Abstract
Central infusion of the Na+/K+-ATPase inhibitor, ouabain in rats serves as an animal model of mania because it leads to hyperactivity, as well as reproduces ion dysregulation and reduced BDNF levels similar to that observed in bipolar disorder. Bipolar disorder is also associated with cognitive inflexibility and working memory deficits. It is unknown whether ouabain treatment in rats leads to similar cognitive flexibility and working memory deficits. The present study examined the effects of an intracerebral ventricular infusion of ouabain in rats on spontaneous alternation, probabilistic reversal learning and BDNF expression levels in the frontal cortex. Ouabain treatment significantly increased locomotor activity, but did not affect alternation performance in a Y-maze. Ouabain treatment selectively impaired reversal learning in a spatial discrimination task using an 80/20 probabilistic reinforcement procedure. The reversal learning deficit in ouabain-treated rats resulted from an impaired ability to maintain a new choice pattern (increased regressive errors). Ouabain treatment also decreased sensitivity to negative feedback during the initial phase of reversal learning. Expression of BDNF mRNA and protein levels was downregulated in the frontal cortex which also negatively correlated with regressive errors. These findings suggest that the ouabain model of mania may be useful in understanding the neuropathophysiology that contributes to cognitive flexibility deficits and test potential treatments to alleviate cognitive deficits in bipolar disorder.
Keywords: bipolar disorder, ouabain, reversal learning, working memory, BDNF, frontal cortex
Introduction
Bipolar disorder is a severe mood disorder that is accompanied by significant cognitive impairments that can include deficits in working memory and cognitive flexibility (Dickstein et al., 2010; Hill et al., 2015; McKirdy et al., 2009; Vrabie et al., 2015). These cognitive deficits are observed in individuals with either type I or type II bipolar disorder (Sole et al, 2011). The cognitive deficits in bipolar disorder are associated with poor work adjustment and reduced daily functioning (Bonnin et al., 2014; Wingo et al., 2009a). Moreover, euthymic bipolar disorder individuals can still exhibit cognitive deficits (Martinez-Aran et al., 2004; Torrent et al., 2012) and treatments effective in stabilizing mood can have very limited effects in reducing cognitive deficits or may even, in certain cases, exacerbate existing impairments (Kozicky et al., 2012; Wingo et al., 2009b). At present, there are no approved treatments to alleviate cognitive deficits in bipolar disorder.
Animal models offer an opportunity to better understand the neuropathophysiology associated with cognitive deficits and test novel treatments to alleviate cognitive deficits in bipolar disorder. The ouabain model of mania is increasingly employed to further understand the neuropathophysiology of bipolar disorder (Decker et al., 2000; Herman et al., 2007; Kim et al., 2008,2013; Souza et al., 2014; Sui et al., 2013; Tonin et al., 2014;Valvassori et al., 2015a; Yu et al., 2010, 2011) and examine treatments that may alleviate defining symptoms in the disorder (Brocardo et al., 2011; Bruning et al., 2012; Gao et al., 2011; Jornada et al., 2011; Souza et al., 2014; Wang et al., 2013; Valvassori et al., 2015b). Ouabain principally acts as a Na+/K+ ATPase inhibitor and is meant to model reduced Na+/K+ ATPase activity observed in bipolar disorder individuals (Banerjee et al., 2012; Goldstein et al., 2009; Huff et al., 2010). Consistent with ouabain treatment serving as a model of mania, an intracerebroventricular (ICV) injection of ouabain increases locomotor activity in rats (Brocardo et al., 2010; Decker et al., 2000; Herman et al., 2007; Souza et al., 2014; Varela et al., 2015). However, few studies have examined whether the ouabain model of mania leads to cognitive deficits (Wang et al., 2013, 2014) and even less is known about whether ICV ouabain treatment leads to impairments in working memory and cognitive flexibility.
An examination of working memory and cognitive flexibility in the ouabain model of mania is of further interest to understand how ouabain-induced changes in brain trophic factors may relate to changes in cognitive function. More specifically, there is accumulating evidence that decreased brain-derived neurotrophic factor (BDNF) is related to bipolar disorder (Cunha et al., 2006; Fernandes et al., 2015; Nassan et al., 2015; Palomino et al., 2006; Pandey et al., 2008). In a comparable fashion, recent studies with ICV treatment of ouabain in rats found reduced BDNF levels in the frontal cortex (Valvassori et al., 2015b; Varela et al., 2015). In addition, other experiments in rodents report that BDNF in the frontal cortex is important for both reversal learning (Graybeal et al., 2011; Kanowski et al., 2007; Xue et al., 2013) and working memory (Li et al., 2010; Xing et al., 2012). However, a relationship between BDNF with reversal learning or working memory has not been established in a rodent model of mania.
To determine whether ouabain treatment affects spatial working memory, rats were tested a spontaneous alternation test in a Y-maze. To understand whether ouabain treatment affects discrimination learning and/or cognitive flexibility, rats were also tested in a two-choice spatial discrimination using 80/20 probabilistic reinforcement. A probabilistic learning and reversal learning test was used because past studies demonstrated that individuals with bipolar disorder or other psychiatric disorders exhibit probabilistic reversal learning deficits (D'Cruz et al., 2013; Dickstein et al., 2010; Waltz & Gold, 2007; Weiler et al., 2009). This study further determined whether ouabain treatment affected BDNF expression in the frontal cortex and was related to behavioral performance.
Materials and Methods
Subjects
Adult male Long Evans rats weighing 320-380 g served as the subjects for this study. The rats were individually housed in plastic cages (26.5 × 50 × 20cm) in a temperature controlled room at 21-23° C and humidity 30%. Rats were on a 12 h light/dark cycle. The animals were food restricted to 85-90% of their body weight during the experiment. A total of 24 rats were used in this experiment. Animal care and use was in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals, and was approved by the Institutional Laboratory Animal Care and Use Committee at the University of Illinois at Chicago.
The specific timeline for the procedures described below are illustrated in Figure 1.
Figure 1.
Illustration of the timeline for the experimental methods.
Surgical Procedure
Stereotaxic surgery was conducted during the light phase of the light/dark cycle. All rats were anesthetized with xylazine (10mg/kg) and ketamine (100mg/kg). Each rat received unilateral implantation of a guide cannula (22 gauge, Plastics One) aimed at the left lateral ventricle. The stereotaxic coordinates were as follows: anterior-posterior = − 0.4 mm from bregma; medial-lateral = −1.5 mm from the midline and ventral = 4.0 mm from skull. The coordinates were based on the stereotaxic atlas by Paxinos and Watson (1998). Four jeweler screws were positioned in the skull surrounding the cannula and secured with dental acrylic. A stylet was placed into the guide cannula to prevent clogging. After surgery, rats received a 1 mg/kg injection of meloxicam to reduce any pain from the surgery. Rats were allowed to recover for 14 d after surgery. For 5 d after surgery, rats were fed ad libitum and left undisturbed. After recovery rats were food restricted as described above and handled 10 min/d.
Microinfusion procedure
Two weeks following surgery each rat received an ICV injection of ouabain (1mM) dissolved in saline or saline alone in a 5 μl volume. The dose was based on past studies using the same concentration of ouabain (Gao et al., 2011; Varela et al., 2015; Wang et al., 2014). To execute the microinfusion procedure the stylet was first removed from the guide cannula while a rat was restrained. Subsequently, an injection cannula (28 gauge) was inserted into the guide cannula that extended 1 mm below the guide cannula. The injection cannula was connected by polyethylene tubing to a 10 μl syringe (Hamilton Company). Each treatment was infused at a rate 1 μl/1.5 min by a microinfusion pump (74900Series; Cole Palmer). A rat was allowed to freely roam in its home cage as the infusion was occurring. The injection cannula was left in place for 1 min after injection to allow for diffusion.
Apparatuses
For the spontaneous alternation test, rats were tested a Y-shaped maze. Each maze arm contained a base (10 × 55 cm), two side walls (15 × 55 cm), and a back wall (8 × 15 cm). A triangular center connected all three arms together. The maze was elevated 72 cm above the floor in a room with various extra maze cues. For the spatial discrimination test, training and testing occurred in a four-arm maze made from black acrylic. The maze was located in a different room than the Y-maze. The maze had a center square base (10 × 10 cm) that connected all four arms. Each maze arm contained a base (10 × 55 cm), two side walls (15 × 55 cm) and a back wall (8 × 15 cm). There was a circular food well (3.2 cm in diameter and 1.6 cm deep) that was placed 3 cm away from the back wall. The maze was placed on table that had a height of 72 cm. The maze was located in a room that had various extramaze cues that could be used to spatially navigate in the maze.
Maze Training
As in past studies (Brown et al., 2010, 2012), rats received training prior to spatial discrimination testing. One week after surgery and one week prior to the ouabain injection, each rat was exposed to the cross-maze and trained to obtain a half piece of Froot Loops cereal (Kellogg, Battle Creek, MI, USA) from each food well. During training, a rat was also picked up after consuming a cereal piece and placed into a different maze arm. This acclimated a rat to being picked up in the maze as would occur in the test phase. After a rat consumed all four cereal pieces from each food well, it was placed in a holding chamber near the maze. The food wells were rebaited and a new trial was started. This phase of training continued until a rat completed at least 6 trials in 15 minutes for two consecutive days. The time range for training lasted 4-6 sessions. Four days after the ICV injection, each rat received a training session as described above. Five days after the ICV injection, a final day of training occurred in which a black plastic block (9 cm wide × 13 cm high × 1 cm thick) was placed at the entrance of one arm, giving the maze a T-shape. A rat was placed in the stem arm and allowed to enter either choice arm to obtain a cereal piece. After the initial choice, a rat was placed back in the stem arm. If a rat chose the same arm as the initial choice, it was returned to the stem arm until it chose the other arm. Once a rat had selected both arms it was placed on top of its home cage while the two choice-arms were rebaited. The session ended after a rat had completed seven of these trials. Testing occurred in the following session.
