Abstract
Regeneration of traumatically injured skeletal muscles is severely limited. Moreover, the regenerative capacity of skeletal muscle declines with aging, further exacerbating the problem. Recent evidence support that delivery of muscle satellite cells to the injured muscles enhances muscle regeneration and reverses features of aging, including reduction in muscle mass and regenerative capacity. However, direct delivery of satellite cells presents a challenge at a translational level due to inflammation and donor cell death, motivating the need to develop engineered matrices for muscle satellite cell delivery. This review will highlight important aspects of satellite cell and their niche biology in the context of muscle regeneration, and examine recent progresses in the development of engineered cell delivery matrices designed for skeletal muscle regeneration. Understanding the interactions of muscle satellite cells and their niche in both native and engineered systems is crucial to developing muscle pathology-specific cell- and biomaterial-based therapies.
Keywords: skeletal muscle, satellite cells, niche, biomaterial, extracellular matrix, aging, stem cell therapy
Introduction
Skeletal muscles function to generate force, enable locomotion, support posture, regulate metabolism, and secrete myokines with autocrine, paracrine and endocrine effects. Muscle has the capacity to repair itself upon minor strains, contusions, and lacerations; however, its native regenerative capacity with an onset of traumatic injury is severely restricted, often leading to further loss of muscle mass, fibrosis, and diminished function [1–3]. Furthermore, this problem is exacerbated in elderly patients, as muscle regenerative capacity declines with aging [4–7]. To date, effective clinical strategies to restore the native muscle tissue structure and function are extremely limited. Collectively, these problems pose significant socioeconomic costs and impact the quality of life [1,2,8,9]. For instance, in the United States, the estimated direct healthcare costs associated with sarcopenia alone exceeded $18.5 billion [8] and combined direct and indirect (i.e., lost productivity) costs associated with traumatic injury exceeded $406 billion in 2000 [10]. Despite the lack of more recent and reliable healthcare cost estimates of sarcopenia and its co-morbidities, the socioeconomic burden is projected to be even more significant with increasing number of individuals aged 65 years and older [9].
Recent advances in the stem cell and niche biology of muscle have identified muscle satellite cells (MuSCs) as one of the crucial components in muscle development and endogenous reparative processes [11,12]. MuSCs, which constitute approximately 2–10% of the total number of myonuclei [13], are quiescent in healthy and resting muscles, but are activated for proliferation and differentiation to form new myofibers in response to mechanical and chemical stimuli resulting from injurious events [11,12]. Indeed, delivery of MuSCs into the injured intramuscular space enhances regenerative outcomes [14–16], indicating that stem cell therapy may be a promising strategy for treating muscle injuries and disorders. Furthermore, it is also important to highlight the role of the MuSC niche in the regenerative process. In particular, recent evidence suggests that biochemical factors (e.g., growth factors) from both systemic and local milieu, as well as biophysical factors (e.g., matrix stiffness, adhesive ligands) significantly influence the bioactivity of the MuSCs and their functional coordination with other resident cells (e.g., inflammatory and fibroadipogenic progenitor cells) [11,17–20], ultimately impacting regenerative outcomes. The intricate interactions of MuSCs and their niche during homeostasis and regeneration are also important considerations in the context of aging, where age-associated alterations in the MuSC niche will negatively influence the function of the skeletal muscle, and further contribute to the progression of sarcopenia [7].
Delivery of stem cells is an effective approach to stimulate and enhance skeletal muscle regeneration in vivo [14–16]. However, direct intramuscular delivery of MuSCs presents challenges at the translational level, as the transplanted MuSCs exhibit limited migration in vivo and are exposed to inflammatory microenvironment consisting of neutrophils, macrophages, reactive oxygen species, and pro-inflammatory cytokines upon injury, where such signals induce cell death and inadequate cellular function [22]. In order to maintain the viability and enhance functional outcomes, engineered biomaterials designed to transiently protect the donor MuSCs from the inflammatory environment and to direct their function in vivo are needed. Specifically, elucidating how MuSCs interact with their niche, as well as incorporating key elements of the niche in the engineered delivery matrices are crucial to developing the optimal bio-functional delivery matrix for skeletal muscle regeneration. Further challenges in cell- and biomaterial-based therapies in the context of skeletal muscle regeneration include having precise control over immune-modulation, as well as effective stimulation of innervation and vascularization. For instance, MuSCs play an important role in forming neuromuscular junctions [23], where quality of neuromuscular junction and motoneuron innervation is important for normal muscle function and susceptibility sarcopenia in rodents and human [24]. This suggests that modulating innervation of the transplanted MuSCs is an important consideration for cell- and biomaterial-based therapy to improve regenerative outcomes in both acute injury and aging contexts. Such considerations to promote vascularization and innervation have been previously reviewed in greater detail [25].
This review will first highlight recent advances in the understanding of MuSCs and their niche in healthy, injured, and aged skeletal muscles. Progress in the development of engineered cell delivery matrices designed for skeletal muscle regeneration will be reviewed. Finally, design considerations for successful MuSC delivery and engraftment, as well as other factors to further improve the functional outcome will be discussed. The current review will primarily focus on biomaterials designed for cell delivery. The findings and conclusions referenced in this review are for rodent models, and not from human data, unless noted otherwise. Tissue engineering strategies of skeletal muscle constructs ex vivo will not be extensively covered here, as such advancements have been recently reviewed elsewhere [25,26].
Satellite cells
MuSCs were first identified and characterized in 1961 in an electron microscope study, and have been implicated to play a role in muscle regeneration [27]. Indeed, recent cell ablation and lineage tracing studies have demonstrated that MuSCs, which express the paired box protein 7 (Pax7) transcription factor when quiescent under resting conditions, are essential to skeletal muscle regeneration [28–31]. In addition, advances in MuSC purification techniques using fluorescence-activated cell sorting enabled transplantation of Pax7+ MuSCs to the injured intramuscular space, where Pax7+ mouse and human MuSC transplantation into mouse recipients enhances regeneration [14–16,21]. Collectively, these transplantation studies demonstrate the importance of MuSC participation in the muscle regeneration process.
Recent studies identified several signaling pathways and mechanisms for maintenance of MuSC quiescence and self-renewal, including expression of microRNA-489 [32], phosphorylation of translation initiation factor eIFα2 [33], increased angiopoietin-1/Tie-2 signaling [34], and expression of EZH2 [35]. In the aged mice, basal autophagy has been identified as a critical regulator for the preservation of MuSC quiescence and prevention of senescence [36]. Within the quiescent MuSC pool, approximately 90% of the quiescent MuSCs express Myf5 and are biased to myogenic differentiation while the rest (~10%) do not [37]. Here, arginine methyltransferase Carm1 methylates arginines of Pax7, and subsequently induces transcription of Myf5 upon asymmetric division [38]. More recently, a study utilizing transgenic Pax7-nuclear GFP mice revealed that MuSCs expressing higher levels of Pax7 are less likely to commit and exhibit lower metabolic activity compared to MuSCs expressing lower levels of Pax7 [39], corroborating the presence of cell heterogeneity within the MuSC population.
With an onset of injury, MuSCs undergo activation, where the myoblast determination protein (MyoD) transcription factor is upregulated [40]. In presence of inflammatory cytokines such as IL-6, MuSCs commit to myogenic lineage progression in a STAT3-dependent manner [41]. One mechanism that regulates MyoD expression involves the histone H4 lysine 20 (H4K20) methyltransferase Suv4-20H, which binds the MyoD locus. When MuSCs are quiescent, H4K20 methyltransferase Suv4-20H1 is bound to the MyoD locus and maintains highly condensed heterochromatin structure [42]. Suv4-20H1 activity is reduced as a consequence of activation, exposing the MyoD locus from the condensed heterochromatin [42]. Furthermore, activated MuSCs undergo metabolic switch, which is characterized by decreased levels of NAD+ and SIRT1 deacetylase activity, subsequently leading to increased level of H4K16 acetylation [43]. This metabolic shift during activation impacts expression of MyoD expression [43]. MyoD+ myoblasts proliferate, migrate, and differentiate into myotubes upregulating myogenin (Myog) and downregulating Pax7 expression via p38/polycomb repressive complex 2 signaling [44–48]. In coordination with Notch signaling [49], Pax7−/Myog+ cells fuse to form multinucleated myotubes and subsequently new regenerating muscle fibers. A recent study identified myomaker, a muscle-specific membrane protein, as one of the key proteins that directly mediates the fusion process [50]. It is also important to note that the intrinsic function and number of MuSCs decline with aging, leading to inferior muscle regenerative capacity [7,51–57]. Comprehensive reviews on intrinsic changes in the MuSCs as a consequence of aging have been recently published [7,51,52].
Recent transplantation studies indicate that Pax7+ MuSCs represent a versatile cell source for treating muscle trauma, disorders, and age-associated muscle dysfunctions [14–16,21,53,58]. Transplantation of Pax7+ MuSCs to injured muscle results in rapid proliferation and engraftment resulting in muscle repair, but transplantation of cultured myoblasts exhibits limited capacity to induce muscle regeneration in vivo [15]. Furthermore, delivery of MuSCs to aged muscle reverses features of aging, including muscle mass, regenerative capacity, and contractile function [58]. Remarkably, delivery of MuSCs into dystrophin-deficient muscles of mdx mice also re-establishes dystrophin expression and enhances muscle function [16]. Embryonic stem and induced pluripotent stem cells that conditionally express Pax7 also result in effective restoration of dystrophin expression and muscle function over 11 months [59]. However, age-associated intrinsic function of MuSC is an important consideration for such translational applications, as the engraftment potential progressively declines with the cellular age [53,60]. Collectively, the evidence demonstrate that Pax7-expressing stem cells indeed are a promising candidate for treating muscle trauma, disorders, and age-associated muscle dysfunctions.
