Abstract
Objectives
To determine whether indocyanine green (ICG)-enhanced near-infrared fluorescence (NIRF) imaging can illuminate high-risk histologic plaque features of human carotid atherosclerosis, and in coronary atheroma of living swine, using intravascular NIRF-optical coherence tomography (OCT) imaging.
Background
New translatable imaging approaches are needed to identify high-risk biological signatures of atheroma. ICG is an FDA-approved NIRF imaging agent that experimentally targets plaque macrophages and lipid in areas of enhanced endothelial permeability, but it is unknown whether ICG can target atheroma in patients.
Methods
Eight patients were enrolled in the BRIGHT-CEA trial (NCT01873716). Five patients were injected intravenously with ICG 99±25 minutes before clinically indicated carotid endarterectomy. Three saline-injected endarterectomy patients served as controls. Excised plaques underwent analysis by intravascular NIRF-OCT, reflectance imaging, microscopy, and histopathology. Next, following ICG intravenous injection, in vivo intracoronary NIRF-OCT and intravascular ultrasound (IVUS) imaged three atheroma-bearing coronary arteries of a diabetic, cholesterol-fed swine.
Results
ICG was well-tolerated; no adverse clinical events occurred up to 30 days post-injection. Multimodal NIRF imaging including intravascular NIRF-OCT revealed that ICG accumulated in all endarterectomy specimens. Plaques from saline-injected control patients exhibited minimal NIRF signal. In the swine experiment, intracoronary NIRF-OCT identified ICG uptake in all IVUS-identified plaques in vivo. On detailed microscopic evaluation, ICG localized to plaque areas exhibiting impaired endothelial integrity, including disrupted fibrous caps, and within areas of neovascularization. Within human plaque areas of endothelial abnormality, ICG was spatially related localized to zones of plaque macrophages and lipid, and notably, intraplaque hemorrhage.
Conclusions
This study demonstrates that ICG targets human plaques exhibiting endothelial abnormalities, and provides new insights into its targeting mechanisms in clinical and experimental atheroma. Intracoronary NIRF-OCT of ICG may offer a novel, clinically-translatable approach to image pathobiological aspects of coronary atherosclerosis.
Clinical Trial Info
The Indocyanine Green Fluorescence Uptake in Human Carotid Artery Plaque Trial [BRIGHT-CEA] NCT01873716
Keywords: atherosclerosis, indocyanine green, endothelium, inflammation, lipid, intraplaque hemorrhage, near-infrared fluorescence, molecular imaging, intravascular imaging
Condensed Abstract
We investigated the ability of indocyanine green (ICG), an FDA-approved near-infrared fluorescence (NIRF) imaging agent, to target and illuminate human carotid atherosclerotic plaques and coronary atheroma of living swine, using translatable intravascular NIRF-optical coherence tomography (OCT). Histopathological assessment of human plaques revealed that ICG illuminated impaired plaque endothelial barrier function, and beneath these areas, localized in regions of macrophages, lipid, and intraplaque hemorrhage. Intracoronary NIRF-OCT further detected ICG in swine coronary atheroma. These results demonstrate that a clinically translatable system and catheter can achieve ICG NIR fluorescence imaging, and that the ICG NIRF signal indicates impaired endothelial integrity in advanced human plaques.
INTRODUCTION
The identification of high-risk atherosclerotic plaques likely to cause myocardial infarction or stroke requires new approaches. In conjunction with near-infrared fluorescence (NIRF) molecular imaging agents, intravascular NIRF imaging is a promising new approach to image biological aspects of high-risk atheroma in coronary artery-sized vessels.(1,2) In addition, the combination of NIRF molecular imaging with optical coherence tomography (OCT), a clinical high-resolution structural imaging approach, offers both molecular and morphological information, as well as quantitative NIRF imaging.(3) Yet a major barrier to the clinical translation of NIRF imaging remains the lack of clinically approved NIRF imaging agents that specifically target high-risk features of atherosclerosis.
Indocyanine green (ICG) remains an intriguing candidate agent to enable targeted NIRF imaging of atherosclerosis. ICG, an amphiphilic near-infrared fluorophore, has received approval by the US Food and Drug Administration (FDA) as a perfusion agent for cardiac output measurements, liver function tests, and ophthalmic angiography, and has an excellent safety profile.(4) Our recent experimental study demonstrated that (i) cultured macrophages and foam cells can internalize ICG, putatively by direct binding to albumin or low density lipoprotein (LDL); (ii) in atherosclerotic rabbits, ICG targeted plaque lipid and macrophages, and deposited in areas of enhanced endothelial permeability delineated by Evans Blue stain; and (iii) ICG enabled rapid intravascular NIRF imaging of rabbit atherosclerosis in vivo (5), a finding subsequently confirmed by Lee et al.(6) Yet given the differences in the complexity and size of human atherosclerosis compared to experimental atherosclerosis, it remains unclear whether ICG can target atherosclerosis in living patients, and if so, which aspects of human atherosclerosis. In addition, the ability of ICG to provide sufficient targeting sensitivity to enable NIRF imaging of atherosclerosis in coronary arteries in vivo requires further investigation.
To advance the potential of intracoronary NIRF molecular imaging, here we report: (i) a first-in-human study of ICG targeting to atheroma in vivo prior to carotid endarterectomy (CEA); and (ii) a first in vivo intracoronary NIRF-OCT imaging study of ICG targeting of coronary atheroma in swine.
METHODS
Human study of ICG plaque targeting (BRIGHT-CEA; ClinicalTrials.gov Identifier: NCT01873716)
The study was approved by the Institutional Review Board of the Partners Human Research Committee (ICG patients, Partners IRB #2012P000895; Control patients for discarded human samples, Partners IRB #2013P002190). Written informed consent was obtained from all patients who received intravenous ICG. Inclusion criteria were: scheduled elective carotid endarterectomy (CEA), age above 18 years, and a signed informed consent. Exclusion criteria were: hemodynamic instability, pregnancy or lactation, any history of iodide/seafood allergy, renal failure, liver failure, bleeding diathesis, or stroke in the preceding 3 months. Five patients scheduled for clinically indicated CEA (significant stenosis and/or signs of cerebral ischemia) were administered ICG in this pilot study, and 3 patient plaques without ICG injection served as controls (received saline).
