Abstract
HIV strains continuously evolve, tend to recombine and new circulating variants are being discovered. Novel strains complicate efforts to develop a vaccine against HIV and may exhibit higher transmission efficiency and virulence, and elevated resistance to antiretroviral agents. The United Nations Joint Programme on HIV/AIDS (UNAIDS) set an ambitious goal to end HIV as a public health threat by 2030 through comprehensive strategies that include epidemiological input as the first step of the process. In this context, molecular epidemiology becomes invaluable as it captures trends in HIV evolution rates that shape epidemiological pictures across several geographical areas.
This review briefly summarizes the molecular epidemiology of HIV among people who inject drugs (PWID) in Europe and Asia. Following high transmission rates of subtype G and CRF14_BG among PWID in Portugal and Spain, two European countries, Greece and Romania, experienced recent HIV outbreaks in PWID that consisted of multiple transmission clusters including subtypes B, A, F1 and recombinants CRF14_BG and CRF35_AD. The latter was first identified in Afghanistan. Russia, Ukraine and other Former Soviet Union (FSU) states are still facing the devastating effects of epidemics in PWID produced by AFSU (also known as IDU-A), BFSU (known as IDU-B), and CRF03_AB. In Asia, CRF01_AE and subtype B (Western B and Thai B) travelled from PWID in Thailand to neighboring countries. Recombination hotspots in South China, Northern Myanmar, and Malaysia have been generating several intersubtype and inter-CRF recombinants (e.g. CRF07_BC, CRF08_BC, CRF33_01B etc.) increasing the complexity of HIV molecular patterns.
Keywords: HIV, molecular epidemiology, subtypes, PWID, drug injecting
1. Introduction
In 1981, unexpected cases of Kaposi sarcoma and Pneumocystis carinii (now known as jirovecii) pneumonia were diagnosed among young men who have sex with men (MSM) (Centers for Disease Control, 1981a, 1981b; Gottlieb et al., 1981). The United States (US) Centers for Disease Control and Prevention (CDC) carried out detailed investigations and soon defined the Acquired Immune Deficiency Syndrome (AIDS) hinting also at a viral etiology of the new disease (Centers for Disease Control, 1982a, 1982b). Human Immunodeficiency Virus (HIV), which causes AIDS, was eventually discovered in 1983 (Barré-Sinoussi et al., 1983; Gallo et al., 1984) but the scientific community soon realized that HIV had been spreading globally before the initial recognition of AIDS in the US. As a matter of fact, a stored sample collected in 1959 in Belgian Congo (now Democratic Republic of the Congo) tested positive for HIV (Nahmias et al., 1986; Zhu et al., 1998) and molecular investigations have shown that HIV was the result of cross-species transmissions from different primates that had taken place long before the first diagnoses (Gao et al., 1999; Keele et al., 2006; Sharp and Hahn, 2011; Van Heuverswyn et al., 2006). The most recent ancestor of the pandemic strain was probably circulating in human populations in Central Africa early in the 20th century (Faria et al., 2014; Korber et al., 2000; Worobey et al., 2008).
The toll of HIV has been high with millions of HIV infections and HIV-related deaths since the early 1980s (Faria et al., 2014; Sharp and Hahn, 2011). However, considerable progress has occurred over the years, which transformed HIV from a lethal disease to a chronic condition. Combinations of potent antiretroviral drugs (antiretroviral treatment - ART) in simplified regimens have significantly reduced morbidity and mortality, and people infected today can anticipate an almost normal life expectancy (Mills et al., 2011; Nsanzimana et al., 2015; Samji et al., 2013; The Antiretroviral Cohort Collaboration, 2008). Following intensified efforts by the World Health Organization (WHO) and the United Nations Joint Programme on HIV/AIDS (UNAIDS), around 17 million people worldwide were on ART in 2015 (UNAIDS, 2016, 2015). In addition, treatment as prevention (TasP) (Cohen et al., 2011a) and pre-exposure prophylaxis (PrEP) (Grant et al., 2010; McCormack et al., 2015; Molina et al., 2015) are promising prevention approaches added to the existing arsenal of effective tools (Giannou et al., 2015) and UNAIDS has thus set an ambitious goal to end the HIV epidemic, as a public health threat, by 2030 (UNAIDS, 2015).
The first important element of a comprehensive strategy to contain HIV transmission and reach UNAIDS targets is public health authorities and other groups in each locale to understand their epidemic (WHO, 2015). Epidemiological input from HIV/AIDS reporting systems, biological surveillance, and behavioral surveys is necessary to get a deep understanding of local epidemics that could then help design, implement, and evaluate appropriate prevention measures (WHO and UNAIDS, 2013). In this context, molecular studies become invaluable as they shed light on parameters that traditional epidemiological techniques fail to capture.
This review summarizes evidence on circulating HIV-1 subtypes in people who inject drugs (PWID) in Europe and Asia. This geographical area includes countries with past or recent PWID-related outbreaks (Nikolopoulos et al., 2015a), countries with mega-epidemics in PWID such as Ukraine, Russia, China, and Malaysia (Wolfe et al., 2010), and hotspots of ongoing recombination processes (Lau and Wong, 2013).
2. Molecular Epidemiology
HIV-1 and HIV-2 comprise two distinct types of HIV. HIV-2 is classified in groups (A to G) and one recombinant (HIV2_CRF01_AB), and is more closely related to Simian Immunodeficiency Viruses (SIV) isolated from sooty mangabeys (Clavel et al., 1986; Gao et al., 1992; Ibe et al., 2010; Sharp and Hahn, 2011). HIV-2 is mostly concentrated in West Africa although it has been detected in other geographical settings, especially in those with historical and political ties to West Africa (Campbell-Yesufu and Gandhi, 2011; Soriano et al., 2000). Between 1 and 2 million people live with HIV-2 in West Africa but both its incidence and prevalence show decreasing trends probably because the transmission efficiency of HIV-2 is lower than that of HIV-1 (Campbell-Yesufu and Gandhi, 2011; Tienen et al., 2010). HIV-2 is less pathogenic than HIV-1 with slower progression to advanced disease (Campbell-Yesufu and Gandhi, 2011). ART administration to the HIV-2 infected population is challenging and probably suboptimal (Ekouevi et al., 2014).
HIV-1 is the pandemic type and consists of 4 groups, M, N, O and P, each of which represent independent cross-species transmission events of SIV from chimpanzees (Pan troglodytes troglodytes) and gorillas (D'arc et al., 2015; Gao et al., 1999; Keele et al., 2006; Sharp and Hahn, 2011; Van Heuverswyn et al., 2006). Group N has been identified in very few cases accounting for approximately 0.1% of infections in Cameroon (Roques et al., 2004; Sharp and Hahn, 2011; Simon et al., 1998; Vallari et al., 2010), while Group O is limited to Cameroon and other adjacent countries having infected around 100,000 individuals (Charneau et al., 1994; D'arc et al., 2015; Gürtler et al., 1994; Peeters et al., 1997; Roques et al., 2002; Sharp and Hahn, 2011). Group P was discovered in 2009 and has been detected insofar in only two persons (D'arc et al., 2015; Plantier et al., 2009; Sharp and Hahn, 2011; Vallari et al., 2011).
Around 1960, Group M transitioned epidemiologically to a faster growth rate outpacing regional population growth and managed to spread globally (Faria et al., 2014). Group M strains are classified into nine genetically distinct subtypes (A-D, F-H, J, K), subsubtypes (A1, A2, F1, F2), circulating recombinant forms (CRFs) that have been detected in at least three epidemiologically unlinked individuals, and unique recombinant forms (URFs) (Peeters et al., 2013). Based on global estimates for 2000-2007, subtype B is dominant in Western and Central Europe, Australia and the Americas but represents around 11% of all circulating strains (Hemelaar et al., 2011; Takebe et al., 2008, 2004). Approximately half (48%) of the HIV-1 infected population carries subtype C, which is prevalent in southern Africa and India (Abecasis et al., 2013; Hemelaar et al., 2011; Takebe et al., 2008, 2004). Almost all subtypes and most CRFs and URFs circulate in the Democratic Republic of the Congo and Cameroon where HIV probably originated (Hemelaar et al., 2011). Globally, A, G, D, CRF02_AG, and CRF01_AE account for 12%, 5%, 2%, 8%, and 5% of infections (Abecasis et al., 2013; Hemelaar et al., 2011). Geographical patterns are not stable and may change over time. The proportions of subtype A, CRF01_AE, and CRF02_AG have increased over time (Hemelaar et al., 2011). Although a biological effect on the different and changing estimates of subtypes prevalence is uncertain, previous research has found, for instance, that subtype A viruses had higher rates of heterosexual transmission and lower rates of disease progression than subtype D viruses in the Rakai district of Uganda (Kiwanuka et al., 2009, 2008). Changes in the distribution of HIV subtypes over time is certainly a complex and multifactorial phenomenon, and includes founder effects, population growth and mixing, migration and interconnectivity between geographical settings (Hemelaar et al., 2011). It should be noted that recent reviews suggest an even higher prevalence for subtype B and much lower for subtype C (Lau and Wong, 2013). Finally, new CRFs are continuously being discovered, especially in recombination hotspots in Africa, Southeast Asia, and South America (Lau and Wong, 2013). The current number of identified CRFs is 79 (http://www.hiv.lanl.gov/; assessed May 28th, 2016).
3. HIV transmission and epidemiology among PWID
An HIV infectee can pass the virus onto susceptible individuals usually through unprotected sexual intercourse, by sharing of injecting equipment, and vertically such as from infected mothers to children during pregnancy, at delivery, and through breastfeeding (Cohen et al., 2011b). The estimated risk of infection following needle/syringe sharing with an HIV positive individual is 0.63% and comes fourth after blood transfusion (92.5%), perinatal transmission (22.6%), and receptive anal intercourse (1.38%) (Patel et al., 2014).
A systematic review has calculated that approximately 16 (11-21) million people worldwide inject drugs. Of these, 4 (3-5) million are in East and Southeast Asia, 3.5 (2.5-4.5) million in Eastern Europe, 2.2 (1.6-3.1) million in Canada and the US, 2 (1.5-2.5) million in Latin America, 1.8 (0.5-3) million in Sub-Saharan Africa, and 1 (0.8-1.3) million in Western Europe (Mathers et al., 2008).
It is estimated that 36.7 million people were living with HIV in 2015 and more than half of them were in Sub-Saharan Africa, mostly infected through unprotected sexual intercourse (UNAIDS, 2016, 2015). Injecting drug use is responsible for nearly 10% of all HIV infections (Mathers et al., 2008; Strathdee and Stockman, 2010). Around 3 million (0.8-6.6) PWID are infected with HIV and most of them are in Eastern Europe (~1 million), in East and Southeast Asia (0.7 million), and in Latin America (0.6 million), while the rest are in Canada and the US (0.35 million), in Sub-Saharan Africa (0.2 million), and in Western Europe (0.1 million) (Mathers et al., 2008).
Management of HIV disease in PWID is challenging. PWID may also suffer from mental illness, co-infection with hepatitis B or C and tuberculosis is common, and they are at increased risk of experiencing drug overdose, violence, and incarceration (Kamarulzaman and Altice, 2015). Although not corroborated by some studies (Wood et al., 2008), drug injectors seem to have suboptimal outcomes along the HIV continuum of care with heightened rates of AIDS and mortality, both overall and liver-related, following initiation of ART (Kamarulzaman and Altice, 2015; Larsen et al., 2010; Murray et al., 2012; Weber et al., 2015). Increased access to treatment, retention to care and adherence to ART, provision of integrated services, fighting stigma and discrimination, reform of harmful policies, and lack of hepatitis C co-infection can improve the outcome of HIV+ PWID (Braitstein et al., 2006; Kamarulzaman and Altice, 2015; Murray et al., 2012; Wolfe et al., 2010).
4. PWID in Former Soviet Union (FSU) states and molecular epidemiology of HIV
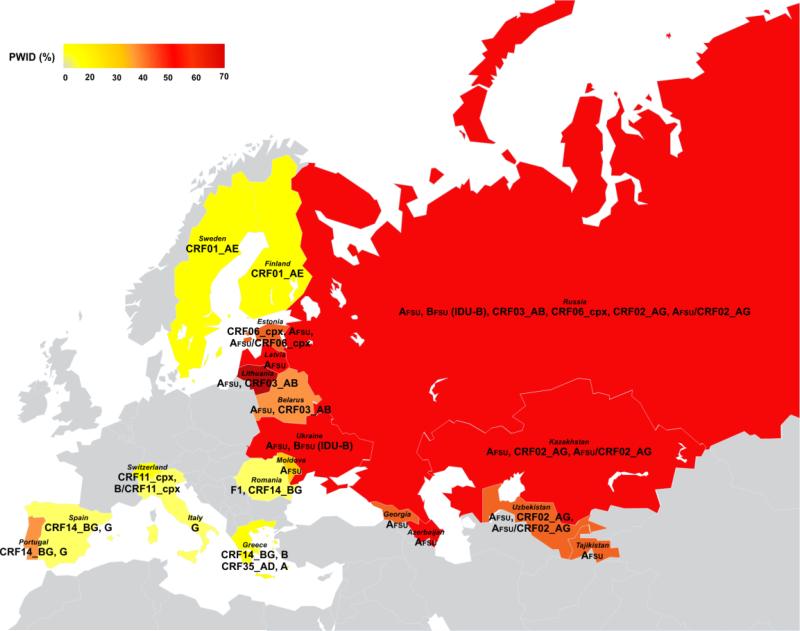
The collapse of Former Soviet Union was followed by economic instability, newly-established networks of drug trafficking, high unemployment rates, serious destruction of norms and values, intergenerational gaps, and youth alienation, which, in turn, contributed to increased rates of injecting drug use and a huge HIV epidemic among PWID (Friedman et al., 2009; Rhodes and Simic, 2005; Strathdee et al., 2006; United Nations Office on Drugs and Crime (UNODC), 2014). Heterosexual transmission of HIV has gradually been increasing and constitutes now the main route of HIV spread in Eastern Europe (European Centre for Disease Prevention and Control (ECDC)/World Health Organization (WHO), 2014). It is estimated that around 1.5 million people live nowadays with HIV in Eastern Europe and Central Asia, and more than 90% of them are in Russia and Ukraine (Bobkova, 2013). Molecular patterns of HIV spread among PWID in the region are shown in Figure 1.
Figure 1.
Distribution of HIV-1 variants among people who inject drugs (PWID) in Europe and Central Asia.
Abbreviations: CRF, circulating recombinant form; FSU, Former Soviet Union; PWID: people who inject drugs.
Note: Colors reflect the proportion of PWID among newly diagnosed cases of HIV infection. The color for Russia is based on 2013 data retrieved from the UNODC (United Nations Office on Drugs and Crime (UNODC), 2014). For the rest of the countries, colors are based on 2014 data retrieved from the ECDC/WHO (European Centre for Disease Prevention and Control (ECDC)/World Health Organization (WHO), 2014). In the gray region, subtype B predominates.
Between the late 1980s and early 1990s, HIV diagnoses in FSU were rather limited, primarily representing sexually-acquired infections (mostly abroad) and nosocomial outbreaks affecting children in southern Russia, and were characterized by substantial molecular diversity (circulating strains included subtypes A-D, F, G, H, and some recombinants) (Bobkov et al., 1994, 2004a; Bobkova, 2013; Lukashov et al., 1995; Thomson and Najera, 2007). However, a big HIV epidemic among PWID started in Ukraine in the mid-1990s and was the result of two single introductions of sub-subtype A1 (called AFSU or IDU-A) and subtype B (labelled here BFSU or, as is widely known, IDU-B) (Bobkova, 2013; Nabatov et al., 2002). AFSU that originated in Africa (Thomson et al., 2007), appeared in Odessa, a major seaport and transportation hub in Southern Ukraine, in 1994-1995 (Novitsky et al., 1998). Research suggests that AFSU originated in the Democratic Republic of the Congo and dates the most recent common ancestor (tMRCA) of AFSU in Odessa in 1984 (Díez-Fuertes et al., 2015). The tMRCA of the sub-cluster of AFSU that caused the PWID-related epidemic was probably also present in Odessa in 1993, a few years before the huge explosion of HIV in PWID (Díez-Fuertes et al., 2015). AFSU spread rapidly to Ukraine (Bobkova, 2013; Saad et al., 2006b), Russia (Bobkov et al., 2001, 2004a), Belarus (Lazouskaya et al., 2005; Lukashov et al., 1998), Kazakhstan (Bobkov et al., 2004b; Eyzaguirre et al., 2007), Uzbekistan (Kurbanov et al., 2003), Georgia (Zarandia et al., 2006), Latvia (Balode et al., 2012, 2004; Ferdats et al., 1999), Lithuania (Caplinskas et al., 2013), Azerbaijan (Saad et al., 2006a), Tajikistan (Beyrer et al., 2009), Armenia (Laga et al., 2015b), Kyrgyzstan (Laga et al., 2015a), and the FSU republic of Moldova (Pandrea et al., 2001). Migratory waves between the FSU states and other countries, and increasing rates of heterosexually-acquired HIV infection have produced some changes in the molecular epidemiological patterns of FSU countries. However, AFSU remains the predominant strain among drug injectors and heterosexually-infected individuals in the above-mentioned countries, including the Russian Far East, with prevalence estimates ranging between 50%-94% (Balode et al., 2012; Bobkova, 2013; Eyzaguirre et al., 2007; Kazennova et al., 2014; Lazouskaya et al., 2005; Rumyantseva et al., 2009; Saad et al., 2006a; Smolskaya et al., 2006; Zarandia et al., 2006).
Among PWID in Estonia, AFSU follows in frequency the predominant CRF06_cpx strains (Adojaan et al., 2005; Avi et al., 2011; Zetterberg et al., 2004). CRF06_cpx has been identified in Africa and meets the complex designation (cpx) because 4 subtypes (A, G, K, J) contribute to genome structure (Montavon et al., 2002). Although CRF06_cpx is constricted to Estonia, it has reportedly increasingly been circulating in Saint Petersburg, the second largest Russian city (Bobkova, 2013). Recombination of AFSU and CRF06_cpx has been observed in Estonia (Adojaan et al., 2005; Avi et al., 2011). CRF02_AG, a common strain in Africa, is the prevailing clade among PWID in Kyrgyzstan followed by AFSU (Laga et al., 2015a). CRF63_02A1, which is a recombination of AFSU and CRF02_AG (Baryshev et al., 2014, 2012), has also been detected in Kyrgyzstan (Laga et al., 2015a). CRF02_AG and its recombinants with AFSU have generally become prevalent in Central Asia (Uzbekistan, Kyrgyzstan, Kazakhstan) (Bobkova, 2013; Carr et al., 2005; Eyzaguirre et al., 2007; Laga et al., 2015a; Lapovok et al., 2014) and also in Asian parts of Russia as drug trafficking routes seem to be changing (Baryshev et al., 2012). It should be mentioned that A1 has been spreading in some Central and Western European countries through sexual or injecting networks (Lai et al., 2016; Parczewski et al., 2016).
