Abstract
Convergent evidence suggests that schizophrenia is a disorder of neurodevelopment with alterations in both early and late developmental processes hypothesized to contribute to the disease process. Abnormalities in certain clinical features of schizophrenia, such as working memory impairments, depend on distributed neural circuitry including the dorsolateral prefrontal cortex (DLPFC) and appear to arise during the protracted maturation of this circuitry across childhood and adolescence. In particular, the neural circuitry substrate for working memory in primates involves the coordinated activity of excitatory pyramidal neurons and a specific population of inhibitory GABA neurons (parvalbumin-containing basket cells) in layer 3 of the DLPFC. Thus, understanding the relationships between the normal development of, and the schizophrenia-associated alterations in, the DLPFC circuitry that subserves working memory could provide new insights into the nature of schizophrenia as a neurodevelopmental disorder. Consequently, in this article, we review 1) recent findings regarding alterations of DLPFC layer 3 circuitry in schizophrenia; 2) the developmental refinements in this circuitry that occur during the period when the working memory alterations in schizophrenia appear to arise and progress; and 3) how various adverse environmental exposures could contribute to developmental disturbances of this circuitry in individuals with schizophrenia.
Keywords: cortical development, dorsolateral prefrontal cortex, excitation/inhibition (E/I) balance, pyramidal cells, parvalbumin interneurons, schizophrenia
Schizophrenia as a developmental disorder
Multiple genetic liabilities (1–5) and perinatal environmental exposures (6;7) suggest that the neural substrate for schizophrenia may be present from early stages of development (8–10). Other environmental exposures, such as urban residence during childhood and repeated cannabis use during adolescence (11), suggest that alterations in later developmental processes may also contribute to the disease process (9;12;13).
The appearance of certain core cognitive features of schizophrenia well before the onset of psychosis also supports the idea that schizophrenia is a neurodevelopmental disorder (14–16). For example, at age 7 individuals who were later diagnosed with schizophrenia displayed deficits in tests indexing reasoning and conceptualization. These individuals subsequently began to fall behind their peers in other aspects of cognitive function, such as working memory (WM), and this developmental lag progressively worsened through puberty (16).
Working memory, the ability to transiently maintain and manipulate a limited amount of information to guide thought or behavior, depends on the activity of a distributed neural network. A number of cortical, thalamic and striatal regions are active during WM tasks (17–19) and alterations in these regions have been reported in schizophrenia (20–22). A key node in this network, the neural circuitry of the dorsolateral prefrontal cortex (DLPFC), has been a major focus of investigation in healthy monkeys and humans (23–25) and in schizophrenia (17;26). In monkeys and humans, both WM performance and DLPFC circuitry undergo protracted patterns of maturation which continue through late adolescence, and greater activation of the DLPFC is associated with improved WM performance during these periods (27–29). Thus, understanding the developmental trajectories of DLPFC circuits that subserve WM may reveal which elements of the circuitry are preferentially vulnerable, and when they are most vulnerable, to the environmental events associated with increased risk for schizophrenia.
Neural substrate for working memory
WM in monkeys appears to require the coordinated activity of excitatory pyramidal neurons and a specific population of inhibitory GABA neurons in DLPFC layer 3 (24). Layer 3 pyramidal cells furnish wide-spreading, horizontal axon collaterals that terminate in stripe-like arrays (30;31). These axon collaterals almost exclusively target the dendritic spines of other pyramidal cells (32). These extensive, reciprocal, glutamatergic connections are thought to provide the anatomical substrate required for the activity of spatially segregated, but functionally related, clusters of pyramidal cells during the maintenance phase of WM tasks (24;33;34).
The axons of DLPFC layer 3 pyramidal cells also give rise to local collaterals that arborize in the vicinity of the cell body; the targets of these terminals are equally divided between pyramidal cell dendritic spines and the dendrites of a subpopulation of GABA interneurons that express the calcium-binding protein parvalbumin (PV) (35;36). These PV interneurons include basket cells (PVBCs), which innervate the perisomatic region (i.e., soma and proximal dendritic shafts and spines) of pyramidal cells. These inputs provide feedback inhibition that shapes the activity of pyramidal cell subpopulations during WM tasks (37–40) (Figure 1A). The divergent connections of PVBCs also result in the coordinated inhibition of multiple pyramidal cells. The fast and simultaneous decay of this inhibition synchronizes the firing of pyramidal cells, producing oscillatory network activity in the γ frequency (30–80 Hz) range (41–44). The potential importance of γ oscillations for supporting WM (45) is suggested by findings that the power of prefrontal γ synchrony increases in proportion to WM load (46;47).
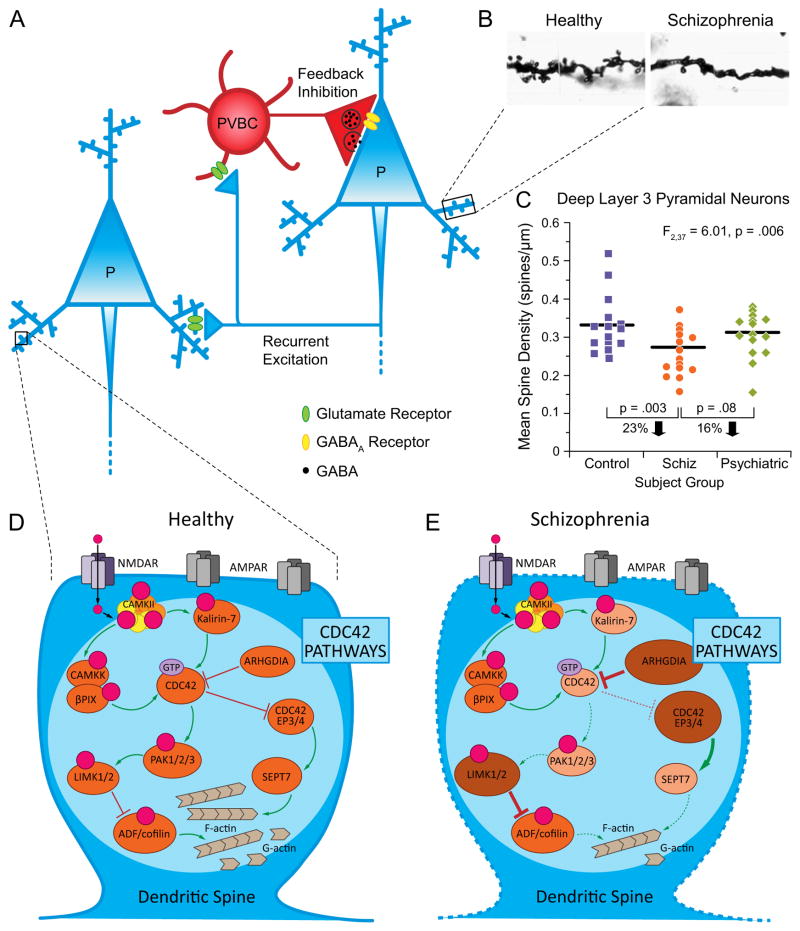
Figure 1.
Alterations in DLPFC layer 3 pyramidal cells in schizophrenia. (A) Schematic diagram illustrating the circuitry in DLPFC layer 3 thought to be crucial for generating γ frequency oscillations required for working memory. Reciprocal connections furnished by local axon collaterals of pyramidal cells provide recurrent excitation, whereas excitatory innervation from pyramidal cells to PVBCs generates feedback inhibition. P, pyramidal cell; PVBC, parvalbumin basket cell. (B) Micrographs of Golgi-impregnated basilar dendritic shafts and spines from a healthy comparison subject (left) and a subject with schizophrenia (right) illustrating a marked decrement in the density of dendritic spines in schizophrenia. Adapted from (58). (C) Scatter plot demonstrating a lower mean spine density on DLPFC deep layer 3 pyramidal neurons in schizophrenia subjects relative to both healthy and psychiatrically-ill comparison subjects. (D) CDC42 signaling pathways that regulate the contribution of F-actin to dendritic spine structure in the healthy brain. The activity of CDC42 is inhibited by ARHGDIA that suppresses intrinsic GTPase activity. CDC42-CDC42EP pathway: Activated CDC42 inhibits CDC42EPs, which dissociate the complex of septin filaments consolidated by SEPT7 in the spine neck, enabling the transient influx of second messengers and molecules from the parent dendrite that facilitate F-actin mediated growth of spines. CDC42-PAK-LIMK pathway: CDC42 activates PAK, which activates LIMK, resulting in downstream regulation of cofilin family of actin depolymerizing proteins necessary for modulating F-actin turnover. Green arrows indicate activation, and red blunted lines indicate inhibition of each target. (E) Molecular alterations and predicted functional consequences of altered CDC42 signaling pathway components in schizophrenia. The multiple alterations in CDC42 signaling pathways are predicted to destabilize actin dynamics and produce spine deficits preferentially in DLPFC layer 3 pyramidal cells in schizophrenia. Larger and darker ovals indicate higher mRNA levels in schizophrenia, while smaller and lighter ovals indicate lower mRNA levels in schizophrenia. Increased thickness of arrows and blunted lines indicate predicted higher activity in schizophrenia, while dashed, thin arrows and blunted lines indicate predicted lower activity in schizophrenia relative to the healthy state shown in panel D.
Of course, other neural elements in the DLPFC might also influence the function of this circuit. For example, the axon terminals of another subpopulation of PV interneurons, chandelier (PVChCs) or axo-axonic cells, form distinctive vertical arrays called cartridges that exclusively target the axon initial segments of pyramidal cells. In contrast to the conventional strongly hyperpolarizing role of PVBCs, the impact of PVChC inputs on pyramidal cell function in rodents appears to depend on stage of development, brain region and level of network activity (48–52). For example, chandelier cell (ChC) inputs can be depolarizing under certain conditions (48). In addition, PVChCs are reported to prevent ectopic action potential back-propagation especially during γ oscillations in vitro (53). Thus, PVBCs and PVChCs appear to uniquely shape the activity of postsynaptic pyramidal cells.