Spontaneous Alternation Test
Six days after the ICV injection, rats were tested for spontaneous alternation performance. A similar procedure was used as in past studies (Ragozzino et al., 1995; Walker et al., 1991). For the test, a rat was placed in one arm and allowed to traverse the maze freely for 15 min. The number and sequence of entries were recorded; an alternation was defined as the consecutive entry into all three arms on overlapping triplet sets. Three consecutive arm choices within the total set of arm choices made up a triplet set. With this procedure, possible alternation sequences are equal to the number of arm entries minus 2, and the percentage of alternation behavior is equal to: (actual alternations/possible alternations) × 100. The number of arm entries was used as a measure of locomotor activity.
Spatial Discrimination Testing with Probabilistic Reinforcement
Approximately 10 minutes after the completion of the spontaneous alternation test, each rat was tested on acquisition of a spatial discrimination. On the subsequent day, rats were tested on reversal learning of the spatial discrimination. A probabilistic learning procedure was used in both test sessions (Brown et al., 2012). In the acquisition phase, one choice arm was designated as the ‘correct’ arm and contained a half piece of cereal on 80% of the trials. On the other 20% of trials, the ‘incorrect’ arm was baited with a half piece of cereal. The first two trials of the test always contained a reinforcement in the ‘correct’ arm. Acquisition criterion was achieved when a rat entered the ‘correct’ arm in 9 out of 10 consecutive trials. Thus, a rat learned to enter the same maze arm based on spatial location for 9 out of 10 consecutive trials. On the second day of testing (reversal learning), the ‘correct’ and ‘incorrect’ arms were reversed from those on acquisition such that a rat was required to enter the arm opposite to that on acquisition. Thus, the new ‘correct’ arm was reinforced on 80% of the trials and the new ‘incorrect’ arm was reinforced on 20% of the trials. The first two trials of the test always contained a reinforcement in the ‘correct’ arm. The criterion for reversal learning was entering the new ‘correct’ arm in 9 out of 10 consecutive trials.
An analysis of errors in the reversal learning session was conducted to determine whether ouabain treatment affected the initial inhibition of a learned choice pattern as measured by perseverative errors and/or the maintenance of a new choice after being initially selected as measured by regressive errors (Brown et al., 2012; Amodeo et al., 2014). To determine the number of perseverative errors, trials were separated into consecutive blocks of four trials. Perseveration was defined as initially selecting the arm that was ‘correct’ during acquisition in three or four trials in a block. Thus, if a rat chose the previously ‘correct’ arm on the majority of trials in a block it was considered to be perseverating. Once a rat made two or more choices in the new, ‘correct’ arm in a block, perseveration was no longer considered to occur. All subsequent entries into the previously ‘correct’ arm were defined as regressive errors. Perseveration is considered a measure of the inability to initially inhibit a previously learned choice pattern. Regressive errors determine the ability to maintain a new choice pattern after being initially selected.
An analysis was also performed to determine whether ouabain treatment altered the sensitivity to reinforcement or no reinforcement on ‘correct’ trials (Amodeo et al., 2012; Bari et al., 2010). A rat's choices in the test were analyzed based on the outcome (reinforcement or no reinforcement) of each preceding trial and expressed as a ratio. For ‘correct’ trials, a win–stay ratio was determined by the number of times a rat received a reinforcement in the ‘correct’ arm and then chose the same ’correct’ arm on the subsequent trial, divided by the total number of reinforced trials for the ‘correct’ trials only. The lose–shift ratio was determined by the number of times a rat changed its choice after not receiving reinforcement in the ‘correct’ arm on the previous trial, divided by the total number of non-reinforced trials for only ‘correct’ trials.
Tissue Preparation and Histology
One day after completion of behavioral testing, each rat received an overdose of sodium pentobarbital (250 mg/kg, i.p.) and was decapitated. The brain was removed and divided into two halves along the anterior-posterior plane at approximately 0.6 mm anterior to bregma (Paxinos & Watson, 1998). The posterior half of the brain was placed in 4% formaldehyde solution and subsequently sectioned to determine the cannula placement. To determine the location of the cannula placements, brains were frozen and cut into 50 micron coronal sections on a cryostat. Sections were placed on slides and examined under a microscope to determine whether the cannula was located in the lateral ventricle.
The anterior half of the brain was transferred to −80°C for storage to be used later for the BDNF assays of the frontal cortex (described below). Prior to the BDNF assays, tissue was removed from storage and the anterior parts of the frontal lobes were dissected in accordance with Paxinos and Watson coordinates (1998), after removal of their basal parts at the level of the rhinal fissures.
Real-Time PCR
BDNF mRNA was determined in the frontal cortex. Total RNA was isolated using TRIzol® reagent (Invitrogen Life Technologies, Carlsbad, CA, USA) according to manufacturer's directions and treated with DNAase 1. The RNA yield was determined by absorbance at 260 nm and the purity of RNA was determined by measuring the optical density with an absorbance ratio of 260/280 using NanoDrop®ND-1000 (NanoDrop Technologies, Montchanin, DE, USA). Only RNA with >1.8 purity was used. The quality of RNA was also assessed using an Agilent Bioanalyzer 2100 and only samples with an RNA integrity number >7 were used.
BDNF mRNA was determined by Real-Time PCR using TaqMan® primers and probes. The PCR reaction was carried out in a final volume of 20 μl, containing 5 μl of cDNA diluted 1:10, 1x of TaqMan primer/probe mix (20x), and 1xTaqMan® Universal PCR Master Mix. For each primer/probe tested, the PCR reaction also included a non-reverse transcription negative control to confirm the absence of genomic DNA, a non-template negative control to check for primer-dimer. All experiments were performed in duplicate as described in TaqMan gene expression protocol. BDNF gene transcript was quantified and normalization with GAPDH as a stable reference transcript (Clancy et al., 2013). The amounts of BDNF genes expressed in a sample were normalized to GAPDH. Fold changes between subject groups were measured using the 2−ΔΔCt method, where ΔΔCT = (CT target - CT normalizer)sample - (CT target - CT endogenous gene)control.
Western blotting
Frontal cortex was homogenized in a buffer containing 100 mM Tris (pH 7.4), 150 mM NaCl, 1% Triton X-100, 1% sodium decanoate, 0.1% SDS, 5 mM EDTA, 1 mM phenylmethylsulfonyl fluoride, 5 μg/ml aprotinin, leupeptin, pepstatin, and 10 μg/ml of 100 mM sodium orthovanadate. The homogenate was centrifuged at 15,000 g for 10 min at 4°C. The supernatant was used to determine the levels of BDNF. Protein levels were determined by Bradford assay.
Immunolabeling of BDNF was determined by Western blot as described earlier in detail (Dwivedi et al., 2003). Equal volumes of tissue lysates (20 μl containing 30 μg protein) were loaded onto sodium dodecyl sulfate (SDS)-polyacrylamide gel. The gels were run and transferred electrophoretically to an enhanced chemiluminescence (ECL) nitrocellulose membrane. The membranes were washed with TBST buffer (10 mM Tris-base, 0.15 M NaCl, and 0.05% [v/v] Tween 20) for 10 min. The blots were blocked by incubating with 5% (w/v) powdered nonfat milk in TBST, 0.02 % nonidet P-40, and 0.02% (w/v) SDS (pH 8.0). Then the blots were incubated overnight at 4°C with polyclonal anti-BDNF antibody (Santa Cruz Biotechnology Inc., Santa Cruz, California, USA) at a dilution of 1:1000. The membranes were washed with TBST buffer and incubated with horseradish-peroxidase-linked secondary antibody (anti-rabbit immunoglobulin G); 1:2000) for 5 hr at room temperature. The membranes were washed with TBST buffer and exposed to ECL autoradiography film. The same nitrocellulose membrane was stripped and re-probed with β-actin antibody (Sigma Chemical Co., St. Louis, Missouri). The bands on the autoradiogram were quantified using the Loats Image Analysis System (Westminister, MD, USA), and the optical density of each sample was corrected by the optical density of the corresponding β-actin band. The values are represented as a percent of control.
Statistical Analysis
A t-test determined whether there was a significant ouabain effect on trials to criterion. A separate t-test was conducted for the acquisition phase and reversal learning phase. In addition, t-tests were conducted to examine differences between the groups on both perseverative and regressive errors, as well as a treatment effect on win–stay and lose–shift performance. An additional analysis was conducted for win-stay and lose-shift probabilities to determine whether there was a group difference for each measure in the first half of trials and/or second half of trials. This was carried out using a two-way analysis of variance with repeated measures. To determine whether ouabain treatment affected frontal cortex BDNF mRNA or protein expression levels, a t-test was employed to determine differences between the groups for each measurement. To determine whether there was a relationship between the activity measure and cognitive performance, Pearson correlations were conducted on number of arm choices with spontaneous alternation performance and reversal learning performance. To determine whether there was a relationship between changes in frontal cortex BDNF expression levels and behavioral performance, Pearson correlations were also conducted on BDNF measures with number of arm choices, reversal learning, regressive errors and lose-shift probability scores. An alpha level of 0.05 was set for significance in all of the statistical analyses unless otherwise noted.
Results
Histology
Histological examination of the cannula placements indicated that the majority of rats (19 of 24) had cannula placements in the lateral ventricle at the level of the fimbria/fornix. Two rats that received saline treatment were excluded from the analyses because of placements outside of the lateral ventricle. One rat had a cannula placement located in the fimbria/fornix and the other had a placement located in the corpus callosum. Two rats that received ouabain treatment were also excluded from the analyses because of cannula misplacements. One rat had a cannula located in the thalamus and the other had a cannula placement in the dorsomedial striatum. An additional rat that received ouabain treatment was excluded from the analyses because the rat stopped performing during acquisition of the spatial discrimination. Thus, a total of 19 rats (10 saline-treated rats and 9 ouabain-treated rats) were included in the behavioral and biochemical analyses.