BFSU (IDU-B) is a common variant in the region (Bobkova, 2013) but failed to spread widely outside of Ukraine creating mainly localized epidemics in Nikolayev, an important transportation junction of Ukraine where the strain was first identified (Nabatov et al., 2002), and in a couple of Ukrainian urban settings, including the capital city of Kiev (Bobkova, 2013; Saad et al., 2006b). BFSU (IDU-B) was rare in Russia but recent analyses showed that has approximately infected 62% of PWID in Vladivostok, the largest sea port of Russian Far East (Kazennova et al., 2014). The increased prevalence of BFSU (IDU-B) in the Far Eastern region of Russia is attributed to labor migrants from Ukraine (Bobkova, 2013; Kazennova et al., 2014). BFSU (IDU-B) strains belong to a monophyletic clade and are distinct from subtype B sequences circulating in Western Europe. The origin of BFSU (IDU-B) remains unknown (Bobkova, 2013).
AFSU and BFSU (IDU-B) were the parental strains of CRF03_AB, a recombinant that infected more than 2,000 PWID in Kaliningrad (Russian exclave between Poland and Lithuania) in mid- to late 1990s (Bobkova, 2013; Liitsola et al., 2000a, 1998). CRF03_AB has occasionally been detected in other Russian areas including Saint Petersburg (Lukashov et al., 1999), has produced an outbreak in Cherepovets (Northern Russia) (Bobkova, 2013; Kazennova et al., 2014; Smolskaya et al., 2006), is very prevalent in Ekaterinburg (Central Russia) comprising 23% of infections (Bobkova, 2013), and has become of epidemiological relevance in Belarus (Eremin et al., 2011) and Lithuania (Caplinskas et al., 2013).
5. Molecular Epidemiology of HIV among PWID in Western and Central Europe
Subtype B travelled from the US to Europe infecting initially MSM, and then PWID and other key populations (Glauser and Francioli, 1984; Kuiken et al., 2000; Thomson and Najera, 2007). PWID in Europe became infected either from local epidemics among MSM (Casado et al., 2000; Kuiken et al., 2000; Thomson and Najera, 2007) or from a variant circulating among PWID in North America (Lukashov et al., 1996; Thomson and Najera, 2007).
Between 70% and 85% of newly diagnosed infections in Western and Central Europe have been caused by subtype B while subtypes A, C and G circulate in less than 20% of infected persons (Abecasis et al., 2013; Bannister et al., 2006; Hemelaar et al., 2011). The prevalence of non-B subtypes has increased over the years, which has been attributed to migration from Sub-Saharan Africa and South America (Holguín et al., 2008; Paraskevis et al., 2007). Among PWID, subtype B is predominant (around 70%) followed by subtype G (10%), subtype A1 (around 5%), and CRF02_AG (3%) (Abecasis et al., 2013; Stanojevic et al., 2012). Circulating subtypes and CRFs among PWID in the region are shown in Figure 1.
Spain and Portugal experienced an enormous HIV spread among PWID in the 1990s and their epidemics are characterized by substantial viral diversity and distinct molecular properties compared to other European countries (Carvalho et al., 2015). Non-B subtypes were present among HIV+ PWID in the early years of the epidemic in Spain (Lospitao et al., 2005). Non-B subtypes, and especially subtype G (21-24%), were also very prevalent among PWID in Portugal in the late 1990s (Esteves et al., 2003, 2002), while subtype G is still found in around 30% of all diagnoses (Carvalho et al., 2015; Palma et al., 2007). Subtype G and other non-B subtypes were introduced in Portugal following intense migration between Portugal and its former African colonies in the late 1970s and early 1980s, especially because of the Portuguese Colonial War that involved multiple theatres of operation including Angola with a high degree of HIV-1 group M genetic diversity (Bártolo et al., 2009; Carvalho et al., 2015; Vermund and Leigh-Brown, 2012). Subtype G, which also circulates among PWID in Spain (Delgado et al., 2002; Pérez-Alvarez et al., 2003), recombined with subtype B, probably in Portugal early in the history of the epidemic, creating CRF14_BG that has been detected in Portugal, at low prevalence in the region of Galicia, Spain, and in some other European settings (Bártolo et al., 2011; Carvalho et al., 2015; Duque et al., 2003; Harris et al., 2005; Thomson et al., 2001). Although CRF14_BG soon became the predominant CRF in Portugal and Spain, its prevalence has been decreasing, a finding that might be associated with its high pathogenicity or its tendency to recombine with other strains, but also to the declining prevalence of HIV-1 among PWID (Bártolo et al., 2011; Carvalho et al., 2015).
Despite decreasing trends in HIV-1 diagnoses among PWID in Western Europe after 2000, Greece and Romania, and in particular their capital cities, Athens and Bucharest respectively, experienced recent PWID-related outbreaks (Nikolopoulos et al., 2015a; Paraskevis et al., 2015). The HIV-1 epidemic in Greece was concentrated in MSM (Nikolopoulos et al., 2008) being the product of multiple introductions of subtype B from other Western countries although the prevalence of subtype A has been increasing over time (Paraskevis et al., 2007). Before 2011, HIV-1 infections among drug injectors in Greece were rather sporadic and not phylogenetically clustered indicating limited networks of transmission in this group (Paraskevis et al., 2013). In parallel with a serious economic crisis, HIV-1 diagnoses among PWID exploded in 2011 and peaked in 2012 (Nikolopoulos et al., 2015b; Paraskevis et al., 2011). The majority of the Greek PWID-related sequences fell within four transmission clusters: CRF14_BG (49%), CRF35_AD (18%), subtype B (12%), and subtype A (6%) (Paraskevis et al., 2015, 2013). The two recombinants had not been identified in samples collected before 2011 and phylodynamic analyses estimated the start of their transmission clusters in 2010-2011 (Paraskevis et al., 2015, 2013). CRF14_BG originated in strains circulating in Romania, while CRF35_AD had its origin in Afghanistan/Iraq (Paraskevis et al., 2015, 2013). Outbreak subtypes A and B originated in Greece; the B cluster begun earlier than the others, probably in 2008 (Paraskevis et al., 2015). HIV transmission among PWID in Greece seems to be subsiding as the number of diagnoses decreased in 2014/2015 (Nikolopoulos et al., 2015b) and effective reproductive numbers estimated by molecular analyses for two of the outbreak clusters had fallen below one by November 2013 (Paraskevis et al., 2015).
Subtype B prevails in HIV infections in central European counties including Poland, Slovakia, the Czech Republic, Hungary, and most Balkan states although some non-B strains including A1 have been identified, especially among persons infected heterosexually or through injecting drug use (Chabadová et al., 2014; Habekova et al., 2010; Linka et al., 2008; Mezei et al., 2011; Reinis et al., 2001; Smoleń-Dzirba et al., 2012; Stanojevic et al., 2012). In Romania, however, HIV spread probably through contaminated needles/syringes used for therapeutic injections and seriously affected newborns and children in orphanages (Hersh et al., 1993, 1991). The Romanian epidemic has been dominated by subtype F1, being a unique case in Europe (Stanojevic et al., 2012). Following decreased support from international funders and heightened injecting rates of stimulants, an HIV-1 outbreak occurred among PWID in Bucharest between 2011 and 2013 with transmission networks including one cluster based on CRF14_BG (23%) and two clusters based on F1 (20% and 50% respectively) (Niculescu et al., 2015; Paraskevis et al., 2015). F1 strains in Romania originated, in contrast to Greece, from locally circulating clades, while CRF14_BG was probably introduced to Romania from Spain/Portugal (Paraskevis et al., 2015). It is estimated that one F1 cluster started earlier (2008) than the others (2010) (Paraskevis et al., 2015).
European countries other than those described before have also experienced HIV outbreaks among PWID in the past. Contrary to the Greek and Romanian cases, previous outbreaks in Western and Central European settings were primarily caused by a single circulating clade although other strains could co-circulate as well (Paraskevis et al., 2015). For example, in the late 1990s-early 2000s in Finland, HIV affected a marginalized population of PWID with high rates of imprisonment and homelessness (Kivelä et al., 2007) and CRF01_AE, a prevalent variant in Southeast Asia that was circulating in Finland in the early 1990s, was the cause of the outbreak (Kivelä et al., 2005; Liitsola et al., 2000b; Skar et al., 2011). CRF01_AE was imported from Helsinki, Finland to Stockholm, Sweden leading there to an outbreak among PWID that started probably in around 2003 and was detected in 2006 (Skar et al., 2011). Similarly, in a PWID-related outbreak in early 2000s in Northern Italy, HIV-1 diagnoses among PWID formed a monophyletic cluster of subtype G with origin in West Africa (Ciccozzi et al., 2007). Finally, CRF11_cpx, of African origin, has been identified in half of PWID with HIV-1 in the western part of Switzerland while B/CRF11 co-infection in that group of PWID was also frequent (5%) (Thomson and Najera, 2007; Yerly et al., 2004).
6. Molecular Epidemiology of HIV among PWID in Asia
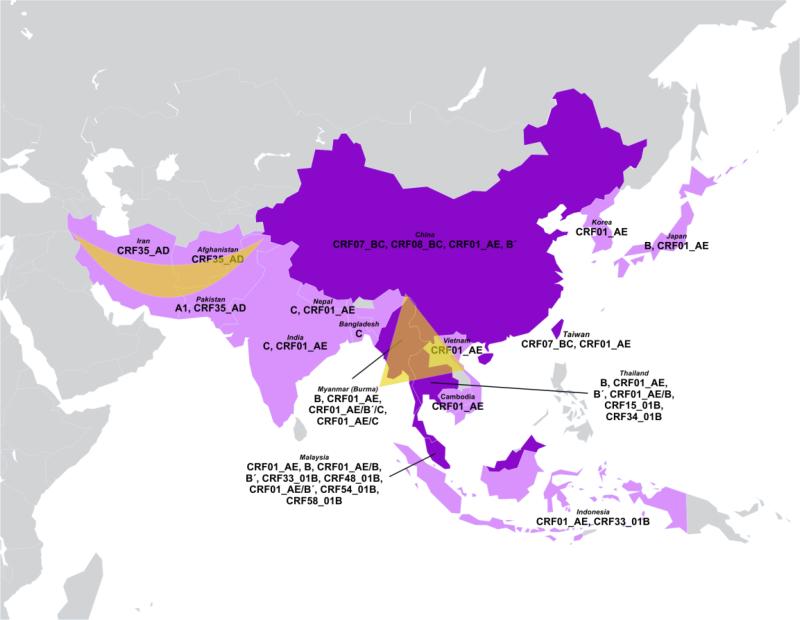
Injecting drug use has been fueling the HIV epidemic in many parts of this region, which includes the two primary opium-producing areas in the world: the Golden Crescent defined by peripheries of mountains in Afghanistan, Iran and Pakistan, and the Golden Triangle than spans Myanmar (Burma), Laos, Vietnam, and Thailand. The major HIV strains that circulate in South and Southeast Asia are subtypes B and C, and CRF01_AE (Lau et al., 2007). Genetic complexity, however, has increased over time as multiple recombination events took place (Figure 2).
Figure 2.
Distribution of HIV-1 variants among people who inject drugs (PWID) in Asia.
Note: Deep purple highlights countries that include hotspots of genetic recombination between HIV-1 strains. Major areas of opium production include the Golden Crescent defined by peripheries of mountains in Afghanistan, Iran, and Pakistan and the Golden Triangle that spans Myanmar (Burma), Laos, Vietnam, and Thailand.
6.1. Counties of South and Southeast Asia with recombination hotspots
In Thailand, during the early years of the epidemic, subtype B (Western type B and Thai B or B’) was the most prevalent clade among PWID while CRF01_AE was frequent in people infected heterosexually (Deng et al., 2008; Kijak et al., 2013; Ou et al., 1993). The ancestor of Thai B or B’ existed around 1985 having diverged from subtype B and became the founder strain in South and Southeast Asia (Deng et al., 2008; Junqueira and Almeida, 2016; Li et al., 2010b). In the mid-1990s and early 2000s, however, CRF01_AE took over in PWID in Thailand (Kijak et al., 2013; Subbarao et al., 1998; Vongsheree et al., 2002) and spread among injecting drug users in other countries of the region including Cambodia, Vietnam, Malaysia, China, Taiwan, Korea, and Japan (Chow et al., 2014; Kato et al., 2001; Lau et al., 2007; Liao et al., 2009; Menu et al., 1996; Shiino et al., 2014). As a matter of fact, Thailand was the source of the CRF01_AE epidemic in the rest of Asia and Europe (Angelis et al., 2015). Some PWID in Thailand were dually infected by CRF01_AE and B (Ramos et al., 2002), and recombinants such as CRF15_01B, and CRF34_01B appeared in Thai injectors (Kijak et al., 2013; Tovanabutra et al., 2007, 2004, 2003, 2001).
Malaysia is home of a PWID-related epidemic with 70% of HIV diagnoses attributed to injecting drug use (Chow et al., 2013). Similarly to Thailand, Malaysia has been experiencing molecular shifts over the years. CRF01_AE and subtype B had been circulating among PWID (Beyrer et al., 1998; Brown et al., 1996; Saraswathy et al., 2000) but, in the early 2000s, CRF01_AE/B recombinants were detected at increasing rates (Tee et al., 2005a, 2005b). Recently, recombinants that originated in Malaysia, CRF33_01B (Tee et al., 2006), and its descendants CRF48_01B (Li et al., 2010a), CRF54_01B (Ng et al., 2012), and CRF58_01B (Chow et al., 2014) have been identified in PWID. A recent study based on samples collected between 2010 and 2011, found that the prevalence of CRF33_01B among PWID in Kuala Lumpur was very high (71%) followed by subtype B' (11%), CRF01_AE (5%), and CRF01_AE/B’ unique recombinants (13%) (Chow et al., 2013). CRF33_01B has also been detected in other countries of the region (Sahbandar et al., 2009).
Major strains circulating in China include subtype B’ (9.6%), CRF01_AE (27.6%), CRF07_BC (35.5%), and CRF08_BC (20.1%) (Han et al., 2015; He et al., 2012; Laeyendecker et al., 2005). Subtype C is currently a minor clade with a prevalence at 1.6% (He et al., 2012). Injecting drug use may still account for around half of HIV transmissions in China (He et al., 2012). Among PWID, the most updated studies showed that the subtype distribution is: CRF01_AE (21.2%), CRF07_BC (48.5%) and CRF08_BC (23.6%) (He et al., 2012). The province of Yunnan that is located in the Southwest part of China and shares borders with Vietnam, Laos, and Burma (Myanmar), has played an important role in HIV spread in China and serves as a hotspot of genetic mixing due to its closeness to the Golden Triangle and the heroin trafficking routes (Beyrer et al., 2000). Subtype C from India and subtype B from Thailand caused the first outbreaks among PWID in Yunnan in the late 1980s and early 1990s (Han et al., 2015; Piyasirisilp et al., 2000; Tee et al., 2008; Zheng et al., 1994). Subtype B', that soon became the predominant B clade in China (Graf et al., 1998; Lau et al., 2007), and subtype C recombined to generate two related but distinct strains, CRF07_BC and CRF08_BC, which trace their origin to Yunnan and fueled the epidemic among PWID in Northwestern, Southeastern and Northeastern China, and to Taiwan (CRF07_BC) (Han et al., 2015; He et al., 2012; Laeyendecker et al., 2005; Lau et al., 2007; Li et al., 2015; Lin et al., 2006; Piyasirisilp et al., 2000; Takebe et al., 2010; Tee et al., 2008). CRF07_BC has also become the predominant strain among PWID (>80%) in Taiwan (Chen et al., 2012, 2010; Huang et al., 2014; Lin et al., 2006). Finally, CRF01_AE, imported from surrounding countries and following drug trafficking routes, was detected in PWID in the provinces of Yunnan, Guangdong, and Guangxi in the mid-1990s (Beyrer et al., 2000; He et al., 2012; Yu et al., 1999). CRF01_AE strains, related to those circulating in Guangxi and other Chinese prefectures, have been identified in transmission clusters containing PWID in Hong Kong (Chen et al., 2009). Molecular analyses have showed that the CRF01_AE epidemics in China and Vietnam were monophyletic suggesting regional dispersal (Angelis et al., 2015).
PWID in Myanmar (Burma) have been infected by subtype B, CRF01_AE, and various recombinants. Subtype B is predominant among PWID in Southern Myanmar but the proportion of CRF01_AE and recombinants is elevated in central regions (Kusagawa et al., 1998; Motomura et al., 2000; Takebe et al., 2003; Zhou et al., 2014). However, the northern part of Myanmar, which shares borders with the Chinese province of Yunnan, is very interesting in epidemiological terms as the majority (>85%) of PWID in the region carry recombinant strains (Liu et al., 2012; Pang et al., 2012; Zhou et al., 2014). Travel, trade and intermarriage across the Myanmar-China borders are common. Dehong prefecture of Yunnan province that includes Ruili district, where the first PWID outbreak in China was detected, has been an important hub of drug trafficking from the Golden Triangle into China and large numbers of PWID, both Burmese and Chinese, cross the Myanmar-China borders to inject drugs (Han et al., 2013; Zhou et al., 2014). Both sides of the borders region have high prevalence of recombinants (>80%) (Han et al., 2013; Pang et al., 2012; Zhou et al., 2014). The proportion of CRF01_AE-related recombinants (CRF01_AE/B'/C or CRF01_AE/C) is higher in Northern Myanmar than in Dehong, China where B’/C recombinants prevail (Han et al., 2013; Pang et al., 2012). This finding suggests that events of genetic mixture in Dehong and Northern Myanmar are, to some degree, independent. Beyond drug injectors who cross the borders and become infected probably by multiple co-circulating strains, other factors may contribute to high levels of genetic heterogeneity in the region. For instance, Burmese long-distance truck drivers who drive between Mandalay, Myanmar and Ruili, China, are engaged in unprotected sex with occasional, multiple partners or sex workers, and some of them also inject drugs (Zhou et al., 2014). These drivers may serve as a bridge of bidirectional transmission between heterosexuals and PWID on both sides of the borders (Zhou et al., 2014).
