Abstract
Purpose
To correlate intraoperative interface fluid dynamics during Descemet stripping automated endothelial keratoplasty (DSAEK) surgery using intraoperative optical coherence tomography (iOCT) in the PIONEER (Prospective Intraoperative and Perioperative Ophthalmic ImagiNg with Optical CohEncE TomogRaphy) study with postoperative outcomes.
Design
Prospective consecutive, interventional, comparative case series
Participants
One hundred and seventy-eight eyes of 173 patients undergoing DSAEK from the Cole Eye Institute, Cleveland, USA.
Methods
Eyes that underwent DSAEK between October 2011 and March 2014 from the PIONEER intraoperative and perioperative OCT study were included. An automated interface fluid segmentation algorithm evaluated intraoperative dynamics of interface fluid before and after surgical manipulations. iOCT images were also captured at multiple intraoperative time points for two different DSAEK techniques, one that used an active air infusion system and one that did not.
Main Outcome Measures
interface fluid metrics, graft non-adherence
Results
iOCT measurements of interface fluid following final surgical manipulations and immediately prior to leaving the OR identified that Total Fluid Volume (p=0.002), Largest Fluid Volume Pocket (p=0.002), Max Fluid Area (p=0.006), Mean Fluid Thickness (p=0.03), and Max Fluid Thickness (p=0.01) significantly correlated with graft non-adherence rates within the first postoperative week. Following placement and optimization of intraoperative lenticle adherence, iOCT revealed a significant difference between the area, volume and thickness of maximum fluid pockets between the two surgical techniques, though both techniques resulted in significant reduction of interface fluid during the procedure.
Conclusions
Larger residual interface fluid volume, area, and thickness at the end of surgery detected with iOCT are associated with early graft non-adherence and can be quantified with an automated algorithm. iOCT imaging can successfully capture technique-dependent differences in fluid dynamics during DSAEK surgery.
Introduction
Endothelial keratoplasty, including Descemet Membrane Endothelial Keratoplasty (DMEK) and Descemet Stripping Automated Endothelial Keratoplasty (DSAEK), has become the choice corneal transplant procedure for the management of corneal endothelial dysfunction.1, 2 It boasts greater tectonic strength, more predictable refractive outcomes, and lower rejection rates compared to traditional penetrating keratoplasty (PK).1 However, these surgeries still carry postoperative complications, among the most concerning of which are graft dislocation and graft failure.1 Many factors may influence complication rates, including residual graft-host interface fluid.1 In response, techniques such as sweeping the recipient stromal bed and pressurizing the anterior chamber were developed to promote graft adherence.3, 4 Limited studies have quantified the efficacy of these maneuvers intraoperatively or correlated perioperative interface fluid with postsurgical outcomes.5, 6
Intraoperative optical coherence tomography (iOCT) is an emerging field that can inform surgical decision-making.7 It presents a method with which to qualitatively and quantitatively evaluate the effects of surgical manipulations on tissues. A number of studies have described the use of iOCT for a variety of conditions and procedures in both the anterior and posterior segment.5–17 iOCT in DSAEK has demonstrated the feasibility to identify and quantify interface fluid not readily apparent through the surgical microscope, and it has been used to confirm graft adherence at the end of the operation.5, 7, 10–12 This is the first and largest study to correlate iOCT measurements of intraoperative fluid dynamics with early graft non-adherence using automated, quantitative values. We also comment on differences in iOCT findings between two surgical techniques and present postoperative findings with one year follow-up for eyes in the PIONEER study undergoing DSAEK.
Methods
The PIONEER study is a prospective intraoperative and perioperative OCT study initiated at the Cleveland Clinic in October 2011 and is registered under U.S. National Institutes of Health/ClinicalTrials.gov (NCT02423161). Briefly, it is a single site, multi-surgeon study whose main focus is to examine the feasibility, utility, and safety of iOCT during anterior and posterior segment surgeries using a microscope-mounted spectral domain OCT system7. The prospective study was approved by the Institutional Review Board at the Cleveland Clinic and is adherent to the principles that were established in the Declaration of Helsinki. All patients were consented. In this investigation, we evaluated 178 consecutive eyes of 173 consecutive patients from the PIONEER study that underwent DSAEK between October 2011 and March 2014.