Satellite cell niche
MuSC function and the inherent regeneration capacity of skeletal muscle are facilitated by intricate interactions between MuSCs and their niche. The microenvironmental niche of MuSCs provides biomechanical and biochemical signals that regulate MuSC functions, including self-renewal, proliferation, differentiation, and migration [61]. MuSCs reside in between the sarcolemma of muscle fibers and the basal lamina matrix, where the basal lamina consists of an array of extracellular matrix proteins. In addition to providing biophysical cues to the cells, these matrix components also provide important sites for soluble growth factor sequestration and presentation [62,63]. Release of growth factors from the matrix, and other resident cells provide additional complexity for controlling MuSC function during homeostasis and repair. Identifying key niche components, and understanding mechanisms by which such factors control MuSC function, is an important engineering consideration for designing translatable and functional synthetic matrices.
Biomechanical signals are an important regulator for directing MuSC function. A seminal study by Gilbert et al. (2008) demonstrated that substrate elasticity is critical in regulating MuSC self-renewal function in vitro, where physiologically relevant stiffness (12 kPa) is essential for directing MuSC self-renewal in a 2D culture [20]. Whereas it remains to be determined whether this also extends to 3D culture systems, it is likely that the mechanical properties of the MuSC niche regulate MuSC function in vivo. For instance, collagen VI, a component of the basal lamina, plays an important role controlling MuSC self-renewal and muscle regeneration by providing structural and mechanical support to the niche [64]. Knockout of collagen VI results in a significantly decreased muscle elastic modulus, but restoration of collagen VI results in reestablishment in the tissue modulus, and more importantly, MuSC functions are rescued [64]. This suggests that any compositional changes arising from damage, aging, and pathology (e.g., muscular dystrophy, cachexia) could alter the physiological mechanical microenvironment, ultimately compromising the optimal MuSC function.
The basal lamina matrix is composed of extracellular matrix proteins, such as laminins, fibronectin, and collagens [65,66]. Notably, laminins are one of the major components of the basal lamina. MuSCs interact with laminins primarily via α7β1 integrin, which is specifically expressed in MuSCs [67,68]. α7β1 integrin binds to the E8 domain of laminin [69] and contributes to proliferation, adhesion, and migration properties [70–73]. Interestingly, mutations in α7 integrin result in congenital myopathies and muscular dystrophies, and furthermore, enhancement in α7 integrin expression delays the progression of muscular pathologies [74,75]. Injection of laminin into mouse models of congenital myopathies and eccentric exercise restores the regenerative capacity of the muscle [76–78], suggesting that interactions between α7 integrin and laminins are essential for the normal physiology of the muscle. Similarly, MuSCs lacking dystroglycan, a laminin receptor, exhibit decreased regenerative capacity [79], and blocking MuSC α6 integrin via RNA interference, thereby inhibiting interaction with the E8 domain of laminin, prevents myogenic differentiation [80], emphasizing the critical role that laminins play in directing MuSC function.
Fibronectin is another key component of the basal lamina that contributes to the regulation of MuSC function. In resting muscle, MuSCs reside in a close proximity to fibronectin-rich areas [81]. Indeed, MuSCs express syndecan-4 and Frizzled-7 co-receptor complexes that bind to fibronectin, where subsequent interactions between the complex and fibronectin promote symmetric division of MuSCs in the presence of Wnt7a [81]. Interestingly, fibronectin expression transiently increases with an onset of injury, and this transient expression induces Wnt7a signaling via syndecan-4 and Frizzled-7 co-receptor complex to expand and maintain the MuSC pool [81]. Furthermore, activated MuSCs gain β3 integrin expression, which interacts with the Arg-Gly-Asp (RGD) motif of fibronectin, and regulates myogenic differentiation and muscle regeneration [82–84]. Activated MuSCs also gain αV and α5 integrin expression [73], which complex with β3 and β1 integrin respectively to interact with the RGD motif of fibronectin. This data suggest that fibronectin is a key extracellular matrix protein involved in the orchestration of skeletal muscle regeneration.
Proteoglycans, such as perlecan, decorin, and biglycans, are also components of the basal lamina matrix [61]. While it remains to be determined whether proteoglycans directly regulate MuSC function, proteoglycans certainly modulate the MuSC niche in an indirect manner. For instance, perlecan-deficient mice exhibit muscle hypertrophy in a myostatin-dependent manner, suggesting that perlecan is crucial for maintaining muscle mass [85]. Furthermore, proteoglycans sequester important soluble growth factors to mediate MuSC function [62,63], such as insulin-like growth factor-1 (IGF-1), hepatocyte growth factor-1 (HGF-1), fibroblast growth factor-2 (FGF-2), vascular endothelial growth factor (VEGF), transforming growth factor beta (TGF- β), and platelet-derived growth factor (PDGF-1). Specifically, such sequestration of growth factors can control the local concentration by releasing the growth factors “on-demand” upon mechanical perturbation.
Inferior regenerative capacity and progression of sarcopenia in aged muscle are due to changes in the MuSC niche [86] and declines in intrinsic MuSC function [7] that occur with aging. A recent study demonstrated that the local microenvironmental stiffness (measured via Atomic Force Microscopy (AFM)) increases with aging [87]. The increased tissue stiffness is attributed to accumulation of advanced glycation end-products and collagen [87]. Ultimately, progressive accumulation of fibrotic matrix proteins leads to chronic fibrosis within the muscle, and results in impaired regeneration [7,89] and progression of sarcopenia [86]. In addition to increased fibrotic tissue accumulation, aged muscles also exhibit impaired expression of Notch ligand Delta on the sarcolemma [90], where Notch signaling regulates critical functions of MuSCs, and therefore, muscle regeneration [91,92]. Finally, systemic alterations in Wnt3a, oxytocin hormone, and IL-6 have also been suggested to impact the muscle regeneration capacity with aging [93–95]. Restoration and augmentation of key niche elements that change as a function of aging through provisional matrices may provide an effective strategy to reinstate the skeletal muscle function upon onset of injuries and age-associated pathology in the aged skeletal muscle.
Roles of inflammation and fibroadipogenic progenitor cells (FAPs) in skeletal muscle regeneration
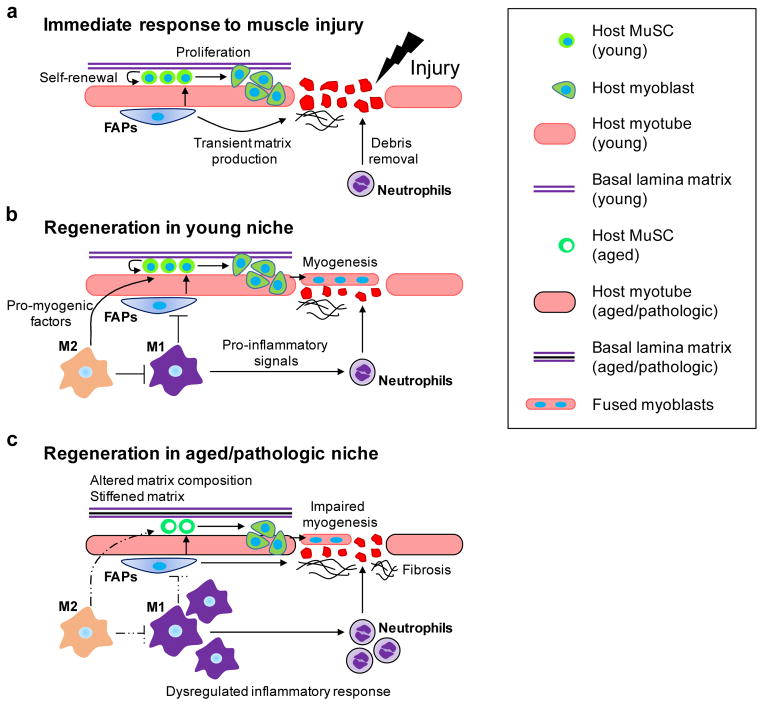
Inflammation is a critical process for adequate muscle regeneration following injury, which is characterized by massive infiltration of macrophages and neutrophils [96]. The initial phase of inflammation involves invasion of neutrophils and macrophages and M1 macrophage activation (Figure 1A and B) [96,97]. Neutrophils remove necrotic tissues and debris at the site of injury. Activated M1 macrophages secrete various pro-inflammatory signals, including IL-1β, IL-12, reactive oxygen species, nitric oxide, as well as insulin-like growth factor 1, which initiate MuSC activation and proliferation (Figure 1A) [96,98,99]. At this stage, fibroadipogenic progenitor cells (FAPs), resident myofibroblasts responsible for laying down transient extracellular matrix for myoblast proliferation and differentiation, are activated (Figure 1A) [18,89]. In rodent muscles, activated FAPs are cleared after ~96 hours post injury, where infiltrating macrophages induce FAP apoptosis via tumor necrosis factor α (TNF-α) [89]. Gradually, the pro-inflammatory M1 macrophages transition into constructive, wound-healing M2 macrophages that promote regeneration and resolution of inflammation through secretion of anti-inflammatory (e.g., IL-10, TGF- β), myogenic (e.g., IGF-1), and angiogenic (e.g., VEGF) factors (Figure 1B) [100–105].
Figure 1.
(a) Immediate response to muscle injury. Upon injury, quiescent muscle satellite cells (MuSCs) activate, self-renew, proliferate, and differentiate into myoblasts. Fibroadipogenic progenitors (FAPs) also activate, differentiate, and subsequently secrete transient extracellular matrix. Neutrophils infiltrate the site of injury and remove debris. (b) Regeneration in young niche. Pro-inflammatory M1 macrophages induce FAP apoptosis and recruit neutrophils. Anti-inflammatory M2 macrophages secrete anti-inflammatory, myogenic, and angiogenic factors. Myoblasts differentiate and fuse to form new muscle fibers. (c) Regeneration in aged/pathologic niche. MuSC niche composition is altered and results in stiffening. Normal inflammatory response is dysregulated, where inflammation persists over a longer period of time. FAPs over-secrete extracellular matrix, leading to progressive fibrosis. Myogenesis is impaired.