Exploring the role of development in DLPFC circuitry dysfunction in schizophrenia
The findings reviewed above suggest that understanding the normal development and alterations in schizophrenia of the DLPFC circuitry that subserves WM could provide new insights into the nature of schizophrenia as a neurodevelopmental disorder. Consequently, here we 1) review evidence of alterations in DLPFC layer 3 circuitry in schizophrenia; 2) consider how these circuitry elements are normally refined during the developmental periods when the WM impairments in schizophrenia arise and progress; and 3) discuss how adverse environmental exposures could produce developmental disturbances in DLPFC layer 3 circuitry underlying WM deficits in schizophrenia.
Altered connectivity of DLPFC layer 3 circuitry in schizophrenia
A number of studies in the postmortem human brain provide convergent evidence that schizophrenia is associated with alterations in multiple elements of the WM circuitry in DLPFC layer 3. The interpretation of postmortem studies of schizophrenia needs to consider the potential effects of antipsychotic medications, nicotine, substances of abuse, suicide and other factors frequently comorbid with schizophrenia. In the findings reviewed below, the effects of these factors have been accounted for using convergent approaches described in detail elsewhere (26;54;55;58).
Alterations in layer 3 pyramidal cells
In subjects with schizophrenia, cortical pyramidal cells exhibited smaller cell bodies selectively in layer 3 (54–56). In addition, basilar dendritic spine density was lower on pyramidal cells in DLPFC layer 3, but not layers 5 or 6, in schizophrenia subjects (57–59) (Figure 1B,C).
Several lines of evidence suggest that the dendritic spine deficit in DLPFC layer 3 pyramidal cells reflects altered regulation of the actin cytoskeleton that is required for the formation and maintenance of spines. First, recent genetic findings implicate convergent signaling pathways that regulate actin dynamics, suggesting a cell-autonomous disturbance in the formation and/or maintenance of dendritic spines (4;5). Second, key molecules regulating actin exhibit altered expression in schizophrenia. For example, the Rho GTPase cell division cycle 42 (CDC42) regulates the actin polymerization required for spine maturation (60;61); CDC42 messenger RNA (mRNA) levels are lower in the DLPFC of subjects with schizophrenia (62). Similarly, mRNA levels of kalirin, a Rho guanine exchange factor that is highly concentrated in spines and that regulates spine integrity through CDC42 signaling pathways (63), were lower in the DLPFC of subjects with schizophrenia (62). Importantly, levels of CDC42 and kalirin mRNAs predicted spine density only on layer 3 pyramidal cells.
The apparent specificity of the spine deficits to DLPFC layer 3 pyramidal neurons might reflect disturbances in molecules that are expressed selectively in these neurons. For example, CDC42 effector proteins (CDC42EP), which inhibit CDC42 activity, are preferentially expressed in layers 2–3 of the DLPFC (64). In schizophrenia, expression levels of CDC42EP3 and CDC42EP4 are upregulated in layer 3 pyramidal cells (65;66). Signaling via the CDC42-CDC42EP complex is thought to regulate the septin barrier in the spine neck that controls the flow of molecules into spines required for F-actin mediated growth of spines and synaptic potentiation. Thus, the combination of lower levels of CDC42 and higher levels of CDC42EPs in schizophrenia might alter the septin filament barrier, impair spine plasticity and result in spine loss (66). In addition, recent gene expression studies specifically in DLPFC layer 3 pyramidal cells suggest that dysfunction in the CDC42-p21-activated serine/threonine protein kinases (PAK)-LIM domain-containing serine/threonine protein kinases (LIMK) signaling pathway could also alter actin dynamics (65) (Figure 1D, E).
In aggregate, these findings support the notion that cell type-specific abnormalities in the molecular regulation of actin dynamics could lead to fewer dendritic spines selectively on layer 3 pyramidal neurons. Because spines are the sites of most excitatory synapses, DLPFC layer 3 circuits would be expected to receive less excitatory drive. As a result, these cells may exist in a hypoactive state and thus would have a reduced need for mitochondria-mediated energy synthesis (67). Consistent with this prediction, transcriptome analyses of layer 3 pyramidal cells revealed a substantial under-expression of nuclear gene products required for mitochondrial energy production (68). Importantly, only a subset of these alterations are present, or found only to a lesser degree, in samples of DLPFC gray matter from the same subjects, suggesting that the pathology is amplified in layer 3 pyramidal cells (68;69).
Alterations in GABAergic components of layer 3 circuitry
Such an upstream deficit in layer 3 pyramidal cell activity suggests that homeostatic mechanisms might be evoked to produce a compensatory downregulation of feedback inhibition (67;70). Supporting this idea, multiple markers of GABAergic inputs to DLPFC layer 3 pyramidal cells are altered in schizophrenia in a manner consistent with lower inhibitory strength (71) (Figure 2A–C). For example, the number of DLPFC PV neurons is not altered in schizophrenia, but these neurons have lower mRNA levels for both PV and the GABA synthesizing enzyme, glutamic acid decarboxylase (GAD67) (72). Moreover, PV and GAD67 protein levels are lower in the axon terminals of PVBCs in DLPFC layer 3 (73;74). Because the expression of both PV and GAD67 is activity-dependent, these findings are consistent with less excitatory drive to PVBCs from a major source of such input, layer 3 pyramidal cells. They also suggest that the synthesis of GABA is lower in PVBCs, and thus the presynaptic strength of PVBC inhibition of layer 3 pyramidal cells is reduced. The synaptic inputs from PVBCs to pyramidal neurons are enriched in postsynaptic GABAA receptors that contain α1 subunits (Figure 2B); mRNA levels for this subunit are selectively lower in DLPFC layer 3 pyramidal neurons in schizophrenia (75;76) (Figure 2C). These changes appear to be specific to 1) PVBCs, as PVChCs show a different pattern of alterations in schizophrenia (Supplemental Material and Figure 2D–F), and 2) to the pathophysiology of schizophrenia as they are not present in other psychiatric disorders.
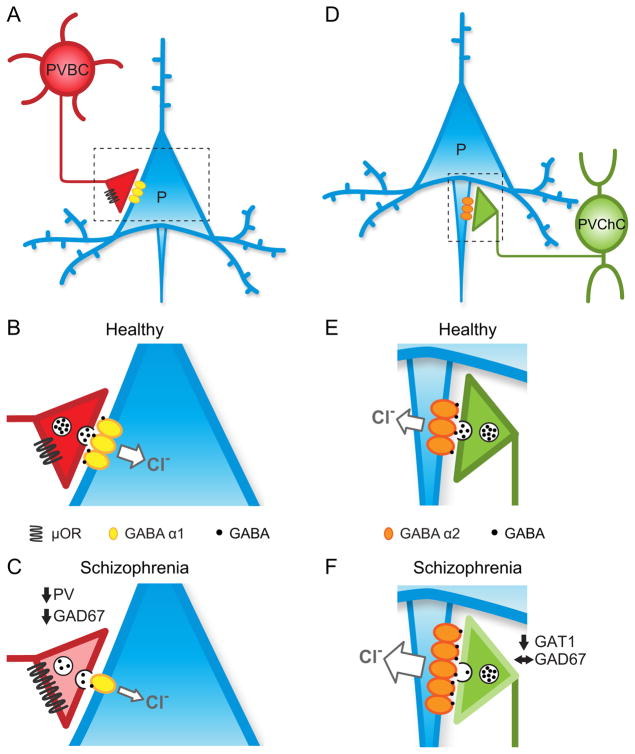
Figure 2.
Schematic summary of alterations in PVBC and PVChC inputs to DLPFC pyramidal cells in schizophrenia. (A) PVBC innervation of the soma and proximal dendrites of pyramidal cells provides strong and fast feedback inhibition. (B) In the healthy state, the strength of PVBC inputs is regulated by both pre- and post-synaptic molecules (See text for details). (C) Schizophrenia is associated with lower strength of PVBC-mediated inhibition due to: 1) downregulation of GAD67 mRNA and protein leading to reduced GABA synthesis and less GABA (black dots) in synaptic vesicles; 2) lower levels of PV expression decrease calcium buffering within PVBC terminals (lighter pink shading); 3) upregulation of “OR lowers presynaptic release of GABA; 4) downregulation of postsynaptic GABAA α1-containing receptors in pyramidal cells which lowers chloride influx and post-synaptic strength of inhibition. Please refer to Supplemental Information for details about PVChCs in D–F. (D) PVChCs innervate the axon initial segments of pyramidal cells. (E) In the healthy state, the strength of PVChC inputs is regulated by both pre- and post-synaptic molecules (See Supplemental Information for details). (F) Schizophrenia might be associated with increased strength of GABA neurotransmission due to: 1) downregulation of GAT1 protein leading to increased spillover of GABA from terminals (depicted as a lighter shade of green on the terminal outline); 2) upregulation of GABAA α2-containing receptors in pyramidal cells which might increase chloride influx and postsynaptic strength of GABA neurotransmission at the axon initial segment. GAD67, glutamic acid decarboxylase, 67kDa; PVBC, parvalbumin basket cell; μOR, “ opioid receptor; GABA α1, GABAA receptor α1 subunit; PVChC, parvalbumin chandelier cell; GAT1, GABA membrane transporter 1; GABA α2, GABAA receptor α2 subunit.
These pre- and post-synaptic findings are consistent with a compensatory downregulation of inhibition from PVBCs in response to lower excitatory output from layer 3 pyramidal cells. This interpretation is supported by findings of upregulated “ opioid receptors (“ORs) in schizophrenia (77). These receptors are localized to the perisomatic compartment and axon boutons of PV interneurons (78;79). Their activation triggers G protein-coupled inwardly rectifying K+ channel activity that hyperpolarizes the membrane potential and suppresses GABA release (80;81). Thus, upregulation of DLPFC “ORs in schizophrenia would be expected to suppress GABA release from PVBCs, further reducing the inhibition of layer 3 pyramidal cells (Figure 2C).