Spontaneous Alternation Performance
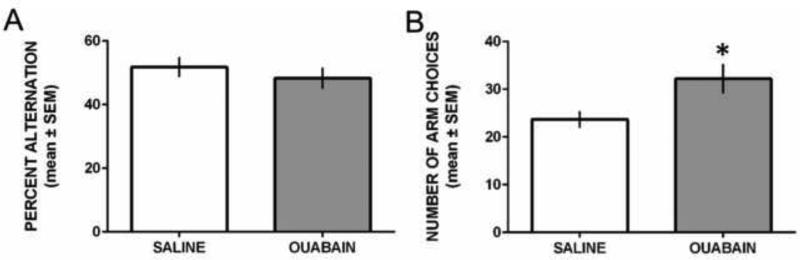
Figure 2 shows the results from spontaneous alternation testing. Both saline- and ouabain-treated rats had alternation scores around 50% (see Figure 2A). The difference in alternation scores between the groups was not significant, t17 = 0.75, P = 0.46. Although both groups exhibited similar alternation scores, ouabain-treated rats displayed a greater number of arm choices compared to that of saline-treated rats (see Figure 2B). The difference in arm choices between the groups was significant, t17 = 2.46, P = 0.025. Thus, ouabain treatment increased locomotor activity during spontaneous alternation performance.
Figure 2.
ICV ouabain injection did not affect spontaneous alternation performance, but increased the number of arm choices in a Y-maze. A) Mean (±SEM) percent alternation during 15 min test. Saline and ouabain treatment led to comparable levels of spontaneous alternation performance. B) Mean (±SEM) number of arm choices during 15 min test. Ouabain-treated rats made significantly more arm choices than saline-treated rats. *P < 0.05 vs. saline treatment.
Spatial discrimination test: Acquisition and Reversal Learning
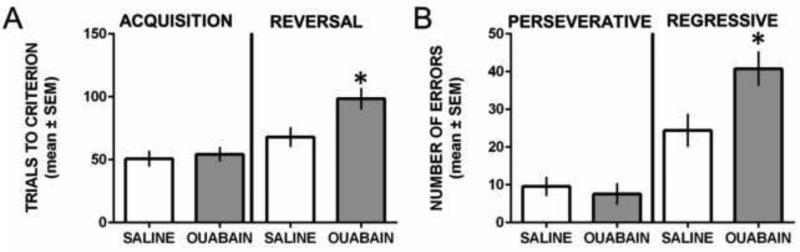
Figure 3 illustrates the results during acquisition and reversal learning of the spatial discrimination. Both groups obtained acquisition criterion in approximately 50 trials (Figure 3A). The difference in trials to criterion during acquisition was not significant, t17 = 0.42, P = 0.68. Both groups also displayed similar retention performance of the acquired spatial discrimination administered requiring 8-9 trials to achieve criterion (data not shown). The difference in retention test performance between the groups was not significant, t17 = 0.72, P = 0.48. In reversal learning, both groups required more trials to achieve criterion than in acquisition, but saline-treated rats required approximately 70 trials to achieve criterion while ouabain-treated rats required approximately 100 trials. The difference in reversal learning performance between the groups was significant, t17 = 2.59, P = 0.019.
Figure 3.
ICV ouabain injection impaired probabilistic reversal learning by selectively regressive errors in a spatial discrimination test. A) Mean (±SEM) trials to criterion on acquisition and reversal learning. Saline- and ouabain-treated rats required similar trials to achieve criterion in acquisition, but ouabain-treated rats required significantly more reversal learning trials compared with that of saline-treated rats. *P < 0.05 vs. saline treatment. B) Mean (±SEM) perseverative and regressive errors committed during reversal learning. Ouabain treatment did not significantly affect perseverative errors, but significantly increased regressive errors compared to that of saline treatment. *P < 0.05 vs. saline treatment.
Perseverative and Regressive Errors during Reversal Learning
In reversal learning, most rats committed perseverative errors in the first two blocks of trials. Subsequently, rats began to choose the new correct spatial location (see Figure 3B). There was no significant difference in the number of perseverative errors between the groups, t17 = 0.54, P = 0.60. There was a significant difference in the number of regressive errors between the groups with ouabain treatment leading to a significantly greater number of regressive errors than that of saline treatment, t17 = 2.58, P = 0.02.
Win-Stay and Lose-Shift Performance during Reversal Learning
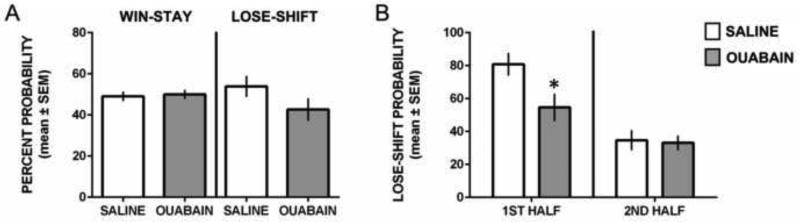
Figure 4 illustrates the win-stay and lose-shift probabilities during reversal learning. An analysis of win-stay performance indicated that both groups had a win-stay probability of approximately 50%. There was not a significant difference in overall win-stay probabilities among the groups, t17 = 0.27, P = 0.79. For lose-shift performance, there was a non-significant trend for saline-treated rats to exhibit a greater probability of lose-shift choices than ouabain-treated rats, t17 = 1.49, P = 0.15.
Figure 4.
Win-stay and lose-shift performance during reversal learning. A) Mean (±SEM) probability for win-stay and lose-shift choices. Both groups exhibited comparable win-stay performance. There was a trend for ouabain treatment to produce lower lose-shift probabilities than saline treatment. B) Mean (±SEM) lose-shift probabilities committed during the first half and second half of reversal learning trials. Ouabain-treated rats had a significantly lower probability of making a lose-shift choice in the first half of trials compared to saline-treated rats. * p < 0.05.
The difference in lose-shift probabilities between the groups was explored in a subsequent analysis. This was to determine whether ouabain-treated rats exhibited a lower lose-shift probability in the first half of the reversal learning test compared to that of saline-treated rats. We hypothesized that the trend for an overall lower lose-shift probability in ouabain-treated rats was due to ouabain treatment decreasing sensitivity to negative feedback on a ‘correct’ trial during the initial trials of reversal learning. This is because all rats eventually achieved reversal learning criterion and thus both groups are likely performing more similar in the second half of trials. To test this hypothesis, we separated the reversal learning trials for each rat in half and calculated the lose-shift probability for the 1st half and 2nd half of reversal learning trials. As shown in Figure 4B, both groups exhibited higher lose-shift probabilities during the initial reversal learning phase than the second half of trials. However, ouabain-treated rats showed a significantly lower lose-shift probability than saline-treated rats when making a ‘correct’ choice, but not receiving a food reward. As both groups learned the new contingency in the 2nd half of reversal learning trials there was a similarly low probability of lose-shift behavior. A two-way analysis of variance with repeated measures revealed that there was not a significant effect for treatment, F1,17 = 3.44, P = 0.11. However, there was a significant effect for time (1st half vs. 2nd half), F1,17 = 35.74, P < 0.0001 and a significant treatment × time interaction, F1,17 = 4.72, P = 0.031. Follow-up analyses of the significant interaction revealed that ouabain-treated rats compared to that of saline-treated rats had a significantly lower lose-shift probability in the first half of trials (p < 0.05), but not in the second half of trials (p > 0.05).
Correlation of Locomotor Activity with Spontaneous Alternation and Reversal Learning
A series of Pearson correlations were conducted to determine whether there was a relationship between locomotor activity with spontaneous alternation or reversal learning. In addition, a Pearson correlation was conducted between spontaneous alternation and reversal learning performance. Correlations on these measures were conducted for each individual group, as well as for the data collapsed across groups. The correlation between the number of arm choices and spontaneous alternation scores for vehicle-treated was r(9) = 0.09, P = 0.80. The same correlation in the ouabain-treated rats was r(8) = 0.32, P = 0.40. A Fisher r to z transformation was applied to determine whether there was a significant difference between the two correlations. The z-score was equal to −0.43, P = 0.67, indicating that there was not a significant difference between the two correlation coefficients. When combining the two groups the correlation between the number of arm choices and spontaneous alternation scores was r(17) = 0.10, P = 0.67. Thus, there was not a significant correlation between locomotor activity and spontaneous alternation performance.
In saline-treated rats, the correlation in the number of arm choices and reversal learning trials was r(9) = 0.18, P = 0.61. A similar positive correlation occurred in ouabain-treated rats, r(8) = 0.15, P = 0.64. A Fisher z transformation led to a z-score of 0.06, P = 0.95 revealing there was not a significant difference in the two correlation coefficients. In addition, when combining the groups there was not a significant correlation between the number of arm choices and reversal learning trials to criterion, r(17) = 0.38, P = 0.11. Therefore, there was not a significant correlation between locomotor activity and reversal learning performance.
Analysis of the relationship between spontaneous alternation and reversal learning performance indicated that in saline-treated rats the correlation between these two measures was r(9) = 0.20, P = 0.58. In the ouabain-treated rats, the correlation between spontaneous alternation and reversal learning was r(8) = 0.38, P = 0.30. Conducting a Fisher r to z transformation indicated that there was not a significant difference in the two correlation coefficients, z = −0.35, P = 0.73. In combining data from the two groups the correlation between spontaneous alternation performance and reversal learning was r(17) = 0.14, P = 0.55. Thus, there was not a significant correlation between spontaneous alternation and reversal learning performance.
BDNF mRNA and protein expression in the Frontal Cortex
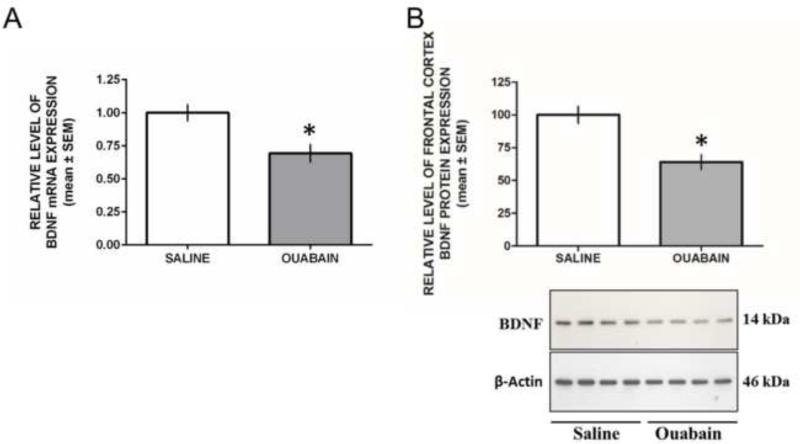
The mRNA level of BDNF was determined in frontal cortex of saline and ouabain-treated rats by qRT-PCR. GAPDH mRNA was determined in this brain area as a housekeeping gene. The findings from frontal cortex BDNF measures are shown in Figure 5. An analysis of frontal cortex BDNF mRNA expression between saline and ouabain treatment revealed that ouabain treatment significantly reduced BDNF mRNA expression, t17 = 3.15, P = 0.006 (Figure 5A). Representative Western blot showing immunolabeling of BDNF protein in frontal cortex is shown in Figure 5B. As shown, the molecular weight of BDNF is 14 kDa. β-Actin protein resolved at 46 kDa. Similar to BDNF mRNA, ouabain treatment significantly reduced BDNF protein levels in the frontal cortex compared to that of saline treatment, t17 = 4.10, P = 0.0007 (Figure 5B).