In the context of chronic muscle injury and aging, coordination of inflammatory cells, FAPs, and MuSCs is disrupted (Figure 1C). For instance, a recent study reported that regulatory T cell accumulation is significantly diminished in aged muscle, which subsequently impacts the activity of IL-33-expressing FAPs [106]. In muscles with chronic injury, apoptosis of FAPs is dysregulated due to elevated levels of TGF-β-producing macrophages, resulting in fibrotic degeneration of the muscle (Figure 1C) [89]. This further highlights that inflammation plays an important role in regulating the MuSC niche, which ultimately governs the regenerative outcome.
Delivery matrices for MuSC engraftment and function
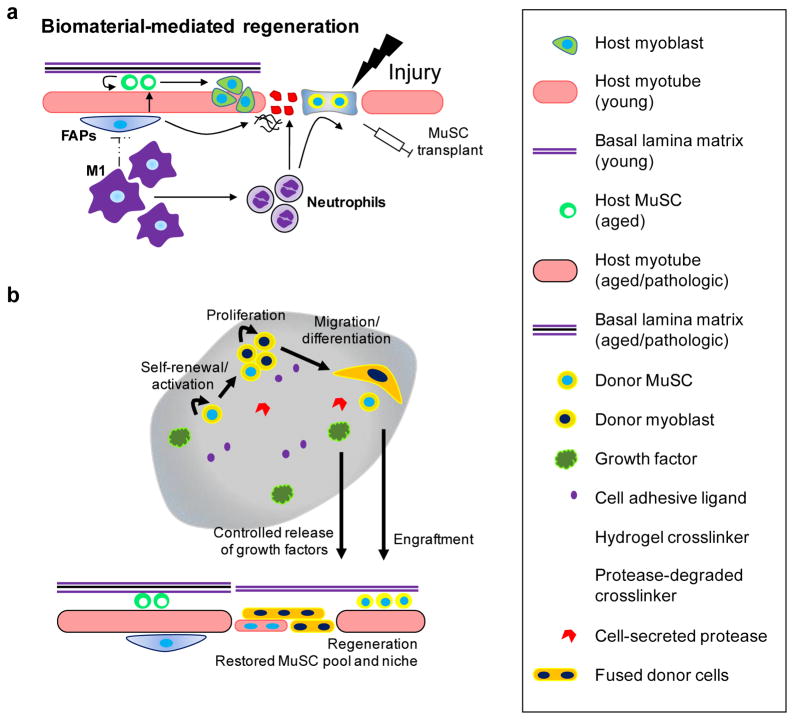
Delivery of Pax7+ MuSCs is an effective strategy to stimulate and accelerate skeletal muscle regeneration, and therefore, to treat muscle injuries [14–16]. However, direct delivery of MuSCs via bolus injection exposes the transplanted cells to a harsh inflammatory environment and compromises their long term viability and function. Furthermore, transplanted MuSCs exhibit limited migration in vivo and thus rescue only a partial muscle volume, significantly limiting their therapeutic efficiency. A potential solution to overcome these hurdles is utilization of delivery matrices that provide protection and guide MuSC function (Figure 2a). In particular, hydrogels exhibit numerous advantageous properties as protein and cell delivery vehicles. For example, hydrogels can be injected in a minimally invasive manner, allow efficient nutrient exchange, and can be tuned to incorporate desirable structural, biophysical, and biochemical cues. Furthermore, such cell-delivery platforms also provide an opportunity to spatiotemporally present important MuSC niche elements that represent the asymmetry of matrix and adhesive components that modulate MuSC function, as MuSCs are exposed to biphasic interactions on the basal side of the matrix and on the apical side of muscle fiber sarcolemma. In this section, hydrogel-based biomaterials, both natural and synthetic, that have been developed to deliver skeletal muscle cells will be reviewed.
Figure 2.
(a) Biomaterial-mediated regeneration. Upon injury, quiescent muscle satellite cells are delivered within a biomaterial. Biomaterial encapsulation provides initial protection of the transplanted cells from inflammation. (b) Biomaterial promotes cell survival by providing integrin attachment sites. Donor cells within the biomaterial activate, self-renew, proliferate, migrate, and differentiate to induce host muscle regeneration. Encapsulated growth factor is released in a controlled manner, as the biomaterial is degraded by the cell-secreted proteases.
Collagen is a naturally derived material that has been used to examine the function of myoblasts. Collagen gels are bioactive, where cells can recognize inherently available cell-binding ligands. Indeed, encapsulating C2C12 or primary skeletal myoblasts in collagen gels promotes proliferation, differentiation, and fusion to form multi-nucleated myotubes in vitro [107–111]. Studies using collagen gels have set an important foundation for studying skeletal muscle cell function and early muscle development in a 3D context. However, there are several limitations associated with collagen gels to be used as a cell delivery vehicle. One limitation is that the major component of the MuSC niche is laminin [65,66], and that MuSC express laminin-specific integrins [67,68,80]. Indeed, culturing MuSCs in Matrigel, solubilized basement membrane protein rich in laminin, led to higher number of Pax7+ and MyoD+ cells than in collagen gels [112]. Furthermore, differentiated cells in laminin-rich Matrigel also resulted in more and larger MyoG+ myotubes than in collagen gels [112], highlighting the importance of the MuSC interaction with the underlying matrix. Here, optimizing the material interaction with MuSCs, rather than myoblasts, is important in the stem cell therapy context, because delivery of myoblasts results in suboptimal engraftment over time compared to MuSCs [15,113]. In particular, replenishing quiescent and self-renewing pool of MuSCs through biomaterials would be important for subsequent rounds of regeneration. Another drawback of using collagen gels is inability to modulate cell adhesive ligands and spatiotemporally present them to both the encapsulated and endogenous cells, which may be powerful tools to precisely control MuSCs.
Alginate is a natural biomaterial derived from brown algae. Alginate gels can be chemically modified to incorporate cell adhesive ligands and to exhibit varying range of stiffness and degradability [114]. Furthermore, due to their cytocompatibility and low toxicity, these materials have been extensively used in the biomedical applications [114]. For example, encapsulated myoblasts in RGD-conjugated alginate gels can proliferate, migrate and fuse into multi-nucleated myotubes in a ligand density-dependent manner in vitro [84,115,116]. Here, outward migration rate of the cells was largely dependent on the structural characteristics of the hydrogel [116]. Furthermore, co-delivery of myoblasts with HGF/FGF2 or VEGF/IGF in RGD-containing alginate gels to injured muscles accelerated regeneration with enhanced muscle mass and contractile function compared to delivering the cells alone [117–119]. It is also important to note that these studies utilized primary myoblasts that have been expanded on tissue culture dishes. Culturing muscle progenitor cells on stiff tissue culture plastics typically abolishes their stemness and results in inefficient engraftment [15,20], and therefore, it is not yet clear how these systems would perform if MuSCs were delivered to the injured muscles. Nonetheless, these investigations have demonstrated that delivery of growth factors in an injury-dependent context (e.g., VEGF/IGF for ischemic/myotoxin injury and HGF/FGF2 for laceration injury) and presentation of cell-adhesive ligands, such as RGD, are effective strategies for augmenting skeletal muscle regeneration.
Hyaluronic acid (HA), an anionic, non-sulfated glycosaminoglycan component of the extracellular matrix, is compatible with myoblasts, suggesting HA gels as a potential vehicle for MuSC delivery [120]. Indeed, delivery of MuSC via UV-crosslinked HA hydrogels to surgically ablated rodent muscles results in enhanced cell engraftment and functional muscle regeneration compared to delivery of myoblasts in HA hydrogel or hydrogel alone [113]. It is important to note that UV-mediated crosslinking is limited due to light scattering and attenuation in biological tissues from a translational perspective. Furthermore, despite HA’s cytocompatibility, most HA-based hydrogels are subject to degradation by hyaluronidases in vivo, where modulation of HA degradation may compromise the desired structural and mechanical properties of the HA hydrogels without proper chemical modification. While the effects of material degradation rate on skeletal muscle regeneration have not been evaluated, it is likely that premature and uncontrolled degradation may expose donor cells to neutrophils and macrophages post-injury, where the inflammation profiles may change with aging and pathologic degeneration. Furthermore, uncontrolled material degradation may induce premature donor cell anoikis - apoptosis induced from loss of cellular attachment to the material [121].
In the context of skeletal muscle engineering, fibrin gels created from fibrinogen and thrombin also have been explored [122–124]. Due to relatively rapid polymerization process and tunabilty of network structure, fibrin has been used in various biomedical applications, including skeletal muscle tissue engineering [125,126]. For instance, delivery of primary myoblasts to the rat skeletal muscle using fibrin gel results in donor cells fusing with the host muscle in a time-dependent and localized manner [122]. Furthermore, cultured myoblast/fibrin constructs can generate contractile force when electrically stimulated [123]. More recently, human muscle cell-seeded fibrin microthreads delivered to injured muscles of immuno-compromised mice induced skeletal muscle regeneration [124]. However, fibrin gels are not without their limitations. One major drawback is that fibrin gels formed at high concentrations exhibit extremely dense microstructure, where cellular infiltration and migratory behavior, as well as nutrient exchange, would be severely obstructed [126]. At low concentrations, fibrin gels become soft and rapidly lose their mechanical integrity. To overcome this limitation, a recent study incorporated gelatin beads into fibrin gels, where subsequent dissolution introduced micron-sized pores [127]. Introduction of macropores within the construct led to improved cell viability and myogenic differentiation of human umbilical cord mesenchymal stem cells [127], demonstrating the importance of material porosity on the cellular function and regenerative outcome. Similar to collagen gels, fibrin gels also contain numerous cell-binding sites for integrins [126], which is another limitation if a systematic approach to build provisional matrix for MuSCs is desired.