ICG, possessing a blood half-life ~3 minutes in subjects with normal liver function, was injected intravenously (0.25 mg/kg, up to 25mg maximum dose over 1 minute; Akorn Pharmaceuticals; Lake Forest, IL; outpatient ophthalmic angiography routinely uses an identical ICG dose). Patients were then transported to the operating room for CEA. Control plaques were collected from the operating room immediately after CEA and transported in cold saline to the imaging facility within 15–30 minutes. All eight patient samples were analyzed.
Near-infrared fluorescence imaging of ICG-enhanced human carotid plaques
Intra-arterial NIRF-OCT and fluorescence reflectance imaging
After surgical resection, the carotid plaque specimen was placed in cold saline for immediate ex vivo intravascular NIRF-OCT. The NIRF-OCT catheter was carefully inserted within the lumen traversing the common and internal carotid arteries. Two NIRF-OCT pullbacks were performed per sample for reproducibility, followed by macroscopic fluorescence reflectance imaging (FRI), as previously described (3,5). In brief, the NIRF-OCT imaging catheter and system acquire simultaneous NIRF and OCT data at a speed of 52 kHz (25.4 frames per second, with 2,048 A-lines per image) and pullback velocity of 10 mm/sec. OCT images have an axial resolution of ~10–15 µm in tissue, a lateral resolution of ~30 µm and signal-to-noise ratio (SNR) >110 dB. NIRF images have a lateral resolution of 100–200 µm with a signal-to-noise ratio (SNR) of 51 dB at a concentration of 100 nM ICG. The NIRF-OCT imaging catheter possesses an external diameter of 0.8 mm (2.4 French), similar to the dimensions of existing clinical single-modality OCT catheters. FRI (Kodak ImageStation 4000, Carestream Health; Rochester, NY) was performed at exposure times of 4 and 64 sec for FITC autofluorescence (excitation/emission 470/535 nm) and NIRF (excitation/emission 740/790 nm), respectively.
Microscopic detection of ICG-enhanced human atheroma
After ex vivo imaging, CEA tissue was embedded fresh into optimal cutting temperature media. Next, serial 8 µm cryostat sections were obtained. Fluorescence and brightfield microscopy was performed with an epifluorescence microscope (Nikon Eclipse 90i; Tokyo, Japan). Autofluorescence was detected with a FITC filter (excitation/emission 480/535 nm) and ICG was detected with a near-infrared filter (excitation/emission 775/845 nm).(7)
Matched histological sections corresponding to the NIRF-OCT cross-sectional images were stained with hematoxylin and eosin (H&E), Masson's trichrome (MT), Oil Red O (ORO), Movat's Pentachrome (MP), or Carstairs’ according to the manufacturer’s recommendations. CD68 immunostaining was performed on fresh frozen cryostat sections fixed in 1:1 acetone:methanol for 10 minutes (−20°C), air-dried, and blocked with protein blocking solution for 30 min. Primary monoclonal mouse anti-human antibodies for macrophages (CD68 clone EBM11, 1:200 dilution; Dako North America; Carpinteria, CA) were applied overnight in a humidified chamber at 4°C. Sections were then incubated with MACH2 labeled AP polymer secondary and visualized with Vulcan fast red chromagen (Biocare Medical; Concord, CA).
Intracoronary NIRF-OCT and histopathology of ICG-enhanced plaques in swine coronary atheroma
Please see Online Supplement 2 for methods.
RESULTS
As part of the BRIGHT-CEA trial (NCT01873716), five electively scheduled patients undergoing CEA received ICG intravenously before surgery (0.25 mg/kg up to 25mg). Three control CEA patients received saline. Surgical resection occurred 99±25 minutes after ICG injection. No adverse events related to ICG were reported during hospitalization or at 30-day follow-up telephone contact.
Intravascular NIRF-OCT detects ICG deposition in human carotid plaques
Immediately after surgical resection, freshly isolated carotid plaques underwent ex vivo intra-arterial NIRF-OCT,(4) fluorescence reflectance imaging (FRI), and correlative histopathology. Distance-corrected, quantitative intravascular NIRF-OCT imaging was performed as before.(8) Focal NIRF plaque signal was evident in all five ICG patients (Figures 1 and 2). ICG NIRF signal was highest in or adjacent to the most stenotic area of the internal carotid artery. Minimally diseased areas yielded scant ICG NIRF signal (Figure 2). Plaques from control patients also exhibited little NIRF signal, consistent with low near-infrared autofluorescence in tissues.(1) Simultaneously acquired co-registered OCT images exhibited bulky plaques with abundant lipid-rich areas.