Figure 5.
Ouabain treatment reduces frontal cortex BDNF mRNA expression and protein levels. A) Mean (±SEM) percent level of frontal cortex BDNF mRNA expression. An ICV injection of ouabain significantly reduced expression of frontal cortex BDNF mRNA expression compared to that of a saline injection. B) Mean (±SEM) percent expression of frontal cortex BDNF protein levels. An ICV injection of ouabain significantly reduced expression of frontal cortex BDNF proteins expression compared to that of a saline injection. * P < 0.05.
Correlation between BDNF mRNA and Behavioral Measures
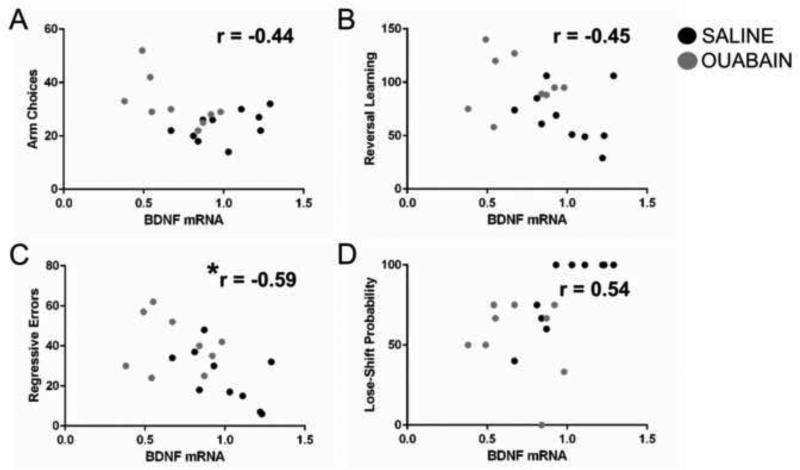
To determine whether there was a relationship between frontal cortex BDNF mRNA and the behavioral measures in which there was a significant difference between the treatment groups, Pearson correlations were conducted between BDNF mRNA expression levels and the following measures: 1) reversal learning trials to criterion; 2) regressive errors; 3) spontaneous alternation arm choices and 4) lose-shift probability in 1st half of reversal learning trials (see Figure 6). Because multiple correlations were conducted a Bonferroni correction was applied such that the significant alpha value was set at 0.0125. The only significant correlation was between BDNF mRNA and regressive errors, r(17) = −0.59, P = 0.008. Thus, lower BDNF mRNA levels were associated with high levels of regressive errors. In contrast, there was not a significant correlation between BDNF mRNA and reversal learning trials to criterion, r(17) = −0.45, P = 0.06. In addition, there was not a significant correlation between BDNF mRNA and the number of arm choices in spontaneous alternation, r(17) = − 0.44, P = 0.06 or lose-shift probability scores in the first half of reversal learning, r(17) = 0.54, P = 0.02. A similar pattern of results were observed when protein levels of BDNF were correlated with these behavioral measures (data not shown).
Figure 6.
Correlation between BDNF mRNA levels and various behavioral measures for all rats included in the behavioral analyses. A) There was not a significant correlation between BDNF mRNA and number of arm choices in spontaneous alternation. B) There was not a significant correlation between BDNF mRNA and reversal learning trials to criterion. C) There was a significant correlation between BDNF mRNA and regressive errors committed during reversal learning. D) There was not a significant correlation between BDNF mRNA and lose-shift probabilities during the 1st half of reversal learning trials. * P < 0.0125.
Discussion
The present experiment investigated the effects of an ICV infusion of the Na+/K+ ATPase inhibitor, ouabain in a working memory and reversal learning test, as well as on frontal cortex BDNF expression levels. Comparable to past studies demonstrating an increase in locomotor activity following ouabain treatment (Brocardo et al., 2010; Decker et al., 2000; Herman et al., 2007; Souza et al., 2014; Varela et al., 2015), a significant increase in the number of arm choices was observed in the spontaneous alternation test. This increase in activity occurred despite ouabain treatment not affecting alternation performance. Furthermore, there was not a relationship between locomotor activity with spontaneous alternation or reversal learning performance. Spontaneous alternation testing in the Y-maze was a single exposure and thus the increase in arm choices with ouabain treatment can be viewed as an augmented exploration to a novel environment. This is similar to that observed in genetic models of mania (Roybal et al., 2007; Young et al., 2010) and also reported in bipolar patients during mania (Minassian et al., 2011; Young et al., 1995).
To explore learning and cognitive flexibility in the ouabain model of mania, rats were tested on acquisition and reversal learning of a spatial discrimination. A central ouabain injection did not affect acquisition of the spatial discrimination. This differs from results reported by Wang and colleagues (2013, 2014) who found that ICV ouabain treatment impaired acquisition in a water maze test. There are various possibilities that may explain the different results. The present study used an appetitive test in which a rat learned to use visuospatial information to locate a food reward. The Morris water maze task also involves the use of visuospatial information, but has an aversive component in which a rat seeks to minimize or avoid swimming in the water (D'Hooge & De Deyn, 2001; Morris, 1984; Sandi et al., 1997). One possibility is that ouabain treatment has differential effects on learning when a task involves appetitive as opposed to aversive motivation as observed with other drug treatments (Cahill & McGaugh, 1990; Salamone, 1994). Another possibility for the different pattern of results is due to a difference in the level of difficulty. Specifically, the spatial discrimination used in the present study has discrete and salient choices. In contrast, the water maze does not have the same type of discrete choices which may make the task more difficult leading to an acquisition deficit. Related, rats were trained and handled in the maze environment for the current study prior to testing, but not in the water maze test. A more extensive use of learning paradigms in the ouabain model of mania can aid in clarifying how ouabain treatment may affect learning.
The present study also examined the effect of ouabain treatment on reversal learning. This was of particular interest because a manic episode can lead to impairments in cognitive flexibility (Daglas et al., 2015; Morice, 1990). A probabilistic learning paradigm was used as past studies showed that individuals with various psychiatric disorders, including bipolar disorder, exhibit deficits in probabilistic reversal learning (D'Cruz et al., 2013; Dickstein et al., 2010; Gorrindo et al., 2005; Waltz & Gold, 2007; Weiler et al., 2009). Similar to that found in studies with bipolar disorder, ouabain treatment did not affect learning of the initial spatial discrimination, but selectively impaired reversal learning. Because rats were tested on spontaneous alternation in a Y-maze, this may have affected reversal learning performance in ouabain-treated rats during reversal learning. However, past studies have shown that a rat which has prior exposure to a working memory test or a different maze environment does not affect pharmacological or brain manipulations on behavioral flexibility tests compared to rats who have not had exposure to a working memory test or a different maze environment (DeCoteau & Kesner, 2000; Gilbert et al., 2001; Ragozzino et al., 1998, 1999, 2002). Similarly, acquisition performance in rats from the current study was comparable to that of rats that had not been previously exposed to a spontaneous alternation test (Brown et al., 2012; Syed et al., 2016). Thus, it is unlikely that exploring a maze in a different context influences subsequent spatial discrimination testing. Moreover, because ouabain-treated rats performed in a comparable manner as controls on acquisition, the pattern of results suggest that ouabain treatment did not affect motivation or have a more general impairing effect on learning when outcomes are uncertain. Instead the findings suggest that when outcomes are uncertain, ouabain treatment impaired the ability to adapt choice patterns with a change in contingencies.
To better understand what behavioral or cognitive processes ouabain may affect which may contribute to the reversal learning deficit, an examination of perseverative and regressive errors was conducted. As in past studies (Amodeo et al., 2014; Ragozzino et al., 2012), perseverative errors measured the ability to initially inhibit a previously learned choice pattern and/or generate a new choice pattern. Regressive errors only occurred after a rat made a correct choice in reversal learning and measured the ability to maintain the new, correct choice pattern. Ouabain treatment impaired reversal learning by selectively increasing regressive errors. The pattern of findings indicate that an ICV infusion of ouabain did not affect initial inhibition and switch away from a learned choice pattern in reversal learning, but affected the ability to maintain a new choice pattern after being initially selected. Several studies in individuals with bipolar disorder have reported perseverative errors using set-shifting tests, e.g. the Wisconsin Card Sort Task (Bora et al., 2009; Krabbendam et al., 2005). However, these studies have not commonly made a distinction between perseverative and regressive errors as in the present study. A recent study did use a similar perseverative and regressive error analysis to study bipolar individuals in a set-shifting test and found that bipolar subjects did not perseverate when required to shift sets, but exhibited an increase in regressive errors compared to that of healthy controls (Hill et al., 2015). Thus, the reversal learning findings with ouabain treatment match the results observed with bipolar patients in two ways. First, both ouabain treatment in rats and bipolar patients exhibit a probabilistic reversal learning deficit. Second, both ouabain treatment in rats and bipolar patients display an increase in regressive errors during cognitive flexibility tests.
The results from probabilistic reversal learning also revealed that ouabain treatment overall did not affect the ability to continue with a correct response immediately following a correct trial in which a food reward was received (win-stay behavior). There was a trend for an overall lower lose-shift probability in the ouabain treatment group. Further analyses revealed that ouabain-treated rats did have a significantly lower lose-shift probability in the first half of trials compared to that of saline-treated rats. In particular, ouabain treatment reduced the probability of switching a response immediately following a correct trial in which no food reward was received in the first half of trials (lose-shift behavior). As rodents are initially exposed to the reversal learning contingency there is a strong tendency to shift back to the original choice when the new correct response does not yield a reward. Ouabain treatment reduced this bias. These findings suggest that ouabain treatment reduced the sensitivity to negative reinforcement early in reversal learning. This result is comparable to findings during a manic episode in which reduced sensitivity to negative feedback occurs (Lembke & Tetter, 2002; Linke et al., 2011).