The signal to downregulate inhibition might be transmitted from DLPFC layer 3 pyramidal cells to PVBCs in the following manner. Pyramidal cells express the immediate early gene, neuronal activity-regulated pentraxin 2 (NARP), in an activity dependent manner (82). NARP protein is secreted at presynaptic glutamatergic axon terminals where it facilitates the clustering of postsynaptic GluR4-containing α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors (AMPARs) on PV interneurons (83;84). Deletion of the NARP gene and its receptor markedly lowered levels of GluR4 AMPARs in PV interneurons, a deficit associated with decreased feedback inhibition to pyramidal neurons and disruptions in WM (85). Consistent with DLPFC layer 3 pyramidal cell hypoactivity in schizophrenia, NARP mRNA expression was lower in these cells in schizophrenia, and NARP mRNA levels were positively correlated with GAD67 mRNA levels (82). The idea that lower GAD67 expression was due to reduced excitatory drive to PVBCs was supported by evidence that expression of the immediate early gene Zif268 was also lower and predicted GAD67 mRNA levels in schizophrenia (86). The GAD67 promoter contains a conserved binding site for Zif268 (87;88), and Zif268 activation is accompanied by increased GAD67 expression (89). Thus, reduced Zif268 expression in response to lower excitatory drive from DLPFC layer 3 pyramidal cells might mediate the reduction in GAD67 mRNA and protein levels in PVBCs, resulting in less GABA synthesis and a compensatory reduction in inhibition of layer 3 pyramidal cells.
In summary, the above findings converge on the idea that schizophrenia is associated with an intrinsic lower excitatory activity of DLPFC layer 3 pyramidal cells that evokes multiple pre- and post-synaptic mechanisms to reduce inhibition by PVBCs (71). These mechanisms could contribute to rebalancing excitation and inhibition in DLPFC layer 3, but at a lower level of both excitation and inhibition that is unable to support γ oscillations at a level required for normal WM function (67). However, these interpretations are based upon observed associations in postmortem human tissue and await proof-of-concept confirmation in relevant model animals.
Postnatal maturation of layer 3 circuitry in monkey DLPFC
Understanding how these components of layer 3 circuitry become vulnerable during development of individuals later diagnosed with schizophrenia requires knowledge of the normal maturation of this circuitry. In nonhuman primates, DLPFC circuitry is significantly refined during postnatal maturation. Thus, as previously proposed (10), when these developmental refinements bring DLPFC layer 3 circuitry “on-line”, the functional consequences of brain alterations that occurred earlier in development are revealed. Alternatively, the timing of developmental refinements in each component of this circuitry may reveal which components are most likely to be disrupted by adverse environmental exposures at a given age (90). To address these alternatives, the following sections review the normal development in nonhuman primates of the DLPFC layer 3 circuitry components that are altered in schizophrenia.
Development of layer 3 pyramidal cells
Pyramidal neurons in primate DLPFC layer 3 substantially change, in nonlinear ways, throughout postnatal development. For example, in both monkeys and humans the density of dendritic spines increases rapidly during the perinatal period, remains at a stable plateau throughout prepuberty, and then declines by ~50% during the peripubertal period (91;92) (Figure 3A). Changes in the density of asymmetric excitatory synapses, most of which target dendritic spines, show very similar developmental patterns in both monkeys and humans (93–95). These age-related developmental refinements in markers of excitatory inputs are more pronounced in layer 3 pyramidal cells than for pyramidal cells in layers 5 or 6 (92;93), a pattern which matches the laminar differences in spine deficits in schizophrenia.
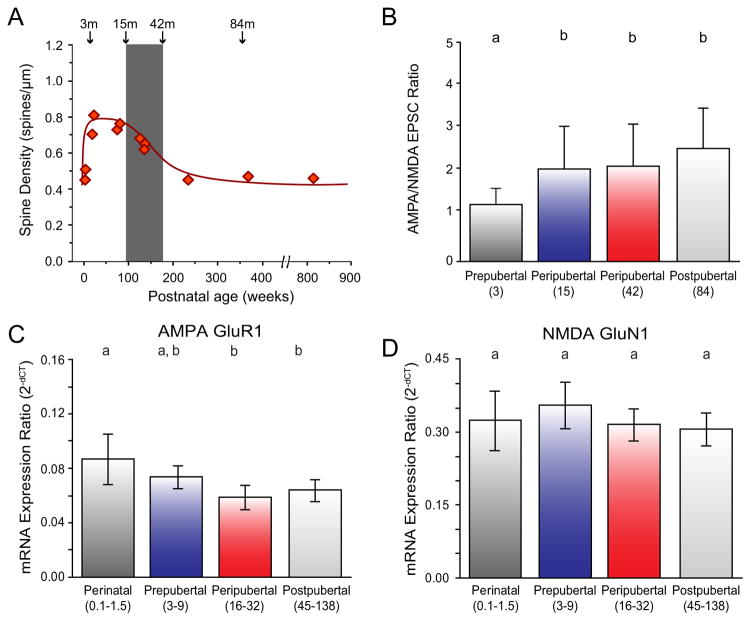
Figure 3.
Postnatal development of DLPFC layer 3 pyramidal cells. (A) Age-related alterations in mean spine density on dendrites of layer 3 pyramidal cells in monkey DLPFC (x-axis in weeks). Gray bar indicates the peripubertal period in monkeys that is roughly analogous to adolescence in humans (93;119). Adapted from (91). (B) Age-related increase in AMPA relative to NMDA receptor contribution to excitatory postsynaptic currents (EPSCs) in DLPFC layer 3 pyramidal cells (x-axis in months). Adapted from (96). (C and D) Age-related changes in mRNA expression of molecular determinants of glutamate neurotransmission (AMPA receptor subunit GluR1 and NMDA receptor subunit GluN1) in DLPFC layer 3 pyramidal cells (x-axis in months). Adapted from (97). In panels B-D, age groups not sharing the same letter are significantly different (p<0.05).
Although the mechanisms that lead to the decline in excitatory inputs to layer 3 pyramidal cells during adolescence remains unknown, the only study that has examined this question in monkeys found that these inputs seemed to be functionally mature well before puberty; a subset of these mature synapses were then pruned during peripuberty. For example, in perinatal monkeys, excitatory inputs to layer 3 pyramidal cells had immature functional properties, such as lower AMPA/N-methyl-D-aspartic acid (NMDA) receptor ratio and high probability of glutamate release (96). In contrast, in peripubertal monkeys, just prior to the onset of synaptic pruning, excitatory inputs to DLPFC layer 3 pyramidal neurons exhibited mature functional properties with higher AMPA/NMDA receptor ratios and low probability of glutamate release (96) (Figure 3B). Similarly, assessment of the postsynaptic molecular determinants of glutamate neurotransmission (expression levels of the AMPA receptor subunit GluA1 and NMDA receptor subunit GluN1) in DLPFC layer 3 pyramidal cells revealed that postpubertal levels of expression were acquired early during development (97) (Figure 3C,D). These findings suggest that the reorganization of excitatory connectivity involves 1) the elimination of mature synapses rather than nascent, immature synapses; and 2) that presynaptic factors (e.g., the source of the excitatory input (98)) and/or postsynaptic signals may tag mature synapses for pruning during peripuberty (96).
Development of layer 3 PVBCs
Both pre- and post-synaptic components of PVBCs undergo marked changes in primate DLPFC layer 3 during postnatal maturation. For example, as measured by PV immunoreactivity, the detection of PVBC boutons (presumed axon terminals) increases steadily from 1 month of age to postpuberty (99). These findings suggest either a developmental rise in bouton density or in PV protein levels per bouton such that they become more evident with age. Recent studies using techniques that can measure both bouton density and protein levels per bouton reported no difference in the density of PVBC boutons between prepubertal and postpubertal monkeys (100;101). In contrast, PV protein levels in PVBC boutons were nearly two-fold higher in postpubertal than in prepubertal monkeys (100;101) (Figure 4A,B). These findings—consistent with anatomical evidence that the axonal arbors of PVBCs remain relatively constant over this same period of development (102)—suggest that the axons of PVBCs mature structurally early in development with progressive changes in their biochemical properties extending across postnatal development.
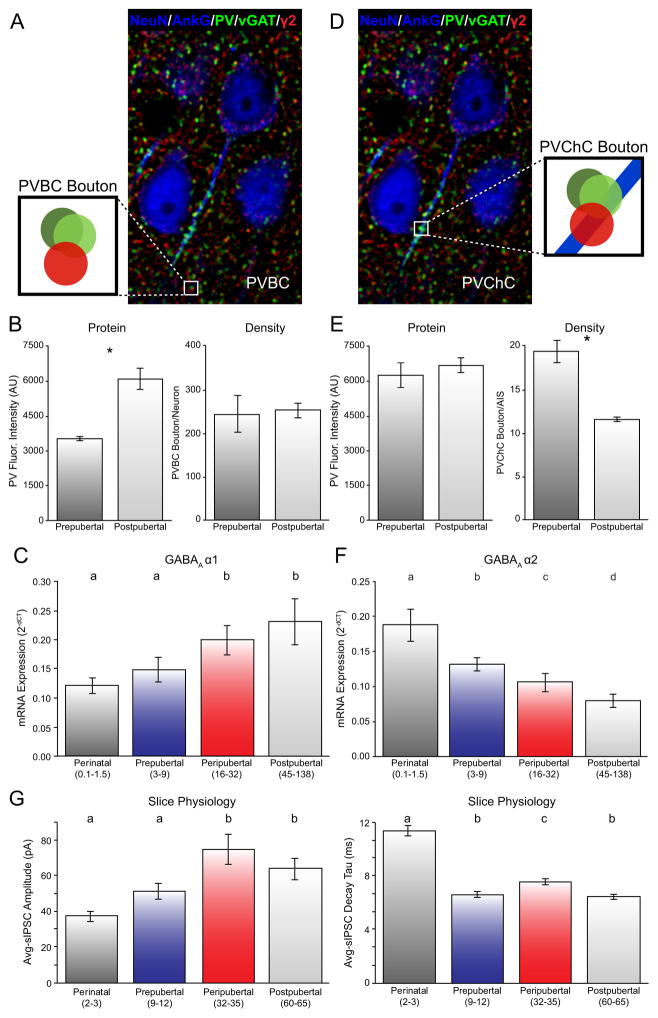
Figure 4.