6.2. Other countries in South and Southeast Asia
CRF01_AE predominates in Cambodia, which has experienced high transmission rates among heterosexuals and HIV prevalence was more than 20% in PWID (Kusagawa et al., 1999; Lau et al., 2007; Menu et al., 1999; Strathdee and Stockman, 2010). In Indonesia, analyses have shown a high prevalence (>90%) of CRF01_AE among drug injectors (Lau et al., 2007; Sahbandar et al., 2009). However, recombinants, including CRF33_01B, have been found (Sahbandar et al., 2009). CRF01_AE, introduced from Thailand to heterosexuals and drug injectors in South Vietnam, is also the major clade circulating among PWID in the country (Lan et al., 2003; Liao et al., 2009). Cross-border transmissions between Northern Vietnam and the Guangxi province of China have been reported (Kato et al., 2001, 1999; Liao et al., 2009). In Japan, drug injectors significantly contributed to CRF01_AE spread, which is the second most prevalent clade after subtype B (Shiino et al., 2014).
6.3. India, Nepal, Bangladesh
India hosts more than 2.5 million people with HIV (Neogi et al., 2012; Shen et al., 2011) and the prevalence in PWID in some settings is as high as 30% with half of the infected being unware of their infection (Armstrong et al., 2015; Goswami et al., 2014). Subtype C is the major strain circulating among HIV-infected people in India and Nepal including drug injectors (Lau et al., 2007; Mandal et al., 2002; Neogi et al., 2012, 2011; Oelrichs et al., 2000; Shahid et al., 2011; Shen et al., 2011). Recombinants of subtypes B and C, and CRF01_AE have been identified in these countries (Bhanja et al., 2005; Mullick et al., 2010; Sarkar et al., 2009; Shahid et al., 2011; Tripathy et al., 2005). HIV prevalence is generally low in the neighboring country of Bangladesh but is more than 5% in PWID in Dhaka, the capital of Bangladesh (Bontell et al., 2013). The epidemic in general and among PWID is subtype C-driven, and strains cluster with viruses from India and Myanmar (Azim et al., 2002; Bontell et al., 2013; Sarker et al., 2008).
6.4. Afghanistan, Iran, Pakistan (Golden Crescent)
Drug injection is the main route of HIV transmission in Iran (~70% of diagnoses) with a prevalence in PWID around 15% (Baesi et al., 2014; Khajehkazemi et al., 2013; Memarnejadian et al., 2015). CRF35_AD is the predominant strain in Iranian drug injectors and clusters with CRF35_AD infections detected in Afghanistan (Baesi et al., 2014; Jahanbakhsh et al., 2013a, 2013b; Memarnejadian et al., 2015; Mousavi et al., 2010). CRF01_AE has rarely been detected in samples of drugs injectors in Iran (Jahanbakhsh et al., 2013b) and subtype A had been identified in the past (Naderi et al., 2006; Sarrami-Forooshani et al., 2006; Tagliamonte et al., 2007).
Afghanistan is the primary producer of opium worldwide but HIV prevalence was relatively low in mid-2000s at around 2-3% (Sanders-Buell et al., 2010, 2007). However, in that period, a novel recombinant, CRF35_AD, was first identified in PWID in Afghanistan and is the major strain in that group (Sanders-Buell et al., 2010, 2007). The genetic relatedness of CRF35_AD strains circulating in Iran and Afghanistan suggests transmission linkages between PWID of these countries due to migration or through drug trafficking as Iran lies on the drug trafficking pathway between Afghanistan and Europe (Baesi et al., 2014).
The HIV epidemic has been growing in Pakistan affecting also the population of injecting drug users with increasing trends in HIV prevalence from around 15% in 2006 to around 30% in 2011 (Altaf et al., 2009; Archibald et al., 2013; Shah et al., 2011). Although CRF35_AD has been found in HIV positives in Pakistan, subtype A1 is the most frequent clade among PWID (Khan et al., 2006; Shah et al., 2011).
7. Conclusion
Injecting drug use is a risky practice associated with increased likelihood of HIV acquisition. Over the last 30 years, millions of PWID became infected with an estimated overall mortality almost 3 times higher among HIV positive than among HIV negative drug injectors (Mathers et al., 2013). In addition, PWID serve as bridges of HIV transmission into other groups including the general population. For instance, nowadays in New York, a city that suffered a huge epidemic among PWID in the 1980s and 1990s but succeeded in containing it in 2000s, especially by increasing access to sterile injecting equipment and ART, HIV prevalence is higher in non-injecting drug users than in PWID (Des Jarlais et al., 2011). High rates of heterosexual transmission of HIV have been observed in FSU countries following the mega-epidemics among PWID during the 1990s (Bobkova, 2013; European Centre for Disease Prevention and Control (ECDC)/World Health Organization (WHO), 2014). However, on the other hand, comprehensive prevention approaches have been developed including scaling-up HIV testing, distribution of clean injection equipment, and increased access to substitution programs and ART that can significantly reduce infection rates in PWID (Abdul-Quader et al., 2013; Aspinall et al., 2014; Des Jarlais et al., 2013; MacArthur et al., 2014).
Molecular epidemiology has revealed underlying patterns of HIV spread that traditional epidemiological methods are inherently unable to capture. For example, we know that starting in Odessa, Ukraine, a major seaport of the Black sea, a particular variant, subtype A1, spread at impressively rapid rates among PWID and to almost every part of the former Soviet Union. Contrary to patterns seen before in Western/Central Europe, Greece and Romania experienced PWID-related outbreaks consisted of multiple transmission clusters. One of the clusters (CRF35_AD) in Greece had its origin in circulating strains in Afghanistan/Iran. This finding indicated the role of population movement in HIV spread, which should be taken into account when public health interventions are designed and implemented. As a matter of fact, in Greece, successful efforts to contain the epidemic (Hatzakis et al., 2015) targeted migrant groups. Finally, the borders of China, Myanmar, Laos, and Thailand comprise a big spot of genetic exchanges between HIV variants circulating in PWID and other key populations of different ethnicities producing novel intersubtype and inter-CRF recombinants. This is a major issue of public health concern as novel strains may further complicate the process of vaccine development, increase transmission and disease progression rates or change patterns of resistance to antiretrovirals (Abecasis et al., 2006; Baeten et al., 2007; Brenner et al., 2003; Renjifo et al., 2004; Stephenson and Barouch, 2013). In addition, molecular epidemiology has shown that HIV spreads in many parts of this region following routes of illicit drugs trafficking, which clearly shows that it might be hard to control HIV unless appropriate measures are taken to limit drug markets and trafficking.
Taken all findings together, it seems that routine systems of collecting, analyzing and interpreting molecular information are urgently needed if we do want to understand better and deeper HIV diversity and its public health implications.
Highlights.
Molecular studies reveal patterns of HIV spread among People who Inject Drugs (PWID).
AFSU, BFSU (IDU-B), and CRF03_AB produced big epidemics in Former Soviet Union states.
Subtype G and CRF14_BG caused big epidemics in Portugal/Spain.
Transmission clusters (B, A, F1, CRF14_BG, CRF35_AD) were observed in Greece/Romania.
CRF35_AD is the most prevalent clade among PWID in Afghanistan/Iran.
Recombination hotspots in South-East Asia have been generating several recombinants.
Acknowledgements
Funding.
GKN acknowledges support from US National Institute on Drug Abuse (grant: DP1 DA034989).
Abbreviations
- AIDS
Acquired Immune Deficiency Syndrome
- ART
antiretroviral treatment
- CDC
United States (US) Centers for Disease Control and Prevention
- CRFs
circulating recombinant forms
- FSU
Former Soviet Union
- HIV
Human Immunodeficiency Virus
- MSM
men who have sex with men
- PrEP
pre-exposure prophylaxis
- PWID
people who inject drugs
- SIV
Simian Immunodeficiency Virus
- TaSP
treatment as prevention
- tMRCA
time of the most recent common ancestor
- UNAIDS
United Nations Joint Programme on HIV/AIDS (UNAIDS)
- URFs
unique recombinant forms
- WHO
World Health Organization
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest.
The authors declare that they have no conflict of interest.
References
- Abdul-Quader AS, Feelemyer J, Modi S, Stein ES, Briceno A, Semaan S, Horvath T, Kennedy GE, Des Jarlais DC. Effectiveness of structural-level needle/syringe programs to reduce HCV and HIV infection among people who inject drugs: a systematic review. AIDS Behav. 2013;17:2878–92. doi: 10.1007/s10461-013-0593-y. doi:10.1007/s10461-013-0593-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abecasis AB, Deforche K, Bacheler LT, McKenna P, Carvalho AP, Gomes P, Vandamme A-M, Camacho RJ. Investigation of baseline susceptibility to protease inhibitors in HIV-1 subtypes C, F, G and CRF02_AG. Antivir. Ther. 2006;11:581–9. [PubMed] [Google Scholar]
- Abecasis AB, Wensing AMJ, Paraskevis D, Vercauteren J, Theys K, Van de Vijver DAMC, Albert J, Asjö B, Balotta C, Beshkov D, Camacho RJ, Clotet B, De Gascun C, Griskevicius A, Grossman Z, Hamouda O, Horban A, Kolupajeva T, Korn K, Kostrikis LG, Kücherer C, Liitsola K, Linka M, Nielsen C, Otelea D, Paredes R, Poljak M, Puchhammer-Stöckl E, Schmit J-C, Sönnerborg A, Stanekova D, Stanojevic M, Struck D, Boucher CAB, Vandamme A-M. HIV-1 subtype distribution and its demographic determinants in newly diagnosed patients in Europe suggest highly compartmentalized epidemics. Retrovirology. 2013;10:7. doi: 10.1186/1742-4690-10-7. doi:10.1186/1742-4690-10-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adojaan M, Kivisild T, Männik A, Krispin T, Ustina V, Zilmer K, Liebert E, Jaroslavtsev N, Priimägi L, Tefanova V, Schmidt J, Krohn K, Villems R, Salminen M, Ustav M. Predominance of a rare type of HIV-1 in Estonia. J. Acquir. Immune Defic. Syndr. 2005;39:598–605. [PubMed] [Google Scholar]
- Altaf A, Saleem N, Abbas S, Muzaffar R. High prevalence of HIV infection among injection drug users (IDUs) in Hyderabad and Sukkur, Pakistan. J. Pak. Med. Assoc. 2009;59:136–40. [PubMed] [Google Scholar]
- Angelis K, Albert J, Mamais I, Magiorkinis G, Hatzakis A, Hamouda O, Struck D, Vercauteren J, Wensing AMJ, Alexiev I, Åsjö B, Balotta C, Camacho RJ, Coughlan S, Griskevicius A, Grossman Z, Horban A, Kostrikis LG, Lepej S, Liitsola K, Linka M, Nielsen C, Otelea D, Paredes R, Poljak M, Puchhammer-Stöckl E, Schmit J-C, Sönnerborg A, Staneková D, Stanojevic M, Boucher CAB, Kaplan L, Vandamme A-M, Paraskevis D. Global Dispersal Pattern of HIV Type 1 Subtype CRF01_AE: A Genetic Trace of Human Mobility Related to Heterosexual Sexual Activities Centralized in Southeast Asia. J. Infect. Dis. 2015;211:1735–44. doi: 10.1093/infdis/jiu666. doi:10.1093/infdis/jiu666. [DOI] [PubMed] [Google Scholar]
- Archibald CP, Shaw SY, Emmanuel F, Otho S, Reza T, Altaf A, Musa N, Thompson LH, Blanchard JF. Geographical and temporal variation of injection drug users in Pakistan. Sex. Transm. Infect. 2013;89(Suppl 2):ii18–28. doi: 10.1136/sextrans-2012-050775. doi:10.1136/sextrans-2012-050775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong G, Medhi GK, Mahanta J, Paranjape RS, Kermode M. Undiagnosed HIV among people who inject drugs in Manipur, India. AIDS Care. 2015;27:288–92. doi: 10.1080/09540121.2014.972322. doi:10.1080/09540121.2014.972322. [DOI] [PubMed] [Google Scholar]
- Aspinall EJ, Nambiar D, Goldberg DJ, Hickman M, Weir A, Van Velzen E, Palmateer N, Doyle JS, Hellard ME, Hutchinson SJ. Are needle and syringe programmes associated with a reduction in HIV transmission among people who inject drugs: a systematic review and meta-analysis. Int. J. Epidemiol. 2014;43:235–48. doi: 10.1093/ije/dyt243. doi:10.1093/ije/dyt243. [DOI] [PubMed] [Google Scholar]
- Avi R, Huik K, Pauskar M, Ustina V, Karki T, Krispin T, Ainsalu K, Paap P, Schmidt J, Nikitina N, Lutsar I. Emerging transmitted drug resistance in treatment-naïve human immunodeficiency virus-1 CRF06_cpx-infected patients in Estonia. Scand. J. Infect. Dis. 2011;43:122–8. doi: 10.3109/00365548.2010.526956. doi:10.3109/00365548.2010.526956. [DOI] [PubMed] [Google Scholar]
- Azim T, Bogaerts J, Yirrell DL, Banerjea AC, Sarker MS, Ahmed G, Amin MMM, Rahman ASMM, Hussain AMZ. Injecting drug users in Bangladesh: prevalence of syphilis, hepatitis, HIV and HIV subtypes. AIDS. 2002;16:121–3. doi: 10.1097/00002030-200201040-00015. [DOI] [PubMed] [Google Scholar]
- Baesi K, Moallemi S, Farrokhi M, Alinaghi SAS, Truong H-HM. Subtype classification of Iranian HIV-1 sequences registered in the HIV databases, 2006-2013. PLoS One. 2014;9:e105098. doi: 10.1371/journal.pone.0105098. doi:10.1371/journal.pone.0105098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baeten JM, Chohan B, Lavreys L, Chohan V, McClelland RS, Certain L, Mandaliya K, Jaoko W, Overbaugh J. HIV-1 subtype D infection is associated with faster disease progression than subtype A in spite of similar plasma HIV-1 loads. J. Infect. Dis. 2007;195:1177–80. doi: 10.1086/512682. doi:10.1086/512682. [DOI] [PubMed] [Google Scholar]
- Balode D, Ferdats A, Dievberna I, Viksna L, Rozentale B, Kolupajeva T, Konicheva V, Leitner T. Rapid epidemic spread of HIV type 1 subtype A1 among intravenous drug users in Latvia and slower spread of subtype B among other risk groups. AIDS Res. Hum. Retroviruses. 2004;20:245–9. doi: 10.1089/088922204773004978. doi:10.1089/088922204773004978. [DOI] [PubMed] [Google Scholar]
- Balode D, Skar H, Mild M, Kolupajeva T, Ferdats A, Rozentale B, Leitner T, Albert J. Phylogenetic analysis of the Latvian HIV-1 epidemic. AIDS Res. Hum. Retroviruses. 2012;28:928–32. doi: 10.1089/aid.2011.0310. doi:10.1089/AID.2011.0310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannister WP, Ruiz L, Loveday C, Vella S, Zilmer K, Kjaer J, Knysz B, Phillips AN, Mocroft A. HIV-1 subtypes and response to combination antiretroviral therapy in Europe. Antivir. Ther. 2006;11:707–15. [PubMed] [Google Scholar]
- Barré-Sinoussi F, Chermann JC, Rey F, Nugeyre MT, Chamaret S, Gruest J, Dauguet C, Axler-Blin C, Vézinet-Brun F, Rouzioux C, Rozenbaum W, Montagnier L. Isolation of a T-lymphotropic retrovirus from a patient at risk for acquired immune deficiency syndrome (AIDS). Science. 1983;220:868–71. doi: 10.1126/science.6189183. [DOI] [PubMed] [Google Scholar]
- Bártolo I, Abecasis AB, Borrego P, Barroso H, McCutchan F, Gomes P, Camacho R, Taveira N. Origin and epidemiological history of HIV-1 CRF14_BG. PLoS One. 2011;6:e24130. doi: 10.1371/journal.pone.0024130. doi:10.1371/journal.pone.0024130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bártolo I, Rocha C, Bartolomeu J, Gama A, Marcelino R, Fonseca M, Mendes A, Epalanga M, Silva PC, Taveira N. Highly divergent subtypes and new recombinant forms prevail in the HIV/AIDS epidemic in Angola: new insights into the origins of the AIDS pandemic. Infect. Genet. Evol. 2009;9:672–82. doi: 10.1016/j.meegid.2008.05.003. doi:10.1016/j.meegid.2008.05.003. [DOI] [PubMed] [Google Scholar]