In contrast to probabilistic reversal learning, an ICV injection of ouabain had no effect on spontaneous alternation performance. The spontaneous alternation test in a Y-maze was used as a short-term or working memory test as in past studies (Mohler et al., 2012; Ragozzino et al., 1995; 2012; Walker et al., 1991). One possibility is that ouabain treatment does not affect working memory in rats. If this is the case, then this would represent a limitation of the ouabain model of mania. Another possibility is that ouabain-treated rats under different test conditions may have exhibited a working memory deficit. For example, past studies have shown that when a delay is inserted between choices in the spontaneous alternation test there can be pharmacological effects on memory (Mohler et al., 2012; Ragozzino et al., 2012). Still other studies report with greater interference, i.e. increased similarity in stimuli to be remembered, working memory deficits can emerge (Gilbert et al., 2001; Ragozzino et al., 1998). Future studies that employ working memory tests that systematically alter delays can address this issue.
Beyond the behavioral measures, the present investigation examined whether ouabain treatment affected frontal cortex BDNF expression levels. As observed in other studies investigating brain BDNF following central ouabain administration using an ELISA method (Varela et al., 2015; Valvassori et al., 2015a), the present experiment found a reduction in frontal cortex BDNF. We measured BDNF by Western blot and qPCR. The findings suggest that BDNF expression is decreased both at the transcriptional (qPCR) and translational (Western blot) levels. With Western blot, we were able to identify the specificity of the antibody and made sure that protein expression is examined for mature BDNF. Some antibodies recognize both pro and mature BDNF and also show bands at higher molecular weight, which can not be tested with ELISA. Because the present results show that BDNF is decreased at the transcriptional level, it suggests that ouabain can have direct or indirect effects on the transcriptional machinery or possibly acting on specific BDNF exon(s), which may be driving the expression of mature BDNF. Future experiments can clarify this issue.
In the present study, rats received ouabain treatment first, which was then followed by multiple behavioral tests. Because rats received multiple behavioral tests following ouabain treatment and the brain tissue was subsequently processed for BDNF levels, maze exposure may have affected the expression of frontal cortex BDNF levels. Findings from past studies, however, do not support this idea (Vedovelli et al., 2011; Xin et al., 2014; Zoladz et al., 2012). Specifically, previous experiments have examined frontal cortex BDNF expression in rodents who have been tested for learning, memory and/or extinction and compared to rodents who have not had those behavioral experiences. The results from those experiments indicate that the behavioral experiences alone do not change frontal BDNF levels compared to controls who were not trained and tested on various learning and memory tests (Vedovelli et al., 2011; Xin et al., 2014; Zoladz et al., 2012). Based on these findings, the most parsimonious explanation for the frontal cortex BDNF changes observed in the current study was that frontal cortex BDNF levels were reduced due to ouabain treatment.
The BDNF findings observed in the current study suggest that altered BDNF signaling in the frontal cortex contributes to the behavioral phenotype in ouabain-treated rats. There was a significant correlation between frontal cortex BDNF mRNA levels and regressive errors committed in probabilistic reversal learning. While this finding demonstrates a relationship between BDNF mRNA expression levels and regressive errors, unknown is whether decreasing BDNF signaling in the frontal cortex with ouabain treatment is sufficient to produce a reversal learning deficit by increasing regressive errors. Previous experiments in rats have demonstrated that frontal cortex BDNF expression is important for reversal learning (Graybeal et al., 2011; Kanowski et al., 2007; Xue et al., 2013). Related, other studies indicate that the prefrontal cortex supports the maintenance of a new choice pattern during reversal learning (Baker et al., 20014a,b), including under conditions in which outcomes are uncertain (Kim & Ragozzino, 2005). Taken together, the behavioral and biochemical findings raise the possibility that central ouabain administration altered BDNF signaling in the frontal cortex that impaired the ability to maintain a new choice pattern during reversal learning.
In this experiment BDNF signaling was measured from the entire frontal cortex. This was carried out because past studies have suggested that multiple areas of the frontal cortex support probabilistic reversal learning (Dalton et al., 2016; Hampton & O'doherty, 2007; Hornak et al., 2004; Morris et al., 2016). However, past studies have also shown that different prefrontal cortex subregions may support distinct processes to enable behavioral flexibility (Hamilton & Brigman, 2015; Ragozzino, 2007). This raises the possibility that ouabain treatment affects BDNF signaling in distinct prefrontal cortex subregions to affect probabilistic reversal learning. Future studies can determine whether ouabain treatment affects BDNF expression in specific frontal cortex subregions.
Although central ouabain administration reduced frontal cortex BDNF and impaired probabilistic reversal learning by increasing regressive errors, BDNF and/or other biochemical mechanisms may have been altered in other brain regions by ouabain treatment. Past investigations have demonstrated that the dorsomedial striatum and ventromedial striatum are brain regions that support behavioral flexibility by selectively facilitating a maintenance of a new choice pattern (Haluk & Floresco, 2009; Palencia & Ragozzino, 2006; Ragozzino & Choi, 2004). Thus, ouabain treatment may also alter BDNF signaling in the striatum to affect reversal learning. In support of this idea, an examination of post-mortem tissue in bipolar disorder revealed a reduction in striatal TrkB mRNA (Reinhart et al., 2015). The TrkB receptor shows the highest affinity for BDNF. Therefore, treatments that modulate corticostriatal circuitry involved in cognitive flexibility may be most effective in alleviating reversal learning deficits manifested in bipolar disorder.
The reduced frontal cortex BDNF levels are comparable to clinical studies indicating altered BDNF levels in bipolar disorder (Cunha et al., 2006; Fernandes et al., 2015; Nassan et al., 2015; Palomino et al., 2006; Pandey et al., 2008). Ouabain-induced decreases in frontal cortex BDNF mRNA and protein levels observed in the present study is consistent with past studies showing ouabain reduced BDNF expression in the frontal cortex (Valvassori et al., 2015a; Varela et al., 2015). Previous experiments have also demonstrated that treatment with mood stabilizers, i.e. lithium or valproate or with BDNF itself correct the reduction in frontal cortex BDNF induced by ICV ouabain treatment (Valvassori et al., 2015a; Varela et al., 2015). Mood stabilizers not only corrected the reduction in frontal cortex BDNF levels, but attenuated the elevated locomotor activity (Varela et al., 2015). Less clear is whether mood stabilizers may be effective in alleviating the type of cognitive deficits observed in the present study. One study examined whether the mood stabilizers, lithium and carbamazepine attenuate spatial learning deficits in the ouabain model of mania (Wang et al., 2013). The findings were mixed with lithium having modest effects in alleviating a spatial learning deficit, while carbamazepine was not effective. Because BDNF is associated with cognitive flexibility (Graybeal et al., 2011; Kanowski et al., 2007; Xue et al., 2013), treatment with this trophic factor may be effective in alleviating a cognitive flexibility deficit. In the ouabain model, a central injection of BDNF attenuates alterations in oxidative stress, but does not affect the hyperactivity induced by ouabain (Valvassori et al., 2015a). Although the present study did not investigate BDNF treatment related to cognitive measures, findings from studies on the serotonin 2A (5HT2A) receptor may suggest modulating BDNF signaling would be effective in treating cognitive flexibility deficits. This is in part due to results indicating that BDNF downregulates 5HT2A receptor expression (Trajkovska et al., 2009) and conversely that treatment with a 5HT2A receptor antagonist increases BDNF levels (Pilar-Cuellar et al., 2012). Furthermore, treatment with a 5HT2A receptor antagonist enhances set-shifting in rats by selectively reducing regressive errors (Baker et al., 2011). While another study found in a mouse model of autism that 5HT2A receptor blockade attenuates a probabilistic reversal learning deficit by selectively reducing regressive errors (Amodeo et al., 2014). Taken together, the results raise the possibility that treatment with a 5HT2A receptor antagonist may be effective in treating cognitive flexibility deficits in bipolar disorder, in part, by enhancing BDNF levels.
In summary, the present study demonstrated that central ouabain treatment in rats impaired probabilistic reversal learning in a spatial discrimination test. The reversal learning deficit was due to an impairment in maintaining a new choice pattern after being initially selected. The findings parallel a probabilistic reversal learning deficit reported in bipolar disorder (Dickstein et al., 2010) and an increase in regressive errors during a cognitive flexibility test in bipolar disorder (Hill et al., 2015). The experiment also found a reduction in frontal cortex BDNF expression levels following ouabain treatment and that BDNF expression levels negatively correlated with regressive errors in reversal learning. Thus, the ouabain model of mania may facilitate our understanding of the neuropathophysiology that underlies cognitive flexibility deficits in bipolar disorder, as well as be useful in testing potential treatments to alleviate the cognitive deficits to improve daily living.
Highlights.