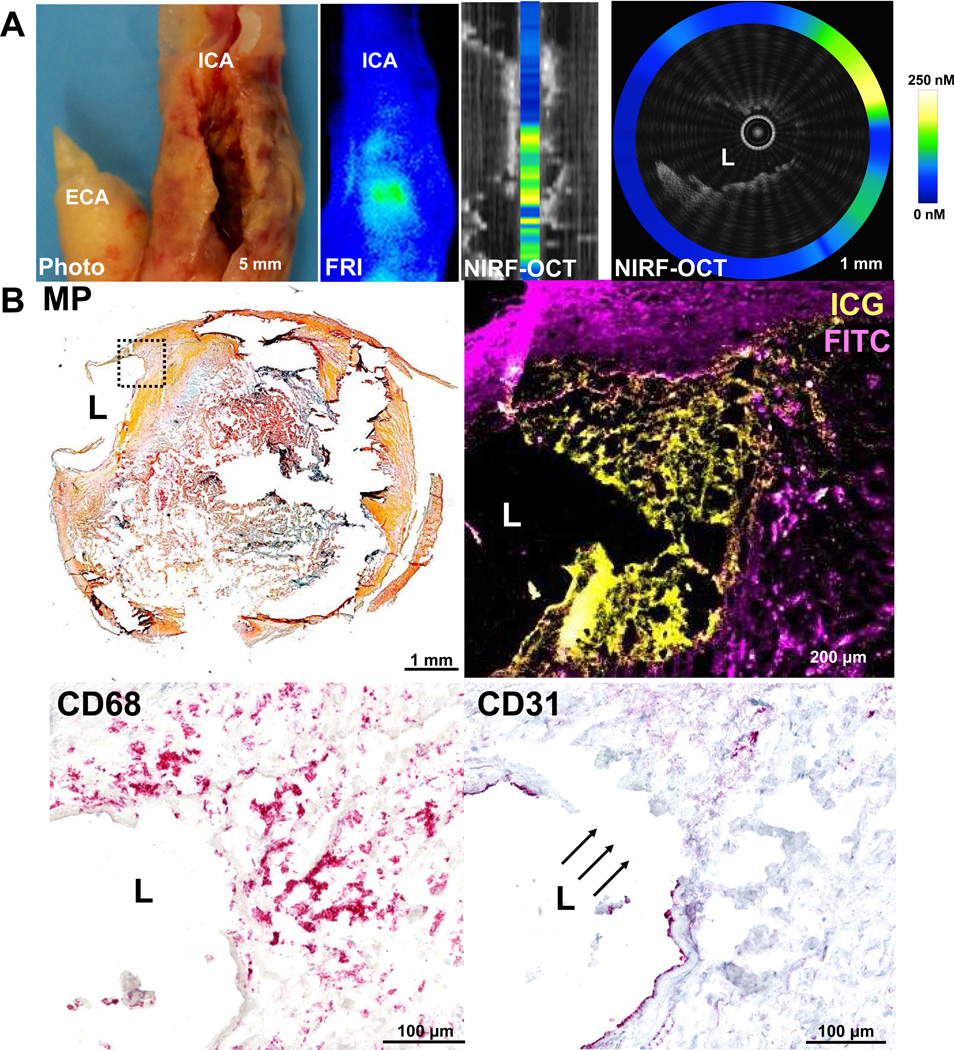
Figure 1. ICG targets human carotid atherosclerosis in vivo in areas of endothelial discontinuity, and NIRF imaging can detect ICG deposition.
ICG was intravenously injected ~1.5 hours before harvest of a representative carotid endarterectomy specimen. (A) Photograph and corresponding near-infrared fluorescence reflectance image (FRI), demonstrating similar morphology and corresponding ICG uptake pattern (light blue=low ICG signal; green-yellow=high ICG signal) at the stenotic region (white arrowheads) in the internal carotid artery (ICA). The upper row middle right image shows an intravascular NIRF-OCT longitudinal fusion image that is anatomically co-registered with the FRI image. The vertical bar inside the lumen in this image depicts the average NIRF signal per cross section. The upper right panel shows a NIRF-OCT cross-sectional fusion image at the area along the white dashed line on the FRI and NIRF-OCT longitudinal fusion images. OCT displays decreased signal intensity at the plaque surface, consistent with a thinned or absent fibrous cap (white dotted box). In this area of diminished OCT signal, increased NIRF signal is evident, represented by the color-scaled circle. (B) Histological analysis of the same area of the cross-sectional image shown in the right image of row A. Movat’s pentachrome (MP) reveals a complex atherosclerotic plaque with a large necrotic core with lipid and cellular infiltration (dotted box). Higher magnification (10×) fluorescence microscopy of the boxed area reveals ICG NIRF signal adjacent to the lumen, which is distinct from FITC-channel autofluorescence. CD68 staining of this same area demonstrates that the ICG NIRF signal (yellow pseudocolor) spatially relates to CD68-defined plaque macrophages beneath the area of intimal disruption. The disruption is confirmed by CD31 staining in this same area. ECA=external carotid artery, FITC=fluorescein isothiocyanate, H&E=Hematoxylin=nuclei (blue), MP=Movat’s Pentachrome, Eosin=eosinophilic structures (pink/red), L=lumen.
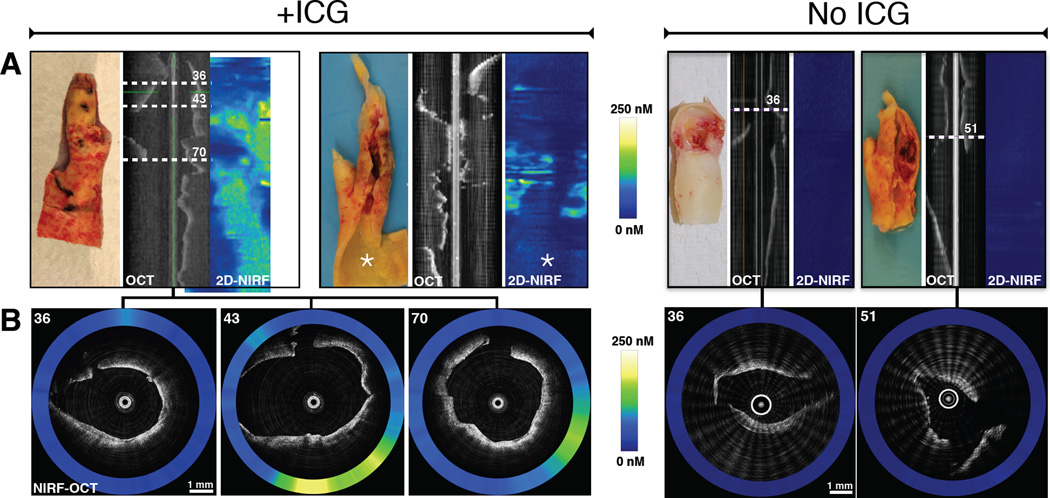
Figure 2. Additional representative ex vivo NIRF imaging examples of two carotid plaques after ICG injection, and two control plaques without ICG.