- Baryshev PB, Bogachev VV, Gashnikova NM. Genetic characterization of an isolate of HIV type 1 AG recombinant form circulating in Siberia, Russia. Arch. Virol. 2012;157:2335–41. doi: 10.1007/s00705-012-1442-4. doi:10.1007/s00705-012-1442-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baryshev PB, Bogachev VV, Gashnikova NM. HIV-1 genetic diversity in Russia: CRF63_02A1, a new HIV type 1 genetic variant spreading in Siberia. AIDS Res. Hum. Retroviruses. 2014;30:592–7. doi: 10.1089/aid.2013.0196. doi:10.1089/aid.2013.0196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyrer C, Patel Z, Stachowiak JA, Tishkova FK, Stibich MA, Eyzaguirre LM, Carr JK, Mogilnii V, Peryshkina A, Latypov A, Strathdee SA. Characterization of the emerging HIV type 1 and HCV epidemics among injecting drug users in Dushanbe, Tajikistan. AIDS Res. Hum. Retroviruses. 2009;25:853–60. doi: 10.1089/aid.2008.0206. doi:10.1089/aid.2008.0206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyrer C, Razak MH, Lisam K, Chen J, Lui W, Yu XF. Overland heroin trafficking routes and HIV-1 spread in south and south-east Asia. AIDS. 2000;14:75–83. doi: 10.1097/00002030-200001070-00009. [DOI] [PubMed] [Google Scholar]
- Beyrer C, Vancott TC, Peng NK, Artenstein A, Duriasamy G, Nagaratnam M, Saw TL, Hegerich PA, Loomis-Price LD, Hallberg PL, Ettore CA, Nelson KE. HIV type 1 subtypes in Malaysia, determined with serologic assays: 1992-1996. AIDS Res. Hum. Retroviruses. 1998;14:1687–91. doi: 10.1089/aid.1998.14.1687. [DOI] [PubMed] [Google Scholar]
- Bhanja P, Sengupta S, Singh NY, Sarkar K, Bhattacharya SK, Chakrabarti S. Determination of gag and env subtypes of HIV-1 detected among injecting drug users (IDUs) in Manipur, India: evidence for intersubtype recombination. Virus Res. 2005;114:149–53. doi: 10.1016/j.virusres.2005.06.008. doi:10.1016/j.virusres.2005.06.008. [DOI] [PubMed] [Google Scholar]
- Bobkov A, Cheingsong-Popov R, Garaev M, Rzhaninova A, Kaleebu P, Beddows S, Bachmann MH, Mullins JI, Louwagie J, Janssens W. Identification of an env G subtype and heterogeneity of HIV-1 strains in the Russian Federation and Belarus. AIDS. 1994;8:1649–55. doi: 10.1097/00002030-199412000-00002. [DOI] [PubMed] [Google Scholar]
- Bobkov A, Kazennova E, Khanina T, Bobkova M, Selimova L, Kravchenko A, Pokrovsky V, Weber J. An HIV type 1 subtype A strain of low genetic diversity continues to spread among injecting drug users in Russia: study of the new local outbreaks in Moscow and Irkutsk. AIDS Res. Hum. Retroviruses. 2001;17:257–61. doi: 10.1089/088922201750063188. doi:10.1089/088922201750063188. [DOI] [PubMed] [Google Scholar]
- Bobkov AF, Kazennova EV, Selimova LM, Khanina TA, Ryabov GS, Bobkova MR, Sukhanova AL, Kravchenko AV, Ladnaya NN, Weber JN, Pokrovsky VV. Temporal trends in the HIV-1 epidemic in Russia: predominance of subtype A. J. Med. Virol. 2004a;74:191–6. doi: 10.1002/jmv.20177. doi:10.1002/jmv.20177. [DOI] [PubMed] [Google Scholar]
- Bobkov AF, Kazennova EV, Sukhanova AL, Bobkova MR, Pokrovsky VV, Zeman VV, Kovtunenko NG, Erasilova IB. An HIV type 1 subtype A outbreak among injecting drug users in Kazakhstan. AIDS Res. Hum. Retroviruses. 2004b;20:1134–6. doi: 10.1089/aid.2004.20.1134. doi:10.1089/aid.2004.20.1134. [DOI] [PubMed] [Google Scholar]
- Bobkova M. Current status of HIV-1 diversity and drug resistance monitoring in the former USSR. AIDS Rev. 2013;15:204–12. [PubMed] [Google Scholar]
- Bontell I, Sarker MS, Rahman M, Afrad MH, Sönnerborg A, Azim T. Molecular dating of HIV-1 subtype C from Bangladesh. PLoS One. 2013;8:e79193. doi: 10.1371/journal.pone.0079193. doi:10.1371/journal.pone.0079193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braitstein P, Justice A, Bangsberg DR, Yip B, Alfonso V, Schechter MT, Hogg RS, Montaner JSG. Hepatitis C coinfection is independently associated with decreased adherence to antiretroviral therapy in a population-based HIV cohort. AIDS. 2006;20:323–31. doi: 10.1097/01.aids.0000198091.70325.f4. doi:10.1097/01.aids.0000198091.70325.f4. [DOI] [PubMed] [Google Scholar]
- Brenner B, Turner D, Oliveira M, Moisi D, Detorio M, Carobene M, Marlink RG, Schapiro J, Roger M, Wainberg MA. A V106M mutation in HIV-1 clade C viruses exposed to efavirenz confers cross-resistance to non-nucleoside reverse transcriptase inhibitors. AIDS. 2003;17:F1–5. doi: 10.1097/00002030-200301030-00001. doi:10.1097/01.aids.0000042957.95433.6c. [DOI] [PubMed] [Google Scholar]
- Brown TM, Robbins KE, Sinniah M, Saraswathy TS, Lee V, Hooi LS, Vijayamalar B, Luo CC, Ou CY, Rapier J, Schochetman G, Kalish ML. HIV type 1 subtypes in Malaysia include B, C, and E. AIDS Res. Hum. Retroviruses. 1996;12:1655–7. doi: 10.1089/aid.1996.12.1655. [DOI] [PubMed] [Google Scholar]
- Campbell-Yesufu OT, Gandhi RT. Update on human immunodeficiency virus (HIV)-2 infection. Clin. Infect. Dis. 2011;52:780–7. doi: 10.1093/cid/ciq248. doi:10.1093/cid/ciq248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caplinskas S, Loukachov VV, Gasich EL, Gilyazova AV, Caplinskiene I, Lukashov VV. Distinct HIV type 1 strains in different risk groups and the absence of new infections by drug-resistant strains in Lithuania. AIDS Res. Hum. Retroviruses. 2013;29:732–7. doi: 10.1089/aid.2012.0312. doi:10.1089/AID.2012.0312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr JK, Nadai Y, Eyzaguirre L, Saad MD, Khakimov MM, Yakubov SK, Birx DL, Graham RR, Wolfe ND, Earhart KC, Sanchez JL. Outbreak of a West African recombinant of HIV-1 in Tashkent, Uzbekistan. J. Acquir. Immune Defic. Syndr. 2005;39:570–5. [PubMed] [Google Scholar]
- Carvalho A, Costa P, Triunfante V, Branca F, Rodrigues F, Santos CL, Correia-Neves M, Saraiva M, Lecour H, Castro AG, Pedrosa J, Osório NS. Analysis of a local HIV-1 epidemic in portugal highlights established transmission of non-B and non-G subtypes. J. Clin. Microbiol. 2015;53:1506–14. doi: 10.1128/JCM.03611-14. doi:10.1128/JCM.03611-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casado C, Urtasun I, Saragosti S, Chaix ML, de Rossi A, Cattelan AM, Dietrich U, López-Galíndez C. Different distribution of HIV type 1 genetic variants in European patients with distinct risk practices. AIDS Res. Hum. Retroviruses. 2000;16:299–304. doi: 10.1089/088922200309403. doi:10.1089/088922200309403. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control Kaposi's sarcoma and Pneumocystis pneumonia among homosexual men--New York City and California. MMWR. Morb. Mortal. Wkly. Rep. 1981a;30:305–8. [PubMed] [Google Scholar]
- Centers for Disease Control Pneumocystis pneumonia--Los Angeles. MMWR. Morb. Mortal. Wkly. Rep. 1981b;30:250–2. [PubMed] [Google Scholar]
- Centers for Disease Control Update on acquired immune deficiency syndrome (AIDS)--United States. MMWR. Morb. Mortal. Wkly. Rep. 1982a;31:507–8. 513–4. [PubMed] [Google Scholar]
- Centers for Disease Control A cluster of Kaposi's sarcoma and Pneumocystis carinii pneumonia among homosexual male residents of Los Angeles and Orange Counties, California. MMWR. Morb. Mortal. Wkly. Rep. 1982b;31:305–7. [PubMed] [Google Scholar]
- Chabadová Z, Habeková M, Truska P, Drobková T, Mojzesová M, Staneková D. Distribution of HIV-1 subtypes circulating in Slovakia (2009-2012). Acta Virol. 2014;58:317–24. doi: 10.4149/av_2014_04_317. [DOI] [PubMed] [Google Scholar]
- Charneau P, Borman AM, Quillent C, Guétard D, Chamaret S, Cohen J, Rémy G, Montagnier L, Clavel F. Isolation and envelope sequence of a highly divergent HIV-1 isolate: definition of a new HIV-1 group. Virology. 1994;205:247–53. doi: 10.1006/viro.1994.1640. doi:10.1006/viro.1994.1640. [DOI] [PubMed] [Google Scholar]
- Chen JHK, Wong KH, Li P, Chan KC, Lee MP, Lam HY, Cheng VCC, Yuen KY, Yam WC. Molecular epidemiological study of HIV-1 CRF01_AE transmission in Hong Kong. J. Acquir. Immune Defic. Syndr. 2009;51:530–5. doi: 10.1097/QAI.0b013e3181aac516. doi:10.1097/QAI.0b013e3181aac516. [DOI] [PubMed] [Google Scholar]
- Chen Y-J, Huang Y-H, Chuang S-Y, Kao DY-T, Lan Y-C, Yang J-Y, Chen Y-MA. Molecular epidemiology of HIV-1 subtype B, CRF01_AE, and CRF07_BC infection among injection drug users in Taiwan. J. Acquir. Immune Defic. Syndr. 2010;53:425–39. doi: 10.1097/QAI.0b013e3181ccba1a. doi:10.1097/QAI.0b013e3181ccba1a. [DOI] [PubMed] [Google Scholar]
- Chen Y-J, Lee C-M, Chen M, Chuang S-Y, Liu H-F, Wong W-W, Lin Y-H, Tsai H-C, Wang J-H, Chen Y-MA. Molecular epidemiology of HIV-1 infection in Taiwan from 2005 to 2008: further spread of CRF07_BC and emergence of CRF07_BC/subtype B dual infection. J. Acquir. Immune Defic. Syndr. 2012;59:438–46. doi: 10.1097/QAI.0b013e3182454ea3. doi:10.1097/QAI.0b013e3182454ea3. [DOI] [PubMed] [Google Scholar]
- Chow WZ, Ong LY, Razak SH, Lee YM, Ng KT, Yong YK, Azmel A, Takebe Y, Al-Darraji HAA, Kamarulzaman A, Tee KK. Molecular diversity of HIV-1 among people who inject drugs in Kuala Lumpur, Malaysia: massive expansion of circulating recombinant form (CRF) 33_01B and emergence of multiple unique recombinant clusters. PLoS One. 2013;8:e62560. doi: 10.1371/journal.pone.0062560. doi:10.1371/journal.pone.0062560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow WZ, Takebe Y, Syafina NE, Prakasa MS, Chan KG, Al-Darraji HAA, Koh C, Kamarulzaman A, Tee KK. A newly emerging HIV-1 recombinant lineage (CRF58_01B) disseminating among people who inject drugs in Malaysia. PLoS One. 2014;9:e85250. doi: 10.1371/journal.pone.0085250. doi:10.1371/journal.pone.0085250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciccozzi M, Montieri S, Salemi M, De Oliveira T, Dorrucci M, Sinicco A, De Luca A, Giuliani M, Balotta C, Rezza G. An outbreak of HIV-1 subtype G among Italian injecting drug users. AIDS. 2007;21:1213–5. doi: 10.1097/QAD.0b013e32813aee1a. doi:10.1097/QAD.0b013e32813aee1a. [DOI] [PubMed] [Google Scholar]
- Clavel F, Guétard D, Brun-Vézinet F, Chamaret S, Rey MA, Santos-Ferreira MO, Laurent AG, Dauguet C, Katlama C, Rouzioux C, Klatzmann D, Champalimaud J, Montagner L. Isolation of a new human retrovirus from West African patients with AIDS. Science. 1986;233:343–6. doi: 10.1126/science.2425430. [DOI] [PubMed] [Google Scholar]
- Cohen MS, Chen YQ, McCauley M, Gamble T, Hosseinipour MC, Kumarasamy N, Hakim JG, Kumwenda J, Grinsztejn B, Pilotto JHS, Godbole SV, Mehendale S, Chariyalertsak S, Santos BR, Mayer KH, Hoffman IF, Eshleman SH, Piwowar-Manning E, Wang L, Makhema J, Mills LA, de Bruyn G, Sanne I, Eron J, Gallant J, Havlir D, Swindells S, Ribaudo H, Elharrar V, Burns D, Taha TE, Nielsen-Saines K, Celentano D, Essex M, Fleming TR. Prevention of HIV-1 infection with early antiretroviral therapy. N. Engl. J. Med. 2011a;365:493–505. doi: 10.1056/NEJMoa1105243. doi:10.1056/NEJMoa1105243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen MS, Shaw GM, McMichael AJ, Haynes BF. Acute HIV-1 Infection. N. Engl. J. Med. 2011b;364:1943–54. doi: 10.1056/NEJMra1011874. doi:10.1056/NEJMra1011874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'arc M, Ayouba A, Esteban A, Learn GH, Boué V, Liegeois F, Etienne L, Tagg N, Leendertz FH, Boesch C, Madinda NF, Robbins MM, Gray M, Cournil A, Ooms M, Letko M, Simon VA, Sharp PM, Hahn BH, Delaporte E, Mpoudi Ngole E, Peeters M. Origin of the HIV-1 group O epidemic in western lowland gorillas. Proc. Natl. Acad. Sci. U. S. A. 2015;112:E1343–52. doi: 10.1073/pnas.1502022112. doi:10.1073/pnas.1502022112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado E, Thomson MM, Villahermosa ML, Sierra M, Ocampo A, Miralles C, Rodríguez-Pérez R, Diz-Aren J, Ojea-de Castro R, Losada E, Cuevas MT, Vázquez-de Parga E, Carmona R, Pérez-Alvarez L, Medrano L, Cuevas L, Taboada JA, Nájera R. Identification of a newly characterized HIV-1 BG intersubtype circulating recombinant form in Galicia, Spain, which exhibits a pseudotype-like virion structure. J. Acquir. Immune Defic. Syndr. 2002;29:536–43. doi: 10.1097/00126334-200204150-00016. [DOI] [PubMed] [Google Scholar]
- Deng X, Liu H, Shao Y, Rayner S, Yang R. The epidemic origin and molecular properties of B': a founder strain of the HIV-1 transmission in Asia. AIDS. 2008;22:1851–8. doi: 10.1097/QAD.0b013e32830f4c62. doi:10.1097/QAD.0b013e32830f4c62. [DOI] [PubMed] [Google Scholar]
- Des Jarlais DC, Arasteh K, Friedman SR. HIV among drug users at Beth Israel Medical Center, New York City, the first 25 years. Subst. Use Misuse. 2011;46:131–9. doi: 10.3109/10826084.2011.521456. doi:10.3109/10826084.2011.521456. [DOI] [PubMed] [Google Scholar]
- Des Jarlais DC, Feelemyer JP, Modi SN, Abdul-Quader A, Hagan H. High coverage needle/syringe programs for people who inject drugs in low and middle income countries: a systematic review. BMC Public Health. 2013;13:53. doi: 10.1186/1471-2458-13-53. doi:10.1186/1471-2458-13-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Díez-Fuertes F, Cabello M, Thomson MM. Bayesian phylogeographic analyses clarify the origin of the HIV-1 subtype A variant circulating in former Soviet Union's countries. Infect. Genet. Evol. 2015;33:197–205. doi: 10.1016/j.meegid.2015.05.003. doi:10.1016/j.meegid.2015.05.003. [DOI] [PubMed] [Google Scholar]
- Duque V, Holguín A, Silvestre M, González-Lahoz J, Soriano V. Human immunodeficiency virus type 1 recombinant B/G subtypes circulating in Coimbra, Portugal. Clin. Microbiol. Infect. 2003;9:422–5. doi: 10.1046/j.1469-0691.2003.00541.x. [DOI] [PubMed] [Google Scholar]
- Ekouevi DK, Tchounga BK, Coffie PA, Tegbe J, Anderson AM, Gottlieb GS, Vitoria M, Dabis F, Eholie SP. Antiretroviral therapy response among HIV-2 infected patients: a systematic review. BMC Infect. Dis. 2014;14:461. doi: 10.1186/1471-2334-14-461. doi:10.1186/1471-2334-14-461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eremin VF, Gasich EL, Sasinovich SV. A new unique recombinant HIV type 1 isolated from a child born to an HIV-infected mother. AIDS Res. Hum. Retroviruses. 2011;27:1323–6. doi: 10.1089/AID.2011.0112. doi:10.1089/AID.2011.0112. [DOI] [PubMed] [Google Scholar]
- Esteves A, Parreira R, Piedade J, Venenno T, Franco M, Germano de Sousa J, Patrício L, Brum P, Costa A, Canas-Ferreira WF. Spreading of HIV-1 subtype G and envB/gagG recombinant strains among injecting drug users in Lisbon, Portugal. AIDS Res. Hum. Retroviruses. 2003;19:511–7. doi: 10.1089/088922203766774568. doi:10.1089/088922203766774568. [DOI] [PubMed] [Google Scholar]
- Esteves A, Parreira R, Venenno T, Franco M, Piedade J, Germano De Sousa J, Canas-Ferreira WF. Molecular epidemiology of HIV type 1 infection in Portugal: high prevalence of non-B subtypes. AIDS Res. Hum. Retroviruses. 2002;18:313–25. doi: 10.1089/088922202753519089. doi:10.1089/088922202753519089. [DOI] [PubMed] [Google Scholar]
- European Centre for Disease Prevention and Control (ECDC)/World Health Organization (WHO) HIV/AIDS Surveilance in Europe. Stockholm, Sweden: 2014. [Google Scholar]
- Eyzaguirre LM, Erasilova IB, Nadai Y, Saad MD, Kovtunenko NG, Gomatos PJ, Zeman VV, Botros BA, Sanchez JL, Birx DL, Earhart KC, Carr JK. Genetic characterization of HIV-1 strains circulating in Kazakhstan. J. Acquir. Immune Defic. Syndr. 2007;46:19–23. doi: 10.1097/QAI.0b013e318073c620. doi:10.1097/QAI.0b013e318073c620. [DOI] [PubMed] [Google Scholar]
- Faria NR, Rambaut A, Suchard MA, Baele G, Bedford T, Ward MJ, Tatem AJ, Sousa JD, Arinaminpathy N, Pepin J, Posada D, Peeters M, Pybus OG, Lemey P. The early spread and epidemic ignition of HIV-1 in human populations. Science (80-. ) 2014;346:56–61. doi: 10.1126/science.1256739. doi:10.1126/science.1256739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferdats A, Konicheva V, Dievberna I, Lilja E, Albert J. An HIV type 1 subtype A outbreak among injecting drug users in Latvia. AIDS Res. Hum. Retroviruses. 1999;15:1487–90. doi: 10.1089/088922299310007. doi:10.1089/088922299310007. [DOI] [PubMed] [Google Scholar]