Ouabain treatment impairs probabilistic reversal learning
Ouabain treatment increases regressive errors during reversal learning
Ouabain treatment reduces BDNF expression levels in the frontal cortex
There is a negative correlation with regressive errors and frontal cortex BDNF levels
Acknowledgements
This research was supported by National Institutes of Health Grants P50 HD055751, RO1MH082802 and R01MH101890.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amodeo DA, Jones JH, Sweeney JA, Ragozzino ME. Differences in BTBR T+ tf/J and C57BL/6J mice on probabilistic reversal learning and stereotyped behaviors. Behavioural Brain Research. 2012;227:64–72. doi: 10.1016/j.bbr.2011.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amodeo DA, Jones JH, Sweeney JA, Ragozzino ME. Risperidone and the 5-HT2A receptor antagonist M100907 improve probabilistic reversal learning in BTBR T + tf/J mice. Autism Res. 2014;7:555–567. doi: 10.1002/aur.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker PM, Thompson JL, Sweeney JA, Ragozzino ME. Differential effects of 5-HT(2A) and 5-HT(2C) receptor blockade on strategy-switching. Behav Brain Res. 2011;219:123–131. doi: 10.1016/j.bbr.2010.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker PM, Ragozzino ME. Contralateral disconnection of the rat prelimbic cortex and dorsomedial striatum impairs cue-guided behavioral switching. Learn Mem. 2014a;21:368–379. doi: 10.1101/lm.034819.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker PM, Ragozzino ME. The prelimbic cortex and subthalamic nucleus contribute to cue-guided behavioral switching. Neurobiol Learn Mem. 2014b;107:65–78. doi: 10.1016/j.nlm.2013.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee U, Dasgupta A, Rout JK, Singh OP. Effects of lithium therapy on Na+-K+-ATPase activity and lipid peroxidation in bipolar disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2012;37:56–61. doi: 10.1016/j.pnpbp.2011.12.006. [DOI] [PubMed] [Google Scholar]
- Bari A, Theobald DE, Caprioli D, Mar AC, Aidoo-Micah A, Dalley JW, Robbins TW. Serotonin modulates sensitivity to reward and negative feedback in a probabilistic reversal learning task in rats. Neuropsychopharmacology. 2010;35:1290–1301. doi: 10.1038/npp.2009.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnín CM, Torrent C, Goikolea JM, Reinares M, Solé B, Valentí M, Sánchez-Moreno J, Hidalgo D, Tabarés-Seisdedos R, Martínez-Arán A, Vieta E. The impact of repeated manic episodes and executive dysfunction on work adjustment in bipolar disorder. Eur Arch Psychiatry Clin Neurosci. 2014;264:247–254. doi: 10.1007/s00406-013-0431-2. [DOI] [PubMed] [Google Scholar]
- Bora E, Yucel M, Pantelis C. Cognitive endophenotypes of bipolar disorder: a meta-analysis of neuropsychological deficits in euthymic patients and their first-degree relatives. Journal of Affective Disorders. 2009;113:1–20. doi: 10.1016/j.jad.2008.06.009. [DOI] [PubMed] [Google Scholar]
- Brocardo PS, Budni J, Pavesi E, Franco JL, Uliano-Silva M, Trevisan R, Terenzi MG, Dafre AL, Rodrigues AL. Folic acid administration prevents ouabain-induced hyperlocomotion and alterations in oxidative stress markers in the rat brain. Bipolar Disord. 2010;12:414–424. doi: 10.1111/j.1399-5618.2010.00827.x. [DOI] [PubMed] [Google Scholar]
- Brown HD, Baker PM, Ragozzino ME. The parafascicular thalamic nucleus concomitantly influences behavioral flexibility and dorsomedial striatal acetylcholine output in rats. J Neurosci. 2010;30:14390–14398. doi: 10.1523/JNEUROSCI.2167-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown HD, Amodeo DA, Sweeney JA, Ragozzino ME. The selective serotonin reuptake inhibitor, escitalopram, enhances inhibition of prepotent responding and spatial reversal learning. J Psychopharmacol. 2012;26:1443–1455. doi: 10.1177/0269881111430749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brüning CA, Prigol M, Luchese C, Pinton S, Nogueira CW. Diphenyl diselenide ameliorates behavioral and oxidative parameters in an animal model of mania induced by ouabain. Prog Neuropsychopharmacol Biol Psychiatry. 2012;38:168–174. doi: 10.1016/j.pnpbp.2012.03.005. [DOI] [PubMed] [Google Scholar]
- Cahill L, McGaugh JL. Amygdaloid complex lesions differentially affect retention of tasks using appetitive and aversive reinforcement. Behavioral Neurosci. 1990;104:532–543. doi: 10.1037//0735-7044.104.4.532. [DOI] [PubMed] [Google Scholar]
- Clancy MA, Rosli HG, Chamala S, Barbazuk WB, Civello PM, Folta KM. Validation of reference transcripts in strawberry (Fragaria spp.). Mol Genet Genomics. 2013;288:671–681. doi: 10.1007/s00438-013-0780-6. [DOI] [PubMed] [Google Scholar]
- Cunha AB, Frey BN, Andreazza AC, Goi JD, Rosa AR, Gonçalves CA, Santin A, Kapczinski F. Serum brain-derived neurotrophic factor is decreased in bipolar disorder during depressive and manic episodes. Neurosci Letters. 2006;398:215–219. doi: 10.1016/j.neulet.2005.12.085. [DOI] [PubMed] [Google Scholar]
- Daglas R, Yücel M, Cotton S, Allott K, Hetrick S, Berk M. Cognitive impairment in first-episode mania: a systematic review of the evidence in the acute and remission phases of the illness. Int J Bipolar Disord. 2015;3:9. doi: 10.1186/s40345-015-0024-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalton GL, Wang NY, Phillips AG, Floresco SB. Multifaceted Contributions by Different Regions of the Orbitofrontal and Medial Prefrontal Cortex to Probabilistic Reversal Learning. J Neurosci. 2016;36:1996–2006. doi: 10.1523/JNEUROSCI.3366-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decker S, Grider G, Cobb M, Li XP, Huff MO, El-Mallakh RS, Levy RS. Open field is more sensitive than automated activity monitor in documenting ouabain-induced hyperlocomotion in the development of an animal model for bipolar illness. Prog Neuropsychopharmacol Biol Psychiatry. 2000;24:455–462. doi: 10.1016/s0278-5846(99)00111-6. [DOI] [PubMed] [Google Scholar]
- DeCoteau WE, Kesner RP. A double dissociation between the rat hippocampus and medial caudoputamen in processing two forms of knowledge. Behav Neurosci. 2000;114:1096–1108. doi: 10.1037//0735-7044.114.6.1096. [DOI] [PubMed] [Google Scholar]
- Dickstein DP, Finger EC, Brotman MA, Rich BA, Pine DS, Blair JR, Leibenluft E. Impaired probabilistic reversal learning in youths with mood and anxiety disorders. Psychol Med. 2010;40:1089–100. doi: 10.1017/S0033291709991462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Cruz AM, Ragozzino ME, Mosconi MW, Shrestha S, Cook EH, Sweeney JA. Reduced behavioral flexibility in autism spectrum disorders. Neuropsychology. 2013;27:152–160. doi: 10.1037/a0031721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Hooge R, De Deyn PP. Applications of the Morris water maze in the study of learning and memory. Brain Res Brain Res Rev. 2001;36:60–90. doi: 10.1016/s0165-0173(01)00067-4. [DOI] [PubMed] [Google Scholar]
- Dwivedi Y, Rizavi HS, Conley RR, Roberts RC, Tamminga CA, Pandey GN. Altered gene expression of brain-derived neurotrophic factor and receptor tyrosine kinase B in postmortem brain of suicide subjects. Arch Gen Psychiatry. 2003;60:804–815. doi: 10.1001/archpsyc.60.8.804. [DOI] [PubMed] [Google Scholar]
- Fernandes BS, Molendijk ML, Köhler CA, Soares JC, Leite CM, Machado-Vieira R, Ribeiro TL, Silva JC, Sales PM, Quevedo J, Oertel-Knöchel V, Vieta E, González-Pinto A, Berk M, Carvalho AF. Peripheral brain-derived neurotrophic factor (BDNF) as a biomarker in bipolar disorder: a meta-analysis of 52 studies. BMC Med. 2015;13:289. doi: 10.1186/s12916-015-0529-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y, Payne RS, Schurr A, Hougland T, Lord J, Herman L, Lei Z, Banerjee P, El-Mallakh RS. Memantine reduces mania-like symptoms in animal models. Psychiatry Res. 2011;188:366–371. doi: 10.1016/j.psychres.2010.12.030. [DOI] [PubMed] [Google Scholar]
- Gilbert PE, Kesner RP, Lee I. Dissociating hippocampal subregions: double dissociation between dentate gyrus and CA1. Hippocampus. 2001;11:626–636. doi: 10.1002/hipo.1077. [DOI] [PubMed] [Google Scholar]
- Goldstein I, Lerer E, Laiba E, Mallet J, Mujaheed M, Laurent C, Rosen H, Ebstein RP, Lichtstein D. Association between sodium- and potassium-activated adenosine triphosphatase alpha isoforms and bipolar disorders. Biol Psychiatry. 2009;65:985–991. doi: 10.1016/j.biopsych.2008.10.033. [DOI] [PubMed] [Google Scholar]
- Gorrindo T, Blair RJ, Budhani S, Dickstein DP, Pine DS, Leibenluft E. Deficits on a probabilistic response-reversal task in patients with pediatric bipolar disorder. Am J Psychiatry. 2005;162:1975–1977. doi: 10.1176/appi.ajp.162.10.1975. [DOI] [PubMed] [Google Scholar]
- Graybeal C, Feyder M, Schulman E, Saksida LM, Bussey TJ, Brigman JL, Holmes A. Paradoxical reversal learning enhancement by stress or prefrontal cortical damage: rescue with BDNF. Nat Neurosci. 2011;14:1507–1509. doi: 10.1038/nn.2954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haluk DM, Floresco SB. Ventral striatal dopamine modulation of different forms of behavioral flexibility. Neuropsychopharmacology. 2009;34:2041–2052. doi: 10.1038/npp.2009.21. [DOI] [PubMed] [Google Scholar]