Poly(ethylene glycol) (PEG)-based synthetic hydrogels are highly cytocompatible, easy to manipulate chemically in order to alter structure, mechanical properties and the presentation of bioactive molecules, and exhibit non-fouling properties, and thus have been used extensively in biomedical applications. Synthetic materials, such as PEG, also are more advantageous than many naturally-derived materials due to diminished risk of pathogen transmission and lot-to-lot variability. Recently, a number of investigations have utilized PEG-based scaffolds (PEG-fibrinogen constructs) to deliver skeletal muscle-derived pericytes [128] and mesoangioblasts [129,130], and demonstrated that cell delivery in such materials promotes myogenic differentiation and skeletal muscle regeneration. Furthermore, controlled delivery of IGF-1 and SDF-1 using PEG-fibrin hydrogels also contributes to skeletal muscle regeneration [131,132]. In order to gain more precise control over building the cellular niche, PEG hydrogels that utilize maleimide crosslinking reaction chemistry also have been explored to culture C2C12 myoblasts in 3D [83]. Here, maleimide reactive groups enable conjugation of desired peptides (e.g., RGD and protease-degradable crosslinking peptides) to the PEG backbone in a highly specific manner. By systematically modulating the characteristics of this modular hydrogel system, myoblast viability and differentiation were found to be dependent on cell seeding density, polymer density, and bioadhesive ligands in 3D culture [83]. The resulting construct containing differentiated myoblasts also exhibited bulk contraction in vitro [83]. Further development and application of MuSC niche-containing synthetic biomaterials remains to be investigated.
Outlook
Recent advances in biomaterials demonstrate that engineered provisional matrices for MuSC may be a promising solution to improve long term regenerative outcome of stem cell therapy of muscle injuries and disorders. While the majority of previous investigations have focused on developing biomaterials for primary myoblasts, the C2C12 myoblast cell line, and other muscle-relevant cells (e.g., pericytes), biomaterials engineered to mimic the MuSC niche remain to be developed. Ideally, transplantation of MuSCs in such engineered biomaterial systems would protect the donor cells from the harsh inflammatory cells and promote cell survival, proliferation and migration by providing integrin attachment sites (Figure 2A). As inflammation resolves over time, the engineered matrix should provide biochemical (e.g., cell adhesive ligands, growth factors) and biophysical (e.g., matrix stiffness) cues necessary for the donor MuSCs to activate, self-renew and proliferate, differentiation, and eventually migrate out towards the sites of injury (Figure 2B). In particular, identification of niche-relevant cell-adhesive ligands (e.g., cell-binding peptides found in laminin, fibronectin, and collagen), integrins/receptors, as well as optimal ligand density that are necessary for MuSC survival and migration will be crucial for developing biomaterial-based approaches to overcome limitations associated with MuSC transplantation. Furthermore, co-delivery of growth factors, such as FGF-2, IGF-1, VEGF, and HGF-1, and other support cell types, such as FAPs, to promote survival, proliferation, differentiation, and migratory behaviors of the donor MuSCs needs to be investigated.
Biomaterial-based cell therapy approaches provide promising opportunities to spatiotemporally present ligands and growth factors to the transplanted cells. In the context of skeletal muscle regeneration, temporal coordination of MuSC expansion, commitment to myogenic progenitors, and differentiation is critical for biomaterial-mediated tissue repair. One possible approach would be to covalently functionalize a combination of caged- (i.e., contains protective chemical group that renders adhesive peptide inactive) and uncaged/active cell-adhesive peptides to the matrices [133]. For example, donor cells could be instructed to proliferate in the presence of uncaged/active α7β1-binding peptides, but directed to myogenic lineage and differentiate upon uncaging αvβ3-binding peptides [82], such as RGD. Presentation of spatially asymmetric provisional matrix, which mimic the physiologic MuSC niche consisting of basal lamina and myofibers-associated components, to the transplanted MuSCs may also be a productive strategy to improve the regenerative outcome. Furthermore, donor cells may also be co-delivered with multiple growth factors that release at varying rates for more effective therapeutic outcome. For instance, factors known to promote MuSC expansion at early time point upon transplantation, such as FGF2 or Prmt5 [134,135], and factors that promote vascularization, such as VEGF [136], can be incorporated into the system to be released at specific rates via a number of strategies, including direct non-covalent encapsulation in the bulk material, encapsulation in microspheres (sustained/delayed release), and covalently immobilizing (continuous/on-demand release) to the material [137]. For this strategy, material-specific control of growth factor release rate will be crucial for successful therapeutic outcomes.
Incorporating cell-demanded, protease-degradable cross-linkers that degrade the material in complement to hydrolytic degradation may also be an important consideration for accelerated MuSC engraftment and tissue regeneration (Figure 2B). Indeed, several types of matrix metalloproteinases (MMPs)-degradable cross-linkers have been previously implemented in synthetic matrices [138,139], where cell-secreted MMPs control the degradation of the material during proliferation, migration, and extracellular matrix synthesis. By using a combination of one or more cross-linkers that exhibit different degree of MMP-sensitivity, the rate of biomaterial degradation and matrix deposition may be more finely balanced, and ultimately enhance tissue regeneration.
Finally, development of engineered provisional matrices for MuSCs may also serve as a powerful tool for elucidating the interactions of MuSC and components of their niche in 3D. Such findings would not only be significant at the basic science level, but also would be critical for informing the development pathology-specific (e.g., traumatic injury, age-associated degeneration, muscular dystrophies) biomaterial-based therapies.
Highlights.
Regenerative capacity of skeletal muscle declines with aging.
Satellite cell delivery enhances regeneration in aging muscles.
Direct delivery of satellite cells presents challenges.
Advances in satellite cell and their niche biology are reviewed.
Advances engineered cell delivery matrices for skeletal muscle are reviewed.
Acknowledgments
This work was funded by National Institutes of Health (NIH) grants R01-AR062368 and R01-AR062920 and the Petit Institute for Bioengineering and Bioscience seed grant program. This research was conducted while Woojin M. Han was a Glenn/AFAR Postdoctoral Fellow.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Järvinen TA, Järvinen M, Kalimo H. Regeneration of injured skeletal muscle after the injury. Muscles Ligaments Tendons J. 2013;3:337–345. [PMC free article] [PubMed] [Google Scholar]
- 2.Järvinen TAH, Järvinen TLN, Kääriäinen M, Kalimo H, Järvinen M. Muscle injuries: biology and treatment. Am J Sports Med. 2005;33:745–764. doi: 10.1177/0363546505274714. [DOI] [PubMed] [Google Scholar]
- 3.Turner NJ, Badylak SF. Regeneration of skeletal muscle. Cell Tissue Res. 2012;347:759–774. doi: 10.1007/s00441-011-1185-7. [DOI] [PubMed] [Google Scholar]
- 4.Ryall JG, Schertzer JD, Lynch GS. Cellular and molecular mechanisms underlying age-related skeletal muscle wasting and weakness. Biogerontology. 2008;9:213–228. doi: 10.1007/s10522-008-9131-0. [DOI] [PubMed] [Google Scholar]
- 5.Carlson BM, Faulkner JA. Muscle transplantation between young and old rats: age of host determines recovery. Am J Physiol. 1989;256:C1262–1266. doi: 10.1152/ajpcell.1989.256.6.C1262. [DOI] [PubMed] [Google Scholar]
- 6.Carosio S, Berardinelli MG, Aucello M, Musarò A. Impact of ageing on muscle cell regeneration. Ageing Res Rev. 2011;10:35–42. doi: 10.1016/j.arr.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 7.Blau HM, Cosgrove BD, Ho ATV. The central role of muscle stem cells in regenerative failure with aging. Nat Med. 2015;21:854–862. doi: 10.1038/nm.3918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Janssen I, Shepard DS, Katzmarzyk PT, Roubenoff R. The healthcare costs of sarcopenia in the United States. J Am Geriatr Soc. 2004;52:80–85. doi: 10.1111/j.1532-5415.2004.52014.x. [DOI] [PubMed] [Google Scholar]