Surgical Techniques
Two surgical techniques were used with a primary difference between them being the use of an active air infusion system (Technique B), while Technique A manually introduced air into the anterior chamber for graft positioning. All Technique A cases were performed by a single surgeon (WJD); Technique B surgeries were also performed by a single surgeon (JMG). Neither patient age, gender distribution, indication for surgery, previous ocular surgery, nor ocular comorbidities significantly differed between groups. Both techniques employed manual corneal sweeping to help eliminate graft-host interface fluid and encourage adherence of the donor lenticle. The differences between Techniques A and B are highlighted in Table 1.
Table 1.
Comparison of Technique A (Sweep Only) and Technique B (Active Air Infusion) for Descemet Stripping Automated Endothelial Keratoplasty
| Technique A | Technique B | |
|---|---|---|
| Incision | Scleral tunnel | Clear Cornea |
| Incision Location | Temporal | Temporal |
| Tissue Preparation | Precut | Surgeon Cut |
| Viscoelastic Use | Yes | Yes |
| Peripheral Roughening | Yes | No |
| Manual Air Pressurization | Yes | No |
| Active Air Infusion System | None | Accurus or VersaVIT |
| Manual Sweep After Air Infusion | Yes | Yes |
Surgeries were performed under monitored anesthesia care using a retrobulbar block. In Technique A, all donor tissue was pre-cut by the local eye bank (Cleveland Eye Bank/Eversight). A temporal, 5mm scleral tunnel incision was used, and the anterior chamber was maintained with sodium hyaluronate (Healon, Abbott Medical Optics, Abbott Park, IL) during Descemet membrane stripping. The peripheral host stroma was roughened with a Terry scraper (Bauch and Lomb Storz, Bridgewater, NJ). 3 The viscoelastic was removed with single port irrigation and aspiration. A thin bead of sodium hyaluronate was injected onto the endothelial surface of all donor tissue prior to folding, and non-coapting forceps were used for graft insertion. The anterior chamber was filled with filtered air, and the lenticle was unfolded and centered. The eye was brought to a high intraocular pressure with additional filtered air and left in place for approximately ten minutes. The recipient stromal bed was manually swept using the side of a cannula to eliminate graft-host interface fluid and encourage graft adherence. Excess air was then removed from the anterior chamber until a physiological intraocular pressure was achieved. Corneal vent incisions were not created.
In Technique B, the surgeon cut his own tissue in the operative room. The donor corneoscleral rims were mounted on an artificial anterior chamber (Moria S.A., Doylestown, PA) and a Moria ATLK microkeratome with a 300 μm head was used to remove an anterior corneal lenticle. The host corneal epithelium was marked with a Weck trephine (Solan Medtronic, Jacksonville, FL) and gentian violet dye, and a clear cornea temporal incision was made with a 2.75 keratome blade. The anterior chamber was filled with a cohesive viscoelastic, and a reverse-bent Price-Sinsky hook scored the host Descemet membrane which was then stripped with a Melles stripper. The viscoelastic was removed with irrigation/aspiration. A Barron-Hessburg trephine (Katena, Denville, JN) was used to punch a posterior corneal lenticle that was folded 60/40 and inserted into the anterior chamber using Utrata forceps through an enlarged 5.2mm temporal incision. Filtered air coupled with a bent 30-gauge needle on a 3cc syringe was used to unfold and center the lenticle.18 Then, an air infusion system (Accurus Surgical System, Alcon Surgical, Forth Worth, TX; or VersaVIT, Synergetics, O’Fallon, MO) was used to pressurize the anterior chamber (40–60 mm Hg) and promote graft adherence to the host stroma. 4 Centripetal corneal sweeps with an irrigating cannula were performed for one minute under peak pressure. An air-fluid exchange was performed with the infusion system to leave the desired air fill at the end of the case. Corneal vent incisions were not created.