Postnatal development of PVBCs and PVChCs in the primate DLPFC. (A) Postpubertal monkey DLPFC multi-labeled for NeuN/AnkG (blue; cell body and axon initial segment (AIS) markers), GABAA receptor γ2 subunit (red; postsynaptic marker), PV and vGAT (green, presynaptic markers). PV/vGAT boutons adjacent to γ2-IR clusters not associated with AnkG-labeled AIS were classified as PVBC (schematic magnification). (B) PV protein levels in PVBC boutons were significantly higher in postpubertal monkeys (left) but the density of PVBC boutons did not differ between prepubertal and postpubertal monkeys (right). (C) GABAA receptor α1 subunit mRNA expression increases in a protracted fashion during postnatal development in DLPFC layer 3 pyramidal cells. Please refer to Supplemental Information for details about PVChCs in D–F. (D) Postpubertal monkey section multi-labeled for NeuN/AnkG, γ2, PV, and vGAT as in panel A. PVChC cartridges were classified as PV/vGAT boutons adjacent to γ2-IR clusters within AnkG-labeled AISs (schematic magnification). (E) PV protein levels in PVChC cartridges did not differ between prepubertal and postpubertal monkeys (left) but the density of PVChC boutons/AIS were significantly lower in postpubertal monkeys (right). Panels A, B, D, E adapted from (100). (F) Protracted age-related decrease in mRNA expression for the GABAA receptor α2 subunit in DLPFC layer 3 pyramidal cells. (G) During postnatal development, the amplitude of inhibitory postsynaptic currents (IPSCs) significantly increases in DLPFC layer 3 pyramidal cells (left), whereas the duration of IPSCs significantly decreases (right). Adapted from (101). In all relevant panels, age groups not sharing the same letter are significantly different (p<0.05).
At the postsynaptic level, expression of mRNAs encoding GABAA receptor α1, β2 and γ2 subunits in monkey DLPFC layer 3 pyramidal neurons exhibit protracted postnatal developmental trajectories. In human DLPFC, mRNA levels for the GABAA α1 (Figure 4C), β2 and γ2 subunits in the gray matter display similar trajectories during postnatal development (103). The GABAA β2 subunit preferentially co-assembles with the α1 and γ2 subunits to mediate GABA neurotransmission at PVBC connections onto pyramidal cells (104). The comparable developmental increases in each of these three GABAA receptor subunits may contribute to important changes in the functional properties of DLPFC circuitry. For example, GABAA receptor α subunits influence the decay rate of inhibitory postsynaptic currents (IPSCs); thus, the progressive increase in expression of GABAA α1 mRNA in layer 3 pyramidal cells across postnatal development is likely to substantially decrease the duration of IPSCs in these neurons with age. Consistent with this prediction, recordings in living slice preparations of monkey DLPFC layer 3 pyramidal cells found that the decay of GABAAR-IPSCs decreased while the amplitude of GABAAR-IPSCs increased across postnatal development, at least until the peripubertal period (101) (Figure 4G). These changes in the molecular and physiological properties of DLPFC layer 3 circuitry elements likely contribute to establishing the network properties required to efficiently generate the γ oscillations associated with WM. In particular, γ oscillations require a fast decay of perisomatic inhibition (e.g., IPSC duration of 25 μsec) in order to generate an oscillation at 40 Hz. Therefore, increasing levels of GABAA α1 subunits at the PVBCs synapses onto pyramidal neurons during postnatal development might contribute to a greater capacity for generating cortical γ oscillations and to the associated developmental improvements in WM performance. However, transcriptional studies of receptor subunit expression cannot 1) discriminate between protein levels of receptor subunits tethered to the plasma membrane or present in the cytoplasm or 2) determine if postnatal developmental changes reflect a switch in subunit composition at particular synaptic sites or a change in total number of receptors containing a particular subunit. Further studies are necessary to distinguish among these possibilities.
Developmental disturbances in DLPFC layer 3 and circuitry alterations in schizophrenia
In sum, the findings reviewed above support the hypothesis that DLPFC dysfunction in schizophrenia could arise, at least in part, from cell-autonomous molecular alterations in layer 3 pyramidal cells. These molecular alterations translate into a deficit in dendritic spines, an associated reduction in excitatory inputs, and a resulting hypoactivity in DLPFC layer 3 circuitry. In response to these alterations, compensatory homeostatic mechanisms are engaged to downregulate feedback inhibition in order to restore excitation/inhibition (E/I) balance in the circuit, albeit at levels of both excitation and inhibition that are too low to maintain the generation of γ oscillations required for normal WM function (67;71). These molecular alterations may arise during postnatal development, given recent evidence that γ oscillations and WM continue developing into childhood-adolescence (67;105;106) and are deficient both in individuals experiencing their first episode of psychosis and in those who will later be diagnosed with schizophrenia (16;107–109). For example, the rapid rise in dendritic spine density during the perinatal period could be disrupted in schizophrenia by intrinsic deficits in the actin cytoskeleton, leading to a reduced complement of dendritic spines. Consequently, decreased excitatory activity of DLPFC layer 3 pyramidal cells in the cortical circuit responsible for WM might drive down the normal developmental increase in presynaptic GAD67 and PV and postsynaptic GABAA α1 containing receptors. At the same time, decreased excitatory activity of these neurons might drive up the normal decline in “OR expression. In concert, these circuit alterations might result in reduced recurrent excitation and feedback inhibition.
Alternatively, it is possible that PV cell alterations drive the circuit abnormalities in schizophrenia. The lower levels of GAD67 mRNA could reflect disturbances in upstream factors that regulate the GAD1 gene. For example, a variant in the GAD1 gene was associated with increased risk for schizophrenia (110). Altered chromatin structures at the GAD1 promoter (111) have been associated with lower GAD67 expression in schizophrenia. Selective NMDA receptor ablation on GABA neurons during postnatal development resulted in schizophrenia-like phenotypes (112). PV cell dysfunction in schizophrenia may also be mediated by alterations in intrinsic molecular cascades. For example, the long non-coding RNA, myocardial infarction associated transcript (MIAT), regulates the splicing of the neuregulin receptor ErbB4 (113), which is expressed in PV cells (114–116). Higher MIAT RNA levels in the DLPFC in schizophrenia are associated with an abnormal shift in ErbB4 splicing from the JM-b to the JM-a variant selectively in PV neurons (114). This shift in splicing is predicted to impair ErbB4 signaling. Specifically, given the role of ErbB4 signaling in the formation of excitatory synapses to PV neurons (117;118), the altered splicing is predicted to contribute to fewer excitatory inputs to PV neurons. Consistent with this prediction, the altered ratio of ErbB4 splicing variants was associated with the activity-dependent down-regulation of PV expression in schizophrenia (114). The idea that an ErbB4-mediated alteration in PV cells is “upstream” in DLPFC layer 3 circuitry alterations in schizophrenia is supported by findings that conditional deletion of ErbB4 in PV neurons in mice resulted in cellular, physiological and behavioral deficits suggestive of schizophrenia, including deficits in dendritic spine density (117).
Many of the components of DLPFC layer 3 circuitry that are altered in schizophrenia differ in the pattern and timing of their developmental trajectories (90;119), suggesting that specific elements of cortical circuitry may be more susceptible to environmental perturbations at specific different stages of development. For example, maternal immune activation could lead to elevated cytokines in the fetal brain (120), which may affect the postnatal development of neural circuits (121;122). Alternatively, postnatal immune dysfunction may interact with peripubertal stress (123) to produce alterations in inflammatory markers observed in schizophrenia (124). Interestingly, model animal studies have shown that peripheral cytokines directly impact dendritic spine morphogenesis and plasticity in a developmentally-mediated fashion (125;126). Furthermore, a potential link between genetic risk, immune activation and developmental spine deficits in schizophrenia has recently been reported (5). Also, frequent cannabis use during early adolescence may disrupt DLPFC layer 3 circuit maturation by altering the balance in perisomatic inhibitory inputs from PVBCs and cholecystokinin-containing basket cells (CCKBCs) and thus dysregulating cortical oscillations (127). Cannabinoid 1 receptors (CB1Rs), the principal target of the key psychoactive agent in cannabis (128), are enriched at CCKBC terminals (129;130). CCKBCs make direct synaptic contacts with PVBCs and pyramidal cells (131), and CCK activates PV cells directly via CCK2 receptors (132). These findings provide a plausible biological mechanism by which repeated exposure to cannabis, resulting in non-physiological activation of CB1Rs, could alter the maturation of PVBCs and shift the balance of perisomatic inhibition to a pathological state thereby disrupting γ oscillations and WM (127).
These associations suggest the existence of multiple sensitive periods during which adverse environmental exposures could alter the postnatal developmental trajectories of, and thus lead to disruptions in, the functional properties of DLPFC layer 3 circuitry in schizophrenia (70). The protracted maturation of this circuitry in primates provides multiple opportunities for adverse environmental factors to have their effects amplified as they alter the trajectories of subsequent developmental events. This amplification process might overwhelm the allostatic processes maintaining homeostasis (133). Indeed, the components of DLPFC circuitry that exhibit marked changes during postnatal development are also altered in schizophrenia, suggesting that the disease-related alterations reflect developmental disturbances. Such a scenario could explain why the status of WM circuitry and function in schizophrenia resembles that of an earlier stage of development (27;134). The cumulative consequences of genetic liabilities and adverse environmental exposures during postnatal development could induce an allostatic load (133) that overwhelms adaptive processes, giving rise to the emergence of the schizophrenia phenotype. Future studies are needed to interrogate how specific, individual stressors operating in discrete, temporal time windows alter circuit components required for WM.
Supplementary Material
Table 1.