- 9.Beaudart C, Rizzoli R, Bruyère O, Reginster J-Y, Biver E. Sarcopenia: burden and challenges for public health. Arch Public Health Arch Belg Santé Publique. 2014;72:45. doi: 10.1186/2049-3258-72-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Corso P, Finkelstein E, Miller T, Fiebelkorn I, Zaloshnja E. Incidence and lifetime costs of injuries in the United States. Inj Prev J Int Soc Child Adolesc Inj Prev. 2006;12:212–218. doi: 10.1136/ip.2005.010983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yin H, Price F, Rudnicki MA. Satellite cells and the muscle stem cell niche. Physiol Rev. 2013;93:23–67. doi: 10.1152/physrev.00043.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Relaix F, Zammit PS. Satellite cells are essential for skeletal muscle regeneration: the cell on the edge returns centre stage. Dev Camb Engl. 2012;139:2845–2856. doi: 10.1242/dev.069088. [DOI] [PubMed] [Google Scholar]
- 13.Dumont NA, Wang YX, Rudnicki MA. Intrinsic and extrinsic mechanisms regulating satellite cell function. Dev Camb Engl. 2015;142:1572–1581. doi: 10.1242/dev.114223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Montarras D, Morgan J, Collins C, Relaix F, Zaffran S, Cumano A, Partridge T, Buckingham M. Direct isolation of satellite cells for skeletal muscle regeneration. Science. 2005;309:2064–2067. doi: 10.1126/science.1114758. [DOI] [PubMed] [Google Scholar]
- 15.Sacco A, Doyonnas R, Kraft P, Vitorovic S, Blau HM. Self-renewal and expansion of single transplanted muscle stem cells. Nature. 2008;456:502–506. doi: 10.1038/nature07384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cerletti M, Jurga S, Witczak CA, Hirshman MF, Shadrach JL, Goodyear LJ, Wagers AJ. Highly efficient, functional engraftment of skeletal muscle stem cells in dystrophic muscles. Cell. 2008;134:37–47. doi: 10.1016/j.cell.2008.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morrissey JB, Cheng RY, Davoudi S, Gilbert PM. Biomechanical Origins of Muscle Stem Cell Signal Transduction. J Mol Biol. 2015 doi: 10.1016/j.jmb.2015.05.004. [DOI] [PubMed] [Google Scholar]
- 18.Joe AWB, Yi L, Natarajan A, Le Grand F, So L, Wang J, Rudnicki MA, Rossi FMV. Muscle injury activates resident fibro/adipogenic progenitors that facilitate myogenesis. Nat Cell Biol. 2010;12:153–163. doi: 10.1038/ncb2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chazaud B, Brigitte M, Yacoub-Youssef H, Arnold L, Gherardi R, Sonnet C, Lafuste P, Chretien F. Dual and beneficial roles of macrophages during skeletal muscle regeneration. Exerc Sport Sci Rev. 2009;37:18–22. doi: 10.1097/JES.0b013e318190ebdb. [DOI] [PubMed] [Google Scholar]
- 20.Gilbert PM, Havenstrite KL, Magnusson KEG, Sacco A, Leonardi NA, Kraft P, Nguyen NK, Thrun S, Lutolf MP, Blau HM. Substrate elasticity regulates skeletal muscle stem cell self-renewal in culture. Science. 2010;329:1078–1081. doi: 10.1126/science.1191035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Charville GW, Cheung TH, Yoo B, Santos PJ, Lee GK, Shrager JB, Rando TA. Ex Vivo Expansion and In Vivo Self-Renewal of Human Muscle Stem Cells. Stem Cell Rep. 2015;5:621–632. doi: 10.1016/j.stemcr.2015.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guérette B, Asselin I, Vilquin JT, Roy R, Tremblay JP. Lymphocyte infiltration following allo- and xenomyoblast transplantation in mdx mice. Muscle Nerve. 1995;18:39–51. doi: 10.1002/mus.880180107. [DOI] [PubMed] [Google Scholar]
- 23.Liu W, Wei-LaPierre L, Klose A, Dirksen RT, Chakkalakal JV. Inducible depletion of adult skeletal muscle stem cells impairs the regeneration of neuromuscular junctions. eLife. 2015;4 doi: 10.7554/eLife.09221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pannérec A, Springer M, Migliavacca E, Ireland A, Piasecki M, Karaz S, Jacot G, Métairon S, Danenberg E, Raymond F, Descombes P, McPhee JS, Feige JN. A robust neuromuscular system protects rat and human skeletal muscle from sarcopenia. Aging. 2016 doi: 10.18632/aging.100926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Qazi TH, Mooney DJ, Pumberger M, Geissler S, Duda GN. Biomaterials based strategies for skeletal muscle tissue engineering: existing technologies and future trends. Biomaterials. 2015;53:502–521. doi: 10.1016/j.biomaterials.2015.02.110. [DOI] [PubMed] [Google Scholar]
- 26.Juhas M, Ye J, Bursac N. Design, Evaluation, and Application of Engineered Skeletal Muscle. Methods San Diego Calif. 2015 doi: 10.1016/j.ymeth.2015.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mauro A. Satellite cell of skeletal muscle fibers. J Biophys Biochem Cytol. 1961;9:493–495. doi: 10.1083/jcb.9.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Seale P, Sabourin LA, Girgis-Gabardo A, Mansouri A, Gruss P, Rudnicki MA. Pax7 is required for the specification of myogenic satellite cells. Cell. 2000;102:777–786. doi: 10.1016/s0092-8674(00)00066-0. [DOI] [PubMed] [Google Scholar]
- 29.Lepper C, Partridge TA, Fan C-M. An absolute requirement for Pax7-positive satellite cells in acute injury-induced skeletal muscle regeneration. Dev Camb Engl. 2011;138:3639–3646. doi: 10.1242/dev.067595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sambasivan R, Yao R, Kissenpfennig A, Van Wittenberghe L, Paldi A, Gayraud-Morel B, Guenou H, Malissen B, Tajbakhsh S, Galy A. Pax7-expressing satellite cells are indispensable for adult skeletal muscle regeneration. Dev Camb Engl. 2011;138:3647–3656. doi: 10.1242/dev.067587. [DOI] [PubMed] [Google Scholar]
- 31.von Maltzahn J, Jones AE, Parks RJ, Rudnicki MA. Pax7 is critical for the normal function of satellite cells in adult skeletal muscle. Proc Natl Acad Sci U S A. 2013;110:16474–16479. doi: 10.1073/pnas.1307680110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cheung TH, Quach NL, Charville GW, Liu L, Park L, Edalati A, Yoo B, Hoang P, Rando TA. Maintenance of muscle stem-cell quiescence by microRNA-489. Nature. 2012;482:524–528. doi: 10.1038/nature10834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zismanov V, Chichkov V, Colangelo V, Jamet S, Wang S, Syme A, Koromilas AE, Crist C. Phosphorylation of eIF2α Is a Translational Control Mechanism Regulating Muscle Stem Cell Quiescence and Self-Renewal. Cell Stem Cell. 2016;18:79–90. doi: 10.1016/j.stem.2015.09.020. [DOI] [PubMed] [Google Scholar]
- 34.Abou-Khalil R, Le Grand F, Pallafacchina G, Valable S, Authier F-J, Rudnicki MA, Gherardi RK, Germain S, Chretien F, Sotiropoulos A, Lafuste P, Montarras D, Chazaud B. Autocrine and paracrine angiopoietin 1/Tie-2 signaling promotes muscle satellite cell self-renewal. Cell Stem Cell. 2009;5:298–309. doi: 10.1016/j.stem.2009.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Juan AH, Derfoul A, Feng X, Ryall JG, Dell’Orso S, Pasut A, Zare H, Simone JM, Rudnicki MA, Sartorelli V. Polycomb EZH2 controls self-renewal and safeguards the transcriptional identity of skeletal muscle stem cells. Genes Dev. 2011;25:789–794. doi: 10.1101/gad.2027911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.García-Prat L, Martínez-Vicente M, Perdiguero E, Ortet L, Rodríguez-Ubreva J, Rebollo E, Ruiz-Bonilla V, Gutarra S, Ballestar E, Serrano AL, Sandri M, Muñoz-Cánoves P. Autophagy maintains stemness by preventing senescence. Nature. 2016;529:37–42. doi: 10.1038/nature16187. [DOI] [PubMed] [Google Scholar]
- 37.Kuang S, Kuroda K, Le Grand F, Rudnicki MA. Asymmetric self-renewal and commitment of satellite stem cells in muscle. Cell. 2007;129:999–1010. doi: 10.1016/j.cell.2007.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kawabe Y-I, Wang YX, McKinnell IW, Bedford MT, Rudnicki MA. Carm1 regulates Pax7 transcriptional activity through MLL1/2 recruitment during asymmetric satellite stem cell divisions. Cell Stem Cell. 2012;11:333–345. doi: 10.1016/j.stem.2012.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rocheteau P, Gayraud-Morel B, Siegl-Cachedenier I, Blasco MA, Tajbakhsh S. A subpopulation of adult skeletal muscle stem cells retains all template DNA strands after cell division. Cell. 2012;148:112–125. doi: 10.1016/j.cell.2011.11.049. [DOI] [PubMed] [Google Scholar]
- 40.Günther S, Kim J, Kostin S, Lepper C, Fan C-M, Braun T. Myf5-positive satellite cells contribute to Pax7-dependent long-term maintenance of adult muscle stem cells. Cell Stem Cell. 2013;13:590–601. doi: 10.1016/j.stem.2013.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tierney MT, Aydogdu T, Sala D, Malecova B, Gatto S, Puri PL, Latella L, Sacco A. STAT3 signaling controls satellite cell expansion and skeletal muscle repair. Nat Med. 2014;20:1182–1186. doi: 10.1038/nm.3656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Boonsanay V, Zhang T, Georgieva A, Kostin S, Qi H, Yuan X, Zhou Y, Braun T. Regulation of Skeletal Muscle Stem Cell Quiescence by Suv4-20h1-Dependent Facultative Heterochromatin Formation. Cell Stem Cell. 2016;18:229–242. doi: 10.1016/j.stem.2015.11.002. [DOI] [PubMed] [Google Scholar]