Intraoperative OCT Imaging
The iOCT image acquisition protocol has been previously described.7, 10 In brief, a portable spectral domain OCT system (Envisu C2200; Bioptigen, Research Triangle Park, NC) with customized microscope mount was used. iOCT images were obtained according to a standard imaging protocol and 12x12mm cube that maximally covered the graft-host interface which was visually confirmed by the surgeon. Surgery was paused for a median time of 1.9 minutes per scan session7. In Technique A, the first image was taken after the graft appeared to be apposed with a full anterior chamber air fill and after initial sweeps had already been performed. In Technique B, the first image was obtained after the graft was unfolded and supported with a manual injection of air but before active infusion and corneal sweeps. Additional images for both techniques were obtained after surgical manipulations based on iOCT appearance or surgeon discretion, but only the first and final image after the last surgical manipulation and prior to leaving the operating room were used for analysis.
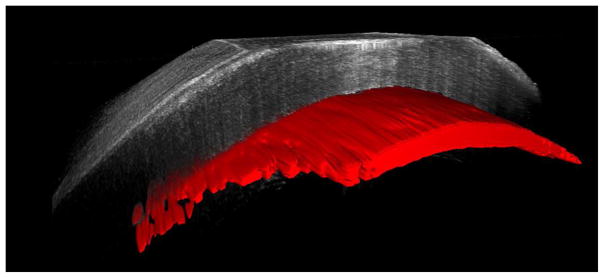
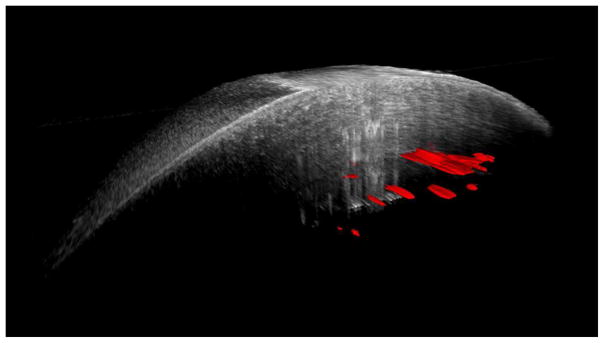
Intraoperative interface fluid was quantified postoperatively using manually validated automated analysis software, as previously described.5 The software calculates multiple parameters from the iOCT images related to interface fluid segmentation, including: total interface fluid volume, largest isolated fluid pocket volume, largest fluid area, mean fluid thickness (i.e., lenticle to host gap), and largest fluid thickness. Figure 1 provides a 3-D reconstruction example of the volumetric and interface segmentation. Parameters were quantified and compared between techniques using a two-tailed Student’s t-test. Univariate analysis was used to correlate iOCT data with early graft nonadherence for all cases.
Figure 1.
Three-dimensional reconstruction of iOCT image (top) before and (bottom) after manipulation of Descemet stripping automated endothelial keratoplasty graft. Automatically segmented interface fluid volume is shown in red. A dramatic reduction in interface fluid is visualized following manipulation of graft.
Clinical Outcomes
Clinical outcome data was collected at postoperative day 1, week 1, month 3, month 6, and up to year 1. All patients had data available for at least one early postoperative visit within the first week, and all but four had at least one late follow-up visit at postoperative month 6 or year 1. Outcome measures included graft nonadherence identified on clinical exam and need for further postoperative intervention (e.g. rebubble or keratoplasty). The correlation of outcome measures with patient characteristics such as primary diagnosis, ocular comorbidities, and previous surgery were assessed by univariate analysis using a 1-tailed Fisher’s exact test for presence of the condition.
Statistical software (JMP Pro 10.0.2, Cary NC) was used for data analysis. Significance of p values was set to <0.05 with adjustments made via Bonferroni corrections for the large number of comparisons made when correlating outcomes with patient characteristics. After Bonferroni corrections, statistical significance was defined as <0.006, <0.005, and <0.003 when stratifying outcome correlations by primary diagnosis, previous intraocular surgery, and ocular comorbidities, respectively.
Results
Patient Demographics and Clinical Characteristics
Patient demographics can be found in Table 2. The most common indication for surgery was Fuchs endothelial corneal dystrophy (66.9%). The most frequent ocular comorbidity was glaucoma (19.7%). The most common prior surgery, excluding cataract or lens extraction, was previous DSAEK (9.0%), vitrectomy (8.4%), or tube shunt (6.2%). Other primary diagnoses, ocular comorbidities, and previous ocular surgeries occurring with less frequency are listed in Table 2.
Table 2.