(A) From left-to-right, gross photograph, aligned OCT longitudinal image and 2D NIRF map (horizontal axis = 0 to 360°, vertical axis = catheter pullback distance), respectively, of two plaques from ICG-injected patients (+ICG) and two control plaques (No ICG). Pullbacks were performed with the NIRF-OCT catheter positioned within the lumen of the resected carotid artery specimens. (B) Representative simultaneously acquired and co-registered NIRF-OCT cross-sectional fusion images from ICG-injected subjects shown on the left panel of images in A. NIRF-OCT demonstrates areas of elevated ICG signal localization within each internal carotid artery plaque (left panel in B, axial white dotted line slices 36, 43, and 70 in the OCT image in A). The right panels in B show two axial NIRF-OCT images from saline-injected control plaques (white dotted lines 36 and 51) that reveal minimal near-infrared autofluorescence signal. The quantitative NIRF scale bars shown (0–250 nM) apply identically to all NIRF images from panels A and B.
ICG deposits beneath areas of impaired endothelial barrier function
Detailed fluorescence microscopy and histopathological analyses revealed new targeting profiles of ICG in human atherosclerosis. ICG deposited beneath areas of endothelial disruption, including plaques with macrophage and lipid infiltration (Figure 1) or frankly disrupted (Figure 3) fibrous caps. Directly beneath these areas of endothelial abnormality, ICG was spatially related to macrophage-rich and lipid-rich zones (Figures 1 and Online Supplemental Figure 1), extending results from our prior experimental study.(5) The human carotid studies also revealed a new target of ICG in plaques: deposition into areas of intraplaque hemorrhage (IPH, as detected by Carstairs’ fibrin stains and Masson’s trichrome, Figure 4, Online supplemental Figure 1A and 1B).
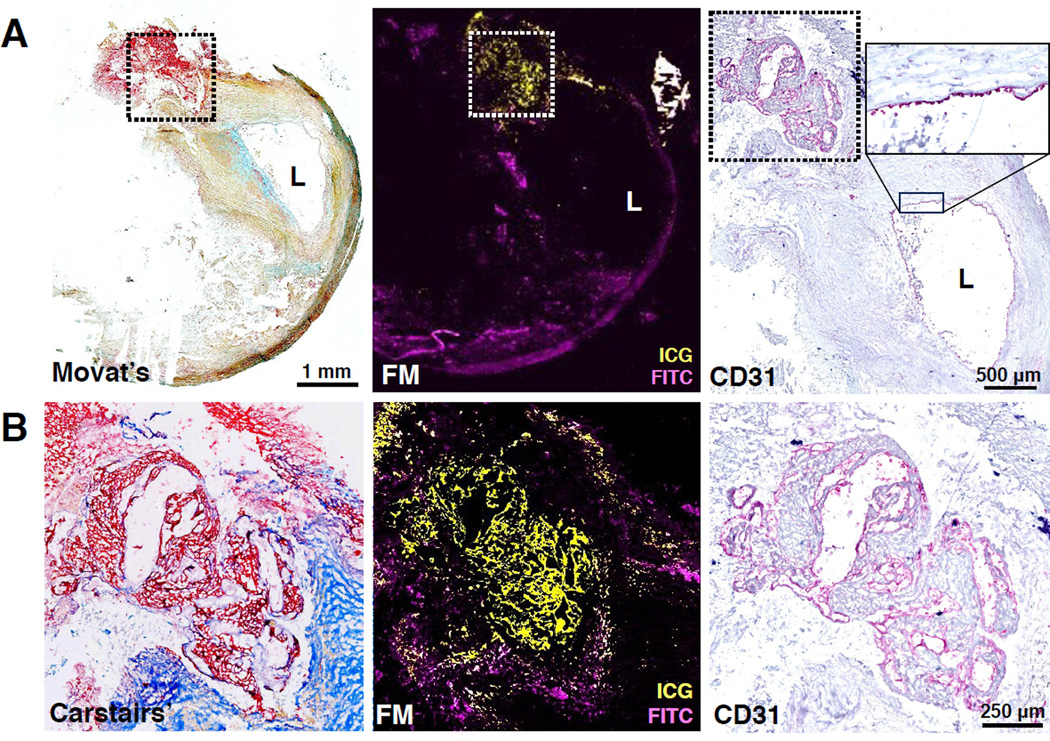
Figure 3. ICG deposits directly beneath an area of subclinical carotid plaque rupture with endothelial discontinuity and atherothrombosis (estimated 1–2 weeks old).
(A) Low-magnification Masson's trichrome (MT) and fluorescence microscopy (purple=FITC-channel autofluorescence, yellow=ICG). The MT stain demonstrates frank plaque rupture (disruption of blue collagen fibers, black arrows) and protrusion of the necrotic core into the lumen. An adjacent FM section (middle image) reveals strong ICG co-localization deposition (yellow) in the region of plaque rupture. The anatomically co-registered NIRF-OCT fusion image (right image) demonstrates high ICG signal by NIRF and plaque surface irregularity by OCT in the regions corresponding to the histological plaque rupture zone (white arrow; quantitative NIRF scale bar 0–250 nM). (B) Low and high magnification H&E staining demonstrated evidence of brown pigment, consistent with hemosiderin within macrophages (arrows) and areas with fibrin. These features indicate that plaque rupture with atherothrombosis occurred subacutely, rather than acute plaque hemorrhage as a direct consequence of surgery.