- 43.Ryall JG, Dell’Orso S, Derfoul A, Juan A, Zare H, Feng X, Clermont D, Koulnis M, Gutierrez-Cruz G, Fulco M, Sartorelli V. The NAD(+)-dependent SIRT1 deacetylase translates a metabolic switch into regulatory epigenetics in skeletal muscle stem cells. Cell Stem Cell. 2015;16:171–183. doi: 10.1016/j.stem.2014.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bentzinger CF, von Maltzahn J, Dumont NA, Stark DA, Wang YX, Nhan K, Frenette J, Cornelison DDW, Rudnicki MA. Wnt7a stimulates myogenic stem cell motility and engraftment resulting in improved muscle strength. J Cell Biol. 2014;205:97–111. doi: 10.1083/jcb.201310035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Otto A, Collins-Hooper H, Patel A, Dash PR, Patel K. Adult skeletal muscle stem cell migration is mediated by a blebbing/amoeboid mechanism. Rejuvenation Res. 2011;14:249–260. doi: 10.1089/rej.2010.1151. [DOI] [PubMed] [Google Scholar]
- 46.Webster MT, Manor U, Lippincott-Schwartz J, Fan C-M. Intravital Imaging Reveals Ghost Fibers as Architectural Units Guiding Myogenic Progenitors during Regeneration. Cell Stem Cell. 2016;18:243–252. doi: 10.1016/j.stem.2015.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Olguin HC, Yang Z, Tapscott SJ, Olwin BB. Reciprocal inhibition between Pax7 and muscle regulatory factors modulates myogenic cell fate determination. J Cell Biol. 2007;177:769–779. doi: 10.1083/jcb.200608122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Palacios D, Mozzetta C, Consalvi S, Caretti G, Saccone V, Proserpio V, Marquez VE, Valente S, Mai A, Forcales SV, Sartorelli V, Puri PL. TNF/p38α/polycomb signaling to Pax7 locus in satellite cells links inflammation to the epigenetic control of muscle regeneration. Cell Stem Cell. 2010;7:455–469. doi: 10.1016/j.stem.2010.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bröhl D, Vasyutina E, Czajkowski MT, Griger J, Rassek C, Rahn H-P, Purfürst B, Wende H, Birchmeier C. Colonization of the satellite cell niche by skeletal muscle progenitor cells depends on Notch signals. Dev Cell. 2012;23:469–481. doi: 10.1016/j.devcel.2012.07.014. [DOI] [PubMed] [Google Scholar]
- 50.Millay DP, O’Rourke JR, Sutherland LB, Bezprozvannaya S, Shelton JM, Bassel-Duby R, Olson EN. Myomaker is a membrane activator of myoblast fusion and muscle formation. Nature. 2013;499:301–305. doi: 10.1038/nature12343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sousa-Victor P, García-Prat L, Serrano AL, Perdiguero E, Muñoz-Cánoves P. Muscle stem cell aging: regulation and rejuvenation. Trends Endocrinol Metab TEM. 2015;26:287–296. doi: 10.1016/j.tem.2015.03.006. [DOI] [PubMed] [Google Scholar]
- 52.Sousa-Victor P, Muñoz-Cánoves P. Regenerative decline of stem cells in sarcopenia. Mol Aspects Med. 2016 doi: 10.1016/j.mam.2016.02.002. [DOI] [PubMed] [Google Scholar]
- 53.Cosgrove BD, Gilbert PM, Porpiglia E, Mourkioti F, Lee SP, Corbel SY, Llewellyn ME, Delp SL, Blau HM. Rejuvenation of the muscle stem cell population restores strength to injured aged muscles. Nat Med. 2014;20:255–264. doi: 10.1038/nm.3464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sinha M, Jang YC, Oh J, Khong D, Wu EY, Manohar R, Miller C, Regalado SG, Loffredo FS, Pancoast JR, Hirshman MF, Lebowitz J, Shadrach JL, Cerletti M, Kim M-J, Serwold T, Goodyear LJ, Rosner B, Lee RT, Wagers AJ. Restoring systemic GDF11 levels reverses age-related dysfunction in mouse skeletal muscle. Science. 2014;344:649–652. doi: 10.1126/science.1251152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liu L, Cheung TH, Charville GW, Hurgo BMC, Leavitt T, Shih J, Brunet A, Rando TA. Chromatin Modifications as Determinants of Muscle Stem Cell Quiescence and Chronological Aging. Cell Rep. 2013;4:189–204. doi: 10.1016/j.celrep.2013.05.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sousa-Victor P, Gutarra S, García-Prat L, Rodriguez-Ubreva J, Ortet L, Ruiz-Bonilla V, Jardí M, Ballestar E, González S, Serrano AL, Perdiguero E, Muñoz-Cánoves P. Geriatric muscle stem cells switch reversible quiescence into senescence. Nature. 2014;506:316–321. doi: 10.1038/nature13013. [DOI] [PubMed] [Google Scholar]
- 57.Price FD, von Maltzahn J, Bentzinger CF, Dumont NA, Yin H, Chang NC, Wilson DH, Frenette J, Rudnicki MA. Inhibition of JAK-STAT signaling stimulates adult satellite cell function. Nat Med. 2014;20:1174–1181. doi: 10.1038/nm.3655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hall JK, Banks GB, Chamberlain JS, Olwin BB. Prevention of muscle aging by myofiber-associated satellite cell transplantation. Sci Transl Med. 2010;2:57ra83. doi: 10.1126/scitranslmed.3001081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Darabi R, Arpke RW, Irion S, Dimos JT, Grskovic M, Kyba M, Perlingeiro RCR. Human ES- and iPS-derived myogenic progenitors restore DYSTROPHIN and improve contractility upon transplantation in dystrophic mice. Cell Stem Cell. 2012;10:610–619. doi: 10.1016/j.stem.2012.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tierney MT, Gromova A, Sesillo FB, Sala D, Spenlé C, Orend G, Sacco A. Autonomous Extracellular Matrix Remodeling Controls a Progressive Adaptation in Muscle Stem Cell Regenerative Capacity during Development. Cell Rep. 2016;14:1940–1952. doi: 10.1016/j.celrep.2016.01.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Thomas K, Engler AJ, Meyer GA. Extracellular matrix regulation in the muscle satellite cell niche. Connect Tissue Res. 2015;56:1–8. doi: 10.3109/03008207.2014.947369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Macri L, Silverstein D, Clark RAF. Growth factor binding to the pericellular matrix and its importance in tissue engineering. Adv Drug Deliv Rev. 2007;59:1366–1381. doi: 10.1016/j.addr.2007.08.015. [DOI] [PubMed] [Google Scholar]
- 63.Schultz GS, Wysocki A. Interactions between extracellular matrix and growth factors in wound healing. Wound Repair Regen Off Publ Wound Heal Soc Eur Tissue Repair Soc. 2009;17:153–162. doi: 10.1111/j.1524-475X.2009.00466.x. [DOI] [PubMed] [Google Scholar]
- 64.Urciuolo A, Quarta M, Morbidoni V, Gattazzo F, Molon S, Grumati P, Montemurro F, Tedesco FS, Blaauw B, Cossu G, Vozzi G, Rando TA, Bonaldo P. Collagen VI regulates satellite cell self-renewal and muscle regeneration. Nat Commun. 2013;4:1964. doi: 10.1038/ncomms2964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sanes JR. Laminin, fibronectin, and collagen in synaptic and extrasynaptic portions of muscle fiber basement membrane. J Cell Biol. 1982;93:442–451. doi: 10.1083/jcb.93.2.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dickson G, Azad A, Morris GE, Simon H, Noursadeghi M, Walsh FS. Co-localization and molecular association of dystrophin with laminin at the surface of mouse and human myotubes. J Cell Sci. 1992;103(Pt 4):1223–1233. doi: 10.1242/jcs.103.4.1223. [DOI] [PubMed] [Google Scholar]
- 67.George-Weinstein M, Foster RF, Gerhart JV, Kaufman SJ. In vitro and in vivo expression of alpha 7 integrin and desmin define the primary and secondary myogenic lineages. Dev Biol. 1993;156:209–229. doi: 10.1006/dbio.1993.1071. [DOI] [PubMed] [Google Scholar]
- 68.Collo G, Starr L, Quaranta V. A new isoform of the laminin receptor integrin alpha 7 beta 1 is developmentally regulated in skeletal muscle. J Biol Chem. 1993;268:19019–19024. [PubMed] [Google Scholar]
- 69.Kramer RH, Vu MP, Cheng YF, Ramos DM, Timpl R, Waleh N. Laminin-binding integrin alpha 7 beta 1: functional characterization and expression in normal and malignant melanocytes. Cell Regul. 1991;2:805–817. doi: 10.1091/mbc.2.10.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yao CC, Ziober BL, Sutherland AE, Mendrick DL, Kramer RH. Laminins promote the locomotion of skeletal myoblasts via the alpha 7 integrin receptor. J Cell Sci. 1996;109(Pt 13):3139–3150. doi: 10.1242/jcs.109.13.3139. [DOI] [PubMed] [Google Scholar]
- 71.Yao CC, Ziober BL, Squillace RM, Kramer RH. Alpha7 integrin mediates cell adhesion and migration on specific laminin isoforms. J Biol Chem. 1996;271:25598–25603. doi: 10.1074/jbc.271.41.25598. [DOI] [PubMed] [Google Scholar]