Clinical Characteristics
| N | % | |
|---|---|---|
| Eyes | 178 | |
|
| ||
| Age (years) | 71.6±12.5 | |
|
| ||
| Sex | ||
| Male | 62 | 34.8% |
| Female | 116 | 65.2% |
|
| ||
| Surgical method | ||
| Technique A | 105 | 59.0% |
| Technique B | 73 | 41.0% |
|
| ||
| Preoperative diagnosis | ||
| Fuchs dystrophy | 119 | 66.9% |
| Bullous Keratopathy | 34 | 19.1% |
| Failed DSAEK | 14 | 7.9% |
| Failed PK | 6 | 3.4% |
| Other | 5 | 2.7% |
|
| ||
| Ocular history/comorbidity | ||
| Glaucoma | 35 | 19.7% |
| Diabetic Retinopathy | 6 | 3.4% |
| Cystoid Macular Edema | 5 | 2.8% |
| Previous Cornea Ulcer | 4 | 2.2% |
| All others < 3% | ||
|
| ||
| Previous ocular surgery | ||
| DSAEK | 16 | 9.0% |
| PPV | 15 | 8.4% |
| Tube Shunt | 11 | 6.2% |
| PK | 11 | 6.2% |
| Trabeculectomy | 6 | 3.4% |
|
| ||
| Lens status at time of surgery | ||
| Pseudophakic | 119 | 66.9% |
| Phakic | 56 | 31.5% |
| Aphakic | 2 | 1.1% |
| Pseudophakic sutured IOL | 1 | 0.5% |
DSAEK: Descemet stripping automated endothelial keratoplasty, PK: penetrating keratoplasty, PPV: pars plana vitrectomy; IOL: intraocular lens
Intraoperative OCT Findings and Early Graft Non-adherence
iOCT yielded excellent visualization of the graft-host interface, and evaluation of the images demonstrated the dynamics of interface fluid reduction over time (Figure 1). iOCT showed significant reduction in fluid following surgical manipulations with both techniques (p<0.01). Final interface fluid measurements at case completion were measured for all cases. Comparative assessment of iOCT parameters between the techniques revealed that there was a trend towards decreased overall fluid volume with Technique B (p=0.06). Technique B also resulted in decreased fluid parameters for largest fluid pocket volume, maximum interface fluid area, mean fluid thickness, and max fluid thickness at the conclusion of the case compared to Technique A (Table 3).
Table 3.
Comparative Final Interface Fluid Parameters on Intraoperative Optical Coherence Tomography
| Technique A | Technique B | P value | |
|---|---|---|---|
|
|
|||
| Number of eyes | 105 | 73 | |
| Final Total Interface Fluid Volume (μm3) | 0.08±0.19 | 0.04±0.09 | 0.06 |
| Final Largest Interface Fluid Pocket Volume (μm3) | 0.07±0.18 | 0.03±0.08 | 0.05* |
| Final Maximum Interface Fluid Area (μm2) | 0.04±0.07 | 0.02±0.03 | 0.04* |
| Final Mean Fluid Thickness (μm) | 18.49±12.90 | 14.24±11.02 | 0.02* |
| Final Maximum Fluid Thickness (μm) | 57.57±45.31 | 39.31±33.03 | 0.002* |
| Need for postoperative intervention (unique eyes) | 8 (7.62%) | 9 (12.32%) | 0.311 |
| Rebubble | 2 (1.90%) | 7 (9.59%) | |
| Repeat DSAEK | 4 (3.81%) | 3 (4.11%) | |
| Penetrating Keratoplasty | 2 (1.90%) | 0 | |
| Boston Keratoprosthesis | 1 (0.95%) | 0 | |
When comparing final iOCT images between groups with different postoperative nonadherence outcomes, we found three parameters significantly differed among the groups. Additionally, higher amounts of interface fluid trended towards larger amount of graft nonadherence. Total fluid volume was significantly higher when comparing grafts that completely dislocated (0.22±0.41 um2) or had partial nonadherence (0.17±0.27 um2) with grafts that had no post-operative interface fluid (0.05±0.12 um2; p=0.002 and p=0.005, respectively). Largest volume in a single pocket of fluid also significantly differed when comparing grafts that completely dislocated (0.20±0.41 um2) or had partial nonadherence (0.14±0.26 um2) with grafts that had no postoperative interface fluid (0.04±0.12 um2; p=0.002 and p=0.005, respectively). Likewise, the maximum area of fluid in the final iOCT image was significantly higher in eyes with subsequent dislocated grafts (0.07±0.11 um) and partial nonadherence grafts (0.06±0.09um2) compared to completely adhered grafts (0.03±0.04 um2, p=0.02 and p=0.03, respectively). Although there were no significant differences between complete dislocation and partial dislocation groups, the values trended larger in the complete dislocation group.
