdrawn from the cell cycle (G0) was reduced, while the percentage of cells in G1 was increased (Figure 9a). These alterations were analogous to those that have been reported for cells with increased GATA-2 levels.10 We also observed a small, non-significant increase in cells in S phase, but no effect on G2/M was apparent. As quiescent cells in G0 do not proliferate, the shift from G0 to G1 and possibly S indicates increased proliferation of progenitors as the mechanism underlying enhanced eosinophil generation when RhoH is lacking. On the other hand, when RhoH is present, a larger fraction of cells was quiescent (i.e., in G0). In order to see whether this phenomenon was detectable only at the onset of eosinophil differentiation or during the entire process, we performed the analysis each second day of the differentiation protocol (Figure 9b). The shift from G0 to G1 (and possibly S) in the absence of RhoH was largely maintained during eosinophil differentiation until day 10 (Figure 9b). These results suggest that RhoH limits eosinophil differentiation through its effect on the cell cycle, that is, by increasing quiescence (G0 residency). As RhoH induces GATA-2 (Figures 3 and 7) and GATA-2 induction has been reported to have the same effect,10 our results are consistent with RhoH limiting eosinophil differentiation by this mechanism.
Figure 9.
Cell cycle analysis in differentiating eosinophils. (a) Freshly isolated bone marrow was stained with 7-AAD (DNA) and pyronin Y (RNA) to determine cell cycle distribution in WT and RhoH−/− mice. Left: FACS gating strategy of stained cells. Right panels: Percentages of cells residing in the different stages of the cell cycle (G0, G1, S and G2/M) in WT and RhoH−/− mouse bone marrow. (b) Cell cycle analysis in bone marrow cultures during eosinophil differentiation. Samples were drawn every 2 days of the differentiation protocol and analyzed as in panel (a). Values represent means +/− S.D. (n=4). *P<0.05
Discussion
Our results identify RhoH as a novel negative regulator of eosinophil differentiation. Reduced eosinophil numbers in the presence of RhoH appear to be due to limited differentiation of eosinophils in the bone marrow rather than enhanced death of eosinophils as has recently been reported for the negative regulator PIR-A.39 Furthermore, RhoH could induce GATA-2, whereas GATA-2 expression was reduced in vivo in the absence of RhoH. RhoH limited eosinophil differentiation and maturation both in vitro and in vivo, likely by regulating GATA-2 levels and the cell cycle.
RhoH appears to have different functions in different hematopoietic cells. Although our data show that eosinophil differentiation is enhanced in the absence of RhoH, T-cell development is impaired and mast cell development unaffected.21, 26 Neutrophil numbers are also unchanged, probably due to the fact that they express little or no RhoH. This observed enhancement results in increased peripheral eosinophil numbers, but the function of mature eosinophils released into the periphery is apparently not impacted, as indicated by the results using eosinophils from IL-5tg and RhoH−/−IL-5tg mice. Similarly, surface expression of the maturation markers CD62L and CCR3 on mature peripheral blood eosinophils from these mice (Figure 2) or from WT and RhoH−/− mice did not differ between genotypes (not shown). The observation that differences in maturation markers are restricted to the bone marrow supports the idea that increased peripheral blood eosinophil numbers in the absence of RhoH are the consequence of increased eosinophil production in the bone marrow rather than enhanced release of immature eosinophils in these mice.
HPC proliferation, survival, engraftment and migration have been reported to be increased by knocking down RhoH,27 and our results using bone marrow chimeras of RhoH overexpressing and control transduced progenitors/stem cells confirm these results. Interestingly, mature eosinophil survival or migration was unaffected in our study. However, our results support the idea that RhoH can negatively regulate proliferation of eosinophils during differentiation, by reducing GATA-2 levels and inducing a shift from G1 to the G0 phase of cell cycle.