- Friedman SR, Rossi D, Braine N. Theorizing “Big Events” as a potential risk environment for drug use, drug-related harm and HIV epidemic outbreaks. Int. J. Drug Policy. 2009;20:283–91. doi: 10.1016/j.drugpo.2008.10.006. doi:10.1016/j.drugpo.2008.10.006. [DOI] [PubMed] [Google Scholar]
- Gallo RC, Salahuddin SZ, Popovic M, Shearer GM, Kaplan M, Haynes BF, Palker TJ, Redfield R, Oleske J, Safai B, White G, Foster P, Markham P. Frequent detection and isolation of cytopathic retroviruses (HTLV-III) from patients with AIDS and at risk for AIDS. Science. 1984;224:500–3. doi: 10.1126/science.6200936. [DOI] [PubMed] [Google Scholar]
- Gao F, Bailes E, Robertson DL, Chen Y, Rodenburg CM, Michael SF, Cummins LB, Arthur LO, Peeters M, Shaw GM, Sharp PM, Hahn BH. Origin of HIV-1 in the chimpanzee Pan troglodytes troglodytes. Nature. 1999;397:436–41. doi: 10.1038/17130. doi:10.1038/17130. [DOI] [PubMed] [Google Scholar]
- Gao F, Yue L, White AT, Pappas PG, Barchue J, Hanson AP, Greene BM, Sharp PM, Shaw GM, Hahn BH. Human infection by genetically diverse SIVSM-related HIV-2 in west Africa. Nature. 1992;358:495–9. doi: 10.1038/358495a0. doi:10.1038/358495a0. [DOI] [PubMed] [Google Scholar]
- Giannou FK, Tsiara CG, Nikolopoulos GK, Talias M, Benetou V, Kantzanou M, Bonovas S, Hatzakis A. Condom effectiveness in reducing heterosexual HIV transmission: a systematic review and meta-analysis of studies on HIV serodiscordant couples. Expert Rev. Pharmacoecon. Outcomes Res. 2015:1–11. doi: 10.1586/14737167.2016.1102635. doi:10.1586/14737167.2016.1102635. [DOI] [PubMed] [Google Scholar]
- Glauser MP, Francioli P. Clinical and epidemiological survey of acquired immune deficiency syndrome in Europe. Eur. J. Clin. Microbiol. 1984;3:55–8. doi: 10.1007/BF02032823. [DOI] [PubMed] [Google Scholar]
- Goswami P, Medhi GK, Armstrong G, Setia MS, Mathew S, Thongamba G, Ramakrishnan L, George B, Singh RK, Paranjape RS, Mahanta J. An assessment of an HIV prevention intervention among people who inject drugs in the states of Manipur and Nagaland, India. Int. J. Drug Policy. 2014;25:853–64. doi: 10.1016/j.drugpo.2014.04.016. doi:10.1016/j.drugpo.2014.04.016. [DOI] [PubMed] [Google Scholar]
- Gottlieb MS, Schroff R, Schanker HM, Weisman JD, Fan PT, Wolf RA, Saxon A. Pneumocystis carinii pneumonia and mucosal candidiasis in previously healthy homosexual men: evidence of a new acquired cellular immunodeficiency. N. Engl. J. Med. 1981;305:1425–31. doi: 10.1056/NEJM198112103052401. doi:10.1056/NEJM198112103052401. [DOI] [PubMed] [Google Scholar]
- Graf M, Shao Y, Zhao Q, Seidl T, Köstler J, Wolf H, Wagner R. Cloning and characterization of a virtually full-length HIV type 1 genome from a subtype B'-Thai strain representing the most prevalent B-clade isolate in China. AIDS Res. Hum. Retroviruses. 1998;14:285–8. doi: 10.1089/aid.1998.14.285. [DOI] [PubMed] [Google Scholar]
- Grant RM, Lama JR, Anderson PL, McMahan V, Liu AY, Vargas L, Goicochea P, Casapía M, Guanira-Carranza JV, Ramirez-Cardich ME, Montoya-Herrera O, Fernández T, Veloso VG, Buchbinder SP, Chariyalertsak S, Schechter M, Bekker L-G, Mayer KH, Kallás EG, Amico KR, Mulligan K, Bushman LR, Hance RJ, Ganoza C, Defechereux P, Postle B, Wang F, McConnell JJ, Zheng J-H, Lee J, Rooney JF, Jaffe HS, Martinez AI, Burns DN, Glidden DV. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N. Engl. J. Med. 2010;363:2587–99. doi: 10.1056/NEJMoa1011205. doi:10.1056/NEJMoa1011205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gürtler LG, Hauser PH, Eberle J, von Brunn A, Knapp S, Zekeng L, Tsague JM, Kaptue L. A new subtype of human immunodeficiency virus type 1 (MVP-5180) from Cameroon. J. Virol. 1994;68:1581–5. doi: 10.1128/jvi.68.3.1581-1585.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habekova M, Takacova M, Lysy J, Mokras M, Camacho R, Truska P, Stanekova D. Genetic subtypes of HIV type 1 circulating in Slovakia. AIDS Res. Hum. Retroviruses. 2010;26:1103–7. doi: 10.1089/aid.2009.0220. doi:10.1089/aid.2009.0220. [DOI] [PubMed] [Google Scholar]
- Han J, Liu S, Guo W, Bao Z, Wang X, Li L, Liu Y, Zhuang D, Li H, Jia L, Gui T, Sui H, Li T, Li J. Development of an HIV-1 Subtype Panel in China: Isolation and Characterization of 30 HIV-1 Primary Strains Circulating in China. PLoS One. 2015;10:e0127696. doi: 10.1371/journal.pone.0127696. doi:10.1371/journal.pone.0127696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han X, An M, Zhao B, Duan S, Yang S, Xu J, Zhang M, McGoogan JM, Takebe Y, Shang H. High prevalence of HIV-1 intersubtype B'/C recombinants among injecting drug users in Dehong, China. PLoS One. 2013;8:e65337. doi: 10.1371/journal.pone.0065337. doi:10.1371/journal.pone.0065337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris B, von Truchsess I, Schätzl HM, Devare SG, Hackett J. Genomic characterization of a novel HIV type 1 B/G intersubtype recombinant strain from an injecting drug user in Germany. AIDS Res. Hum. Retroviruses. 2005;21:654–60. doi: 10.1089/aid.2005.21.654. doi:10.1089/aid.2005.21.654. [DOI] [PubMed] [Google Scholar]
- Hatzakis A, Sypsa V, Paraskevis D, Nikolopoulos G, Tsiara C, Micha K, Panopoulos A, Malliori M, Psichogiou M, Pharris A, Wiessing L, van de Laar M, Donoghoe M, Heckathorn DD, Friedman SR, Des Jarlais DC. Design and baseline findings of a large-scale rapid response to an HIV outbreak in people who inject drugs in Athens, Greece: the ARISTOTLE programme. Addiction. 2015;110:1453–67. doi: 10.1111/add.12999. doi:10.1111/add.12999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He X, Xing H, Ruan Y, Hong K, Cheng C, Hu Y, Xin R, Wei J, Feng Y, Hsi JH, Takebe Y, Shao Y. A comprehensive mapping of HIV-1 genotypes in various risk groups and regions across China based on a nationwide molecular epidemiologic survey. PLoS One. 2012;7:e47289. doi: 10.1371/journal.pone.0047289. doi:10.1371/journal.pone.0047289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemelaar J, Gouws E, Ghys PD, Osmanov S. Global trends in molecular epidemiology of HIV-1 during 2000-2007. AIDS. 2011;25:679–89. doi: 10.1097/QAD.0b013e328342ff93. doi:10.1097/QAD.0b013e328342ff93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hersh BS, Popovici F, Apetrei RC, Zolotusca L, Beldescu N, Calomfirescu A, Jezek Z, Oxtoby MJ, Gromyko A, Heymann DL. Acquired immunodeficiency syndrome in Romania. Lancet (London, England) 1991;338:645–9. doi: 10.1016/0140-6736(91)91230-r. [DOI] [PubMed] [Google Scholar]
- Hersh BS, Popovici F, Jezek Z, Satten GA, Apetrei RC, Beldescu N, George JR, Shapiro CN, Gayle HD, Heymann DL. Risk factors for HIV infection among abandoned Romanian children. AIDS. 1993;7:1617–24. doi: 10.1097/00002030-199312000-00012. [DOI] [PubMed] [Google Scholar]
- Holguín A, de Mulder M, Yebra G, López M, Soriano V. Increase of non-B subtypes and recombinants among newly diagnosed HIV-1 native Spaniards and immigrants in Spain. Curr. HIV Res. 2008;6:327–34. doi: 10.2174/157016208785132455. [DOI] [PubMed] [Google Scholar]
- Huang S-W, Wang S-F, Lin Y-T, Yen C-H, Lee C-H, Wong W-W, Tsai H-C, Yang C-J, Hu B-S, Lin Y-H, Wang C-T, Wang J-J, Hu Z, Kuritzkes DR, Chen Y-H, Chen Y-MA. Patients infected with CRF07_BC have significantly lower viral loads than patients with HIV-1 subtype B: mechanism and impact on disease progression. PLoS One. 2014;9:e114441. doi: 10.1371/journal.pone.0114441. doi:10.1371/journal.pone.0114441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibe S, Yokomaku Y, Shiino T, Tanaka R, Hattori J, Fujisaki S, Iwatani Y, Mamiya N, Utsumi M, Kato S, Hamaguchi M, Sugiura W. HIV-2 CRF01_AB: first circulating recombinant form of HIV-2. J. Acquir. Immune Defic. Syndr. 2010;54:241–7. doi: 10.1097/QAI.0b013e3181dc98c1. doi:10.1097/QAI.0b013e3181dc98c1. [DOI] [PubMed] [Google Scholar]
- Jahanbakhsh F, Hattori J, Matsuda M, Ibe S, Monavari S-HR, Memarnejadian A, Aghasadeghi MR, Mostafavi E, Mohraz M, Jabbari H, Kamali K, Keyvani H, Azadmanesh K, Sugiura W. Prevalence of transmitted HIV drug resistance in Iran between 2010 and 2011. PLoS One. 2013a;8:e61864. doi: 10.1371/journal.pone.0061864. doi:10.1371/journal.pone.0061864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahanbakhsh F, Ibe S, Hattori J, Monavari SHR, Matsuda M, Maejima M, Iwatani Y, Memarnejadian A, Keyvani H, Azadmanesh K, Sugiura W. Molecular epidemiology of HIV type 1 infection in Iran: genomic evidence of CRF35_AD predominance and CRF01_AE infection among individuals associated with injection drug use. AIDS Res. Hum. Retroviruses. 2013b;29:198–203. doi: 10.1089/AID.2012.0186. doi:10.1089/AID.2012.0186. [DOI] [PubMed] [Google Scholar]
- Junqueira DM, Almeida SE, de M. HIV-1 subtype B: Traces of a pandemic. Virology. 2016;495:173–184. doi: 10.1016/j.virol.2016.05.003. doi:10.1016/j.virol.2016.05.003. [DOI] [PubMed] [Google Scholar]
- Kamarulzaman A, Altice FL. Challenges in managing HIV in people who use drugs. Curr. Opin. Infect. Dis. 2015;28:10–6. doi: 10.1097/QCO.0000000000000125. doi:10.1097/QCO.0000000000000125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato K, Kusagawa S, Motomura K, Yang R, Shiino T, Nohtomi K, Sato H, Shibamura K, Nguyen TH, Pham KC, Pham HT, Duong CT, Bui DT, Hoang TL, Nagai Y, Takebe Y. Closely related HIV-1 CRF01_AE variant among injecting drug users in northern Vietnam: evidence of HIV spread across the Vietnam-China border. AIDS Res. Hum. Retroviruses. 2001;17:113–23. doi: 10.1089/08892220150217201. doi:10.1089/08892220150217201. [DOI] [PubMed] [Google Scholar]
- Kato K, Shiino T, Kusagawa S, Sato H, Nohtomi K, Shibamura K, Nguyen TH, Pham KC, Truong XL, Mai HA, Hoang TL, Bunyaraksyotin G, Fukushima Y, Honda M, Wasi C, Yamazaki S, Nagai Y, Takebe Y. Genetic similarity of HIV type 1 subtype E in a recent outbreak among injecting drug users in northern Vietnam to strains in Guangxi Province of southern China. AIDS Res. Hum. Retroviruses. 1999;15:1157–68. doi: 10.1089/088922299310250. doi:10.1089/088922299310250. [DOI] [PubMed] [Google Scholar]
- Kazennova E, Laga V, Lapovok I, Glushchenko N, Neshumaev D, Vasilyev A, Bobkova M. HIV-1 genetic variants in the Russian Far East. AIDS Res. Hum. Retroviruses. 2014;30:742–52. doi: 10.1089/aid.2013.0194. doi:10.1089/AID.2013.0194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keele BF, Van Heuverswyn F, Li Y, Bailes E, Takehisa J, Santiago ML, Bibollet-Ruche F, Chen Y, Wain LV, Liegeois F, Loul S, Ngole EM, Bienvenue Y, Delaporte E, Brookfield JFY, Sharp PM, Shaw GM, Peeters M, Hahn BH. Chimpanzee reservoirs of pandemic and nonpandemic HIV-1. Science. 2006;313:523–6. doi: 10.1126/science.1126531. doi:10.1126/science.1126531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khajehkazemi R, Osooli M, Sajadi L, Karamouzian M, Sedaghat A, Fahimfar N, Safaie A, Mostafavi E, Haghdoost A-A. HIV prevalence and risk behaviours among people who inject drugs in Iran: the 2010 National Surveillance Survey. Sex. Transm. Infect. 2013;89(Suppl 3):iii29–32. doi: 10.1136/sextrans-2013-051204. doi:10.1136/sextrans-2013-051204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan S, Rai MA, Khanani MR, Khan MN, Ali SH. HIV-1 subtype A infection in a community of intravenous drug users in Pakistan. BMC Infect. Dis. 2006;6:164. doi: 10.1186/1471-2334-6-164. doi:10.1186/1471-2334-6-164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kijak GH, Tovanabutra S, Rerks-Ngarm S, Nitayaphan S, Eamsila C, Kunasol P, Khamboonruang C, Thongcharoen P, Namwat C, Premsri N, Benenson M, Morgan P, Bose M, Sanders-Buell E, Paris R, Robb ML, Birx DL, De Souza MS, McCutchan FE, Michael NL, Kim JH. Molecular evolution of the HIV-1 Thai epidemic between the time of RV144 immunogen selection to the execution of the vaccine efficacy trial. J. Virol. 2013;87:7265–81. doi: 10.1128/JVI.03070-12. doi:10.1128/JVI.03070-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kivelä P, Krol A, Simola S, Vaattovaara M, Tuomola P, Brummer-Korvenkontio H, Ristola M. HIV outbreak among injecting drug users in the Helsinki region: social and geographical pockets. Eur. J. Public Health. 2007;17:381–6. doi: 10.1093/eurpub/ckl252. doi:10.1093/eurpub/ckl252. [DOI] [PubMed] [Google Scholar]
- Kivelä PS, Krol A, Salminen MO, Geskus RB, Suni JI, Anttila V-J, Liitsola K, Zetterberg V, Miedema F, Berkhout B, Coutinho RA, Ristola MA. High plasma HIV load in the CRF01-AE outbreak among injecting drug users in Finland. Scand. J. Infect. Dis. 2005;37:276–83. [PubMed] [Google Scholar]
- Kiwanuka N, Laeyendecker O, Quinn TC, Wawer MJ, Shepherd J, Robb M, Kigozi G, Kagaayi J, Serwadda D, Makumbi FE, Reynolds SJ, Gray RH. HIV-1 subtypes and differences in heterosexual HIV transmission among HIV-discordant couples in Rakai, Uganda. AIDS. 2009;23:2479–84. doi: 10.1097/QAD.0b013e328330cc08. doi:10.1097/QAD.0b013e328330cc08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiwanuka N, Laeyendecker O, Robb M, Kigozi G, Arroyo M, McCutchan F, Eller LA, Eller M, Makumbi F, Birx D, Wabwire-Mangen F, Serwadda D, Sewankambo NK, Quinn TC, Wawer M, Gray R. Effect of human immunodeficiency virus Type 1 (HIV-1) subtype on disease progression in persons from Rakai, Uganda, with incident HIV-1 infection. J. Infect. Dis. 2008;197:707–13. doi: 10.1086/527416. doi:10.1086/527416. [DOI] [PubMed] [Google Scholar]
- Korber B, Muldoon M, Theiler J, Gao F, Gupta R, Lapedes A, Hahn BH, Wolinsky S, Bhattacharya T. Timing the ancestor of the HIV-1 pandemic strains. Science. 2000;288:1789–96. doi: 10.1126/science.288.5472.1789. [DOI] [PubMed] [Google Scholar]
- Kuiken C, Thakallapalli R, Esklid A, de Ronde A. Genetic analysis reveals epidemiologic patterns in the spread of human immunodeficiency virus. Am. J. Epidemiol. 2000;152:814–22. doi: 10.1093/aje/152.9.814. [DOI] [PubMed] [Google Scholar]
- Kurbanov F, Kondo M, Tanaka Y, Zalalieva M, Giasova G, Shima T, Jounai N, Yuldasheva N, Ruzibakiev R, Mizokami M, Imai M. Human immunodeficiency virus in Uzbekistan: epidemiological and genetic analyses. AIDS Res. Hum. Retroviruses. 2003;19:731–8. doi: 10.1089/088922203769232520. doi:10.1089/088922203769232520. [DOI] [PubMed] [Google Scholar]
- Kusagawa S, Sato H, Kato K, Nohtomi K, Shiino T, Samrith C, Leng HB, Phalla T, Heng MB, Takebe Y. HIV type 1 env subtype E in Cambodia. AIDS Res. Hum. Retroviruses. 1999;15:91–4. doi: 10.1089/088922299311772. doi:10.1089/088922299311772. [DOI] [PubMed] [Google Scholar]
- Kusagawa S, Sato H, Watanabe S, Nohtomi K, Kato K, Shino T, Thwe M, OO KY, Lwin S, Mra R, Kywe B, Yamazaki S, Takebe Y. Genetic and serologic characterization of HIV type 1 prevailing in Myanmar (Burma). AIDS Res. Hum. Retroviruses. 1998;14:1379–85. doi: 10.1089/aid.1998.14.1379. [DOI] [PubMed] [Google Scholar]
- Laeyendecker O, Zhang GW, Quinn TC, Garten R, Ray SC, Lai S, Liu W, Chen J, Yu X-F. Molecular epidemiology of HIV-1 subtypes in southern China. J. Acquir. Immune Defic. Syndr. 2005;38:356–62. [PubMed] [Google Scholar]
- Laga V, Lapovok I, Kazennova E, Ismailova A, Beisheeva N, Asybalieva N, Glushchenko N, Bobkova M. The Genetic Variability of HIV-1 in Kyrgyzstan: The Spread of CRF02_AG and Subtype A1 Recombinants. J HIV AIDS. 2015a;1 doi: http://dx.doi.org/10.16966/2380-5536.106. [Google Scholar]