Several iOCT measurements were associated with total and partial graft non-adherence within the first postoperative week. These included: increased final interface fluid volume (p=0.002); increased maximum isolated interface fluid pocket volume (p=0.002); increased maximum fluid area (p=0.006); increased mean interface fluid thickness (p=0.03); increased max interface fluid thickness (p=0.01). No iOCT parameter significantly correlated with the need for graft rebubbling nor in cases that had a rebubble followed by a regrafting procedure.
Graft Non-adherence, Dislocation, and Failure
Of 178 cases, a total of 21 grafts had partial or total non-adherence during the postoperative period. Six of these resolved spontaneously. Nine eyes required a rebubble. Of these nine rebubbles, three needed a repeat DSAEK. The remaining six eyes with lenticle dislocations underwent repeat DSAEK or PK without prior rebubble. In all, long-term follow-up identified 12 total graft failures, defined as the need for repeat keratoplasty (n=11) or persistent edema (n = 1) in the late postoperative period at year 1.
Comparative assessment between the two techniques found no difference in postoperative intervention rates (Table 3). The type of intervention per technique is further broken down in Table 3, but may vary related to numerous confounding variables including patient characteristics and individual surgeon practice patterns. Other features that were associated with non-adherence included glaucoma (p=0.03) and previously failed penetrating keratoplasty (p=0.007)
Discussion
Endothelial keratoplasty has shown consistent growth in its use, and DSAEK remains the most common subtype of endothelial transplant.2 While DSAEK boasts numerous advantages over PK in select cases, complications such as graft dislocation and graft failure exist.1 iOCT shows promise in providing additional information to the surgeon regarding graft manipulation and final graft-host interface fluid which may influence postoperative outcomes.
Previous studies have examined the effects of surgical manipulation in DSAEK using iOCT but in smaller case series, and few quantified the actual intraoperative interface fluid dynamics. The first use of iOCT in DSAEK was published in 2010 by Knecht. 6 They showed serially decreasing interface fluid, measured manually as the broadest interface width between the graft and host tissue, after multiple surgical steps. The average width at the end of surgery was 0.040 mm between two patients; four patients had no interface fluid upon surgical conclusion.6 Other case series have successfully used iOCT to show interface fluid drainage after corneal stab incisions.11, 12 Similarly, Ide et. al. successfully recorded intraoperative fluid dynamics in DSAEK.16 Our lab has previously reported that transient interface fluid was identifiable on iOCT and that it correlated with the appearance of textural interface opacity.10 In that report and in our experience, iOCT imaging was not able to differentiate between fluid and viscoelastic in this space. However, transient interface fluid did not impact the likelihood of postoperative donor adherence in this series. 10 The results of the study herein present new information that correlates various geometric interface fluid measurements on iOCT images with select outcomes. We also comment on iOCT findings between two DSAEK surgical techniques.
We believe that this study is the first and largest to correlate intraoperative interface fluid measurements with early graft nonadherence rates. We found that the final interface fluid parameters of total fluid volume, largest volume in a single pocket of interface fluid, and higher maximum fluid area trended with a larger extent of postoperative graft nonadherence. These parameters were significantly higher when comparing full dislocation and partial nonadherence to grafts that had no postoperative interface fluid. However, the large variability in measurements makes it challenging to identify threshold values for these parameters. Although there were no significant differences in values between the complete and partial dislocation groups, the complete dislocation group did trend towards larger values of interface fluid. No interface fluid parameters were associated with graft rebubbling followed by regrafting. This finding may be due to the small number of cases (i.e., 3) that underwent this course of events.