- 72.Liu J, Burkin DJ, Kaufman SJ. Increasing alpha 7 beta 1-integrin promotes muscle cell proliferation, adhesion, and resistance to apoptosis without changing gene expression. Am J Physiol Cell Physiol. 2008;294:C627–640. doi: 10.1152/ajpcell.00329.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Siegel AL, Atchison K, Fisher KE, Davis GE, Cornelison DDW. 3D timelapse analysis of muscle satellite cell motility. Stem Cells Dayt Ohio. 2009;27:2527–2538. doi: 10.1002/stem.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Boppart MD, Burkin DJ, Kaufman SJ. Activation of AKT signaling promotes cell growth and survival in α7β1 integrin-mediated alleviation of muscular dystrophy. Biochim Biophys Acta. 2011;1812:439–446. doi: 10.1016/j.bbadis.2011.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Burkin DJ, Wallace GQ, Nicol KJ, Kaufman DJ, Kaufman SJ. Enhanced expression of the alpha 7 beta 1 integrin reduces muscular dystrophy and restores viability in dystrophic mice. J Cell Biol. 2001;152:1207–1218. doi: 10.1083/jcb.152.6.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rooney JE, Gurpur PB, Yablonka-Reuveni Z, Burkin DJ. Laminin-111 restores regenerative capacity in a mouse model for alpha7 integrin congenital myopathy. Am J Pathol. 2009;174:256–264. doi: 10.2353/ajpath.2009.080522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Van Ry PM, Minogue P, Hodges BL, Burkin DJ. Laminin-111 improves muscle repair in a mouse model of merosin-deficient congenital muscular dystrophy. Hum Mol Genet. 2014;23:383–396. doi: 10.1093/hmg/ddt428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zou K, De Lisio M, Huntsman HD, Pincu Y, Mahmassani Z, Miller M, Olatunbosun D, Jensen T, Boppart MD. Laminin-111 improves skeletal muscle stem cell quantity and function following eccentric exercise. Stem Cells Transl Med. 2014;3:1013–1022. doi: 10.5966/sctm.2014-0044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cohn RD, Henry MD, Michele DE, Barresi R, Saito F, Moore SA, Flanagan JD, Skwarchuk MW, Robbins ME, Mendell JR, Williamson RA, Campbell KP. Disruption of DAG1 in differentiated skeletal muscle reveals a role for dystroglycan in muscle regeneration. Cell. 2002;110:639–648. doi: 10.1016/s0092-8674(02)00907-8. [DOI] [PubMed] [Google Scholar]
- 80.Wilschut KJ, van Tol HTA, Arkesteijn GJA, Haagsman HP, Roelen BAJ. Alpha 6 integrin is important for myogenic stem cell differentiation. Stem Cell Res. 2011;7:112–123. doi: 10.1016/j.scr.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 81.Bentzinger CF, Wang YX, von Maltzahn J, Soleimani VD, Yin H, Rudnicki MA. Fibronectin regulates Wnt7a signaling and satellite cell expansion. Cell Stem Cell. 2013;12:75–87. doi: 10.1016/j.stem.2012.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Liu H, Niu A, Chen S-E, Li Y-P. Beta3-integrin mediates satellite cell differentiation in regenerating mouse muscle. FASEB J Off Publ Fed Am Soc Exp Biol. 2011;25:1914–1921. doi: 10.1096/fj.10-170449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Salimath AS, García AJ. Biofunctional hydrogels for skeletal muscle constructs. J Tissue Eng Regen Med. 2014 doi: 10.1002/term.1881. [DOI] [PubMed] [Google Scholar]
- 84.Rowley JA, Mooney DJ. Alginate type and RGD density control myoblast phenotype. J Biomed Mater Res. 2002;60:217–223. doi: 10.1002/jbm.1287. [DOI] [PubMed] [Google Scholar]
- 85.Xu Z, Ichikawa N, Kosaki K, Yamada Y, Sasaki T, Sakai LY, Kurosawa H, Hattori N, Arikawa-Hirasawa E. Perlecan deficiency causes muscle hypertrophy, a decrease in myostatin expression, and changes in muscle fiber composition. Matrix Biol J Int Soc Matrix Biol. 2010;29:461–470. doi: 10.1016/j.matbio.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Fry CS, Lee JD, Mula J, Kirby TJ, Jackson JR, Liu F, Yang L, Mendias CL, Dupont-Versteegden EE, McCarthy JJ, Peterson CA. Inducible depletion of satellite cells in adult, sedentary mice impairs muscle regenerative capacity without affecting sarcopenia. Nat Med. 2015;21:76–80. doi: 10.1038/nm.3710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lacraz G, Rouleau A-J, Couture V, Söllrald T, Drouin G, Veillette N, Grandbois M, Grenier G. Increased Stiffness in Aged Skeletal Muscle Impairs Muscle Progenitor Cell Proliferative Activity. PloS One. 2015;10:e0136217. doi: 10.1371/journal.pone.0136217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kovanen V, Suominen H, Risteli J, Risteli L. Type IV collagen and laminin in slow and fast skeletal muscle in rats--effects of age and life-time endurance training. Coll Relat Res. 1988;8:145–153. doi: 10.1016/s0174-173x(88)80026-8. [DOI] [PubMed] [Google Scholar]
- 89.Lemos DR, Babaeijandaghi F, Low M, Chang C-K, Lee ST, Fiore D, Zhang R-H, Natarajan A, Nedospasov SA, Rossi FMV. Nilotinib reduces muscle fibrosis in chronic muscle injury by promoting TNF-mediated apoptosis of fibro/adipogenic progenitors. Nat Med. 2015;21:786–794. doi: 10.1038/nm.3869. [DOI] [PubMed] [Google Scholar]
- 90.Conboy IM, Conboy MJ, Smythe GM, Rando TA. Notch-mediated restoration of regenerative potential to aged muscle. Science. 2003;302:1575–1577. doi: 10.1126/science.1087573. [DOI] [PubMed] [Google Scholar]
- 91.Bjornson CRR, Cheung TH, Liu L, Tripathi PV, Steeper KM, Rando TA. Notch signaling is necessary to maintain quiescence in adult muscle stem cells. Stem Cells Dayt Ohio. 2012;30:232–242. doi: 10.1002/stem.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mourikis P, Sambasivan R, Castel D, Rocheteau P, Bizzarro V, Tajbakhsh S. A critical requirement for notch signaling in maintenance of the quiescent skeletal muscle stem cell state. Stem Cells Dayt Ohio. 2012;30:243–252. doi: 10.1002/stem.775. [DOI] [PubMed] [Google Scholar]
- 93.Brack AS, Conboy MJ, Roy S, Lee M, Kuo CJ, Keller C, Rando TA. Increased Wnt signaling during aging alters muscle stem cell fate and increases fibrosis. Science. 2007;317:807–810. doi: 10.1126/science.1144090. [DOI] [PubMed] [Google Scholar]
- 94.Elabd C, Cousin W, Upadhyayula P, Chen RY, Chooljian MS, Li J, Kung S, Jiang KP, Conboy IM. Oxytocin is an age-specific circulating hormone that is necessary for muscle maintenance and regeneration. Nat Commun. 2014;5:4082. doi: 10.1038/ncomms5082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ershler WB, Keller ET. Age-associated increased interleukin-6 gene expression, late-life diseases, and frailty. Annu Rev Med. 2000;51:245–270. doi: 10.1146/annurev.med.51.1.245. [DOI] [PubMed] [Google Scholar]
- 96.Forbes SJ, Rosenthal N. Preparing the ground for tissue regeneration: from mechanism to therapy. Nat Med. 2014;20:857–869. doi: 10.1038/nm.3653. [DOI] [PubMed] [Google Scholar]
- 97.Tidball JG. Inflammatory processes in muscle injury and repair. Am J Physiol Regul Integr Comp Physiol. 2005;288:R345–353. doi: 10.1152/ajpregu.00454.2004. [DOI] [PubMed] [Google Scholar]
- 98.Lu H, Huang D, Saederup N, Charo IF, Ransohoff RM, Zhou L. Macrophages recruited via CCR2 produce insulin-like growth factor-1 to repair acute skeletal muscle injury. FASEB J Off Publ Fed Am Soc Exp Biol. 2011;25:358–369. doi: 10.1096/fj.10-171579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Philippou A, Halapas A, Maridaki M, Koutsilieris M. Type I insulin-like growth factor receptor signaling in skeletal muscle regeneration and hypertrophy. J Musculoskelet Neuronal Interact. 2007;7:208–218. [PubMed] [Google Scholar]
- 100.Massimino ML, Rapizzi E, Cantini M, Libera LD, Mazzoleni F, Arslan P, Carraro U. ED2+ macrophages increase selectively myoblast proliferation in muscle cultures. Biochem Biophys Res Commun. 1997;235:754–759. doi: 10.1006/bbrc.1997.6823. [DOI] [PubMed] [Google Scholar]
- 101.Saclier M, Yacoub-Youssef H, Mackey AL, Arnold L, Ardjoune H, Magnan M, Sailhan F, Chelly J, Pavlath GK, Mounier R, Kjaer M, Chazaud B. Differentially activated macrophages orchestrate myogenic precursor cell fate during human skeletal muscle regeneration. Stem Cells Dayt Ohio. 2013;31:384–396. doi: 10.1002/stem.1288. [DOI] [PubMed] [Google Scholar]
- 102.Arnold L, Henry A, Poron F, Baba-Amer Y, van Rooijen N, Plonquet A, Gherardi RK, Chazaud B. Inflammatory monocytes recruited after skeletal muscle injury switch into antiinflammatory macrophages to support myogenesis. J Exp Med. 2007;204:1057–1069. doi: 10.1084/jem.20070075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ruffell D, Mourkioti F, Gambardella A, Kirstetter P, Lopez RG, Rosenthal N, Nerlov C. A CREB-C/EBPbeta cascade induces M2 macrophage-specific gene expression and promotes muscle injury repair. Proc Natl Acad Sci U S A. 2009;106:17475–17480. doi: 10.1073/pnas.0908641106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Cattin A-L, Burden JJ, Van Emmenis L, Mackenzie FE, Hoving JJA, Garcia Calavia N, Guo Y, McLaughlin M, Rosenberg LH, Quereda V, Jamecna D, Napoli I, Parrinello S, Enver T, Ruhrberg C, Lloyd AC. Macrophage-Induced Blood Vessels Guide Schwann Cell-Mediated Regeneration of Peripheral Nerves. Cell. 2015;162:1127–1139. doi: 10.1016/j.cell.2015.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sicari BM, Dziki JL, Siu BF, Medberry CJ, Dearth CL, Badylak SF. The promotion of a constructive macrophage phenotype by solubilized extracellular matrix. Biomaterials. 2014;35:8605–8612. doi: 10.1016/j.biomaterials.2014.06.060. [DOI] [PubMed] [Google Scholar]
- 106.Kuswanto W, Burzyn D, Panduro M, Wang KK, Jang YC, Wagers AJ, Benoist C, Mathis D. Poor Repair of Skeletal Muscle in Aging Mice Reflects a Defect in Local, Interleukin-33-Dependent Accumulation of Regulatory T Cells. Immunity. 2016;44:355–367. doi: 10.1016/j.immuni.2016.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Vandenburgh HH, Karlisch P, Farr L. Maintenance of highly contractile tissue-cultured avian skeletal myotubes in collagen gel. Vitro Cell Dev Biol J Tissue Cult Assoc. 1988;24:166–174. doi: 10.1007/BF02623542. [DOI] [PubMed] [Google Scholar]