- Hamilton DA, Brigman JL. Behavioral flexibility in rats and mice: contributions of distinct frontocortical regions. Genes Brain Behav. 2015;14:4–21. doi: 10.1111/gbb.12191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampton AN, O'Doherty JP. Decoding the neural substrates of reward-related decision making with functional MRI. Proc Natl Acad Sci. 2007;104:1377–1382. doi: 10.1073/pnas.0606297104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman L, Hougland T, El-Mallakh RS. Mimicking human bipolar ion dysregulation models mania in rats. Neurosci Biobehav Rev. 2007;31:874–881. doi: 10.1016/j.neubiorev.2007.04.001. [DOI] [PubMed] [Google Scholar]
- Hill SK, Reilly JL, Ragozzino ME, Rubin LH, Bishop JR, Gur RC, Gershon ES, Tamminga CA, Pearlson GD, Keshavan MS, Keefe RS, Sweeney JA. Regressing to Prior Response Preference After Set Switching Implicates Striatal Dysfunction Across Psychotic Disorders: Findings From the B-SNIP Study. Schizophr Bull. 2015;41:940–950. doi: 10.1093/schbul/sbu130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornak J, O'Doherty J, Bramham J, Rolls ET, Morris RG, Bullock PR, Polkey CE. Reward-related reversal learning after surgical excisions in orbito-frontal or dorsolateral prefrontal cortex in humans. J Cogn Neurosci. 2004;16:463–478. doi: 10.1162/089892904322926791. [DOI] [PubMed] [Google Scholar]
- Huff MO, Li XP, Ginns E, El-Mallakh RS. Effect of ethacrynic acid on the sodium- and potassium-activated adenosine triphosphatase activity and expression in Old Order Amish bipolar individuals. J Affect Disord. 2010;123:303–307. doi: 10.1016/j.jad.2009.09.018. [DOI] [PubMed] [Google Scholar]
- Jornada LK, Valvassori SS, Steckert AV, Moretti M, Mina F, Ferreira CL, Arent CO, Dal-Pizzol F, Quevedo J. Lithium and valproate modulate antioxidant enzymes and prevent ouabain-induced oxidative damage in an animal model of mania. J Psychiatr Res. 2011;45:162–168. doi: 10.1016/j.jpsychires.2010.05.011. [DOI] [PubMed] [Google Scholar]
- Kanoski SE, Meisel RL, Mullins AJ, Davidson TL. The effects of energy-rich diets on discrimination reversal learning and on BDNF in the hippocampus and prefrontal cortex of the rat. Behav Brain Res. 2007;182:57–66. doi: 10.1016/j.bbr.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Ragozzino ME. The involvement of the orbitofrontal cortex in learning under changing task contingencies. Neurobiol Learn Mem. 2005;83:125–133. doi: 10.1016/j.nlm.2004.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SH, Yu HS, Park HG, Jeon WJ, Song JY, Kang UG, Ahn YM, Lee YH, Kim YS. Dose-dependent effect of intracerebroventricular injection of ouabain on the phosphorylation of the MEK1/2-ERK1/2-p90RSK pathway in the rat brain related to locomotor activity. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:1637–1642. doi: 10.1016/j.pnpbp.2008.05.027. [DOI] [PubMed] [Google Scholar]
- Kim SH, Yu HS, Park HG, Ha K, Kim YS, Shin SY, Ahn YM. Intracerebroventricular administration of ouabain, a Na/K-ATPase inhibitor, activates mTOR signal pathways and protein translation in the rat frontal cortex. Prog Neuropsychopharmacol Biol Psychiatry. 2013;45:73–82. doi: 10.1016/j.pnpbp.2013.04.018. [DOI] [PubMed] [Google Scholar]
- Kozicky JM, Torres IJ, Bond DJ, Lam RW, Yatham LN. Comparison of neuropsychological effects of adjunctive risperidone or quetiapine in euthymic patients with bipolar I disorder. Int Clin Psychopharmacol. 2012;27:91–99. doi: 10.1097/YIC.0b013e32834e3bea. [DOI] [PubMed] [Google Scholar]
- Krabbendam L, Arts B, van Os J, Aleman A. Cognitive functioning in patients with schizophrenia and bipolar disorder: a quantitative review. Schizophrenia Research. 2005;80:137–149. doi: 10.1016/j.schres.2005.08.004. [DOI] [PubMed] [Google Scholar]
- Lembke A, Ketter TA. Impaired recognition of facial emotion in mania. Am J Psychiatry. 2002;159:302–304. doi: 10.1176/appi.ajp.159.2.302. [DOI] [PubMed] [Google Scholar]
- Li B, Arime Y, Hall FS, Uhl GR, Sora I. Impaired spatial working memory and decreased frontal cortex BDNF protein level in dopamine transporter knockout mice. Eur J Pharmacol. 2010;628:104–107. doi: 10.1016/j.ejphar.2009.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linke J, Sönnekes C, Wessa M. Sensitivity to positive and negative feedback in euthymic patients with bipolar I disorder: the last episode makes the difference. Bipolar Disorders. 2011;13:638–650. doi: 10.1111/j.1399-5618.2011.00956.x. [DOI] [PubMed] [Google Scholar]
- Martínez-Arán A, Vieta E, Colom F, Torrent C, Sánchez-Moreno J, Reinares M, Benabarre A, Goikolea JM, Brugué E, Daban C, Salamero M. Cognitive impairment in euthymic bipolar patients: implications for clinical and functional outcome. Bipolar Disord. 2004;6:224–232. doi: 10.1111/j.1399-5618.2004.00111.x. [DOI] [PubMed] [Google Scholar]
- McKirdy J, Sussmann JE, Hall J, Lawrie SM, Johnstone EC, McIntosh AM. Set shifting and reversal learning in patients with bipolar disorder or schizophrenia. Psychol Med. 2009;39:1289–1293. doi: 10.1017/S0033291708004935. [DOI] [PubMed] [Google Scholar]
- Minassian A, Henry BL, Young JW, Masten V, Geyer MA, Perry W. Repeated assessment of exploration and novelty seeking in the human behavioral pattern monitor in bipolar disorder patients and healthy individuals. PloS One. 2011;6:e24185. doi: 10.1371/journal.pone.0024185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohler EG, Baker PM, Gannon KS, Jones SS, Shacham S, Sweeney JA, Ragozzino ME. The effects of PRX-07034, a novel 5-HT6 antagonist, on cognitive flexibility and working memory in rats. Psychopharmacology. 2012;220:687–696. doi: 10.1007/s00213-011-2518-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morice R. Cognitive inflexibility and pre-frontal dysfunction in schizophrenia and mania. The British Journal of Psychiatry. 1990;157:50–54. doi: 10.1192/bjp.157.1.50. [DOI] [PubMed] [Google Scholar]
- Morris R. Developments of a water-maze procedure for studying spatial learning in the rat. J Neurosci Meth. 1984;11:47–60. doi: 10.1016/0165-0270(84)90007-4. [DOI] [PubMed] [Google Scholar]
- Morris LS, Kundu P, Dowell N, Mechelmans DJ, Favre P, Irvine MA, Robbins TW, Daw N, Bullmore ET, Harrison NA, Voon V. Fronto-striatal organization: Defining functional and microstructural substrates of behavioural flexibility. Cortex. 2016;74:118–133. doi: 10.1016/j.cortex.2015.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nassan M, Croarkin PE, Luby JL, Veldic M, Joshi PT, McElroy SL, Post RM, Walkup JT, Cercy K, Geske JR, Wagner KD, Cuellar-Barboza AB, Casuto L, Lavebratt C, Schalling M, Jensen PS, Biernacka JM, Frye MA. Association of brain-derived neurotrophic factor (BDNF) Val66Met polymorphism with early-onset bipolar disorder. Bipolar Disord. 2015;17:645–652. doi: 10.1111/bdi.12323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palencia CA, Ragozzino ME. The effect of N-methyl-D-aspartate receptor blockade on acetylcholine efflux in the dorsomedial striatum during response reversal learning. Neuroscience. 2006;143:671–678. doi: 10.1016/j.neuroscience.2006.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palomino Aitor, Vallejo-Illarramendi Ainara, González-Pinto Ana, Aldama Ana, González-Gómez Cristina, Mosquera Fernando, González-García Gixane, Matute Carlos. Decreased levels of plasma BDNF in first-episode schizophrenia and bipolar disorder patients.“. Schizophrenia research. 2006;86:321–322. doi: 10.1016/j.schres.2006.05.028. [DOI] [PubMed] [Google Scholar]
- Pandey GN, Rizavi HS, Dwivedi Y, Pavuluri MN. Brain-derived neurotrophic factor gene expression in pediatric bipolar disorder: effects of treatment and clinical response. J Am Acad Child Adolesc Psychiatry. 2008;47:1077–1085. doi: 10.1097/CHI.0b013e31817eecd9. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates, New York. 1998 doi: 10.1016/0165-0270(80)90021-7. [DOI] [PubMed] [Google Scholar]
- Pilar-Cuéllar F, Vidal R, Pazos A. Subchronic treatment with fluoxetine and ketanserin increases hippocampal brain-derived neurotrophic factor, -catenin and antidepressant-like effects. Br J Pharmacol. 2012;165:1046–1057. doi: 10.1111/j.1476-5381.2011.01516.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragozzino ME, Hellems K, Lennartz RC, Gold PE. Pyruvate infusions into the septal area attenuate spontaneous alternation impairments induced by intraseptal morphine injections. Behav Neurosci. 1995;109:1074–1080. doi: 10.1037//0735-7044.109.6.1074. [DOI] [PubMed] [Google Scholar]
- Ragozzino ME, Adams S, Kesner RP. Differential involvement of the dorsal anterior cingulate and prelimbic-infralimbic areas of the rodent prefrontal cortex in spatial working memory. Behav Neurosci. 1998;112:293–303. doi: 10.1037//0735-7044.112.2.293. [DOI] [PubMed] [Google Scholar]