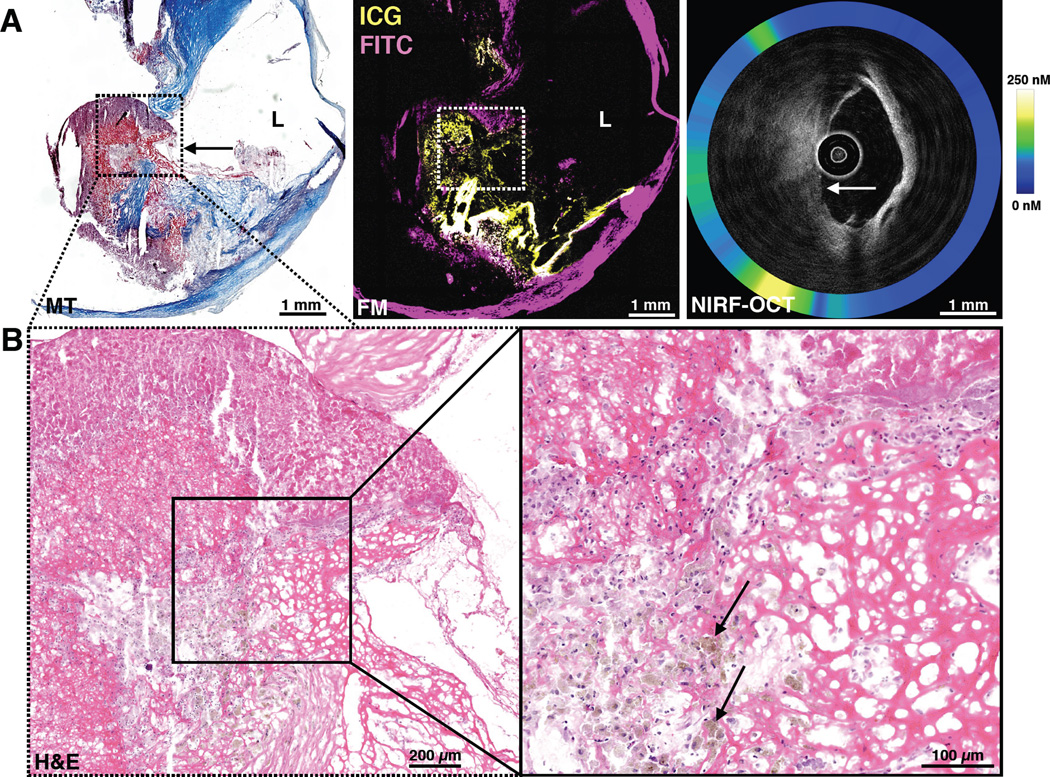
Figure 4. ICG deposits in areas of intraplaque hemorrhage (IPH) in a human atheroma.
(A) Low magnification Movat’s pentachrome staining and FM (purple=FITC-channel autofluorescence, yellow=ICG) reveal strong ICG uptake at a location of plaque hemorrhage beneath the highly stenotic plaque lumen (L), that was clearly demarcated by CD31 (right top, high magnification box). (B) Higher magnification (5×) FM of the dashed box area in A) demonstrates a large circumscribed zone of ICG-positive signal that colocalizes with intraplaque hemorrhage (Carstairs’ fibrin staining, red) and CD31 staining demonstrates that neovessels are present in the area of intraplaque hemorrhage, offering a potential pathway for ICG extravasation.
ICG enables intravascular NIRF-OCT imaging of coronary atherosclerosis in vivo
In the second part of this translational study, we investigated whether ICG could target intracoronary NIRF plaque imaging in vivo. One of the four swine investigated developed coronary atheroma detectable by IVUS. This animal received ICG (0.25 mg/kg IV). Five hours later, intracoronary NIRF-OCT demonstrated focal NIRF signal in areas of lipid-rich plaque in the left anterior descending artery (Figure 5). NIRF microscopy revealed that ICG was spatially related to a deep calcified nodule in this swine model of atherosclerosis.
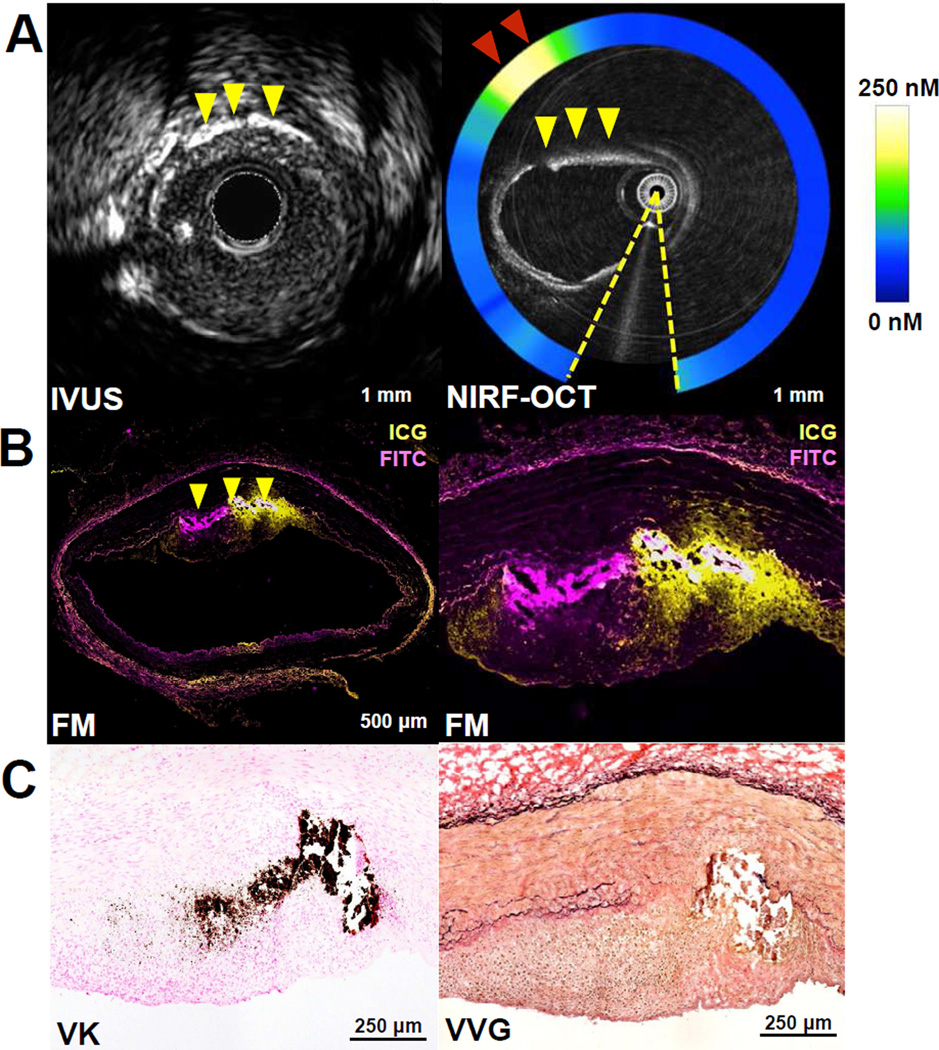
Figure 5. Intracoronary NIRF-OCT and histological assessment of ICG deposition in swine atherosclerosis.