Reports on the role of GATA-2 in eosinophil differentiation and function have shown that it can have positive, as well as negative regulatory functions.8, 9, 10, 11, 12, 13, 14 As GATA-2 cooperates and competes with a number of transcription factors, these apparently contrasting results are likely due to differences in experimental systems and corresponding differences in the relative levels of GATA-2 and its interaction partners or competitors. Our results indicate that a partial reduction in physiological GATA-2 levels as a result of RhoH deficiency promotes differentiation into the eosinophil lineage. Importantly, GATA-1 levels were unaffected in RhoH−/− mice, suggesting that a shift in the GATA-1/GATA-2 balance could alleviate GATA-2 antagonism of GATA-1-mediated gene expression during eosinophil development.
Interestingly, we found that ROCK activity was required for RhoH-induced GATA-2 upregulation. ROCK is a downstream effector of several Rho proteins and has critical roles in cell cycle progression, cell survival and proliferation.40 Although RhoH has so far not been described to affect ROCK activity, it has been reported to antagonize Rac, and Rac can block Rho/ROCK signalling.27, 40 Whether RhoH affects ROCK directly, or indirectly, for example, through relieving Rac-mediated antagonism of ROCK, remains to be investigated. Although ROCK has been shown to be important for cell cycle progression, it appears to affect the G1 to S transition rather than, like GATA-2, the G0 to G1 transition.41 Our results are, therefore, more compatible with the notion that ROCK-induced GATA-2 expression, rather than ROCK activity per se, is involved in the observed phenotype.
Besides its role in eosinophil differentiation, RhoH likely also has a role in mature eosinophils, as it is strongly and rapidly upregulated upon cytokine exposure in vitro and highly expressed in some HES patients, probably as a consequence of in vivo cytokine exposure. This rapid increase in RhoH protein might be facilitated by the comparatively high levels of mRNA stored in eosinophils (Figure 1a). In neutrophils, RhoH has been reported to limit cytokine-induced LTB4 production.29 Although this suggests that in mature eosinophils, RhoH could also act as a negative feedback regulator of cytokine signalling, we found no evidence for differences in IL-5-mediated survival or eotaxin-induced migration between mouse eosinophils with or without RhoH expression.
As eosinophils play major roles in asthma and allergy, eosinophil-directed therapies are currently being tested.18 While many of these successfully reduce peripheral blood eosinophil counts, they fail to eradicate tissue eosinophils. Therefore, treatments targeting de novo generation in the bone marrow and, therefore, long-term depletion might be promising. However, development of such treatments is clearly hampered by a lack of understanding of eosinophilopoiesis. For most of the genes found to be upregulated in the microarray analysis, a role in granulopoiesis/eosinophilopoiesis has not been investigated so far. As very few factors governing eosinophil generation and maturation are known (and even fewer negative regulators), and as RhoH negatively regulates eosinophil differentiation, our data warrant further studies to elucidate the exact molecular mechanisms underlying these processes.
The notion that RhoH influences transcription factors governing leukocyte differentiation is also highly interesting in a slightly different context. RhoH is frequently mutated in lymphomas and leukemias and mutations often correlate with prognosis, either poor or favorable, depending on the specific malignancy, precluding a role for RhoH as classical tumor suppressor or oncogene.19 The molecular basis for these apparently contradictory observations is unclear. However, shifts in the balance of transcription factors related to hematopoietic differentiation have been implicated in the pathogenesis of leukemia and therapeutic agents affecting that balance are used successfully in the clinic (e.g., all-trans retinoic acid for acute myeloid leukemia).42 A clearer view of how this network is regulated might also open up new possibilities for interventions to treat hematopoietic malignancies.
Materials and Methods
Antibodies and flow cytometry
Details are provided in the Supplementary Information.
Leukocyte isolation and culture from human blood and bone marrow
Leukocyte subsets were isolated from Li-heparinized blood as outlined in the Supplementary Information. For some experiments, eosinophils were cultured in RPMI 5% FCS with or without 10 ng/ml IL-5, IL-3 (R&D Systems, Minneapolis, MN, USA), or GM-CSF (Novartis, Basel, Switzerland, Leukomax 300).