- 108.Cheema U, Yang S-Y, Mudera V, Goldspink GG, Brown RA. 3-D in vitro model of early skeletal muscle development. Cell Motil Cytoskeleton. 2003;54:226–236. doi: 10.1002/cm.10095. [DOI] [PubMed] [Google Scholar]
- 109.Cheema U, Brown R, Mudera V, Yang SY, McGrouther G, Goldspink G. Mechanical signals and IGF-I gene splicing in vitro in relation to development of skeletal muscle. J Cell Physiol. 2005;202:67–75. doi: 10.1002/jcp.20107. [DOI] [PubMed] [Google Scholar]
- 110.Rhim C, Lowell DA, Reedy MC, Slentz DH, Zhang SJ, Kraus WE, Truskey GA. Morphology and ultrastructure of differentiating three-dimensional mammalian skeletal muscle in a collagen gel. Muscle Nerve. 2007;36:71–80. doi: 10.1002/mus.20788. [DOI] [PubMed] [Google Scholar]
- 111.Smith AST, Passey S, Greensmith L, Mudera V, Lewis MP. Characterization and optimization of a simple, repeatable system for the long term in vitro culture of aligned myotubes in 3D. J Cell Biochem. 2012;113:1044–1053. doi: 10.1002/jcb.23437. [DOI] [PubMed] [Google Scholar]
- 112.Grefte S, Vullinghs S, Kuijpers-Jagtman AM, Torensma R, Von den Hoff JW. Matrigel, but not collagen I, maintains the differentiation capacity of muscle derived cells in vitro. Biomed Mater Bristol Engl. 2012;7:055004. doi: 10.1088/1748-6041/7/5/055004. [DOI] [PubMed] [Google Scholar]
- 113.Rossi CA, Flaibani M, Blaauw B, Pozzobon M, Figallo E, Reggiani C, Vitiello L, Elvassore N, De Coppi P. In vivo tissue engineering of functional skeletal muscle by freshly isolated satellite cells embedded in a photopolymerizable hydrogel. FASEB J Off Publ Fed Am Soc Exp Biol. 2011;25:2296–2304. doi: 10.1096/fj.10-174755. [DOI] [PubMed] [Google Scholar]
- 114.Lee KY, Mooney DJ. Alginate: properties and biomedical applications. Prog Polym Sci. 2012;37:106–126. doi: 10.1016/j.progpolymsci.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Rowley JA, Madlambayan G, Mooney DJ. Alginate hydrogels as synthetic extracellular matrix materials. Biomaterials. 1999;20:45–53. doi: 10.1016/s0142-9612(98)00107-0. [DOI] [PubMed] [Google Scholar]
- 116.Hill E, Boontheekul T, Mooney DJ. Designing scaffolds to enhance transplanted myoblast survival and migration. Tissue Eng. 2006;12:1295–1304. doi: 10.1089/ten.2006.12.1295. [DOI] [PubMed] [Google Scholar]
- 117.Hill E, Boontheekul T, Mooney DJ. Regulating activation of transplanted cells controls tissue regeneration. Proc Natl Acad Sci U S A. 2006;103:2494–2499. doi: 10.1073/pnas.0506004103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Borselli C, Cezar CA, Shvartsman D, Vandenburgh HH, Mooney DJ. The role of multifunctional delivery scaffold in the ability of cultured myoblasts to promote muscle regeneration. Biomaterials. 2011;32:8905–8914. doi: 10.1016/j.biomaterials.2011.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Wang L, Cao L, Shansky J, Wang Z, Mooney D, Vandenburgh H. Minimally invasive approach to the repair of injured skeletal muscle with a shape-memory scaffold. Mol Ther J Am Soc Gene Ther. 2014;22:1441–1449. doi: 10.1038/mt.2014.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Wang W, Fan M, Zhang L, Liu S, Sun L, Wang C. Compatibility of hyaluronic acid hydrogel and skeletal muscle myoblasts. Biomed Mater Bristol Engl. 2009;4:025011. doi: 10.1088/1748-6041/4/2/025011. [DOI] [PubMed] [Google Scholar]
- 121.Gilmore AP. Anoikis. Cell Death Differ. 2005;12:1473–1477. doi: 10.1038/sj.cdd.4401723. [DOI] [PubMed] [Google Scholar]
- 122.Beier JP, Stern-Straeter J, Foerster VT, Kneser U, Stark GB, Bach AD. Tissue engineering of injectable muscle: three-dimensional myoblast-fibrin injection in the syngeneic rat animal model. Plast Reconstr Surg. 2006;118:1113–1121. doi: 10.1097/01.prs.0000221007.97115.1d. discussion 1122–1124. [DOI] [PubMed] [Google Scholar]
- 123.Borschel GH, Dow DE, Dennis RG, Brown DL. Tissue-engineered axially vascularized contractile skeletal muscle. Plast Reconstr Surg. 2006;117:2235–2242. doi: 10.1097/01.prs.0000224295.54073.49. [DOI] [PubMed] [Google Scholar]
- 124.Page RL, Malcuit C, Vilner L, Vojtic I, Shaw S, Hedblom E, Hu J, Pins GD, Rolle MW, Dominko T. Restoration of skeletal muscle defects with adult human cells delivered on fibrin microthreads. Tissue Eng Part A. 2011;17:2629–2640. doi: 10.1089/ten.TEA.2011.0024. [DOI] [PubMed] [Google Scholar]
- 125.Janmey PA, Winer JP, Weisel JW. Fibrin gels and their clinical and bioengineering applications. J R Soc Interface R Soc. 2009;6:1–10. doi: 10.1098/rsif.2008.0327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Brown AC, Barker TH. Fibrin-based biomaterials: modulation of macroscopic properties through rational design at the molecular level. Acta Biomater. 2014;10:1502–1514. doi: 10.1016/j.actbio.2013.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Liu J, Xu HHK, Zhou H, Weir MD, Chen Q, Trotman CA. Human umbilical cord stem cell encapsulation in novel macroporous and injectable fibrin for muscle tissue engineering. Acta Biomater. 2013;9:4688–4697. doi: 10.1016/j.actbio.2012.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Fuoco C, Sangalli E, Vono R, Testa S, Sacchetti B, Latronico MVG, Bernardini S, Madeddu P, Cesareni G, Seliktar D, Rizzi R, Bearzi C, Cannata SM, Spinetti G, Gargioli C. 3D hydrogel environment rejuvenates aged pericytes for skeletal muscle tissue engineering. Front Physiol. 2014;5:203. doi: 10.3389/fphys.2014.00203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Fuoco C, Salvatori ML, Biondo A, Shapira-Schweitzer K, Santoleri S, Antonini S, Bernardini S, Tedesco FS, Cannata S, Seliktar D, Cossu G, Gargioli C. Injectable polyethylene glycol-fibrinogen hydrogel adjuvant improves survival and differentiation of transplanted mesoangioblasts in acute and chronic skeletal-muscle degeneration. Skelet Muscle. 2012;2:24. doi: 10.1186/2044-5040-2-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Fuoco C, Rizzi R, Biondo A, Longa E, Mascaro A, Shapira-Schweitzer K, Kossovar O, Benedetti S, Salvatori ML, Santoleri S, Testa S, Bernardini S, Bottinelli R, Bearzi C, Cannata SM, Seliktar D, Cossu G, Gargioli C. In vivo generation of a mature and functional artificial skeletal muscle. EMBO Mol Med. 2015;7:411–422. doi: 10.15252/emmm.201404062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Hammers DW, Sarathy A, Pham CB, Drinnan CT, Farrar RP, Suggs LJ. Controlled release of IGF-I from a biodegradable matrix improves functional recovery of skeletal muscle from ischemia/reperfusion. Biotechnol Bioeng. 2012;109:1051–1059. doi: 10.1002/bit.24382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Rybalko VY, Pham CB, Hsieh P-L, Hammers DW, Merscham-Banda M, Suggs LJ, Farrar RP. Controlled delivery of SDF-1α and IGF-1: CXCR4(+) cell recruitment and functional skeletal muscle recovery. Biomater Sci. 2015;3:1475–1486. doi: 10.1039/c5bm00233h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Lee TT, García JR, Paez JI, Singh A, Phelps EA, Weis S, Shafiq Z, Shekaran A, Del Campo A, García AJ. Light-triggered in vivo activation of adhesive peptides regulates cell adhesion, inflammation and vascularization of biomaterials. Nat Mater. 2015;14:352–360. doi: 10.1038/nmat4157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Chakkalakal JV, Jones KM, Basson MA, Brack AS. The aged niche disrupts muscle stem cell quiescence. Nature. 2012;490:355–360. doi: 10.1038/nature11438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Zhang T, Günther S, Looso M, Künne C, Krüger M, Kim J, Zhou Y, Braun T. Prmt5 is a regulator of muscle stem cell expansion in adult mice. Nat Commun. 2015;6:7140. doi: 10.1038/ncomms8140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Phelps EA, Landázuri N, Thulé PM, Taylor WR, García AJ. Bioartificial matrices for therapeutic vascularization. Proc Natl Acad Sci U S A. 2010;107:3323–3328. doi: 10.1073/pnas.0905447107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Chen F-M, Zhang M, Wu Z-F. Toward delivery of multiple growth factors in tissue engineering. Biomaterials. 2010;31:6279–6308. doi: 10.1016/j.biomaterials.2010.04.053. [DOI] [PubMed] [Google Scholar]
- 138.Adelöw C, Segura T, Hubbell JA, Frey P. The effect of enzymatically degradable poly(ethylene glycol) hydrogels on smooth muscle cell phenotype. Biomaterials. 2008;29:314–326. doi: 10.1016/j.biomaterials.2007.09.036. [DOI] [PubMed] [Google Scholar]
- 139.Phelps EA, Enemchukwu NO, Fiore VF, Sy JC, Murthy N, Sulchek TA, Barker TH, García AJ. Maleimide cross-linked bioactive PEG hydrogel exhibits improved reaction kinetics and cross-linking for cell encapsulation and in situ delivery. Adv Mater Deerfield Beach Fla. 2012;24:64–70. 2. doi: 10.1002/adma.201103574. [DOI] [PMC free article] [PubMed] [Google Scholar]