(A) Co-registered IVUS and NIRF-OCT cross-sectional fusion images in the left anterior descending artery, with NIRF signal detecting up to 250nM of ICG (red arrowheads denote high ICG NIRF signal (yellow/white color at 10 o’clock). Coronary atheroma demonstrates heterogeneous ICG plaque uptake in a lesion with intimal and medial calcification (yellow arrowheads). The plaque morphology and calcified region allowed precise co-registration among IVUS, NIRF-OCT, and histology. Artifact from the intracoronary guidewire (dashed yellow lines, NIRF-OCT) was excluded from the NIRF signal ring, OCT catheter dimensions: inner bright circle, 0.5mm diameter; outer faint circle, 0.8mm diameter. (B) Fluorescence microscopy (FM; purple=FITC-channel autofluorescence, yellow=ICG) and histological assessment of the calcified coronary atheroma in panel A. Low and high magnification FM (2nd row, left) shows ICG plaque uptake in the same region of the plaque with calcification (yellow arrowheads). (C) von Kossa calcification stain (VK, dark brown) and Verhoeff-Van Gieson (VVG) stain) confirm the OCT and IVUS images by demonstrating that the calcium resides within the deep intima at the medial border.
DISCUSSION
In this first-in-human investigation of targeted ICG NIRF imaging of human atherosclerosis, we found that FDA-approved ICG (i) deposits in human carotid plaques and is detectable by NIRF imaging; (ii) illuminates the plaque feature of impaired endothelial integrity; and (iii) beneath such areas of endothelial compromise, localizes in zones of macrophages, lipid, and notably, intraplaque hemorrhage.
Impaired endothelial barrier function promotes atherogenesis by facilitating lipid transport(9), leukocyte transmigration(10), and intraplaque hemorrhage.(11) Here we show that ICG-enhanced NIRF imaging provides a novel approach for imaging impaired endothelial integrity, a form of endothelial dysfunction (12,13), in advanced human plaques. ICG did not diffusely illuminate all areas of atherosclerosis (Figures 1A and 2A), but rather deposited adjacent to areas of endothelial compromise with advanced features such as plaque disruption or intraplaque hemorrhage. ICG deposited in regions of overt endothelial disruption (Figure 1 and 3) overlying areas of macrophage infiltration (Figure 1, Supplemental Figure 1), and areas of intraplaque hemorrhage (Figures 4, Supplemental Figure 1). While ICG is a small molecule (MW 775 kDa), on injection into blood, ICG primarily binds albumin(14) and secondarily to lipoproteins, as previously showed directly by size exclusion chromatography.(5) Therefore, ICG is anticipated to follow a deposition pattern similar to albumin, which can localize to damaged intimal layers in atheroma and in areas of injured endothelium.(15,16) The overall results demonstrate that ICG deposition demarcates plaque areas that harbor a compromised endothelial barrier.
The ICG targeting pattern observed in this human atherosclerosis study merits discussion in comparison to prior experimental results. In rabbit atherosclerosis, ICG localized to macrophage-rich and lipid-rich atheroma,(5,6) and in vitro human studies demonstrated that ICG could label human macrophages, and bind acetylated LDL.(5) Of note one prior study (5) demonstrated that ICG also deposited in areas of impaired endothelial barrier function, highlighted by Evans Blue extravasation into ICG-positive plaques. In the current human study, we found that ICG could bind zones of atheroma governed by endothelial compromise. We postulate that the greater size of human carotid plaques (~10-fold thicker than rabbit atheroma) revealed that ICG has diffusion limits as it interacts with larger atheroma, a finding potentiated by ICG’s relatively short blood half-life of 3–5 minutes. While deposition of ICG occurred in certain regions of human plaque macrophages and lipid, binding was not as specific as in the prior rabbit study, suggesting the presence of additional ICG binding targets in human atheroma. To this point, we discovered that ICG could deposit in areas of human intraplaque hemorrhage (fibrin-positive areas in Figure 4 and Supplemental Figure 1), a new finding compared to prior studies of atherosclerotic rabbits, whose lesions do not routinely develop intraplaque hemorrhage. IPH is a high-risk plaque feature that occurs in the setting of plaque neovascularization and can drive plaque progression and adverse clinical events (11,17). Mechanisms underlying the retention of ICG in regions of intraplaque hemorrhage merit further investigation. Finally, imaging of ICG occurred on average 99 minutes after ICG injection and surgical resection, a longer period than in rabbits where detection of ICG occurred within 20 minutes after injection. The ability of ICG imaging in human plaques at earlier timepoints using intravascular NIRF-OCT merits future clinical studies. Despite the relatively small volume of plaque labeled by ICG, the targeted volume sufficed to enable intravascular NIRF-OCT of human plaques ex vivo and swine coronary plaques in vivo. The detected concentration of the ICG NIR fluorophore in human plaques ex vivo and swine plaques in vivo, ranging up to 250 nM, is a similar concentration that permitted in vivo NIRF-OCT imaging of rabbit atherosclerosis.(3) The overall study results thus lend support for an intracoronary NIRF imaging trial of ICG for detecting high-risk features of coronary plaques characterized by abnormal endothelial barrier function, a feature that conventional approaches cannot detect in vivo in human coronary plaques. Therefore ICG NIRF provides additional information beyond OCT: detecting regions with plaque disruption, impaired endothelial integrity, and intraplaque hemorrhage not visualized by OCT. We thus postulate that ICG, through its capacity to detect regions of endothelial compromise, provides additional information that can in principle be used to differentiate high-risk from low-risk fibroatheroma. For example, a subset of thin cap fibroatheromas (TCFAs) that exhibits ICG-NIRF signal might have higher risk of provoking a clinical thrombotic event than a subset of ICG-negative TCFAs. The current study provides a foundation for formal testing of this hypothesis. Translationally, intracoronary NIRF molecular imaging is accelerating into the clinical arena(18,19) and encouragingly, a recent clinical study demonstrates the ability to perform human intracoronary NIRF-OCT detection of coronary plaque NIR autofluorescence.(20) This study provides a foundation for targeted NIRF-OCT molecular imaging in humans in the near future.