- Laga V, Vasilyev A, Lapovok I, Grigoryan S, Papoyan A, Glushchenko N, Kazennova E, Bobkova M. HIV Type 1 Subtype A1 Dominates in Armenia. Curr. HIV Res. 2015b;13:219–25. doi: 10.2174/1570162x13666150407142834. [DOI] [PubMed] [Google Scholar]
- Lai A, Bozzi G, Franzetti M, Binda F, Simonetti FR, De Luca A, Micheli V, Meraviglia P, Bagnarelli P, Di Biagio A, Monno L, Saladini F, Zazzi M, Zehender G, Ciccozzi M, Balotta C. HIV-1 A1 Subtype Epidemic in Italy Originated from Africa and Eastern Europe and Shows a High Frequency of Transmission Chains Involving Intravenous Drug Users. PLoS One. 2016;11:e0146097. doi: 10.1371/journal.pone.0146097. doi:10.1371/journal.pone.0146097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan NTH, Recordon-Pinson P, Hung P, Van, Uyen NTV, Lien TTX, Tien HT, Garrigue I, Schrive M-H, Pellegrin I, Lafon M-E, Aboulker J-P, Barré-Sinousi F, Fleury HJ. HIV type 1 isolates from 200 untreated individuals in Ho Chi Minh City (Vietnam): ANRS 1257 Study. Large predominance of CRF01_AE and presence of major resistance mutations to antiretroviral drugs. AIDS Res. Hum. Retroviruses. 2003;19:925–8. doi: 10.1089/088922203322493111. doi:10.1089/088922203322493111. [DOI] [PubMed] [Google Scholar]
- Lapovok I, Kazennova E, Laga V, Vasilyev A, Utegenova A, Abishev A, Dzissyuk N, Tukeev M, Bobkova M. Short communication: molecular epidemiology of HIV type 1 infection in Kazakhstan: CRF02_AG prevalence is increasing in the southeastern provinces. AIDS Res. Hum. Retroviruses. 2014;30:769–74. doi: 10.1089/aid.2013.0291. doi:10.1089/AID.2013.0291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen MV, Omland LH, Gerstoft J, Røge BT, Larsen CS, Pedersen G, Obel N, Kronborg G. Impact of injecting drug use on response to highly active antiretroviral treatment in HIV-1-infected patients: a nationwide population-based cohort study. Scand. J. Infect. Dis. 2010;42:917–23. doi: 10.3109/00365548.2010.511258. doi:10.3109/00365548.2010.511258. [DOI] [PubMed] [Google Scholar]
- Lau KA, Wang B, Saksena NK. Emerging trends of HIV epidemiology in Asia. AIDS Rev. 2007;9:218–29. [PubMed] [Google Scholar]
- Lau KA, Wong JJL. Current Trends of HIV Recombination Worldwide. Infect. Dis. Rep. 2013;5:e4. doi: 10.4081/idr.2013.s1.e4. doi:10.4081/idr.2013.s1.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazouskaya NV, Eremin VF, Adema KW, Gasich EL, Baan E, Lukashov VV. The HIV type 1 epidemic in Belarus: predominance of Eastern European subtype A strains and circulation of subtype B viruses. AIDS Res. Hum. Retroviruses. 2005;21:830–3. doi: 10.1089/aid.2005.21.830. doi:10.1089/aid.2005.21.830. [DOI] [PubMed] [Google Scholar]
- Li L, Wei D, Hsu W-L, Li T, Gui T, Wood C, Liu Y, Li H, Bao Z, Liu S, Wang X, Li J. CRF07_BC Strain Dominates the HIV-1 Epidemic in Injection Drug Users in Liangshan Prefecture of Sichuan, China. AIDS Res. Hum. Retroviruses. 2015;31:479–87. doi: 10.1089/aid.2014.0120. doi:10.1089/AID.2014.0120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Tee KK, Liao H, Hase S, Uenishi R, Li X-J, Tsuchiura T, Yang R, Govindasamy S, Yong YK, Tan HY, Pybus OG, Kamarulzaman A, Takebe Y. Identification of a novel second-generation circulating recombinant form (CRF48_01B) in Malaysia: a descendant of the previously identified CRF33_01B. J. Acquir. Immune Defic. Syndr. 2010a;54:129–36. doi: 10.1097/QAI.0b013e3181d82ce5. doi:10.1097/QAI.0b013e3181d82ce5. [DOI] [PubMed] [Google Scholar]
- Li Y, Uenishi R, Hase S, Liao H, Li X-J, Tsuchiura T, Tee KK, Pybus OG, Takebe Y. Explosive HIV-1 subtype B' epidemics in Asia driven by geographic and risk group founder events. Virology. 2010b;402:223–7. doi: 10.1016/j.virol.2010.03.048. doi:10.1016/j.virol.2010.03.048. [DOI] [PubMed] [Google Scholar]
- Liao H, Tee KK, Hase S, Uenishi R, Li X-J, Kusagawa S, Thang PH, Hien NT, Pybus OG, Takebe Y. Phylodynamic analysis of the dissemination of HIV-1 CRF01_AE in Vietnam. Virology. 2009;391:51–6. doi: 10.1016/j.virol.2009.05.023. doi:10.1016/j.virol.2009.05.023. [DOI] [PubMed] [Google Scholar]
- Liitsola K, Holm K, Bobkov A, Pokrovsky V, Smolskaya T, Leinikki P, Osmanov S, Salminen M. An AB recombinant and its parental HIV type 1 strains in the area of the former Soviet Union: low requirements for sequence identity in recombination. UNAIDS Virus Isolation Network. AIDS Res. Hum. Retroviruses. 2000a;16:1047–53. doi: 10.1089/08892220050075309. doi:10.1089/08892220050075309. [DOI] [PubMed] [Google Scholar]
- Liitsola K, Ristola M, Holmström P, Salminen M, Brummer-Korvenkontio H, Simola S, Suni J, Leinikki P. An outbreak of the circulating recombinant form AECM240 HIV-1 in the Finnish injection drug user population. AIDS. 2000b;14:2613–5. doi: 10.1097/00002030-200011100-00028. [DOI] [PubMed] [Google Scholar]
- Liitsola K, Tashkinova I, Laukkanen T, Korovina G, Smolskaja T, Momot O, Mashkilleyson N, Chaplinskas S, Brummer-Korvenkontio H, Vanhatalo J, Leinikki P, Salminen MO. HIV-1 genetic subtype A/B recombinant strain causing an explosive epidemic in injecting drug users in Kaliningrad. AIDS. 1998;12:1907–19. doi: 10.1097/00002030-199814000-00023. [DOI] [PubMed] [Google Scholar]
- Lin H-H, Shih Y-L, Liu Y-C, Lee SS-J, Huang C-K, Chen Y-L, Chin C, Lai C-H, Tsai H-C, Guo Y-C, Zhang L. An epidemic of HIV type I CRF07_BC infection among injection drug users in Taiwan. J. Acquir. Immune Defic. Syndr. 2006;42:248–55. doi: 10.1097/01.qai.0000214818.80539.da. doi:10.1097/01.qai.0000214818.80539.da. [DOI] [PubMed] [Google Scholar]
- Linka M, Brůcková M, Malý M, Vandasová J, Stanková M, Reinis M. A study of HIV-1 genetic diversity in the Czech Republic: 1986-2007. Cent. Eur. J. Public Health. 2008;16:175–7. doi: 10.21101/cejph.a3500. [DOI] [PubMed] [Google Scholar]
- Liu J, Jia Y, Xu Q, Zheng Y-T, Zhang C. Phylodynamics of HIV-1 unique recombinant forms in China-Myanmar border: implication for HIV-1 transmission to Myanmar from Dehong, China. Infect. Genet. Evol. 2012;12:1944–8. doi: 10.1016/j.meegid.2012.08.001. doi:10.1016/j.meegid.2012.08.001. [DOI] [PubMed] [Google Scholar]
- Lospitao E, Alvarez A, Soriano V, Holguín A. HIV-1 subtypes in Spain: a retrospective analysis from 1995 to 2003. HIV Med. 2005;6:313–20. doi: 10.1111/j.1468-1293.2005.00313.x. doi:10.1111/j.1468-1293.2005.00313.x. [DOI] [PubMed] [Google Scholar]
- Lukashov VV, Cornelissen MT, Goudsmit J, Papuashvilli MN, Rytik PG, Khaitov RM, Karamov EV, de Wolf F. Simultaneous introduction of distinct HIV-1 subtypes into different risk groups in Russia, Byelorussia and Lithuania. AIDS. 1995;9:435–9. [PubMed] [Google Scholar]
- Lukashov VV, Huismans R, Rakhmanova AG, Lisitsina ZN, Akhtyrskaya NA, Vlasov NN, Melnick OB, Goudsmit J. Circulation of subtype A and gagA/envB recombinant HIV type 1 strains among injecting drug users in St. Petersburg, Russia, correlates with geographical origin of infections. AIDS Res. Hum. Retroviruses. 1999;15:1577–83. doi: 10.1089/088922299309874. doi:10.1089/088922299309874. [DOI] [PubMed] [Google Scholar]
- Lukashov VV, Karamov EV, Eremin VF, Titov LP, Goudsmit J. Extreme founder effect in an HIV type 1 subtype A epidemic among drug users in Svetlogorsk, Belarus. AIDS Res. Hum. Retroviruses. 1998;14:1299–303. doi: 10.1089/aid.1998.14.1299. [DOI] [PubMed] [Google Scholar]
- Lukashov VV, Kuiken CL, Vlahov D, Coutinho RA, Goudsmit J. Evidence for HIV type 1 strains of U.S. intravenous drug users as founders of AIDS epidemic among intravenous drug users in northern Europe. AIDS Res. Hum. Retroviruses. 1996;12:1179–83. doi: 10.1089/aid.1996.12.1179. [DOI] [PubMed] [Google Scholar]
- MacArthur GJ, van Velzen E, Palmateer N, Kimber J, Pharris A, Hope V, Taylor A, Roy K, Aspinall E, Goldberg D, Rhodes T, Hedrich D, Salminen M, Hickman M, Hutchinson SJ. Interventions to prevent HIV and Hepatitis C in people who inject drugs: a review of reviews to assess evidence of effectiveness. Int. J. Drug Policy. 2014;25:34–52. doi: 10.1016/j.drugpo.2013.07.001. doi:10.1016/j.drugpo.2013.07.001. [DOI] [PubMed] [Google Scholar]
- Mandal D, Jana S, Bhattacharya SK, Chakrabarti S. HIV type 1 subtypes circulating in eastern and northeastern regions of India. AIDS Res. Hum. Retroviruses. 2002;18:1219–27. doi: 10.1089/08892220260387968. doi:10.1089/08892220260387968. [DOI] [PubMed] [Google Scholar]
- Mathers BM, Degenhardt L, Bucello C, Lemon J, Wiessing L, Hickman M. Mortality among people who inject drugs: a systematic review and meta-analysis. Bull. World Health Organ. 2013;91:102–23. doi: 10.2471/BLT.12.108282. doi:10.2471/BLT.12.108282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathers BM, Degenhardt L, Phillips B, Wiessing L, Hickman M, Strathdee SA, Wodak A, Panda S, Tyndall M, Toufik A, Mattick RP. Global epidemiology of injecting drug use and HIV among people who inject drugs: a systematic review. Lancet (London, England) 2008;372:1733–45. doi: 10.1016/S0140-6736(08)61311-2. doi:10.1016/S0140-6736(08)61311-2. [DOI] [PubMed] [Google Scholar]
- McCormack S, Dunn DT, Desai M, Dolling DI, Gafos M, Gilson R, Sullivan AK, Clarke A, Reeves I, Schembri G, Mackie N, Bowman C, Lacey CJ, Apea V, Brady M, Fox J, Taylor S, Antonucci S, Khoo SH, Rooney J, Nardone A, Fisher M, McOwan A, Phillips AN, Johnson AM, Gazzard B, Gill ON. Pre-exposure prophylaxis to prevent the acquisition of HIV-1 infection (PROUD): effectiveness results from the pilot phase of a pragmatic open-label randomised trial. Lancet (London, England) 2015 doi: 10.1016/S0140-6736(15)00056-2. doi:10.1016/S0140-6736(15)00056-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Memarnejadian A, Menbari S, Mansouri SA, Sadeghi L, Vahabpour R, Aghasadeghi MR, Mostafavi E, Abdi M. Transmitted Drug Resistance Mutations in Antiretroviral-Naïve Injection Drug Users with Chronic HIV-1 Infection in Iran. PLoS One. 2015;10:e0126955. doi: 10.1371/journal.pone.0126955. doi:10.1371/journal.pone.0126955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menu E, Reynes JM, Müller-Trutwin MC, Guillemot L, Versmisse P, Chiron M, An S, Trouplin V, Charneau P, Fleury H, Barré-Sinoussi F, Sainte Marie FF. Predominance of CCR5-dependent HIV-1 subtype E isolates in Cambodia. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 1999;20:481–7. doi: 10.1097/00042560-199904150-00011. [DOI] [PubMed] [Google Scholar]
- Menu E, Truong TX, Lafon ME, Nguyen TH, Müller-Trutwin MC, Nguyen TT, Deslandres A, Chaouat G, Duong QT, Ha BK, Fleury HJ, Barré-Sinoussi F. HIV type 1 Thai subtype E is predominant in South Vietnam. AIDS Res. Hum. Retroviruses. 1996;12:629–33. doi: 10.1089/aid.1996.12.629. [DOI] [PubMed] [Google Scholar]
- Mezei M, Ay E, Koroknai A, Tóth R, Balázs A, Bakos A, Gyori Z, Bánáti F, Marschalkó M, Kárpáti S, Minárovits J. Molecular epidemiological analysis of env and pol sequences in newly diagnosed HIV type 1-infected, untreated patients in Hungary. AIDS Res. Hum. Retroviruses. 2011;27:1243–7. doi: 10.1089/aid.2011.0077. doi:10.1089/aid.2011.0077. [DOI] [PubMed] [Google Scholar]
- Mills EJ, Bakanda C, Birungi J, Chan K, Ford N, Cooper CL, Nachega JB, Dybul M, Hogg RS. Life expectancy of persons receiving combination antiretroviral therapy in low-income countries: a cohort analysis from Uganda. Ann. Intern. Med. 2011;155:209–16. doi: 10.7326/0003-4819-155-4-201108160-00358. doi:10.7326/0003-4819-155-4-201108160-00358. [DOI] [PubMed] [Google Scholar]
- Molina J-M, Capitant C, Spire B, Pialoux G, Cotte L, Charreau I, Tremblay C, Le Gall J-M, Cua E, Pasquet A, Raffi F, Pintado C, Chidiac C, Chas J, Charbonneau P, Delaugerre C, Suzan-Monti M, Loze B, Fonsart J, Peytavin G, Cheret A, Timsit J, Girard G, Lorente N, Préau M, Rooney JF, Wainberg MA, Thompson D, Rozenbaum W, Doré V, Marchand L, Simon M-C, Etien N, Aboulker J-P, Meyer L, Delfraissy J-F. On-Demand Preexposure Prophylaxis in Men at High Risk for HIV-1 Infection. N. Engl. J. Med. 2015;373:2237–46. doi: 10.1056/NEJMoa1506273. doi:10.1056/NEJMoa1506273. [DOI] [PubMed] [Google Scholar]
- Montavon C, Toure-Kane C, Nkengasong JN, Vergne L, Hertogs K, Mboup S, Delaporte E, Peeters M. CRF06-cpx: a new circulating recombinant form of HIV-1 in West Africa involving subtypes A, G, K, and J. J. Acquir. Immune Defic. Syndr. 2002;29:522–30. doi: 10.1097/00126334-200204150-00014. [DOI] [PubMed] [Google Scholar]
- Motomura K, Kusagawa S, Kato K, Nohtomi K, Lwin HH, Tun KM, Thwe M, Oo KY, Lwin S, Kyaw O, Zaw M, Nagai Y, Takebe Y. Emergence of new forms of human immunodeficiency virus type 1 intersubtype recombinants in central Myanmar. AIDS Res. Hum. Retroviruses. 2000;16:1831–43. doi: 10.1089/08892220050195793. doi:10.1089/08892220050195793. [DOI] [PubMed] [Google Scholar]
- Mousavi SM, Hamkar R, Gouya MM, Safaie A, Zahraei SM, Yazdani Z, Asbaghi Namini S, Bertagnolio S, Sutherland D, Sandstrom P, Brooks J. Surveillance of HIV drug resistance transmission in Iran: experience gained from a pilot study. Arch. Virol. 2010;155:329–34. doi: 10.1007/s00705-009-0583-6. doi:10.1007/s00705-009-0583-6. [DOI] [PubMed] [Google Scholar]
- Mullick R, Sengupta S, Sarkar K, Chakrabarti S. Molecular characterization of tat gene and long terminal repeat region of human immunodeficiency virus type-1 detected among the injecting drug users (IDUs) of Manipur, India: identification of BC recombinants. Virus Res. 2010;147:195–201. doi: 10.1016/j.virusres.2009.10.024. doi:10.1016/j.virusres.2009.10.024. [DOI] [PubMed] [Google Scholar]
- Murray M, Hogg RS, Lima VD, May MT, Moore DM, Abgrall S, Bruyand M, D'Arminio Monforte A, Tural C, Gill MJ, Harris RJ, Reiss P, Justice A, Kirk O, Saag M, Smith CJ, Weber R, Rockstroh J, Khaykin P, Sterne JAC. The effect of injecting drug use history on disease progression and death among HIV-positive individuals initiating combination antiretroviral therapy: collaborative cohort analysis. HIV Med. 2012;13:89–97. doi: 10.1111/j.1468-1293.2011.00940.x. doi:10.1111/j.1468-1293.2011.00940.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabatov AA, Kravchenko ON, Lyulchuk MG, Shcherbinskaya AM, Lukashov VV. Simultaneous introduction of HIV type 1 subtype A and B viruses into injecting drug users in southern Ukraine at the beginning of the epidemic in the former Soviet Union. AIDS Res. Hum. Retroviruses. 2002;18:891–5. doi: 10.1089/08892220260190380. doi:10.1089/08892220260190380. [DOI] [PubMed] [Google Scholar]
- Naderi HR, Tagliamonte M, Tornesello ML, Ciccozzi M, Rezza G, Farid R, Buonaguro FM, Buonaguro L. Molecular and phylogenetic analysis of HIV-1 variants circulating among injecting drug users in Mashhad-Iran. Infect. Agent. Cancer. 2006;1:4. doi: 10.1186/1750-9378-1-4. doi:10.1186/1750-9378-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nahmias AJ, Weiss J, Yao X, Lee F, Kodsi R, Schanfield M, Matthews T, Bolognesi D, Durack D, Motulsky A. Evidence for human infection with an HTLV III/LAV-like virus in Central Africa, 1959. Lancet (London, England) 1986;1:1279–80. doi: 10.1016/s0140-6736(86)91422-4. [DOI] [PubMed] [Google Scholar]