Previous studies have reported that dislocations most often occur within the first postoperative week, with few exceptions. 1, 19, 20 We found that volumetric and area assessments of final interface fluid were strongly associated with graft nonadherence within postoperative week one (all p<0.006). Linear parameters, such as fluid thickness, were also significantly associated, but to a lesser extent (all p<0.03). These parameters reflect the amount of fluid along the anterior-posterior axis between the graft and host tissue. While challenging to confirm, this may imply that overall interface fluid burden (e.g., area, volume) may be more important than a single linear measurement. Though a previous case series has shown that spontaneous reattachment is possible in lenticles with only central interface fluid and in lenticles that are fully detached, it is thought that location of the interface fluid may correlate with reattachment rates.21 Future studies should examine the location of interface fluid, such as peripheral fluid that is contiguous with the anterior chamber, and its correlation with graft nonadherence. Further research is needed with larger sample sizes to better understand the impact of fluid geometry on graft non-adherence and dislocation rates.
We also observed differences in iOCT-measured interface fluid between two different DSAEK surgical techniques. Final fluid assessment revealed Technique B to have less final interface fluid compared to Technique A. No statistically significant difference was found between groups regarding the need for further postoperative interventions such as graft rebubble or repeat keratoplasty. A comparison of techniques was not the objective of the current study, however, and given the low number of reoperation events to compare between groups and potential confounding factors beyond differences in technique alone, a comparison of re-operation rates between techniques is inconclusive. Future studies aimed at a systematic comparison of techniques would need to experimentally isolate maneuvers of interest and control for factors such as surgeon, tissue preparation method and use of ocular viscoelastic device. Moreover, a sufficiently powered study should examine iOCT images at pre-designated time points within each surgical technique in order to evaluate the efficacy of individual manipulations in eliminating graft-host interface fluid.
Another limitation of this study includes the influence that iOCT imaging may have on surgical maneuvers. The disconnect between surgeons’ en face impression and the actual interface fluid present on iOCT has been shown in the PIONEER study examining iOCT utility. In fact, iOCT visualization definitively changed surgical management in 9% of lamellar keratoplasty cases where the image demonstrated a finding disparate from the surgeon’s intraoperative assessment.7
While this study presents significant new data, the authors recognize its limitations. Surgeons may be biased towards less aggressive early manipulations knowing they can rely on iOCT imaging. Likewise, surgeons may be more aggressive in later manipulations until subclinical interface fluid only recognized on iOCT is eliminated. A randomized, masked controlled study may be able to delineate the influence of iOCT in the operating room and allow a more direct comparison of surgical techniques. Additionally, comparing images at identical timepoints after each step in Technique A and B would provide more insight into the fluid dynamics of differing DSAEK approaches. Finally, this study utilized a microscope-mounted portable SD-OCT system, rather than a microscope-integrated system. The dynamics and visualization of an integrated system may also impact the surgeon’s approach to minimize interface fluid.
To our knowledge, we present the largest study that prospectively examines and quantifies intraoperative fluid dynamics in DSAEK and correlates novel, automated calculations of fluid parameters with graft non-adherence within the first week. Further research is needed to better understand the role of iOCT in DSAEK and the utility of automated fluid analysis in minimizing risk of graft non-adherence and potential repeat surgical interventions. Overall, iOCT is a useful method to enhance our knowledge of the intraoperative and postoperative effects of various DSAEK surgical maneuvers.
Supplementary Material
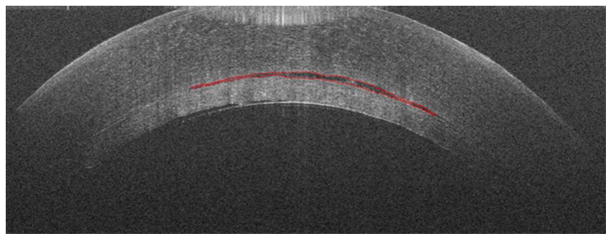
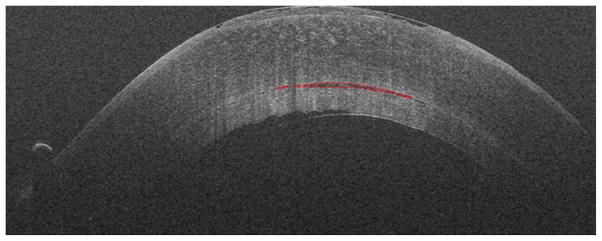
Figure 2.