DLPFC circuitry: Cell type-specific functions, alterations in schizophrenia and developmental trajectories
| Proposed circuit function in working memory | Alterations in schizophrenia | Postnatal developmental trajectories | References | |
|---|---|---|---|---|
| Pyramidal Neurons | Recurrent excitation among layer 3 pyramidal neurons | ↓ Dendritic spine density ↓ Somal and dendritic arbor size ↓ GABAA α1 mRNA ↑ GABAA α2-IR AIS; ↑ GABAA α2 mRNA |
↑ Perinatal dendritic spine density ↓ Peripubertal dendritic spine density ↑ GABAA α1 mRNA ↓ GABAA α2 mRNA |
(57–59), (91–93) (54–56), (91–93) (75;76;135;136) (75;137) |
| PVBCs | Feedback inhibition of layer 3 pyramidal neurons | ↑ “OR mRNA ↓ PV mRNA and protein |
↓ “OR mRNA ↑ PV protein levels per terminal ↔ Terminal density during postnatal development |
(138) (72;74;139) (100) |
| PVChCs | Regulation of pyramidal neurons: -Blockade of back- propagating action potentials -Depolarization |
↓ GAT1 immunoreactive cartridge terminals ? PV protein levels in PVChC terminals currently unknown |
↓ Terminal density during postnatal development ↔ PV protein levels per terminal |
(100;137) (100) |
Acknowledgments
We would like to thank Mary Brady for her exceptional technical assistance in preparing the figures for this manuscript.
Studies by the authors cited in this article were supported by National Institutes of Health Grants MH043784 and MH103204.
Footnotes
Disclosures
David A. Lewis currently receives investigator-initiated research support from Pfizer and in 2013–2015 served as a consultant in the areas of target identification and validation and new compound development to Autifony, Bristol-Myers Squibb, Concert Pharmaceuticals, and Sunovion. Gil D. Hoftman and Dibyadeep Datta reported no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Arnedo J, Svrakic DM, Del Val C, Romero-Zaliz R, Hernandez-Cuervo H C Molecular Genetics of Schizophrenia, et al. Uncovering the hidden risk architecture of the schizophrenias: confirmation in three independent genome-wide association studies. Am J Psychiatry. 2015;172(2):139–53. doi: 10.1176/appi.ajp.2014.14040435. [DOI] [PubMed] [Google Scholar]
- 2.Karayiorgou M, Simon TJ, Gogos JA. 22q11.2 microdeletions: linking DNA structural variation to brain dysfunction and schizophrenia. Nat Rev Neurosci. 2010;11(6):402–16. doi: 10.1038/nrn2841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schizophrenia Working Group of the Psychiatric Genomics C. Biological insights from 108 schizophrenia-associated genetic loci. Nature. 2014;511(7510):421–7. doi: 10.1038/nature13595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Loh PR, Bhatia G, Gusev A, Finucane HK, Bulik-Sullivan BK, Pollack SJ, et al. Contrasting genetic architectures of schizophrenia and other complex diseases using fast variance-components analysis. Nat Genet. 2015;47(12):1385–92. doi: 10.1038/ng.3431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sekar A, Bialas AR, de Rivera H, Davis A, Hammond TR, Kamitaki N, et al. Schizophrenia risk from complex variation of complement component 4. Nature. 2016;530(7589):177–83. doi: 10.1038/nature16549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown AS. Further evidence of infectious insults in the pathogenesis and pathophysiology of schizophrenia. Am J Psychiatry. 2011;168(8):764–6. doi: 10.1176/appi.ajp.2011.11050722. [DOI] [PubMed] [Google Scholar]
- 7.Brown AS, Derkits EJ. Prenatal infection and schizophrenia: a review of epidemiologic and translational studies. Am J Psychiatry. 2010;167(3):261–80. doi: 10.1176/appi.ajp.2009.09030361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lewis DA, Levitt P. Schizophrenia as a disorder of neurodevelopment. Annu Rev Neurosci. 2002;25:409–32. doi: 10.1146/annurev.neuro.25.112701.142754. [DOI] [PubMed] [Google Scholar]
- 9.Keshavan MS, Anderson S, Pettegrew JW. Is schizophrenia due to excessive synaptic pruning in the prefrontal cortex? The Feinberg hypothesis revisited. J Psychiatr Res. 1994;28(3):239–65. doi: 10.1016/0022-3956(94)90009-4. [DOI] [PubMed] [Google Scholar]
- 10.Weinberger DR. Implications of normal brain development for the pathogenesis of schizophrenia. Arch Gen Psychiatry. 1987;44(7):660–9. doi: 10.1001/archpsyc.1987.01800190080012. [DOI] [PubMed] [Google Scholar]
- 11.van Os J, Kenis G, Rutten BP. The environment and schizophrenia. Nature. 2010;468(7321):203–12. doi: 10.1038/nature09563. [DOI] [PubMed] [Google Scholar]
- 12.Feinberg I. Schizophrenia: caused by a fault in programmed synaptic elimination during adolescence? J Psychiatr Res. 1982;17(4):319–34. doi: 10.1016/0022-3956(82)90038-3. [DOI] [PubMed] [Google Scholar]
- 13.Thompson BL, Levitt P. Now you see it, now you don’t--closing in on allostasis and developmental basis of psychiatric disorders. Neuron. 2010;65(4):437–9. doi: 10.1016/j.neuron.2010.02.010. [DOI] [PubMed] [Google Scholar]
- 14.Gur RC, Calkins ME, Satterthwaite TD, Ruparel K, Bilker WB, Moore TM, et al. Neurocognitive growth charting in psychosis spectrum youths. JAMA Psychiatry. 2014;71(4):366–74. doi: 10.1001/jamapsychiatry.2013.4190. [DOI] [PubMed] [Google Scholar]
- 15.MacCabe JH, Wicks S, Lofving S, David AS, Berndtsson A, Gustafsson JE, et al. Decline in cognitive performance between ages 13 and 18 years and the risk for psychosis in adulthood: a Swedish longitudinal cohort study in males. JAMA Psychiatry. 2013;70(3):261–70. doi: 10.1001/2013.jamapsychiatry.43. [DOI] [PubMed] [Google Scholar]
- 16.Reichenberg A, Caspi A, Harrington H, Houts R, Keefe RS, Murray RM, et al. Static and dynamic cognitive deficits in childhood preceding adult schizophrenia: a 30-year study. Am J Psychiatry. 2010;167(2):160–9. doi: 10.1176/appi.ajp.2009.09040574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arnsten AF. Stress weakens prefrontal networks: molecular insults to higher cognition. Nat Neurosci. 2015;18(10):1376–85. doi: 10.1038/nn.4087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- 19.Linden DE. The working memory networks of the human brain. Neuroscientist. 2007;13(3):257–67. doi: 10.1177/1073858406298480. [DOI] [PubMed] [Google Scholar]
- 20.Simpson EH, Kellendonk C, Kandel E. A possible role for the striatum in the pathogenesis of the cognitive symptoms of schizophrenia. Neuron. 2010;65(5):585–96. doi: 10.1016/j.neuron.2010.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lesh TA, Niendam TA, Minzenberg MJ, Carter CS. Cognitive control deficits in schizophrenia: mechanisms and meaning. Neuropsychopharmacology. 2011;36(1):316–38. doi: 10.1038/npp.2010.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alelu-Paz R, Gimenez-Amaya JM. The mediodorsal thalamic nucleus and schizophrenia. J Psychiatry Neurosci. 2008;33(6):489–98. [PMC free article] [PubMed] [Google Scholar]
- 23.Constantinidis C, Wang XJ. A neural circuit basis for spatial working memory. Neuroscientist. 2004;10(6):553–65. doi: 10.1177/1073858404268742. [DOI] [PubMed] [Google Scholar]
- 24.Goldman-Rakic PS. Cellular basis of working memory. Neuron. 1995;14(3):477–85. doi: 10.1016/0896-6273(95)90304-6. [DOI] [PubMed] [Google Scholar]
- 25.Arnsten AF, Wang MJ, Paspalas CD. Neuromodulation of thought: flexibilities and vulnerabilities in prefrontal cortical network synapses. Neuron. 2012;76(1):223–39. doi: 10.1016/j.neuron.2012.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lewis DA, Gonzalez-Burgos G. Neuroplasticity of neocortical circuits in schizophrenia. Neuropsychopharmacology. 2008;33(1):141–65. doi: 10.1038/sj.npp.1301563. [DOI] [PubMed] [Google Scholar]
- 27.Luna B. Developmental changes in cognitive control through adolescence. Adv Child Dev Behav. 2009;37:233–78. doi: 10.1016/s0065-2407(09)03706-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fuster JM, Alexander GE. Neuron activity related to short-term memory. Science. 1971;173(3997):652–4. doi: 10.1126/science.173.3997.652. [DOI] [PubMed] [Google Scholar]
- 29.Alexander GE. Functional development of frontal association cortex in monkeys: behavioural and electrophysiological studies. Neurosci Res Program Bull. 1982;20(4):471–9. [PubMed] [Google Scholar]
- 30.Pucak ML, Levitt JB, Lund JS, Lewis DA. Patterns of intrinsic and associational circuitry in monkey prefrontal cortex. J Comp Neurol. 1996;376(4):614–30. doi: 10.1002/(SICI)1096-9861(19961223)376:4<614::AID-CNE9>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 31.Kritzer MF, Goldman-Rakic PS. Intrinsic circuit organization of the major layers and sublayers of the dorsolateral prefrontal cortex in the rhesus monkey. J Comp Neurol. 1995;359(1):131–43. doi: 10.1002/cne.903590109. [DOI] [PubMed] [Google Scholar]