HPC and immature myeloid precursors were isolated from human bone marrow as previously described.43 Precursors were differentiated with 25 ng/ml G-CSF (neutrophils) or 10 ng/ml IL-5 (eosinophils). For details, see Supplementary Information.
Generation and culture of stably transduced HL-60 clone 15 cells
For generation of cell lines inducibly overexpressing RhoH see Supplementary Information. For inhibitor experiments, cells were pre-treated with SB772077B dihydrochloride (1 μM, Tocris Bioscience, Bristol, UK), PD169316, LY294002 (both 10 μM, Calbiochem, Darmstadt, Germany), PD98059 (50 μM, Calbiochem) or FIPI hydrochloride (1 μM, R&D Systems) 45 min before induction with 100 nM 4-OHT.
RT-qPCR, microarray and bioinformatics analysis
pPCR or microarray analysis using the Affimetrix Human Gene 2.0 ST Array (Affimetrix, Santa Clara, CA, USA) were performed as outlined in the Supplementary Information.
Mice and isolation of mouse eosinophils
Animal studies were approved by the Veterinary Office of the Canton of Bern and conducted in accordance with Swiss federal legislation on animal welfare. RhoH−/− mice24 were provided by Cord Brakebusch (University of Copenhagen, Denmark) and WT C57/B6 mice by the in-house facility. For functional tests, eosinophils were isolated from IL-5tg (C57BL/6J-Tg(Il5)1638Jlee),16 provided by Jamie Lee (Mayo Clinic, Scottsdale, Arizona), and RhoH−/−IL-5tg mice, which were generated by crossing IL-5tg mice to RhoH−/− mice. For eosinophil isolation, spleens were mechanically dissociated and RBC lysed with ACK buffer. T cells, B cells and macrophages were depleted with antibodies against CD8α, CD90.2 and CD19 (Miltenyi Biotec, Bergisch Gladbach, Germany) to obtain eosinophils (>90% pure by Diff-Quik).
Differentiation of eosinophils from bone marrow of 12–24-week-old mice was performed as described.38 Briefly, bone marrow of age- and sex-matched mice was cultured in the presence of Flt3L and SCF (Peprotech) for 4 days, and subsequently with IL-5 (R&D systems) for 10 days with medium replacement every 2 days.
Bone marrow chimera
To generate mixed bone marrow chimeras, mouse bone marrow HSPCs from CD45.1 mice were transduced with lentiviral vector expressing full-length mouse RhoH (see Supplementary Information). IL-5tg (n=3) and GFP+IL-5tg (n=4) recipient mice (both CD45.2) were lethally irradiated with 10 Gy from a 137Cs source (Gammacell 40 Exactor, Theratronics, Ottawa, Ontario, Canada). A 1 : 1 mixture of CD45.1 and CD45.2 transduced cells (2 × 105) were injected into the retroorbital venous sinus of recipient mice 18 h after irradiation. IL-5tg mice received a mixture of CD45.1 and GFP+CD45.2 HSPCs, whereas GFP+IL-5tg mice received a mixture of CD45.1 and GFPnegCD45.2 HSPCs. Antibiotics (1% Cotrim; Spirig Pharma AG, Egerkingen, Switzerland) were added to the drinking water for 4 weeks. Leukocyte subsets in blood were analyzed 4 and 8 weeks after transfer. Reconstitution efficiency was evaluated by flow cytometry analysis of recipient cells (CD45.1, CD45.2 or GFP).
Acknowledgments
We wish to thank Jamie Lee and Cord Brakebusch for providing mice, John Silke for lentiviral constructs, Benjamin Gantenbein for providing human bone marrow, Kevin Oberson for experimental animal support and flow cytometry, staff of the GTF Lausanne for support with microarray data analysis, and doctors, patients and donors for providing blood samples. This study was supported by grants of the Swiss National Science Foundation (grant no. 310030_146181 and 310030_166473 to HUS and 310030_749790 to CB), Deutsche Forschungsgemeinschaft (grant no. STO-906/1-1 to CS) and Novartis Stiftung für medizinisch-biologische Forschung (grant no. 14B074 to CS).