Cross-sectional iOCT imaging showing progression of decreasing interface fluid with each surgical manipulation after insertion of the graft (top, middle, bottom). Segmentation of interface fluid is noted in red.
Acknowledgments
A. Funding and support: NIH/NEI K23-EY022947-01A1 (JPE); Ohio Department of Development TECH-13-059 (JPE, WJD, SKS); Research to Prevent Blindness (Cole Eye Institutional)
B. Financial Disclosures:
KH: None;
BC: None;
JMG: None;
WJD: Zeiss (R), Avedro (R, C), Ziemer (C);
SKS: Bausch and Lomb (C, R); Bioptigen (P); Allergan (R); Synergetics (P); Leica (C), Carl Zeiss Meditec (C);
JPE: Bioptigen (C, P), Thrombogenics (C, R), Synergetics (P), Genentech (R), Leica (C), Santen (C), Regeneron (R), Zeiss (C), Alcon (C);
C. Author Contributions: Design of the study (JPE, WJD, SKS, PKK); Conduct of the study (All authors); Data collection (All authors); Data management (JPE; KH); Data analysis (All authors); Data Interpretation (All authors); Preparation of the manuscript (KH); Review and approval of the manuscript (All authors); Provision of patients (JMG, WJD)
D. This study was HIPAA compliant, IRB-approved by the Cleveland Clinic Foundation Institutional Review Board, and was prospective in nature.
E. Other Contributors: The authors thank Jamie Reese and Carmen Calabrise for their extensive contributions for study coordination and image acquisition. The authors would like to acknowledge Amit Vasanji, PhD for his support for figure development.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lee W, Jacobs D, Musch D, Kaufman SC, Reinhart WJ, Shtein RM. Descemet’s Stripping Endothelial Keratoplasty: Safety and Outcomes. Ophthalmology. 2009;116(9):1818–30. doi: 10.1016/j.ophtha.2009.06.021. [DOI] [PubMed] [Google Scholar]
- 2.Van Meter WS. Eye Banking Statistical Report. Vol. 2015 Eye Bank of America; 2012. [Google Scholar]
- 3.Terry MA, Shamie N, Chen ES, Hoar KL, Friend DJ. Endothelial Keratoplasty: A Simplified Technique to Minimize Graft Dislocation, Iatrogenic Graft Failure, and Pupillary Block. Ophthalmology. 2008;117(7):1179–86. doi: 10.1016/j.ophtha.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 4.Meisler D, Dupps W, Jr, Covert D, Koenig S. Use of an air-fluid exchange system to promote graft adhesion during Descemet’s stripping automated endothelial keratoplasty. J Cataract Refract Surg. 2007;33(5):770–2. doi: 10.1016/j.jcrs.2006.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xu D, Dupps W, Jr, Srivastava S, Ehlers J. Automated-volumetric Analysis of Interface Fluid in Descemet Stripping Automated Endothelial Keratoplasty Using Intraoperative Optical Coherence Tomography. Invest Ophthalmol Vis Scie. 2014;55(9):5610–5. doi: 10.1167/iovs.14-14346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Knecht P, Kaufmann C, Menke MN, Watson SL, Bosch MM. Use of Intraoperative Fourier Domain Anterior Segment Optical Coherence Tomography During Descemet Stripping Endothelial Keratoplasty. Am J Ophthalmol. 2010;150(3):360–5. doi: 10.1016/j.ajo.2010.04.017. [DOI] [PubMed] [Google Scholar]
- 7.Ehlers J, Dupps W, Kaiser P, et al. The Prospective Intraoperative and Perioperative Ophthalmic ImagiNg with Optical CoherEncE TomogRaphy (PIONEER) Study: 2-Year Results. Am J Ophthalmol. 2014;158(5):999–1007. doi: 10.1016/j.ajo.2014.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Scorcia V, Busin M, Lucisano A, Beltz J, Carta A, Scorcia G. Anterior Segment Optical Coherence Tomography-Guided Big-Bubble Technique. Ophthalmology. 2013;120(3):471–6. doi: 10.1016/j.ophtha.2012.08.041. [DOI] [PubMed] [Google Scholar]