In summary, ICG enables targeted intravascular NIRF imaging of impaired endothelial integrity in human plaques and in vivo in swine coronary plaques. Within these areas of impaired endothelial barrier function, ICG deposits in accessible zones of human plaques, including regions containing macrophages, lipid, and intraplaque hemorrhage. Intravascular NIRF imaging of ICG may therefore offer a new approach to assess the pathobiology of human coronary plaques.
Supplementary Material
Fluorescence microscopy (purple=FITC-channel autofluorescence, yellow=ICG) and histological analysis of two human carotid artery plaques from patients after receiving intravenous ICG (A and B), and one control patient without ICG (C). Column 1 shows low magnification Movat's pentachrome staining (MP) followed by higher magnification (5×) fluorescence microscopy (FM), CD68 immunohistochemistry, Oil Red O (ORO), Masson's trichrome (MT), and Carstairs' fibrin staining highlighting the dashed boxed area in column 1. (A) A stenotic internal carotid artery plaques reveals ICG uptake in the area of CD68-positive macrophages (pink). (B) ICG deposits in an area of CD68+ macrophages and ORO+ lipid (orange-red), as well as intraplaque hemorrhage identified by Carstairs' fibrin staining (red). (C) Control plaque (no ICG injection) demonstrates minimal near-infrared autofluorescence. L=lumen.
Clinical Perspectives.
Competency in Medical Knowledge
Patients with carotid plaques possessing an impaired plaque endothelial barrier bind indocyanine green (ICG), an FDA-approved injectable near-infrared fluorescence (NIRF) imaging agent. In plaques with an impaired endothelial barrier, ICG deposits in zones of macrophages, lipid, and intraplaque hemorrhage.
Translational Outlook
Translation of ICG-enhanced intracoronary near-infrared fluorescence imaging and optical coherence tomography may offer a new approach to the simultaneous assessment of plaque pathobiology and microstructure in clinical subjects presenting to the cardiac catheterization laboratory.
Acknowledgments
Disclosures: Dr. Libby has sponsored research grants from General Electric, GlaxoSmithKline, and Novartis. Massachusetts General Hospital has a patent licensing arrangement with Terumo and Canon Corporations. Dr. Tearney (Terumo, Canon, MIT) and Dr. Jaffer (Canon) have the right to receive licensing royalties. Dr. Tearney receives sponsored research from Canon Inc. and Ardea Biosciences and catheter components from Terumo. Dr. Jaffer has sponsored research grants from Kowa, Siemens, and Canon, has a consulting agreement with Boston Scientific and Abbott Vascular.
Funding Sources
-
-
NIH R01 HL108229 and R01 HL122388-01A1 (FAJ), R01 HL093717 (development of imaging console and catheter, GT), R01 HL080472 (PL), Bethesda MA;
-
-
American Heart Association (#13GRNT17060040, FAJ), Dallas, TX;
-
-
MGH SPARK Award (FAJ), MGH ECOR Support Fund (FAJ), George D. Behrakis Cardiovascular Research Fellowship (PHS, APA, MIP), Harvard Catalyst NIH KL2 TR001100 (EAO), Boston MA;
-
-
Rubicon Grant 825.12.013 / Netherlands Organization for Scientific Research (JWV), the Hague, the Netherlands.
The authors acknowledge Dr. Mireille Rosenberg, Heather Marino, Luke Stone and Constance Crittenden, Massachusetts General Hospital Research Study Coordinators, for study assistance; Dr. James Stone and Zakir Siddiquee, Massachusetts General Hospital for assistance with histopathology; Dr. Ahmet Umit Coskun, Brigham and Women’s Hospital and Northeastern University, for advice on in vivo swine experiments; Dr. Jenny Zhao, Massachusetts General Hospital, for assistance with histopathology; Dr. Joseph Boyle, Imperial College, for helpful discussions; and Dr. Evangelos Gragoudas, Massachusetts General Hospital, for observation of ICG-based retinal angiography
Abbreviation list
- ICG
Indocyanine green
- FDA
Food and Drug Administration
- NIRF
Near-infrared fluorescence
- OCT
Optical coherence tomography
- FM
Fluorescence microscopy
- IRB
Institutional review board
- CEA
Carotid endarterectomy
- SNR
Signal-to-noise ratio
- FRI
Fluorescence reflectance imaging
- H&E
Hematoxylin & eosin
- ORO
Oil Red O
- IVUS
Intravascular ultrasound
- vWF
von Willebrand factor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Jaffer FA, Calfon MA, Rosenthal A, et al. Two-dimensional intravascular near-infrared fluorescence molecular imaging of inflammation in atherosclerosis and stent-induced vascular injury. J Am Coll Cardiol. 2011;57:2516–2526. doi: 10.1016/j.jacc.2011.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mulder WJ, Jaffer FA, Fayad ZA, Nahrendorf M. Imaging and nanomedicine in inflammatory atherosclerosis. Sci Transl Med. 2014;6:239sr1. doi: 10.1126/scitranslmed.3005101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yoo H, Kim JW, Shishkov M, et al. Intra-arterial catheter for simultaneous microstructural and molecular imaging in vivo. Nat Med. 2011;17:1680–1684. doi: 10.1038/nm.2555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hope-Ross M, Yannuzzi LA, Gragoudas ES, et al. Adverse reactions due to indocyanine green. Ophthalmology. 1994;101:529–533. doi: 10.1016/s0161-6420(94)31303-0. [DOI] [PubMed] [Google Scholar]