Glossary
- ECP
eosinophil cationic protein
- EDN
eosinophil-derived neurotoxin
- FACS
fluorescence-activated cell sorting
- Flt3L
Fms-related tyrosine kinase 3 ligand
- G-CSF
granulocyte colony-stimulating factor
- GM-CSF
granulocyte/macrophage colony-stimulating factor
- HES
hypereosinophilic syndrome
- HPC
hematopoietic progenitor cells
- 4-OHT
4-hydroxytamoxifen
- IL
interleukin
- MEK
mitogen/extracellular signal-regulated kinase
- PBMC
peripheral blood mononuclear cells
- PCR
polymerase chain reaction
- PI3K
phosphoinositide 3-kinase
- ROCK
Rho-associated protein kinase
- SCF
stem cell factor
- STAT
signal transducer and activator of transcription
- WT
wild-type
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies this paper on Cell Death and Differentiation website (http://www.nature.com/cdd)
Edited by P Salomoni
Supplementary Material
References
- Rosenberg HF, Dyer KD, Foster PS. Eosinophils: changing perspectives in health and disease. Nat Rev Immunol 2013; 13: 9–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soragni A, Yousefi S, Stoeckle C, Soriaga AB, Sawaya MR, Kozlowski E et al. Toxicity of eosinophil MBP is repressed by intracellular crystallization and promoted by extracellular aggregation. Mol Cell 2015; 57: 1011–1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon D, Simon HU. Eosinophilic disorders. J Allergy Clin Immunol 2007; 119: 1291–1300. [DOI] [PubMed] [Google Scholar]
- Valent P, Klion AD, Horny HP, Roufosse F, Gotlib J, Weller PF et al. Contemporary consensus proposal on criteria and classification of eosinophilic disorders and related syndromes. J Allergy Clin Immunol 2012; 130: 607–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammel V, Stoeckle C, Padberg B, Zweifel R, Kienle DL, Reinhart WH et al. Hypereosinophilia driven by GM-CSF in large-cell carcinoma of the lung. Lung Cancer 2012; 76: 493–495. [DOI] [PubMed] [Google Scholar]
- Rothenberg ME, Hogan SP. The eosinophil. Annu Rev Immunol 2006; 24: 147–174. [DOI] [PubMed] [Google Scholar]
- McNagny K., Graf T. Making eosinophils through subtle shifts in transcription factor expression. J Exp Med 2002; 195: F43–F47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai FY, Keller G, Kuo FC, Weiss M, Chen J, Rosenblatt M et al. An early haematopoietic defect in mice lacking the transcription factor GATA-2. Nature 1994; 371: 221–226. [DOI] [PubMed] [Google Scholar]
- Persons DA, Allay JA, Allay ER, Ashmun RA, Orlic D, Jane SM et al. Enforced expression of the GATA-2 transcription factor blocks normal hematopoiesis. Blood 1999; 93: 488–499. [PubMed] [Google Scholar]
- Tipping AJ, Pina C, Castor A, Hong D, Rodrigues NP, Lazzari L et al. High GATA-2 expression inhibits human hematopoietic stem and progenitor cell function by effects on cell cycle. Blood 2009; 113: 2661–2672. [DOI] [PubMed] [Google Scholar]
- Iwasaki H, Mizuno S, Arinobu Y, Ozawa H, Mori Y, Shigematsu H et al. The order of expression of transcription factors directs hierarchical specification of hematopoietic lineages. Genes Dev 2006; 20: 3010–3021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu Z, Dyer KD, Xie Z, Radinger M, Rosenberg HF. GATA transcription factors regulate the expression of the human eosinophil-derived neurotoxin (RNase 2) gene. J Biol Chem 2009; 284: 13099–13109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi Y, Ackerman SJ, Minegishi N, Takiguchi M, Yamamoto M, Suda T. Mechanisms of transcription in eosinophils: GATA-1, but not GATA-2, transactivates the promoter of the eosinophil granule major basic protein gene. Blood 1998; 91: 3447–3458. [PubMed] [Google Scholar]