- Neogi U, Bontell I, Shet A, De Costa A, Gupta S, Diwan V, Laishram RS, Wanchu A, Ranga U, Banerjea AC, Sönnerborg A. Molecular epidemiology of HIV-1 subtypes in India: origin and evolutionary history of the predominant subtype C. PLoS One. 2012;7:e39819. doi: 10.1371/journal.pone.0039819. doi:10.1371/journal.pone.0039819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neogi U, Sood V, Ronsard L, Singh J, Lata S, Ramachandran VG, Das S, Wanchu A, Banerjea AC. Genetic architecture of HIV-1 genes circulating in north India & their functional implications. Indian J. Med. Res. 2011;134:769–78. doi: 10.4103/0971-5916.92624. doi:10.4103/0971-5916.92624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng KT, Ong LY, Takebe Y, Kamarulzaman A, Tee KK. Genome sequence of a novel HIV-1 circulating recombinant form 54_01B from Malaysia. J. Virol. 2012;86:11405–6. doi: 10.1128/JVI.01949-12. doi:10.1128/JVI.01949-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niculescu I, Paraschiv S, Paraskevis D, Abagiu A, Batan I, Banica L, Otelea D. Recent HIV-1 Outbreak Among Intravenous Drug Users in Romania: Evidence for Cocirculation of CRF14_BG and Subtype F1 Strains. AIDS Res. Hum. Retroviruses. 2015;31:488–95. doi: 10.1089/aid.2014.0189. doi:10.1089/aid.2014.0189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolopoulos G, Paraskevis D, Hatzakis A. HIV epidemiology in Greece. Future Microbiol. 2008;3:507–16. doi: 10.2217/17460913.3.5.507. doi:10.2217/17460913.3.5.507. [DOI] [PubMed] [Google Scholar]
- Nikolopoulos GK, Fotiou A, Kanavou E, Richardson C, Detsis M, Pharris A, Suk JE, Semenza JC, Costa-Storti C, Paraskevis D, Sypsa V, Malliori M-M, Friedman SR, Hatzakis A. National Income Inequality and Declining GDP Growth Rates Are Associated with Increases in HIV Diagnoses among People Who Inject Drugs in Europe: A Panel Data Analysis. PLoS One. 2015a;10:e0122367. doi: 10.1371/journal.pone.0122367. doi:10.1371/journal.pone.0122367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolopoulos GK, Sypsa V, Bonovas S, Paraskevis D, Malliori-Minerva M, Hatzakis A, Friedman SR. Big Events in Greece and HIV Infection Among People Who Inject Drugs. Subst. Use Misuse. 2015b;50:825–38. doi: 10.3109/10826084.2015.978659. doi:10.3109/10826084.2015.978659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novitsky VA, Montano MA, Essex M. Molecular epidemiology of an HIV-1 subtype A subcluster among injection drug users in the Southern Ukraine. AIDS Res. Hum. Retroviruses. 1998;14:1079–85. doi: 10.1089/aid.1998.14.1079. [DOI] [PubMed] [Google Scholar]
- Nsanzimana S, Remera E, Kanters S, Chan K, Forrest JI, Ford N, Condo J, Binagwaho A, Mills EJ. Life expectancy among HIV-positive patients in Rwanda: a retrospective observational cohort study. Lancet. Glob. Heal. 2015;3:e169–77. doi: 10.1016/S2214-109X(14)70364-X. doi:10.1016/S2214-109X(14)70364-X. [DOI] [PubMed] [Google Scholar]
- Oelrichs RB, Shrestha IL, Anderson DA, Deacon NJ. The explosive human immunodeficiency virus type 1 epidemic among injecting drug users of Kathmandu, Nepal, is caused by a subtype C virus of restricted genetic diversity. J. Virol. 2000;74:1149–57. doi: 10.1128/jvi.74.3.1149-1157.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou CY, Takebe Y, Weniger BG, Luo CC, Kalish ML, Auwanit W, Yamazaki S, Gayle HD, Young NL, Schochetman G. Independent introduction of two major HIV-1 genotypes into distinct high-risk populations in Thailand. Lancet (London, England) 1993;341:1171–4. doi: 10.1016/0140-6736(93)91001-3. [DOI] [PubMed] [Google Scholar]
- Palma AC, Araújo F, Duque V, Borges F, Paixão MT, Camacho R. Molecular epidemiology and prevalence of drug resistance-associated mutations in newly diagnosed HIV-1 patients in Portugal. Infect. Genet. Evol. 2007;7:391–8. doi: 10.1016/j.meegid.2007.01.009. doi:10.1016/j.meegid.2007.01.009. [DOI] [PubMed] [Google Scholar]
- Pandrea I, Descamps D, Collin G, Robertson DL, Damond F, Dimitrienco V, Gheorghita S, Pecec M, Simon F, Brun-Vézinet F, Apetrei C. HIV type 1 genetic diversity and genotypic drug susceptibility in the Republic of Moldova. AIDS Res. Hum. Retroviruses. 2001;17:1297–304. doi: 10.1089/088922201750461375. doi:10.1089/088922201750461375. [DOI] [PubMed] [Google Scholar]
- Pang W, Zhang C, Duo L, Zhou Y-H, Yao Z-H, Liu F-L, Li H, Tu Y-Q, Zheng Y-T. Extensive and complex HIV-1 recombination between B', C and CRF01_AE among IDUs in south-east Asia. AIDS. 2012;26:1121–9. doi: 10.1097/QAD.0b013e3283522c97. doi:10.1097/QAD.0b013e3283522c97. [DOI] [PubMed] [Google Scholar]
- Paraskevis D, Magiorkinis E, Magiorkinis G, Sypsa V, Paparizos V, Lazanas M, Gargalianos P, Antoniadou A, Panos G, Chrysos G, Sambatakou H, Karafoulidou A, Skoutelis A, Kordossis T, Koratzanis G, Theodoridou M, Daikos GL, Nikolopoulos G, Pybus OG, Hatzakis A. Increasing prevalence of HIV-1 subtype A in Greece: estimating epidemic history and origin. J. Infect. Dis. 2007;196:1167–76. doi: 10.1086/521677. doi:10.1086/521677. [DOI] [PubMed] [Google Scholar]
- Paraskevis D, Nikolopoulos G, Fotiou A, Tsiara C, Paraskeva D, Sypsa V, Lazanas M, Gargalianos P, Psichogiou M, Skoutelis A, Wiessing L, Friedman SR, Jarlais DCDES, Terzidou M, Kremastinou J, Malliori M, Hatzakis A. Economic recession and emergence of an HIV-1 outbreak among drug injectors in athens metropolitan area: a longitudinal study. PLoS One. 2013;8:e78941. doi: 10.1371/journal.pone.0078941. doi:10.1371/journal.pone.0078941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paraskevis D, Nikolopoulos G, Tsiara C, Paraskeva D, Antoniadou A, Lazanas M, Gargalianos P, Psychogiou M, Malliori M, Kremastinou J, Hatzakis A. HIV-1 outbreak among injecting drug users in Greece, 2011: a preliminary report. Euro Surveill. 2011;16 doi: 10.2807/ese.16.36.19962-en. [DOI] [PubMed] [Google Scholar]
- Paraskevis D, Paraschiv S, Sypsa V, Nikolopoulos G, Tsiara C, Magiorkinis G, Psichogiou M, Flampouris A, Mardarescu M, Niculescu I, Batan I, Malliori M, Otelea D, Hatzakis A. Enhanced HIV-1 surveillance using molecular epidemiology to study and monitor HIV-1 outbreaks among intravenous drug users (IDUs) in Athens and Bucharest. Infect. Genet. Evol. 2015;35:109–121. doi: 10.1016/j.meegid.2015.08.004. doi:10.1016/j.meegid.2015.08.004. [DOI] [PubMed] [Google Scholar]
- Parczewski M, Leszczyszyn-Pynka M, Witak-Jędra M, Rymer W, Zalewska M, Gąsiorowski J, Bociąga-Jasik M, Kalinowska-Nowak A, Garlicki A, Grzeszczuk A, Jankowska M, Lemańska M, Barałkiewicz G, Mozer-Lisewska I, Łojewski W, Grąbczewska E, Olczak A, Jabłonowska E, Urbańska A. Distribution and time trends of HIV-1 variants in Poland: Characteristics of non-B clades and recombinant viruses. Infect. Genet. Evol. 2016;39:232–40. doi: 10.1016/j.meegid.2016.02.001. doi:10.1016/j.meegid.2016.02.001. [DOI] [PubMed] [Google Scholar]
- Patel P, Borkowf CB, Brooks JT, Lasry A, Lansky A, Mermin J. Estimating per-act HIV transmission risk: a systematic review. AIDS. 2014;28:1509–19. doi: 10.1097/QAD.0000000000000298. doi:10.1097/QAD.0000000000000298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peeters M, Gueye A, Mboup S, Bibollet-Ruche F, Ekaza E, Mulanga C, Ouedrago R, Gandji R, Mpele P, Dibanga G, Koumare B, Saidou M, Esu-Williams E, Lombart JP, Badombena W, Luo N, Vanden Haesevelde M, Delaporte E. Geographical distribution of HIV-1 group O viruses in Africa. AIDS. 1997;11:493–8. doi: 10.1097/00002030-199704000-00013. [DOI] [PubMed] [Google Scholar]
- Peeters M, Jung M, Ayouba A. The origin and molecular epidemiology of HIV. Expert Rev. Anti. Infect. Ther. 2013;11:885–96. doi: 10.1586/14787210.2013.825443. doi:10.1586/14787210.2013.825443. [DOI] [PubMed] [Google Scholar]
- Pérez-Alvarez L, Carmona R, Muñoz M, Delgado E, Thomson MM, Contreras G, Pedreira JD, Rodríguez Real R, Vázquez de Parga E, Medrano L, Taboada JA, Nájera R. High incidence of non-B and recombinant HIV-1 strains in newly diagnosed patients in Galicia, Spain: study of genotypic resistance. Antivir. Ther. 2003;8:355–60. [PubMed] [Google Scholar]
- Piyasirisilp S, McCutchan FE, Carr JK, Sanders-Buell E, Liu W, Chen J, Wagner R, Wolf H, Shao Y, Lai S, Beyrer C, Yu XF. A recent outbreak of human immunodeficiency virus type 1 infection in southern China was initiated by two highly homogeneous, geographically separated strains, circulating recombinant form AE and a novel BC recombinant. J. Virol. 2000;74:11286–95. doi: 10.1128/jvi.74.23.11286-11295.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plantier J-C, Leoz M, Dickerson JE, De Oliveira F, Cordonnier F, Lemée V, Damond F, Robertson DL, Simon F. A new human immunodeficiency virus derived from gorillas. Nat. Med. 2009;15:871–2. doi: 10.1038/nm.2016. doi:10.1038/nm.2016. [DOI] [PubMed] [Google Scholar]
- Ramos A, Hu DJ, Nguyen L, Phan K-O, Vanichseni S, Promadej N, Choopanya K, Callahan M, Young NL, McNicholl J, Mastro TD, Folks TM, Subbarao S. Intersubtype human immunodeficiency virus type 1 superinfection following seroconversion to primary infection in two injection drug users. J. Virol. 2002;76:7444–52. doi: 10.1128/JVI.76.15.7444-7452.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinis M, Brucková M, Graham RR, Vandasová J, Stanková M, Carr JK. Genetic subtypes of HIV type 1 viruses circulating in the Czech Republic. AIDS Res. Hum. Retroviruses. 2001;17:1305–10. doi: 10.1089/088922201750461384. doi:10.1089/088922201750461384. [DOI] [PubMed] [Google Scholar]
- Renjifo B, Gilbert P, Chaplin B, Msamanga G, Mwakagile D, Fawzi W, Essex M. Preferential in-utero transmission of HIV-1 subtype C as compared to HIV-1 subtype A or D. AIDS. 2004;18:1629–36. doi: 10.1097/01.aids.0000131392.68597.34. [DOI] [PubMed] [Google Scholar]
- Rhodes T, Simic M. Transition and the HIV risk environment. BMJ. 2005;331:220–3. doi: 10.1136/bmj.331.7510.220. doi:10.1136/bmj.331.7510.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roques P, Robertson DL, Souquière S, Apetrei C, Nerrienet E, Barré-Sinoussi F, Müller-Trutwin M, Simon F. Phylogenetic characteristics of three new HIV-1 N strains and implications for the origin of group N. AIDS. 2004;18:1371–81. doi: 10.1097/01.aids.0000125990.86904.28. [DOI] [PubMed] [Google Scholar]
- Roques P, Robertson DL, Souquière S, Damond F, Ayouba A, Farfara I, Depienne C, Nerrienet E, Dormont D, Brun-Vézinet F, Simon F, Mauclère P. Phylogenetic analysis of 49 newly derived HIV-1 group O strains: high viral diversity but no group M-like subtype structure. Virology. 2002;302:259–73. doi: 10.1006/viro.2002.1430. [DOI] [PubMed] [Google Scholar]
- Rumyantseva OA, Olkhovskiy IA, Malysheva MA, Ruzaeva LA, Vasiliev AV, Kazennova EV, Bobkova MR, Lukashov VV. Epidemiological networks and drug resistance of HIV type 1 in Krasnoyarsk region, Russia. AIDS Res. Hum. Retroviruses. 2009;25:931–6. doi: 10.1089/aid.2009.0075. doi:10.1089/aid.2009.0075. [DOI] [PubMed] [Google Scholar]
- Saad MD, Aliev Q, Botros BAM, Carr JK, Gomatos PJ, Nadai Y, Michael AA, Nasibov Z, Sanchez JL, Brix DI, Earhart KC. Genetic forms of HIV Type 1 in the former Soviet Union dominate the epidemic in Azerbaijan. AIDS Res. Hum. Retroviruses. 2006a;22:796–800. doi: 10.1089/aid.2006.22.796. doi:10.1089/aid.2006.22.796. [DOI] [PubMed] [Google Scholar]
- Saad MD, Shcherbinskaya AM, Nadai Y, Kruglov YV, Antonenko SV, Lyullchuk MG, Kravchenko ON, Earhart KC, Sanchez JL, Birx DL, Carr JK. Molecular epidemiology of HIV Type 1 in Ukraine: birthplace of an epidemic. AIDS Res. Hum. Retroviruses. 2006b;22:709–14. doi: 10.1089/aid.2006.22.709. doi:10.1089/aid.2006.22.709. [DOI] [PubMed] [Google Scholar]
- Sahbandar IN, Takahashi K, Djoerban Z, Firmansyah I, Naganawa S, Motomura K, Sato H, Kitamura K, Pohan HT, Sato S. Current HIV type 1 molecular epidemiology profile and identification of unique recombinant forms in Jakarta, Indonesia. AIDS Res. Hum. Retroviruses. 2009;25:637–46. doi: 10.1089/aid.2008.0266. doi:10.1089/aid.2008.0266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samji H, Cescon A, Hogg RS, Modur SP, Althoff KN, Buchacz K, Burchell AN, Cohen M, Gebo KA, Gill MJ, Justice A, Kirk G, Klein MB, Korthuis PT, Martin J, Napravnik S, Rourke SB, Sterling TR, Silverberg MJ, Deeks S, Jacobson LP, Bosch RJ, Kitahata MM, Goedert JJ, Moore R, Gange SJ. Closing the Gap: Increases in Life Expectancy among Treated HIV-Positive Individuals in the United States and Canada. PLoS One. 2013;8:e81355. doi: 10.1371/journal.pone.0081355. doi:10.1371/journal.pone.0081355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders-Buell E, Bose M, Nasir A, Todd CS, Stanekzai MR, Tovanabutra S, Scott PT, Strathdee SA, Tjaden J, Michael NL, McCutchan FE. Distinct circulating recombinant HIV-1 strains among injecting drug users and sex workers in Afghanistan. AIDS Res. Hum. Retroviruses. 2010;26:605–8. doi: 10.1089/aid.2009.0226. doi:10.1089/aid.2009.0226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders-Buell E, Saad MD, Abed AM, Bose M, Todd CS, Strathdee SA, Botros BA, Safi N, Earhart KC, Scott PT, Michael N, McCutchan FE. A nascent HIV type 1 epidemic among injecting drug users in Kabul, Afghanistan is dominated by complex AD recombinant strain, CRF35_AD. AIDS Res. Hum. Retroviruses. 2007;23:834–9. doi: 10.1089/aid.2006.0299. doi:10.1089/aid.2006.0299. [DOI] [PubMed] [Google Scholar]
- Saraswathy TS, Ng KP, Sinniah M. Human immunodeficiency virus type 1 subtypes among Malaysian intravenous drug users. Southeast Asian J. Trop. Med. Public Health. 2000;31:283–6. [PubMed] [Google Scholar]
- Sarkar R, Sengupta S, Mullick R, Singh NB, Sarkar K, Chakrabarti S. Implementation of a multiregion hybridization assay to characterize HIV-1 strains detected among injecting drug users in Manipur, India. Intervirology. 2009;52:175–8. doi: 10.1159/000224645. doi:10.1159/000224645. [DOI] [PubMed] [Google Scholar]
- Sarker MS, Rahman M, Yirrell D, Campbell E, Rahman ASMM, Islam LN, Azim T. Molecular evidence for polyphyletic origin of human immunodeficiency virus type 1 subtype C in Bangladesh. Virus Res. 2008;135:89–94. doi: 10.1016/j.virusres.2008.02.010. doi:10.1016/j.virusres.2008.02.010. [DOI] [PubMed] [Google Scholar]
- Sarrami-Forooshani R, Das SR, Sabahi F, Adeli A, Esmaeili R, Wahren B, Mohraz M, Haji-Abdolbaghi M, Rasoolinejad M, Jameel S, Mahboudi F. Molecular analysis and phylogenetic characterization of HIV in Iran. J. Med. Virol. 2006;78:853–63. doi: 10.1002/jmv.20634. doi:10.1002/jmv.20634. [DOI] [PubMed] [Google Scholar]
- Shah S, Xing H, Altaf A, Chen B, Liao L, Jia Y, Vermund SH, Shao Y. Antiretroviral drug resistance mutations among treated and treatment-naive patients in Pakistan: diversity of the HIV type 1 pol gene in Pakistan. AIDS Res. Hum. Retroviruses. 2011;27:1277–82. doi: 10.1089/aid.2010.0324. doi:10.1089/AID.2010.0324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahid A, Dixit SM, Gurbacharya VL, Karmacharya D, Ali S. HIV-1 circulating recombinant form in Nepal. J. Virol. 2011;85:8458. doi: 10.1128/JVI.05108-11. doi:10.1128/JVI.05108-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp PM, Hahn BH. Origins of HIV and the AIDS pandemic. Cold Spring Harb. Perspect. Med. 2011;1:a006841. doi: 10.1101/cshperspect.a006841. doi:10.1101/cshperspect.a006841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen C, Craigo J, Ding M, Chen Y, Gupta P. Origin and dynamics of HIV-1 subtype C infection in India. PLoS One. 2011;6:e25956. doi: 10.1371/journal.pone.0025956. doi:10.1371/journal.pone.0025956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiino T, Hattori J, Yokomaku Y, Iwatani Y, Sugiura W. Phylodynamic analysis reveals CRF01_AE dissemination between Japan and neighboring Asian countries and the role of intravenous drug use in transmission. PLoS One. 2014;9:e102633. doi: 10.1371/journal.pone.0102633. doi:10.1371/journal.pone.0102633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon F, Mauclère P, Roques P, Loussert-Ajaka I, Müller-Trutwin MC, Saragosti S, Georges-Courbot MC, Barré-Sinoussi F, Brun-Vézinet F. Identification of a new human immunodeficiency virus type 1 distinct from group M and group O. Nat. Med. 1998;4:1032–7. doi: 10.1038/2017. doi:10.1038/2017. [DOI] [PubMed] [Google Scholar]