- 32.Melchitzky DS, Sesack SR, Pucak ML, Lewis DA. Synaptic targets of pyramidal neurons providing intrinsic horizontal connections in monkey prefrontal cortex. J Comp Neurol. 1998;390(2):211–24. doi: 10.1002/(sici)1096-9861(19980112)390:2<211::aid-cne4>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 33.Wang M, Yang Y, Wang CJ, Gamo NJ, Jin LE, Mazer JA, et al. NMDA receptors subserve persistent neuronal firing during working memory in dorsolateral prefrontal cortex. Neuron. 2013;77(4):736–49. doi: 10.1016/j.neuron.2012.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang XJ. Synaptic basis of cortical persistent activity: the importance of NMDA receptors to working memory. J Neurosci. 1999;19(21):9587–603. doi: 10.1523/JNEUROSCI.19-21-09587.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Melchitzky DS, Gonzalez-Burgos G, Barrionuevo G, Lewis DA. Synaptic targets of the intrinsic axon collaterals of supragranular pyramidal neurons in monkey prefrontal cortex. J Comp Neurol. 2001;430(2):209–21. doi: 10.1002/1096-9861(20010205)430:2<209::aid-cne1026>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 36.Melchitzky DS, Lewis DA. Pyramidal neuron local axon terminals in monkey prefrontal cortex: differential targeting of subclasses of GABA neurons. Cereb Cortex. 2003;13(5):452–60. doi: 10.1093/cercor/13.5.452. [DOI] [PubMed] [Google Scholar]
- 37.Constantinidis C, Goldman-Rakic PS. Correlated discharges among putative pyramidal neurons and interneurons in the primate prefrontal cortex. J Neurophysiol. 2002;88(6):3487–97. doi: 10.1152/jn.00188.2002. [DOI] [PubMed] [Google Scholar]
- 38.Funahashi S, Bruce CJ, Goldman-Rakic PS. Mnemonic coding of visual space in the monkey’s dorsolateral prefrontal cortex. J Neurophysiol. 1989;61(2):331–49. doi: 10.1152/jn.1989.61.2.331. [DOI] [PubMed] [Google Scholar]
- 39.Funahashi S, Bruce CJ, Goldman-Rakic PS. Neuronal activity related to saccadic eye movements in the monkey’s dorsolateral prefrontal cortex. J Neurophysiol. 1991;65(6):1464–83. doi: 10.1152/jn.1991.65.6.1464. [DOI] [PubMed] [Google Scholar]
- 40.Funahashi S, Chafee MV, Goldman-Rakic PS. Prefrontal neuronal activity in rhesus monkeys performing a delayed anti-saccade task. Nature. 1993;365(6448):753–6. doi: 10.1038/365753a0. [DOI] [PubMed] [Google Scholar]
- 41.Bartos M, Vida I, Jonas P. Synaptic mechanisms of synchronized gamma oscillations in inhibitory interneuron networks. Nat Rev Neurosci. 2007;8(1):45–56. doi: 10.1038/nrn2044. [DOI] [PubMed] [Google Scholar]
- 42.Cardin JA, Carlen M, Meletis K, Knoblich U, Zhang F, Deisseroth K, et al. Driving fast-spiking cells induces gamma rhythm and controls sensory responses. Nature. 2009;459(7247):663–7. doi: 10.1038/nature08002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sohal VS, Zhang F, Yizhar O, Deisseroth K. Parvalbumin neurons and gamma rhythms enhance cortical circuit performance. Nature. 2009;459(7247):698–702. doi: 10.1038/nature07991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fries P. Neuronal gamma-band synchronization as a fundamental process in cortical computation. Annu Rev Neurosci. 2009;32:209–24. doi: 10.1146/annurev.neuro.051508.135603. [DOI] [PubMed] [Google Scholar]
- 45.Tallon-Baudry C, Bertrand O, Peronnet F, Pernier J. Induced gamma-band activity during the delay of a visual short-term memory task in humans. J Neurosci. 1998;18(11):4244–54. doi: 10.1523/JNEUROSCI.18-11-04244.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Howard MW, Rizzuto DS, Caplan JB, Madsen JR, Lisman J, Aschenbrenner-Scheibe R, et al. Gamma oscillations correlate with working memory load in humans. Cereb Cortex. 2003;13(12):1369–74. doi: 10.1093/cercor/bhg084. [DOI] [PubMed] [Google Scholar]
- 47.Cho KK, Hoch R, Lee AT, Patel T, Rubenstein JL, Sohal VS. Gamma rhythms link prefrontal interneuron dysfunction with cognitive inflexibility in Dlx5/6(+/−) mice. Neuron. 2015;85(6):1332–43. doi: 10.1016/j.neuron.2015.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Szabadics J, Varga C, Molnar G, Olah S, Barzo P, Tamas G. Excitatory effect of GABAergic axo-axonic cells in cortical microcircuits. Science. 2006;311(5758):233–5. doi: 10.1126/science.1121325. [DOI] [PubMed] [Google Scholar]
- 49.Woodruff A, Xu Q, Anderson SA, Yuste R. Depolarizing effect of neocortical chandelier neurons. Front Neural Circuits. 2009;3:15. doi: 10.3389/neuro.04.015.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Woodruff AR, McGarry LM, Vogels TP, Inan M, Anderson SA, Yuste R. State-dependent function of neocortical chandelier cells. J Neurosci. 2011;31(49):17872–86. doi: 10.1523/JNEUROSCI.3894-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hajos N, Karlocai MR, Nemeth B, Ulbert I, Monyer H, Szabo G, et al. Input-output features of anatomically identified CA3 neurons during hippocampal sharp wave/ripple oscillation in vitro. J Neurosci. 2013;33(28):11677–91. doi: 10.1523/JNEUROSCI.5729-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Le Magueresse C, Monyer H. GABAergic interneurons shape the functional maturation of the cortex. Neuron. 2013;77(3):388–405. doi: 10.1016/j.neuron.2013.01.011. [DOI] [PubMed] [Google Scholar]
- 53.Dugladze T, Schmitz D, Whittington MA, Vida I, Gloveli T. Segregation of axonal and somatic activity during fast network oscillations. Science. 2012;336(6087):1458–61. doi: 10.1126/science.1222017. [DOI] [PubMed] [Google Scholar]
- 54.Pierri JN, Volk CL, Auh S, Sampson A, Lewis DA. Decreased somal size of deep layer 3 pyramidal neurons in the prefrontal cortex of subjects with schizophrenia. Arch Gen Psychiatry. 2001;58(5):466–73. doi: 10.1001/archpsyc.58.5.466. [DOI] [PubMed] [Google Scholar]
- 55.Rajkowska G, Selemon LD, Goldman-Rakic PS. Neuronal and glial somal size in the prefrontal cortex: a postmortem morphometric study of schizophrenia and Huntington disease. Arch Gen Psychiatry. 1998;55(3):215–24. doi: 10.1001/archpsyc.55.3.215. [DOI] [PubMed] [Google Scholar]
- 56.Sweet RA, Bergen SE, Sun Z, Marcsisin MJ, Sampson AR, Lewis DA. Anatomical evidence of impaired feedforward auditory processing in schizophrenia. Biol Psychiatry. 2007;61(7):854–64. doi: 10.1016/j.biopsych.2006.07.033. [DOI] [PubMed] [Google Scholar]
- 57.Garey LJ, Ong WY, Patel TS, Kanani M, Davis A, Mortimer AM, et al. Reduced dendritic spine density on cerebral cortical pyramidal neurons in schizophrenia. J Neurol Neurosurg Psychiatry. 1998;65(4):446–53. doi: 10.1136/jnnp.65.4.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Glantz LA, Lewis DA. Decreased dendritic spine density on prefrontal cortical pyramidal neurons in schizophrenia. Arch Gen Psychiatry. 2000;57(1):65–73. doi: 10.1001/archpsyc.57.1.65. [DOI] [PubMed] [Google Scholar]
- 59.Konopaske GT, Lange N, Coyle JT, Benes FM. Prefrontal cortical dendritic spine pathology in schizophrenia and bipolar disorder. JAMA Psychiatry. 2014;71(12):1323–31. doi: 10.1001/jamapsychiatry.2014.1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Saneyoshi T, Fortin DA, Soderling TR. Regulation of spine and synapse formation by activity-dependent intracellular signaling pathways. Curr Opin Neurobiol. 2010;20(1):108–15. doi: 10.1016/j.conb.2009.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Scott EK, Reuter JE, Luo L. Small GTPase Cdc42 is required for multiple aspects of dendritic morphogenesis. J Neurosci. 2003;23(8):3118–23. doi: 10.1523/JNEUROSCI.23-08-03118.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hill JJ, Hashimoto T, Lewis DA. Molecular mechanisms contributing to dendritic spine alterations in the prefrontal cortex of subjects with schizophrenia. Mol Psychiatry. 2006;11(6):557–66. doi: 10.1038/sj.mp.4001792. [DOI] [PubMed] [Google Scholar]
- 63.Cahill ME, Xie Z, Day M, Photowala H, Barbolina MV, Miller CA, et al. Kalirin regulates cortical spine morphogenesis and disease-related behavioral phenotypes. Proc Natl Acad Sci U S A. 2009;106(31):13058–63. doi: 10.1073/pnas.0904636106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Arion D, Unger T, Lewis DA, Mirnics K. Molecular markers distinguishing supragranular and infragranular layers in the human prefrontal cortex. Eur J Neurosci. 2007;25(6):1843–54. doi: 10.1111/j.1460-9568.2007.05396.x. [DOI] [PubMed] [Google Scholar]
- 65.Datta D, Arion D, Corradi JP, Lewis DA. Altered Expression of CDC42 Signaling Pathway Components in Cortical Layer 3 Pyramidal Cells in Schizophrenia. Biol Psychiatry. 2015;78(11):775–85. doi: 10.1016/j.biopsych.2015.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ide M, Lewis DA. Altered cortical CDC42 signaling pathways in schizophrenia: implications for dendritic spine deficits. Biol Psychiatry. 2010;68(1):25–32. doi: 10.1016/j.biopsych.2010.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gonzalez-Burgos G, Cho RY, Lewis DA. Alterations in cortical network oscillations and parvalbumin neurons in schizophrenia. Biol Psychiatry. 2015;77(12):1031–40. doi: 10.1016/j.biopsych.2015.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Arion D, Corradi JP, Tang S, Datta D, Boothe F, He A, et al. Distinctive transcriptome alterations of prefrontal pyramidal neurons in schizophrenia and schizoaffective disorder. Mol Psychiatry. 2015;20(11):1397–405. doi: 10.1038/mp.2014.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Middleton FA, Mirnics K, Pierri JN, Lewis DA, Levitt P. Gene expression profiling reveals alterations of specific metabolic pathways in schizophrenia. J Neurosci. 2002;22(7):2718–29. doi: 10.1523/JNEUROSCI.22-07-02718.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lewis DA. Cortical circuit dysfunction and cognitive deficits in schizophrenia--implications for preemptive interventions. Eur J Neurosci. 