- Ragozzino ME, Detrick S, Kesner RP. Involvement of the prelimbic-infralimbic areas of the rodent prefrontal cortex in behavioral flexibility for place and response learning. J Neurosci. 1999;19:4585–4594. doi: 10.1523/JNEUROSCI.19-11-04585.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragozzino ME, Ragozzino KE, Mizumori SJ, Kesner RP. Role of the dorsomedial striatum in behavioral flexibility for response and visual cue discrimination learning. Behav Neurosci. 2002;116:105–15. doi: 10.1037//0735-7044.116.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragozzino ME, Choi D. Dynamic changes in acetylcholine output in the medial striatum during place reversal learning. Learn Mem. 2004;11:70–77. doi: 10.1101/lm.65404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragozzino ME, Artis S, Singh A, Twose TM, Beck JE, Messer WS., Jr The selective M1 muscarinic cholinergic agonist CDD-0102A enhances working memory and cognitive flexibility. J Pharmacol Exp Ther. 2012;340:588–594. doi: 10.1124/jpet.111.187625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhart V, Bove SE, Volfson D, Lewis DA, Kleiman RJ, Lanz TA. Evaluation of TrkB and BDNF transcripts in prefrontal cortex, hippocampus, and striatum from subjects with schizophrenia, bipolar disorder, and major depressive disorder. Neurobiol Dis. 2015;77:220–227. doi: 10.1016/j.nbd.2015.03.011. [DOI] [PubMed] [Google Scholar]
- Roybal K, Theobold D, Graham A, DiNieri JA, Russo SJ, Krishnan V, Chakravarty S, Peevey J, Oehrlein N, Birnbaum S, Vitaterna MH. Mania-like behavior induced by disruption of CLOCK. Proc Natl Acad Sci. 2007;104:6406–6411. doi: 10.1073/pnas.0609625104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salamone JD. The involvement of nucleus accumbens dopamine in appetitive and aversive motivation. Behav Brain Res. 1994;61:117–133. doi: 10.1016/0166-4328(94)90153-8. [DOI] [PubMed] [Google Scholar]
- Sandi C, Loscertales M, Guaza C. Experience-dependent facilitating effect of corticosterone on spatial memory formation in the water maze. Eur J Neurosci. 1997;9:637–642. doi: 10.1111/j.1460-9568.1997.tb01412.x. [DOI] [PubMed] [Google Scholar]
- Solé B, Martínez-Arán A, Torrent C, Bonnin CM, Reinares M, Popovic D, Sánchez-Moreno J, Vieta E. Are bipolar II patients cognitively impaired? A systematic review. Psychol Med. 2011;41:1791–1803. doi: 10.1017/S0033291711000018. [DOI] [PubMed] [Google Scholar]
- Souza LC, Wilhelm EA, Bortolatto CF, Nogueira CW, Boeira SP, Jesse CR. The protective effect of melatonin against brain oxidative stress and hyperlocomotion in a rat model of mania induced by ouabain. Behav Brain Res. 2014;271:316–324. doi: 10.1016/j.bbr.2014.06.030. [DOI] [PubMed] [Google Scholar]
- Sui L, Song XJ, Ren J, Ju LH, Wang Y. Intracerebroventricular administration of ouabain alters synaptic plasticity and dopamine release in rat medial prefrontal cortex. J Neural Transm. 2013;120:1191–1199. doi: 10.1007/s00702-013-0973-5. [DOI] [PubMed] [Google Scholar]
- Syed A, Baker PM, Ragozzino ME. Pedunculopontine tegmental nucleus lesions impair probabilistic reversal learning by reducing sensitivity to positive reward feedback. Neurobiology of Learning and Memory. 2016 doi: 10.1016/j.nlm.2016.03.010. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonin PT, Valvassori SS, Lopes-Borges J, Mariot E, Varela RB, Teixeira AL, Quevedo J. Effects of ouabain on cytokine/chemokine levels in an animal model of mania. J Neuroimmunol. 2014;276:236–239. doi: 10.1016/j.jneuroim.2014.09.007. [DOI] [PubMed] [Google Scholar]
- Torrent C, Martinez-Arán A, del Mar Bonnin C, Reinares M, Daban C, Solé B, Rosa AR, Tabarés-Seisdedos R, Popovic D, Salamero M, Vieta E. Long-term outcome of cognitive impairment in bipolar disorder. J Clin Psychiatry. 2012;73:e899–905. doi: 10.4088/JCP.11m07471. [DOI] [PubMed] [Google Scholar]
- Trajkovska V, Santini MA, Marcussen AB, Thomsen MS, Hansen HH, Mikkelsen JD, Arneberg L, Kokaia M, Knudsen GM, Aznar S. BDNF downregulates 5-HT(2A) receptor protein levels in hippocampal cultures. Neurochem Int. 2009;55:697–702. doi: 10.1016/j.neuint.2009.06.013. [DOI] [PubMed] [Google Scholar]
- Valvassori SS, Arent CO, Steckert AV, Varela RB, Jornada LK, Tonin PT, Budni J, Mariot E, Kapczinski F, Quevedo J. Intracerebral Administration of BDNF Protects Rat Brain Against Oxidative Stress Induced by Ouabain in an Animal Model of Mania. Mol Neurobiol. 2015a;52:353–362. doi: 10.1007/s12035-014-8873-8. [DOI] [PubMed] [Google Scholar]
- Valvassori SS, Dal-Pont GC, Steckert AV, Varela RB, Lopes-Borges J, Mariot E, Resende WR, Arent CO, Carvalho AF, Quevedo J. Sodium butyrate has an antimanic effect and protects the brain against oxidative stress in an animal model of mania induced by ouabain. Psychiatry Res. 2015b doi: 10.1016/j.psychres.2015.11.017. in press. [DOI] [PubMed] [Google Scholar]
- Varela RB, Valvassori SS, Lopes-Borges J, Mariot E, Dal-Pont GC, Amboni RT, Bianchini G, Quevedo J. Sodium butyrate and mood stabilizers block ouabain-induced hyperlocomotion and increase BDNF, NGF and GDNF levels in brain of Wistar rats. J Psychiatr Res. 2015;61:114–121. doi: 10.1016/j.jpsychires.2014.11.003. [DOI] [PubMed] [Google Scholar]
- Vedovelli K, Silveira E, Velho E, Stertz L, Kapczinski F, Schröder N. Bromberg E. Effects of increased opportunity for physical exercise and learning experiences on recognition memory and brain-derived neurotrophic factor levels in brain and serum of rats. Neurosci. 2011;199:284–291. doi: 10.1016/j.neuroscience.2011.08.012. [DOI] [PubMed] [Google Scholar]
- Vrabie M, Marinescu V, Tala man A, Tǎutu O, Drima E, Micluţia I. Cognitive impairment in manic bipolar patients: important, understated, significant aspects. Ann Gen Psychiatry. 2015;14:41. doi: 10.1186/s12991-015-0080-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker DL, McGlynn T, Grey C, Ragozzino M, Gold PE. Naloxone modulates the behavioral effects of cholinergic agonists and antagonists. Psychopharm. 1991;105:57–62. doi: 10.1007/BF02316864. [DOI] [PubMed] [Google Scholar]
- Waltz JA, Gold JM. Probabilistic reversal learning impairments in schizophrenia: further evidence of orbitofrontal dysfunction. Schizophrenia research. 2007;93:296–303. doi: 10.1016/j.schres.2007.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YC, Wang EN, Wang CC, Huang CL, Huang AC. Effects of lithium and carbamazepine on spatial learning and depressive behavior in a rat model of bipolar disorder induced by ouabain. Pharmacol Biochem Behav. 2013;105:118–127. doi: 10.1016/j.pbb.2013.02.004. [DOI] [PubMed] [Google Scholar]
- Wang YC, Wang EN, Wang CC, Huang CL, Huang AC. Dissociating effects of spatial learning from locomotor activity for ouabain-induced bipolar disorder-like rats. Psychiatry Res. 2014;216:432–437. doi: 10.1016/j.psychres.2014.03.003. [DOI] [PubMed] [Google Scholar]
- Weiler JA, Bellebaum C, Brüne M, Juckel G, Daum I. Impairment of probabilistic reward-based learning in schizophrenia. Neuropsychology. 2009;23:571–580. doi: 10.1037/a0016166. [DOI] [PubMed] [Google Scholar]
- Wingo AP, Harvey PD, Baldessarini RJ. Neurocognitive impairment in bipolar disorder patients: functional implications. Bipolar Disord. 2009a;11:113–125. doi: 10.1111/j.1399-5618.2009.00665.x. [DOI] [PubMed] [Google Scholar]
- Wingo AP, Wingo TS, Harvey PD, Baldessarini RJ. Effects of lithium on cognitive performance: a meta-analysis. J Clin Psychiatry. 2009b;70:1588–1597. doi: 10.4088/JCP.08r04972. [DOI] [PubMed] [Google Scholar]
- Xin J, Ma L, Zhang TY, Yu H, Wang Y, Kong L, Chen ZY. Involvement of BDNF signaling transmission from basolateral amygdala to infralimbic prefrontal cortex in conditioned taste aversion extinction. J Neurosci. 2014;34:7302–7313. doi: 10.1523/JNEUROSCI.5030-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing B, Guo J, Meng X, Wei SG, Li SB. The dopamine D1 but not D3 receptor plays a fundamental role in spatial working memory and BDNF expression in prefrontal cortex of mice. Behav Brain Res. 2012;235:36–41. doi: 10.1016/j.bbr.2012.06.035. [DOI] [PubMed] [Google Scholar]
- Xue X, Shao S, Wang W, Shao F. Maternal separation induces alterations in reversal learning and brain-derived neurotrophic factor expression in adult rats. Neuropsychobiology. 2013;68:243–249. doi: 10.1159/000356188. [DOI] [PubMed] [Google Scholar]
- Young JW, Goey AK, Minassian A, Perry W, Paulus MP, Geyer MA. The mania-like exploratory profile in genetic dopamine transporter mouse models is diminished in a familiar environment and reinstated by subthreshold psychostimulant administration. Pharmacol Biochem Behav. 2010;96:7–15. doi: 10.1016/j.pbb.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young LT, Bagby RM, Cooke RG, Parker JD, Levitt AJ, Joffe RT. A comparison of Tridimensional Personality Questionnaire dimensions in bipolar disorder and unipolar depression. Psychiatry Research. 1995;58:139–143. doi: 10.1016/0165-1781(95)02684-o. [DOI] [PubMed] [Google Scholar]
- Yu HS, Kim SH, Park HG, Kim YS, Ahn YM. Activation of Akt signaling in rat brain by intracerebroventricular injection of ouabain: a rat model for mania. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34:888–894. doi: 10.1016/j.pnpbp.2010.04.010. [DOI] [PubMed] [Google Scholar]
- Yu HS, Kim SH, Park HG, Kim YS, Ahn YM. Intracerebroventricular administration of ouabain, a Na/K-ATPase inhibitor, activates tyrosine hydroxylase through extracellular signal-regulated kinase in rat striatum. Neurochem Int. 2011;59:779–786. doi: 10.1016/j.neuint.2011.08.011. [DOI] [PubMed] [Google Scholar]
- Zoladz PR, Park CR, Halonen JD, Salim S, Alzoubi KH, Srivareerat M, Fleshner M, Alkadhi KA, Diamond DM. Differential expression of molecular markers of synaptic plasticity in the hippocampus, prefrontal cortex, and amygdala in response to spatial learning, predator exposure, and stress-induced amnesia. Hippocampus. 2012;22:577–589. doi: 10.1002/hipo.20922. [DOI] [PubMed] [Google Scholar]