- 5.Vinegoni C, Botnaru I, Aikawa E, et al. Indocyanine green enables near-infrared fluorescence imaging of lipid-rich, inflamed atherosclerotic plaques. Sci Transl Med. 2011;3:84ra45. doi: 10.1126/scitranslmed.3001577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee S, Lee MW, Cho HS, et al. Fully integrated high-speed intravascular optical coherence tomography/near-infrared fluorescence structural/molecular imaging in vivo using a clinically available near-infrared fluorescence-emitting indocyanine green to detect inflamed lipid-rich atheromata in coronary-sized vessels. Circ Cardiovasc Interv. 2014;7:560–569. doi: 10.1161/CIRCINTERVENTIONS.114.001498. [DOI] [PubMed] [Google Scholar]
- 7.Hara T, Bhayana B, Thompson B, et al. Molecular imaging of fibrin deposition in deep vein thrombosis using fibrin-targeted near-infrared fluorescence. JACC Cardiovasc Imaging. 2012 Jun;5(6):607–615. doi: 10.1016/j.jcmg.2012.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ughi GJ, Verjans J, Fard AM, et al. Dual modality intravascular optical coherence tomography (OCT) and near-infrared fluorescence (NIRF) imaging: a fully automated algorithm for the distance-calibration of NIRF signal intensity for quantitative molecular imaging. Int J Cardiovasc Imaging. 2015;31:259–268. doi: 10.1007/s10554-014-0556-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rutledge JC, Curry FR, Lenz JF, Davis PA. Low density lipoprotein transport across a microvascular endothelial barrier after permeability is increased. Circ Res. 1990;66:486–495. doi: 10.1161/01.res.66.2.486. [DOI] [PubMed] [Google Scholar]
- 10.Rao RM, Yang L, Garcia-Cardena G, Luscinskas FW. Endothelial-dependent mechanisms of leukocyte recruitment to the vascular wall. Circ Res. 2007;101:234–247. doi: 10.1161/CIRCRESAHA.107.151860b. [DOI] [PubMed] [Google Scholar]
- 11.Kolodgie FD, Gold HK, Burke AP, et al. Intraplaque hemorrhage and progression of coronary atheroma. N Engl J Med. 2003;349:2316–2325. doi: 10.1056/NEJMoa035655. [DOI] [PubMed] [Google Scholar]
- 12.Hirase T, Node K. Endothelial dysfunction as a cellular mechanism for vascular failure. Am J Physiol Heart Circ Physiol. 2012;302:H499–H505. doi: 10.1152/ajpheart.00325.2011. [DOI] [PubMed] [Google Scholar]
- 13.Fuster V, Moreno PR, Fayad ZA, Corti R, Badimon JJ. Atherothrombosis and high-risk plaque: part I: evolving concepts. J Am Coll Cardiol. 2005;46:937–954. doi: 10.1016/j.jacc.2005.03.074. [DOI] [PubMed] [Google Scholar]
- 14.Akorn-Pharmaceuticals. Product Sell Sheet - Indocyanine Green. [Accessed March 5th 2015];2008 Available at: http://akorn.com/documents/catalog/sell_sheets/17478-701-02.pdf. [Google Scholar]
- 15.Adams CW, Morgan RS, Bayliss OB. The differential entry of [125-I] albumin into mildly and severely atheromatous rabbit aortas. Atherosclerosis. 1970;11:119–124. doi: 10.1016/0021-9150(70)90010-9. [DOI] [PubMed] [Google Scholar]
- 16.Ramirez CA, Colton CK, Smith KA, Stemerman MB, Lees RS. Transport of 125I-albumin across normal and deendothelialized rabbit thoracic aorta in vivo. Arteriosclerosis. 1984;4:283–291. doi: 10.1161/01.atv.4.3.283. [DOI] [PubMed] [Google Scholar]
- 17.Saam T, Hetterich H, Hoffmann V, et al. Meta-analysis and systematic review of the predictive value of carotid plaque hemorrhage on cerebrovascular events by magnetic resonance imaging. J Am Coll Cardiol. 2013;62:1081–1091. doi: 10.1016/j.jacc.2013.06.015. [DOI] [PubMed] [Google Scholar]
- 18.Osborn EA, Jaffer FA. The advancing clinical impact of molecular imaging in CVD. JACC Cardiovasc Imaging. 2013 Dec;6(12):1327–1341. doi: 10.1016/j.jcmg.2013.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jaffer FA, Verjans JW. Molecular Imaging of Atherosclerosis: Clinical State-of-the-Art. Heart. 2014;100:1469–1477. doi: 10.1136/heartjnl-2011-301370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ughi GJ, Wang H, Gerbaud E, Gardecki JA, Fard AM, Hamidi E, Vacas-Jacques P, Rosenberg M, Jaffer FA, Tearney GJ. Clinical Characterization of Coronary Atherosclerosis With Dual-Modality OCT and Near-Infrared Autofluorescence Imaging. JACC Cardiovasc Imaging. 2016 Nov;9(11):1304–1314. doi: 10.1016/j.jcmg.2015.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fluorescence microscopy (purple=FITC-channel autofluorescence, yellow=ICG) and histological analysis of two human carotid artery plaques from patients after receiving intravenous ICG (A and B), and one control patient without ICG (C). Column 1 shows low magnification Movat's pentachrome staining (MP) followed by higher magnification (5×) fluorescence microscopy (FM), CD68 immunohistochemistry, Oil Red O (ORO), Masson's trichrome (MT), and Carstairs' fibrin staining highlighting the dashed boxed area in column 1. (A) A stenotic internal carotid artery plaques reveals ICG uptake in the area of CD68-positive macrophages (pink). (B) ICG deposits in an area of CD68+ macrophages and ORO+ lipid (orange-red), as well as intraplaque hemorrhage identified by Carstairs' fibrin staining (red). (C) Control plaque (no ICG injection) demonstrates minimal near-infrared autofluorescence. L=lumen.