2012;35(12):1871–8. doi: 10.1111/j.1460-9568.2012.08156.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lewis DA, Curley AA, Glausier JR, Volk DW. Cortical parvalbumin interneurons and cognitive dysfunction in schizophrenia. Trends Neurosci. 2012;35(1):57–67. doi: 10.1016/j.tins.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hashimoto T, Volk DW, Eggan SM, Mirnics K, Pierri JN, Sun Z, et al. Gene expression deficits in a subclass of GABA neurons in the prefrontal cortex of subjects with schizophrenia. J Neurosci. 2003;23(15):6315–26. doi: 10.1523/JNEUROSCI.23-15-06315.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Curley AA, Arion D, Volk DW, Asafu-Adjei JK, Sampson AR, Fish KN, et al. Cortical deficits of glutamic acid decarboxylase 67 expression in schizophrenia: clinical, protein, and cell type-specific features. Am J Psychiatry. 2011;168(9):921–9. doi: 10.1176/appi.ajp.2011.11010052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Glausier JR, Fish KN, Lewis DA. Altered parvalbumin basket cell inputs in the dorsolateral prefrontal cortex of schizophrenia subjects. Mol Psychiatry. 2014;19(1):30–6. doi: 10.1038/mp.2013.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Beneyto M, Abbott A, Hashimoto T, Lewis DA. Lamina-specific alterations in cortical GABA(A) receptor subunit expression in schizophrenia. Cereb Cortex. 2011;21(5):999–1011. doi: 10.1093/cercor/bhq169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Glausier JR, Lewis DA. Selective pyramidal cell reduction of GABA(A) receptor alpha1 subunit messenger RNA expression in schizophrenia. Neuropsychopharmacology. 2011;36(10):2103–10. doi: 10.1038/npp.2011.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Volk DW, Matsubara T, Li S, Sengupta EJ, Georgiev D, Minabe Y, et al. Deficits in transcriptional regulators of cortical parvalbumin neurons in schizophrenia. Am J Psychiatry. 2012;169(10):1082–91. doi: 10.1176/appi.ajp.2012.12030305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Drake CT, Milner TA. Mu opioid receptors are in discrete hippocampal interneuron subpopulations. Hippocampus. 2002;12(2):119–36. doi: 10.1002/hipo.1107. [DOI] [PubMed] [Google Scholar]
- 79.Stumm RK, Zhou C, Schulz S, Hollt V. Neuronal types expressing mu- and delta-opioid receptor mRNA in the rat hippocampal formation. J Comp Neurol. 2004;469(1):107–18. doi: 10.1002/cne.10997. [DOI] [PubMed] [Google Scholar]
- 80.Capogna M, Gahwiler BH, Thompson SM. Mechanism of mu-opioid receptor-mediated presynaptic inhibition in the rat hippocampus in vitro. J Physiol. 1993;470:539–58. doi: 10.1113/jphysiol.1993.sp019874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Glickfeld LL, Atallah BV, Scanziani M. Complementary modulation of somatic inhibition by opioids and cannabinoids. J Neurosci. 2008;28(8):1824–32. doi: 10.1523/JNEUROSCI.4700-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kimoto S, Zaki MM, Bazmi HH, Lewis DA. Altered Markers of Cortical gamma-Aminobutyric Acid Neuronal Activity in Schizophrenia: Role of the NARP Gene. JAMA Psychiatry. 2015;72(8):747–56. doi: 10.1001/jamapsychiatry.2015.0533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chang MC, Park JM, Pelkey KA, Grabenstatter HL, Xu D, Linden DJ, et al. Narp regulates homeostatic scaling of excitatory synapses on parvalbumin-expressing interneurons. Nat Neurosci. 2010;13(9):1090–7. doi: 10.1038/nn.2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gu Y, Huang S, Chang MC, Worley P, Kirkwood A, Quinlan EM. Obligatory role for the immediate early gene NARP in critical period plasticity. Neuron. 2013;79(2):335–46. doi: 10.1016/j.neuron.2013.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pelkey KA, Barksdale E, Craig MT, Yuan X, Sukumaran M, Vargish GA, et al. Pentraxins coordinate excitatory synapse maturation and circuit integration of parvalbumin interneurons. Neuron. 2015;85(6):1257–72. doi: 10.1016/j.neuron.2015.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kimoto S, Bazmi HH, Lewis DA. Lower expression of glutamic acid decarboxylase 67 in the prefrontal cortex in schizophrenia: contribution of altered regulation by Zif268. Am J Psychiatry. 2014;171(9):969–78. doi: 10.1176/appi.ajp.2014.14010004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Szabo G, Katarova Z, Kortvely E, Greenspan RJ, Urban Z. Structure and the promoter region of the mouse gene encoding the 67-kD form of glutamic acid decarboxylase. DNA Cell Biol. 1996;15(12):1081–91. doi: 10.1089/dna.1996.15.1081. [DOI] [PubMed] [Google Scholar]
- 88.Yanagawa Y, Kobayashi T, Kamei T, Ishii K, Nishijima M, Takaku A, et al. Structure and alternative promoters of the mouse glutamic acid decarboxylase 67 gene. Biochem J. 1997;326(Pt 2):573–8. doi: 10.1042/bj3260573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Luo AH, Georges FE, Aston-Jones GS. Novel neurons in ventral tegmental area fire selectively during the active phase of the diurnal cycle. Eur J Neurosci. 2008;27(2):408–22. doi: 10.1111/j.1460-9568.2007.05985.x. [DOI] [PubMed] [Google Scholar]
- 90.Hoftman GD, Lewis DA. Postnatal developmental trajectories of neural circuits in the primate prefrontal cortex: identifying sensitive periods for vulnerability to schizophrenia. Schizophr Bull. 2011;37(3):493–503. doi: 10.1093/schbul/sbr029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Anderson SA, Classey JD, Conde F, Lund JS, Lewis DA. Synchronous development of pyramidal neuron dendritic spines and parvalbumin-immunoreactive chandelier neuron axon terminals in layer III of monkey prefrontal cortex. Neuroscience. 1995;67(1):7–22. doi: 10.1016/0306-4522(95)00051-j. [DOI] [PubMed] [Google Scholar]
- 92.Petanjek Z, Judas M, Simic G, Rasin MR, Uylings HB, Rakic P, et al. Extraordinary neoteny of synaptic spines in the human prefrontal cortex. Proc Natl Acad Sci U S A. 2011;108(32):13281–6. doi: 10.1073/pnas.1105108108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bourgeois JP, Goldman-Rakic PS, Rakic P. Synaptogenesis in the prefrontal cortex of rhesus monkeys. Cereb Cortex. 1994;4(1):78–96. doi: 10.1093/cercor/4.1.78. [DOI] [PubMed] [Google Scholar]
- 94.Huttenlocher PR. Synaptic density in human frontal cortex - developmental changes and effects of aging. Brain Res. 1979;163(2):195–205. doi: 10.1016/0006-8993(79)90349-4. [DOI] [PubMed] [Google Scholar]
- 95.Huttenlocher PR, Dabholkar AS. Regional differences in synaptogenesis in human cerebral cortex. J Comp Neurol. 1997;387(2):167–78. doi: 10.1002/(sici)1096-9861(19971020)387:2<167::aid-cne1>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 96.Gonzalez-Burgos G, Kroener S, Zaitsev AV, Povysheva NV, Krimer LS, Barrionuevo G, et al. Functional maturation of excitatory synapses in layer 3 pyramidal neurons during postnatal development of the primate prefrontal cortex. Cereb Cortex. 2008;18(3):626–37. doi: 10.1093/cercor/bhm095. [DOI] [PubMed] [Google Scholar]
- 97.Datta D, Arion D, Lewis DA. Developmental Expression Patterns of GABAA Receptor Subunits in Layer 3 and 5 Pyramidal Cells of Monkey Prefrontal Cortex. Cereb Cortex. 2015;25(8):2295–305. doi: 10.1093/cercor/bhu040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Woo TU, Pucak ML, Kye CH, Matus CV, Lewis DA. Peripubertal refinement of the intrinsic and associational circuitry in monkey prefrontal cortex. Neuroscience. 1997;80(4):1149–58. doi: 10.1016/s0306-4522(97)00059-6. [DOI] [PubMed] [Google Scholar]
- 99.Erickson SL, Lewis DA. Postnatal development of parvalbumin- and GABA transporter-immunoreactive axon terminals in monkey prefrontal cortex. J Comp Neurol. 2002;448(2):186–202. doi: 10.1002/cne.10249. [DOI] [PubMed] [Google Scholar]
- 100.Fish KN, Hoftman GD, Sheikh W, Kitchens M, Lewis DA. Parvalbumin-containing chandelier and basket cell boutons have distinctive modes of maturation in monkey prefrontal cortex. J Neurosci. 2013;33(19):8352–8. doi: 10.1523/JNEUROSCI.0306-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Gonzalez-Burgos G, Miyamae T, Pafundo DE, Yoshino H, Rotaru DC, Hoftman G, et al. Functional Maturation of GABA Synapses During Postnatal Development of the Monkey Dorsolateral Prefrontal Cortex. Cereb Cortex. 2015;25(11):4076–93. doi: 10.1093/cercor/bhu122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lund JS, Lewis DA. Local circuit neurons of developing and mature macaque prefrontal cortex: Golgi and immunocytochemical characteristics. J Comp Neurol. 1993;328(2):282–312. doi: 10.1002/cne.903280209. [DOI] [PubMed] [Google Scholar]
- 103.Fillman SG, Duncan CE, Webster MJ, Elashoff M, Weickert CS. Developmental co-regulation of the beta and gamma GABAA receptor subunits with distinct alpha subunits in the human dorsolateral prefrontal cortex. Int J Dev Neurosci. 2010;28(6):513–9. doi: 10.1016/j.ijdevneu.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 104.Fritschy JM, Mohler H. GABAA-receptor heterogeneity in the adult rat brain: differential regional and cellular distribution of seven major subunits. J Comp Neurol. 1995;359(1):154–94. doi: 10.1002/cne.903590111. [DOI] [PubMed] [Google Scholar]