- Skar H, Axelsson M, Berggren I, Thalme A, Gyllensten K, Liitsola K, Brummer-Korvenkontio H, Kivelä P, Spångberg E, Leitner T, Albert J. Dynamics of two separate but linked HIV-1 CRF01_AE outbreaks among injection drug users in Stockholm, Sweden, and Helsinki, Finland. J. Virol. 2011;85:510–8. doi: 10.1128/JVI.01413-10. doi:10.1128/JVI.01413-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smoleń-Dzirba J, Rosińska M, Kruszyński P, Bratosiewicz-Wąsik J, Janiec J, Beniowski M, Bociąga-Jasik M, Jabłonowska E, Szetela B, Porter K, Wąsik TJ. Molecular epidemiology of recent HIV-1 infections in southern Poland. J. Med. Virol. 2012;84:1857–68. doi: 10.1002/jmv.23395. doi:10.1002/jmv.23395. [DOI] [PubMed] [Google Scholar]
- Smolskaya T, Liitsola K, Zetterberg V, Golovanova E, Kevlova N, Konovalova N, Sevastianova K, Brummer-Korvenkontio H, Salminen M. HIV epidemiology in the Northwestern Federal District of Russia: dominance of HIV type 1 subtype A. AIDS Res. Hum. Retroviruses. 2006;22:1074–80. doi: 10.1089/aid.2006.22.1074. doi:10.1089/aid.2006.22.1074. [DOI] [PubMed] [Google Scholar]
- Soriano V, Gomes P, Heneine W, Holguín A, Doruana M, Antunes R, Mansinho K, Switzer WM, Araujo C, Shanmugam V, Lourenço H, González-Lahoz J, Antunes F. Human immunodeficiency virus type 2 (HIV-2) in Portugal: clinical spectrum, circulating subtypes, virus isolation, and plasma viral load. J. Med. Virol. 2000;61:111–6. [PubMed] [Google Scholar]
- Stanojevic M, Alexiev I, Beshkov D, Gökengin D, Mezei M, Minarovits J, Otelea D, Paraschiv S, Poljak M, Zidovec-Lepej S, Paraskevis D. HIV-1 molecular epidemiology in the Balkans: a melting pot for high genetic diversity. AIDS Rev. 2012;14:28–36. [PubMed] [Google Scholar]
- Stephenson KE, Barouch DH. A global approach to HIV-1 vaccine development. Immunol. Rev. 2013;254:295–304. doi: 10.1111/imr.12073. doi:10.1111/imr.12073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strathdee SA, Stachowiak JA, Todd CS, Al-Delaimy WK, Wiebel W, Hankins C, Patterson TL. Complex emergencies, HIV, and substance use: no “big easy” solution. Subst. Use Misuse. 2006;41:1637–51. doi: 10.1080/10826080600848116. doi:10.1080/10826080600848116. [DOI] [PubMed] [Google Scholar]
- Strathdee SA, Stockman JK. Epidemiology of HIV among injecting and non-injecting drug users: current trends and implications for interventions. Curr. HIV/AIDS Rep. 2010;7:99–106. doi: 10.1007/s11904-010-0043-7. doi:10.1007/s11904-010-0043-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subbarao S, Limpakarnjanarat K, Mastro TD, Bhumisawasdi J, Warachit P, Jayavasu C, Young NL, Luo CC, Shaffer N, Kalish ML, Schochetman G. HIV type 1 in Thailand, 1994-1995: persistence of two subtypes with low genetic diversity. AIDS Res. Hum. Retroviruses. 1998;14:319–27. doi: 10.1089/aid.1998.14.319. [DOI] [PubMed] [Google Scholar]
- Tagliamonte M, Naderi HR, Tornesello ML, Farid R, Buonaguro FM, Buonaguro L. HIV type 1 subtype A epidemic in injecting drug user (IDU) communities in Iran. AIDS Res. Hum. Retroviruses. 2007;23:1569–74. doi: 10.1089/aid.2007.0169. doi:10.1089/aid.2007.0169. [DOI] [PubMed] [Google Scholar]
- Takebe Y, Kusagawa S, Motomura K. Molecular epidemiology of HIV: tracking AIDS pandemic. Pediatr. Int. 2004;46:236–44. doi: 10.1046/j.1442-200x.2004.01869.x. doi:10.1046/j.1442-200x.2004.01869.x. [DOI] [PubMed] [Google Scholar]
- Takebe Y, Liao H, Hase S, Uenishi R, Li Y, Li X-J, Han X, Shang H, Kamarulzaman A, Yamamoto N, Pybus OG, Tee KK. Reconstructing the epidemic history of HIV-1 circulating recombinant forms CRF07_BC and CRF08_BC in East Asia: the relevance of genetic diversity and phylodynamics for vaccine strategies. Vaccine. 2010;28(Suppl 2):B39–44. doi: 10.1016/j.vaccine.2009.07.101. doi:10.1016/j.vaccine.2009.07.101. [DOI] [PubMed] [Google Scholar]
- Takebe Y, Motomura K, Tatsumi M, Lwin HH, Zaw M, Kusagawa S. High prevalence of diverse forms of HIV-1 intersubtype recombinants in Central Myanmar: geographical hot spot of extensive recombination. AIDS. 2003;17:2077–87. doi: 10.1097/00002030-200309260-00009. doi:10.1097/01.aids.0000076350.42412.a5. [DOI] [PubMed] [Google Scholar]
- Takebe Y, Uenishi R, Li X. Global molecular epidemiology of HIV: understanding the genesis of AIDS pandemic. Adv. Pharmacol. 2008;56:1–25. doi: 10.1016/S1054-3589(07)56001-1. doi:10.1016/S1054-3589(07)56001-1. [DOI] [PubMed] [Google Scholar]
- Tee KK, Li X-J, Nohtomi K, Ng KP, Kamarulzaman A, Takebe Y. Identification of a novel circulating recombinant form (CRF33_01B) disseminating widely among various risk populations in Kuala Lumpur, Malaysia. J. Acquir. Immune Defic. Syndr. 2006;43:523–9. doi: 10.1097/01.qai.0000242451.74779.a7. doi:10.1097/01.qai.0000242451.74779.a7. [DOI] [PubMed] [Google Scholar]
- Tee KK, Pon CK, Kamarulzaman A, Ng KP. Emergence of HIV-1 CRF01_AE/B unique recombinant forms in Kuala Lumpur, Malaysia. AIDS. 2005a;19:119–26. doi: 10.1097/00002030-200501280-00003. [DOI] [PubMed] [Google Scholar]
- Tee KK, Pybus OG, Li X-J, Han X, Shang H, Kamarulzaman A, Takebe Y. Temporal and spatial dynamics of human immunodeficiency virus type 1 circulating recombinant forms 08_BC and 07_BC in Asia. J. Virol. 2008;82:9206–15. doi: 10.1128/JVI.00399-08. doi:10.1128/JVI.00399-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tee KK, Saw TL, Pon CK, Kamarulzaman A, Ng KP. The evolving molecular epidemiology of HIV type 1 among injecting drug users (IDUs) in Malaysia. AIDS Res. Hum. Retroviruses. 2005b;21:1046–50. doi: 10.1089/aid.2005.21.1046. doi:10.1089/aid.2005.21.1046. [DOI] [PubMed] [Google Scholar]
- The Antiretroviral Cohort Collaboration Life expectancy of individuals on combination antiretroviral therapy in high-income countries: a collaborative analysis of 14 cohort studies. Lancet (London, England) 2008;372:293–9. doi: 10.1016/S0140-6736(08)61113-7. doi:10.1016/S0140-6736(08)61113-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson MM, de Parga EV, Vinogradova A, Sierra M, Yakovlev A, Rakhmanova A, Delgado E, Casado G, Muñoz M, Carmona R, Vega Y, Pérez-Alvarez L, Contreras G, Medrano L, Osmanov S, Nájera R. New insights into the origin of the HIV type 1 subtype A epidemic in former Soviet Union's countries derived from sequence analyses of preepidemically transmitted viruses. AIDS Res. Hum. Retroviruses. 2007;23:1599–604. doi: 10.1089/aid.2007.0166. doi:10.1089/aid.2007.0166. [DOI] [PubMed] [Google Scholar]
- Thomson MM, Delgado E, Manjón N, Ocampo A, Villahermosa ML, Mariño A, Herrero I, Cuevas MT, Vázquez-de Parga E, Pérez-Alvarez L, Medrano L, Taboada JA, Nájera R. HIV-1 genetic diversity in Galicia Spain: BG intersubtype recombinant viruses circulating among injecting drug users. AIDS. 2001;15:509–16. doi: 10.1097/00002030-200103090-00010. [DOI] [PubMed] [Google Scholar]
- Thomson MM, Najera R. Increasing HIV-1 genetic diversity in Europe. J. Infect. Dis. 2007;196:1120–4. doi: 10.1086/521683. doi:10.1086/521683. [DOI] [PubMed] [Google Scholar]
- Tienen C. van, van der Loeff MS, Zaman SMA, Vincent T, Sarge-Njie R, Peterson I, Leligdowicz A, Jaye A, Rowland-Jones S, Aaby P, Whittle H. Two distinct epidemics: the rise of HIV-1 and decline of HIV-2 infection between 1990 and 2007 in rural Guinea-Bissau. J. Acquir. Immune Defic. Syndr. 2010;53:640–7. doi: 10.1097/QAI.0b013e3181bf1a25. doi:10.1097/QAI.0b013e3181bf1a25. [DOI] [PubMed] [Google Scholar]
- Tovanabutra S, Beyrer C, Sakkhachornphop S, Razak MH, Ramos GL, Vongchak T, Rungruengthanakit K, Saokhieo P, Tejafong K, Kim B, De Souza M, Robb ML, Birx DL, Jittiwutikarn J, Suriyanon V, Celentano DD, McCutchan FE. The changing molecular epidemiology of HIV type 1 among northern Thai drug users, 1999 to 2002. AIDS Res. Hum. Retroviruses. 2004;20:465–75. doi: 10.1089/088922204323087705. doi:10.1089/088922204323087705. [DOI] [PubMed] [Google Scholar]
- Tovanabutra S, Kijak GH, Beyrer C, Gammon-Richardson C, Sakkhachornphop S, Vongchak T, Jittiwutikarn J, Razak MH, Sanders-Buell E, Robb ML, Suriyanon V, Birx DL, Michael NL, Celentano DD, McCutchan FE. Identification of CRF34_01B, a second circulating recombinant form unrelated to and more complex than CRF15_01B, among injecting drug users in northern Thailand. AIDS Res. Hum. Retroviruses. 2007;23:829–33. doi: 10.1089/aid.2006.0300. doi:10.1089/aid.2006.0300. [DOI] [PubMed] [Google Scholar]
- Tovanabutra S, Polonis V, De Souza M, Trichavaroj R, Chanbancherd P, Kim B, Sanders-Buell E, Nitayaphan S, Brown A, Robb MR, Birx DL, McCutchan FE, Carr JK. First CRF01_AE/B recombinant of HIV-1 is found in Thailand. AIDS. 2001;15:1063–5. doi: 10.1097/00002030-200105250-00018. [DOI] [PubMed] [Google Scholar]
- Tovanabutra S, Watanaveeradej V, Viputtikul K, De Souza M, Razak MH, Suriyanon V, Jittiwutikarn J, Sriplienchan S, Nitayaphan S, Benenson MW, Sirisopana N, Renzullo PO, Brown AE, Robb ML, Beyrer C, Celentano DD, McNeil JG, Birx DL, Carr JK, McCutchan FE. A new circulating recombinant form, CRF15_01B, reinforces the linkage between IDU and heterosexual epidemics in Thailand. AIDS Res. Hum. Retroviruses. 2003;19:561–7. doi: 10.1089/088922203322230923. doi:10.1089/088922203322230923. [DOI] [PubMed] [Google Scholar]
- Tripathy SP, Kulkarni SS, Jadhav SD, Agnihotri KD, Jere AJ, Kurle SN, Bhattacharya SK, Singh K, Tripathy SP, Paranjape RS. Subtype B and subtype C HIV type 1 recombinants in the northeastern state of Manipur, India. AIDS Res. Hum. Retroviruses. 2005;21:152–7. doi: 10.1089/aid.2005.21.152. doi:10.1089/aid.2005.21.152. [DOI] [PubMed] [Google Scholar]
- UNAIDS . AIDS by the numbers, 2015. Geneva, Switzerland: 2015. [Google Scholar]
- UNAIDS . Global AIDS update. Geneva, Switzerland: 2016. [Google Scholar]
- United Nations Office on Drugs and Crime (UNODC) World Drug Report. Vienna, Austria: 2014. [Google Scholar]
- Vallari A, Bodelle P, Ngansop C, Makamche F, Ndembi N, Mbanya D, Kaptué L, Gürtler LG, McArthur CP, Devare SG, Brennan CA. Four new HIV-1 group N isolates from Cameroon: Prevalence continues to be low. AIDS Res. Hum. Retroviruses. 2010;26:109–15. doi: 10.1089/aid.2009.0178. doi:10.1089/aid.2009.0178. [DOI] [PubMed] [Google Scholar]
- Vallari A, Holzmayer V, Harris B, Yamaguchi J, Ngansop C, Makamche F, Mbanya D, Kaptué L, Ndembi N, Gürtler L, Devare S, Brennan CA. Confirmation of putative HIV-1 group P in Cameroon. J. Virol. 2011;85:1403–7. doi: 10.1128/JVI.02005-10. doi:10.1128/JVI.02005-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Heuverswyn F, Li Y, Neel C, Bailes E, Keele BF, Liu W, Loul S, Butel C, Liegeois F, Bienvenue Y, Ngolle EM, Sharp PM, Shaw GM, Delaporte E, Hahn BH, Peeters M. Human immunodeficiency viruses: SIV infection in wild gorillas. Nature. 2006;444:164. doi: 10.1038/444164a. doi:10.1038/444164a. [DOI] [PubMed] [Google Scholar]
- Vermund SH, Leigh-Brown AJ. The HIV Epidemic: High-Income Countries. Cold Spring Harb. Perspect. Med. 2012;2:a007195. doi: 10.1101/cshperspect.a007195. doi:10.1101/cshperspect.a007195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vongsheree S, Phutiprawan T, Sri-ngam P, Thaisri H, Puangtabtim W, Sawanpanyalert P. Co-existence of HIV-1 subtypes B' and E infections among Thai injecting drug users. Asian Pac. J. Allergy Immunol. 2002;20:29–35. [PubMed] [Google Scholar]
- Weber R, Huber M, Battegay M, Stähelin C, Castro Batanjer E, Calmy A, Bregenzer A, Bernasconi E, Schoeni-Affolter F, Ledergerber B. Influence of noninjecting and injecting drug use on mortality, retention in the cohort, and antiretroviral therapy, in participants in the Swiss HIV Cohort Study. HIV Med. 2015;16:137–51. doi: 10.1111/hiv.12184. doi:10.1111/hiv.12184. [DOI] [PubMed] [Google Scholar]
- WHO . Consolidated strategic information guidelines for HIV in the health sector. World Health Organization; Geneva, Switzerland: 2015. [PubMed] [Google Scholar]
- WHO, UNAIDS . Guidelenes for second generation HIV surveillance: an Update: Know your epidemic. Geneva: 2013. [PubMed] [Google Scholar]
- Wolfe D, Carrieri MP, Shepard D. Treatment and care for injecting drug users with HIV infection: a review of barriers and ways forward. Lancet (London, England) 2010;376:355–66. doi: 10.1016/S0140-6736(10)60832-X. doi:10.1016/S0140-6736(10)60832-X. [DOI] [PubMed] [Google Scholar]
- Wood E, Hogg RS, Lima VD, Kerr T, Yip B, Marshall BDL, Montaner JSG. Highly active antiretroviral therapy and survival in HIV-infected injection drug users. JAMA. 2008;300:550–4. doi: 10.1001/jama.300.5.550. doi:10.1001/jama.300.5.550. [DOI] [PubMed] [Google Scholar]
- Worobey M, Gemmel M, Teuwen DE, Haselkorn T, Kunstman K, Bunce M, Muyembe J-J, Kabongo J-MM, Kalengayi RM, Van Marck E, Gilbert MTP, Wolinsky SM. Direct evidence of extensive diversity of HIV-1 in Kinshasa by 1960. Nature. 2008;455:661–4. doi: 10.1038/nature07390. doi:10.1038/nature07390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yerly S, Jost S, Monnat M, Telenti A, Cavassini M, Chave J-P, Kaiser L, Burgisser P, Perrin L. HIV-1 co/super-infection in intravenous drug users. AIDS. 2004;18:1413–21. doi: 10.1097/01.aids.0000131330.28762.0c. [DOI] [PubMed] [Google Scholar]
- Yu XF, Chen J, Shao Y, Beyrer C, Liu B, Wang Z, Liu W, Yang J, Liang S, Viscidi RP, Gu J, Gurri-Glass G, Lai S. Emerging HIV infections with distinct subtypes of HIV-1 infection among injection drug users from geographically separate locations in Guangxi Province, China. J. Acquir. Immune Defic. Syndr. 1999;22:180–8. doi: 10.1097/00126334-199910010-00011. [DOI] [PubMed] [Google Scholar]
- Zarandia M, Tsertsvadze T, Carr JK, Nadai Y, Sanchez JL, Nelson AK. HIV-1 genetic diversity and genotypic drug susceptibility in the Republic of Georgia. AIDS Res. Hum. Retroviruses. 2006;22:470–6. doi: 10.1089/aid.2006.22.470. doi:10.1089/aid.2006.22.470. [DOI] [PubMed] [Google Scholar]
- Zetterberg V, Ustina V, Liitsola K, Zilmer K, Kalikova N, Sevastianova K, Brummer-Korvenkontio H, Leinikki P, Salminen MO. Two viral strains and a possible novel recombinant are responsible for the explosive injecting drug use-associated HIV type 1 epidemic in Estonia. AIDS Res. Hum. Retroviruses. 2004;20:1148–56. doi: 10.1089/aid.2004.20.1148. doi:10.1089/aid.2004.20.1148. [DOI] [PubMed] [Google Scholar]
- Zheng X, Tian C, Choi KH, Zhang J, Cheng H, Yang X, Li D, Lin J, Qu S, Sun X. Injecting drug use and HIV infection in southwest China. AIDS. 1994;8:1141–7. doi: 10.1097/00002030-199408000-00017. [DOI] [PubMed] [Google Scholar]
- Zhou Y-H, Liang Y-B, Pang W, Qin W-H, Yao Z-H, Chen X, Zhang C, Zheng Y-T. Diverse forms of HIV-1 among Burmese long-distance truck drivers imply their contribution to HIV-1 cross-border transmission. BMC Infect. Dis. 2014;14:463. doi: 10.1186/1471-2334-14-463. doi:10.1186/1471-2334-14-463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu T, Korber BT, Nahmias AJ, Hooper E, Sharp PM, Ho DD. An African HIV-1 sequence from 1959 and implications for the origin of the epidemic. Nature. 1998;391:594–7. doi: 10.1038/35400. doi:10.1038/35400. [DOI] [PubMed] [Google Scholar]