- 9.Lee C, Kim K, Han S, et al. Stimulated-penetrating keratoplasty using real-time virtual intraoperative surgical optical coherence tomography. Journal of Biomedical Optics. 2014;19(3):030502-1–3. doi: 10.1117/1.JBO.19.3.030502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Juthani V, Goshe J, Srivastava S, Ehlers J. Association Between Transient Interface Fluid on Intraoperative OCT and Textural Interface Opacity After DSAEK Surgery in the PIONEER Study. Cornea. 2014;33(9):887–92. doi: 10.1097/ICO.0000000000000209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miyakoshi A, Okazi H, Otsuka M, Hayashi A. Efficacy of Intraoperative Anterior Segment Optical Coherence Tomography during Descemet’s Stripping Automated Endothelial Keratoplasty. ISRN Ophthalmology. 2014:1–4. doi: 10.1155/2014/562062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wylegala E, Nowinska A, Wroblewska-Czajka E, Janiszewska D. Donor Disc Attachment Assessment with Intraoperative Spectral Optical Coherence Tomography during Descement Stipping Automated Endothelial Keratoplasty. Indian J Ophthalmol. 2013;61(9):511–3. doi: 10.4103/0301-4738.119440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De Benito-Llopis L, Mehta J, Angunawela R, Ang M, Tan DT. Intraoperative Anterior Segment Optical Coherence Tomography: A Novel Assessment Tool During Deep Anterior Keratoplasty. Am J Ophthalmol. 2014;157(2):334–41. doi: 10.1016/j.ajo.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 14.Steven P, Le Blanc C, Velten K, et al. Optimizing Descement Membrane Endothelial Keratoplasty Using Intraoperative Optical Coherence Tomography. JAMA Ophthalmol. 2013;131(9):1135–242. doi: 10.1001/jamaophthalmol.2013.4672. [DOI] [PubMed] [Google Scholar]
- 15.Ray R, Baranano D, Fortun J, et al. Intraoperative Microscope-mounted Spectral Domain Optical Coherence Tomography for Evaluation of Retinal Anatomy During Macular Surgery. Ophthalmology. 2011;118(11):2212–7. doi: 10.1016/j.ophtha.2011.04.012. [DOI] [PubMed] [Google Scholar]
- 16.Ide J, Wang J, Tao A, et al. Intraoperative Uuse of three-dimensional spectral-domain optical coherence tomography. Ophthalmic Surg Lasers Imaging. 2010;41(2):250–4. doi: 10.3928/15428877-20100303-15. [DOI] [PubMed] [Google Scholar]
- 17.Ye C, Yu M, Jhanji V. Stormal Bed Thickness Measurement During Laser in Situ Keratomileusis Using Intraoperative Optical Coherence Tomography. Cornea. 2015;34(4):387–91. doi: 10.1097/ICO.0000000000000345. [DOI] [PubMed] [Google Scholar]
- 18.Koenig SB, Dupps WJ, Jr, Covert DJ, Meisler DM. Simple technique to unfold the donor corneal lenticule during Descemet’s stripping and automated endothelial keratoplasty. J Cataract Refract Surg. 2007;33(2):189–90. doi: 10.1016/j.jcrs.2006.09.035. [DOI] [PubMed] [Google Scholar]
- 19.Busin M, Bhatt P. Late detachment of donor graft after Descemet stripping automated endothelial keratoplasty. J Cataract Refract Surg. 2008;34(1):159–60. doi: 10.1016/j.jcrs.2007.08.027. [DOI] [PubMed] [Google Scholar]
- 20.Gorovoy M, Meisler D, Dupps W., Jr Late repeat Descemet stripping automated endothelial keratoplasty. Cornea. 2008;27(2):238–40. doi: 10.1097/ICO.0b013e31815b82e0. [DOI] [PubMed] [Google Scholar]
- 21.Hayes DD, Shih CY, Shamie N, Terry MA, Price FW, Price MO, et al. Spontaneous reattachment of descemet stripping automated endothelial keratoplasty lenticles: a case series of 12 patients. Am J Ophthalmol. 2010;150(6):790–7. doi: 10.1016/j.ajo.2010.06.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.