- Yang D, Suzuki S, Hao LJ, Fujii Y, Yamauchi A, Yamamoto M et al. Eosinophil-specific regulation of gp91(phox) gene expression by transcription factors GATA-1 and GATA-2. J Biol Chem 2000; 275: 9425–9432. [DOI] [PubMed] [Google Scholar]
- Geering B, Stoeckle C, Conus S, Simon HU. Living and dying for inflammation: neutrophils, eosinophils, basophils. Trends Immunol 2013; 34: 398–409. [DOI] [PubMed] [Google Scholar]
- Lee NA, McGarry MP, Larson KA, Horton MA, Kristensen AB, Lee JJ. Expression of IL-5 in thymocytes/T cells leads to the development of a massive eosinophilia, extramedullary eosinophilopoiesis, and unique histopathologies. J Immunol 1997; 158: 1332–1344. [PubMed] [Google Scholar]
- Stoeckle C, Simon HU. CD8+ T cells producing IL-3 and IL-5 in non-IgE-mediated eosinophilic diseases. Allergy 2013; 68: 1622–1625. [DOI] [PubMed] [Google Scholar]
- Radonjic-Hoesli S, Valent P, Klion AD, Wechsler ME, Simon HU. Novel targeted therapies for eosinophil-associated diseases and allergy. Annu Rev Pharmacol Toxicol 2015; 55: 633–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troeger A, Williams DA. Hematopoietic-specific Rho GTPases Rac2 and RhoH and human blood disorders. Exp Cell Res 2013; 319: 2375–2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chae HD, Siefring JE, Hildeman DA, Gu Y, Williams DA. RhoH regulates subcellular localization of ZAP-70 and Lck in T cell receptor signaling. PLoS One 2010; 5: e13970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Y, Chae HD, Siefring JE, Jasti AC, Hildeman DA, Williams DA. RhoH GTPase recruits and activates Zap70 required for T cell receptor signaling and thymocyte development. Nat Immunol 2006; 7: 1182–1190. [DOI] [PubMed] [Google Scholar]
- Wang H, Zeng X, Fan Z, Lim B. RhoH modulates pre-TCR and TCR signalling by regulating LCK. Cell Signal 2011; 23: 249–258. [DOI] [PubMed] [Google Scholar]
- Schmidt-Mende J, Geering B, Yousefi S, Simon HU. Lysosomal degradation of RhoH protein upon antigen receptor activation in T but not B cells. Eur J Immunol 2010; 40: 525–529. [DOI] [PubMed] [Google Scholar]
- Dorn T, Kuhn U, Bungartz G, Stiller S, Bauer M, Ellwart J et al. RhoH is important for positive thymocyte selection and T-cell receptor signaling. Blood 2007; 109: 2346–2355. [DOI] [PubMed] [Google Scholar]
- Crequer A, Troeger A, Patin E, Ma CS, Picard C, Pedergnana V et al. Human RHOH deficiency causes T cell defects and susceptibility to EV-HPV infections. J Clin Invest 2012; 122: 3239–3247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oda H, Fujimoto M, Patrick MS, Chida D, Sato Y, Azuma Y et al. RhoH plays critical roles in Fc epsilon RI-dependent signal transduction in mast cells. J Immunol 2009; 182: 957–962. [DOI] [PubMed] [Google Scholar]
- Gu Y, Jasti AC, Jansen M, Siefring JE. RhoH, a hematopoietic-specific Rho GTPase, regulates proliferation, survival, migration, and engraftment of hematopoietic progenitor cells. Blood 2005; 105: 1467–1475. [DOI] [PubMed] [Google Scholar]
- Gundogdu MS, Liu H, Metzdorf D, Hildebrand D, Aigner M, Aktories K et al. The haematopoietic GTPase RhoH modulates IL3 signalling through regulation of STAT activity and IL3 receptor expression. Mol Cancer 2010; 9: 225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daryadel A, Yousefi S, Troi D, Schmid I, Schmidt-Mende J, Mordasini C et al. RhoH/TTF negatively regulates leukotriene production in neutrophils. J Immunol 2009; 182: 6527–6532. [DOI] [PubMed] [Google Scholar]