- 105.Uhlhaas PJ, Singer W. Abnormal neural oscillations and synchrony in schizophrenia. Nat Rev Neurosci. 2010;11(2):100–13. doi: 10.1038/nrn2774. [DOI] [PubMed] [Google Scholar]
- 106.Cho RY, Walker CP, Polizzotto NR, Wozny TA, Fissell C, Chen CM, et al. Development of sensory gamma oscillations and cross-frequency coupling from childhood to early adulthood. Cereb Cortex. 2015;25(6):1509–18. doi: 10.1093/cercor/bht341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Symond MP, Harris AW, Gordon E, Williams LM. “Gamma synchrony” in first-episode schizophrenia: a disorder of temporal connectivity? Am J Psychiatry. 2005;162(3):459–65. doi: 10.1176/appi.ajp.162.3.459. [DOI] [PubMed] [Google Scholar]
- 108.Minzenberg MJ, Firl AJ, Yoon JH, Gomes GC, Reinking C, Carter CS. Gamma oscillatory power is impaired during cognitive control independent of medication status in first-episode schizophrenia. Neuropsychopharmacology. 2010;35(13):2590–9. doi: 10.1038/npp.2010.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Spencer KM, Salisbury DF, Shenton ME, McCarley RW. Gamma-band auditory steady-state responses are impaired in first episode psychosis. Biol Psychiatry. 2008;64(5):369–75. doi: 10.1016/j.biopsych.2008.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Straub RE, Lipska BK, Egan MF, Goldberg TE, Callicott JH, Mayhew MB, et al. Allelic variation in GAD1 (GAD67) is associated with schizophrenia and influences cortical function and gene expression. Mol Psychiatry. 2007;12(9):854–69. doi: 10.1038/sj.mp.4001988. [DOI] [PubMed] [Google Scholar]
- 111.Huang HS, Matevossian A, Whittle C, Kim SY, Schumacher A, Baker SP, et al. Prefrontal dysfunction in schizophrenia involves mixed-lineage leukemia 1-regulated histone methylation at GABAergic gene promoters. J Neurosci. 2007;27(42):11254–62. doi: 10.1523/JNEUROSCI.3272-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Belforte JE, Zsiros V, Sklar ER, Jiang Z, Yu G, Li Y, et al. Postnatal NMDA receptor ablation in corticolimbic interneurons confers schizophrenia-like phenotypes. Nat Neurosci. 2010;13(1):76–83. doi: 10.1038/nn.2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Barry G, Briggs JA, Vanichkina DP, Poth EM, Beveridge NJ, Ratnu VS, et al. The long non-coding RNA Gomafu is acutely regulated in response to neuronal activation and involved in schizophrenia-associated alternative splicing. Mol Psychiatry. 2014;19(4):486–94. doi: 10.1038/mp.2013.45. [DOI] [PubMed] [Google Scholar]
- 114.Chung DW, Volk DW, Arion D, Zhang Y, Sampson AR, Lewis DA. Dysregulated ErbB4 Splicing in Schizophrenia: Selective Effects on Parvalbumin Expression. Am J Psychiatry. 2015 doi: 10.1176/appi.ajp.2015.15020150. appiajp201515020150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Neddens J, Fish KN, Tricoire L, Vullhorst D, Shamir A, Chung W, et al. Conserved Interneuron-Specific ErbB4 Expression in Frontal Cortex of Rodents, Monkeys, and Humans: Implications for Schizophrenia. Biol Psychiatry. 2011 doi: 10.1016/j.biopsych.2011.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Fazzari P, Paternain AV, Valiente M, Pla R, Lujan R, Lloyd K, et al. Control of cortical GABA circuitry development by Nrg1 and ErbB4 signalling. Nature. 2010;464(7293):1376–80. doi: 10.1038/nature08928. [DOI] [PubMed] [Google Scholar]
- 117.Del Pino I, Garcia-Frigola C, Dehorter N, Brotons-Mas JR, Alvarez-Salvado E, Martinez de Lagran M, et al. Erbb4 deletion from fast-spiking interneurons causes schizophrenia-like phenotypes. Neuron. 2013;79(6):1152–68. doi: 10.1016/j.neuron.2013.07.010. [DOI] [PubMed] [Google Scholar]
- 118.Ting AK, Chen Y, Wen L, Yin DM, Shen C, Tao Y, et al. Neuregulin 1 promotes excitatory synapse development and function in GABAergic interneurons. J Neurosci. 2011;31(1):15–25. doi: 10.1523/JNEUROSCI.2538-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Lewis DA. Development of the prefrontal cortex during adolescence: insights into vulnerable neural circuits in schizophrenia. Neuropsychopharmacology. 1997;16(6):385–98. doi: 10.1016/S0893-133X(96)00277-1. [DOI] [PubMed] [Google Scholar]
- 120.Meyer U, Nyffeler M, Engler A, Urwyler A, Schedlowski M, Knuesel I, et al. The time of prenatal immune challenge determines the specificity of inflammation-mediated brain and behavioral pathology. J Neurosci. 2006;26(18):4752–62. doi: 10.1523/JNEUROSCI.0099-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Meyer U, Nyffeler M, Yee BK, Knuesel I, Feldon J. Adult brain and behavioral pathological markers of prenatal immune challenge during early/middle and late fetal development in mice. Brain Behav Immun. 2008;22(4):469–86. doi: 10.1016/j.bbi.2007.09.012. [DOI] [PubMed] [Google Scholar]
- 122.Richetto J, Calabrese F, Riva MA, Meyer U. Prenatal immune activation induces maturation-dependent alterations in the prefrontal GABAergic transcriptome. Schizophr Bull. 2014;40(2):351–61. doi: 10.1093/schbul/sbs195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Giovanoli S, Engler H, Engler A, Richetto J, Voget M, Willi R, et al. Stress in puberty unmasks latent neuropathological consequences of prenatal immune activation in mice. Science. 2013;339(6123):1095–9. doi: 10.1126/science.1228261. [DOI] [PubMed] [Google Scholar]
- 124.Volk DW, Chitrapu A, Edelson JR, Roman KM, Moroco AE, Lewis DA. Molecular Mechanisms and Timing of Cortical Immune Activation in Schizophrenia. Am J Psychiatry. 2015;172(11):1112–21. doi: 10.1176/appi.ajp.2015.15010019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Dietz DM, Sun H, Lobo MK, Cahill ME, Chadwick B, Gao V, et al. Rac1 is essential in cocaine-induced structural plasticity of nucleus accumbens neurons. Nat Neurosci. 2012;15(6):891–6. doi: 10.1038/nn.3094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Hering H, Sheng M. Dendritic spines: structure, dynamics and regulation. Nat Rev Neurosci. 2001;2(12):880–8. doi: 10.1038/35104061. [DOI] [PubMed] [Google Scholar]
- 127.Curley AA, Lewis DA. Cortical basket cell dysfunction in schizophrenia. J Physiol. 2012;590(Pt 4):715–24. doi: 10.1113/jphysiol.2011.224659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Freund TF, Katona I, Piomelli D. Role of endogenous cannabinoids in synaptic signaling. Physiol Rev. 2003;83(3):1017–66. doi: 10.1152/physrev.00004.2003. [DOI] [PubMed] [Google Scholar]
- 129.Alger BE. Endocannabinoids and their implications for epilepsy. Epilepsy Curr. 2004;4(5):169–73. doi: 10.1111/j.1535-7597.2004.04501.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Eggan SM, Melchitzky DS, Sesack SR, Fish KN, Lewis DA. Relationship of cannabinoid CB1 receptor and cholecystokinin immunoreactivity in monkey dorsolateral prefrontal cortex. Neuroscience. 2010;169(4):1651–61. doi: 10.1016/j.neuroscience.2010.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Karson MA, Tang AH, Milner TA, Alger BE. Synaptic cross talk between perisomatic-targeting interneuron classes expressing cholecystokinin and parvalbumin in hippocampus. J Neurosci. 2009;29(13):4140–54. doi: 10.1523/JNEUROSCI.5264-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Foldy C, Lee SY, Szabadics J, Neu A, Soltesz I. Cell type-specific gating of perisomatic inhibition by cholecystokinin. Nat Neurosci. 2007;10(9):1128–30. doi: 10.1038/nn1952. [DOI] [PubMed] [Google Scholar]
- 133.McEwen BS. Stress, adaptation, and disease. Allostasis and allostatic load. Ann N Y Acad Sci. 1998;840:33–44. doi: 10.1111/j.1749-6632.1998.tb09546.x. [DOI] [PubMed] [Google Scholar]
- 134.Crone EA, Wendelken C, Donohue S, van Leijenhorst L, Bunge SA. Neurocognitive development of the ability to manipulate information in working memory. Proc Natl Acad Sci U S A. 2006;103(24):9315–20. doi: 10.1073/pnas.0510088103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Duncan CE, Webster MJ, Rothmond DA, Bahn S, Elashoff M, Shannon Weickert C. Prefrontal GABA(A) receptor alpha-subunit expression in normal postnatal human development and schizophrenia. J Psychiatr Res. 2010;44(10):673–81. doi: 10.1016/j.jpsychires.2009.12.007. [DOI] [PubMed] [Google Scholar]
- 136.Hashimoto T, Nguyen QL, Rotaru D, Keenan T, Arion D, Beneyto M, et al. Protracted developmental trajectories of GABAA receptor alpha1 and alpha2 subunit expression in primate prefrontal cortex. Biol Psychiatry. 2009;65(12):1015–23. doi: 10.1016/j.biopsych.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Volk DW, Pierri JN, Fritschy JM, Auh S, Sampson AR, Lewis DA. Reciprocal alterations in pre- and postsynaptic inhibitory markers at chandelier cell inputs to pyramidal neurons in schizophrenia. Cereb Cortex. 2002;12(10):1063–70. doi: 10.1093/cercor/12.10.1063. [DOI] [PubMed] [Google Scholar]
- 138.Volk DW, Radchenkova PV, Walker EM, Sengupta EJ, Lewis DA. Cortical opioid markers in schizophrenia and across postnatal development. Cereb Cortex. 2012;22(5):1215–23. doi: 10.1093/cercor/bhr202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Hoftman GD, Volk DW, Bazmi HH, Li S, Sampson AR, Lewis DA. Altered cortical expression of GABA-related genes in schizophrenia: illness progression vs developmental disturbance. Schizophr Bull. 2015;41(1):180–91. doi: 10.1093/schbul/sbt178. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.