- Geering B, Schmidt-Mende J, Federzoni E, Stoeckle C, Simon HU. Protein overexpression following lentiviral infection of primary mature neutrophils is due to pseudotransduction. J Immunol Methods 2011; 373: 209–218. [DOI] [PubMed] [Google Scholar]
- Xu W, Wang P, Petri B, Zhang Y, Tang W, Sun L et al. Integrin-induced PIP5K1C kinase polarization regulates neutrophil polarization, directionality, and in vivo infiltration. Immunity 2010; 33: 340–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamassia N, Bazzoni F, Le Moigne V, Calzetti F, Masala C, Grisendi G et al. IFN-beta expression is directly activated in human neutrophils transfected with plasmid DNA and is further increased via TLR-4-mediated signaling. J Immunol 2012; 189: 1500–1509. [DOI] [PubMed] [Google Scholar]
- Vince JE, Wong WW, Khan N, Feltham R, Chau D, Ahmed AU et al. IAP antagonists target cIAP1 to induce TNFalpha-dependent apoptosis. Cell 2007; 131: 682–693. [DOI] [PubMed] [Google Scholar]
- Voehringer D, van Rooijen N, Locksley RM. Eosinophils develop in distinct stages and are recruited to peripheral sites by alternatively activated macrophages. J Leukoc Biol 2007; 81: 1434–1444. [DOI] [PubMed] [Google Scholar]
- Waterstrat A, Liang Y, Swiderski CF, Shelton BJ, van Zant G. Congenic interval of CD45/Ly-5 congenic mice contains multiple genes that may influence hematopoietic stem cell engraftment. Blood 2010; 115: 408–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu S, Ray A, Dittel BN. Differential representation of B cell subsets in mixed bone marrow chimera mice due to expression of allelic variants of CD45. J Immunol Methods 2013; 396: 163–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercier FE, Sykes DB, Scadden DT. Single targeted exon mutation creates a true congenic mouse for competitive hematopoietic stem cell transplantation: The C57BL/6-CD45.1STEM mouse. Stem Cell Reports 2016; 6: 985–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyer KD, Moser JM, Czapiga M, Siegel SJ, Percopo CM, Rosenberg HF. Functionally competent eosinophils differentiated ex vivo in high purity from normal mouse bone marrow. J Immunol 2008; 181: 4004–4009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben Baruch-Morgenstern N, Shik D, Moshkovits I, Itan M, Karo-Atar D, Bouffi C et al. Paired immunoglobulin-like receptor A is an intrinsic, self-limiting suppressor of IL-5-induced eosinophil development. Nat Immunol 2014; 15: 36–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amin E, Dubey BN, Zhang SC, Gremer L, Dvorsky R, Moll JM et al. Rho-kinase: regulation, (dys)function, and inhibition. Biol Chem 2013; 394: 1399–1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Tang Q, Xu F, Xue Y, Zhen Z, Deng Y et al. RhoA regulates G1-S progression of gastric cancer cells by modulation of multiple INK4 family tumor suppressors. Mol Cancer Res 2009; 7: 570–580. [DOI] [PubMed] [Google Scholar]
- Martelli MP, Gionfriddo I, Mezzasoma F, Milano F, Pierangeli S, Mulas F et al. Arsenic trioxide and all-trans retinoic acid target NPM1 mutant oncoprotein levels and induce apoptosis in NPM1-mutated AML cells. Blood 2015; 125: 3455–3465. [DOI] [PubMed] [Google Scholar]
- Geering B, Stoeckle C, Rozman S, Oberson K, Benarafa C, Simon HU. DAPK2 positively regulates motility of neutrophils and eosinophils in response to intermediary chemoattractants. J Leukoc Biol 2014; 95: 293–303. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.