Abstract
Group-level cooperation often poses a social dilemma in which joint action may be difficult to achieve. Theoretical models and experimental work on humans show that social incentives, such as punishment of defectors and rewarding of cooperators, can promote cooperation in groups of unrelated individuals. Here, we demonstrate that these processes can operate in a non-human animal species, and be used to effectively promote the production of a public good. We took advantage of the fact that intergroup fights in vervet monkeys (Chlorocebus aethiops pygerythrus) are characterized by episodes of intergroup aggression with pauses in-between. During pauses, females selectively groomed males that had participated in the previous aggressive episode, but aggressed male group members that had not. In subsequent (i.e. future) episodes, males who had received either aggression or grooming participated above their personal base-line level. Therefore, female–male aggression and grooming both appear to function as social incentives that effectively promote male participation in intergroup fights. Importantly, females stood to gain much from recruiting males as the probability of winning intergroup fights was dependent on the number of active participants, relative to the number of fighters in the opposing group. Furthermore, females appear to maximize the benefits gained from recruiting males as they primarily used social incentives where and when high-quality food resources, which are the resources primarily limiting to female fitness, were at stake.
Keywords: public good, n-player games, social dilemma, social incentives, punishment, reward
1. Introduction
Helping among unrelated individuals has attracted major research efforts among evolutionarily minded scientists as it has to be reconciled with a theory that strongly emphasizes competition [1–7]. In group-living species, actions like predator vigilance, cooperative hunting or the defence of territories often produce a public good, where individuals that do not contribute receive greater pay-offs than those that do. The former are called free-riders or defectors while the latter are called cooperators. Thus, group-level cooperation often poses a social dilemma in which cooperators, who contribute to the production of the public good, are vulnerable to exploitation by their free-riding group members [3]. Such dilemmas are often modelled as an n-player Prisoner's Dilemma, where the self-serving decision of individual group members is to defect, even if this does not result in the maximum possible pay-off at the level of the group [8,9]. However, many social dilemmas in nature may better fit the framework of a volunteer's dilemma (an n-player Snowdrift), in which individuals still prefer to free-ride, unless not enough cooperators are present to secure the production of the public good, in which case cooperation becomes the self-serving strategy (despite a certain degree of exploitation) [10,11]. Besides such negative frequency dependencies in a volunteer's dilemmas, spatial population structure and social incentives can also favour contributions to public goods based on direct fitness benefits [12–17]. For example, public goods experiments conducted on humans in a laboratory setting show that social incentives like the punishment of defectors and rewarding of cooperators can effectively promote cooperative behaviour [18,19].
One of the riskiest joint actions that humans engage in is warfare, and social incentives are thought to have been important in promoting the participation (i.e. cooperation) of male warriors in primitive warfare [16,20,21]. The majority of animal species, however, do not engage in warfare [22,23] but group members still cooperatively defend a territory, or parts of their home range. Cooperative intergroup aggression in non-warring animals is nevertheless a high-risk activity, potentially resulting in injury or death [24–29], and is prone to social dilemmas (e.g. the collective action problem [3,30–33]). Unlike in humans, there is little empirical evidence that animals use social incentives to manipulate the participation of their group members and overcome social dilemmas during intergroup fights. In fact, studies clearly demonstrating that non-human animals use punishment or rewards to manipulate the cooperative behaviour of conspecifics in any context are remarkably limited [34,35].
We conducted a field study of intergroup aggression in vervet monkeys (Chlorocebus aethiops pygerythrus), a species in which both sexes participate aggressively during intergroup fights, even though males are approximately 1.5 times larger than females [36,37]. Patterns of intergroup aggression in this species follow the predictions of the volunteer's dilemma, in which the pay-offs of home range defence are nonlinear [10,11]. That is to say, production of the public good (i.e. home range defence) does not increase linearly with the number of participants, rather a certain number of volunteers are required to successfully secure the public good [10,11]. In vervet monkeys, typically only a small proportion of group members participate in a given intergroup fight, and although individual participation is highly variable, the average number of individuals who participate in intergroup fights is similar among groups [17]. Given that defending access to food resources can have significant fitness benefits for female primates [38–42], females probably have a strong incentive to participate in intergroup fights when valuable food resources are at stake. Furthermore, because they are the philopatric sex, female vervet monkeys stand to gain long-term direct and indirect fitness benefits from effective home range defence [25,43]. However, high-ranking females are more likely to participate in intergroup fights and low-ranking individuals are more likely to free-ride on the efforts of others [17,36], which suggests that those who have priority of access to defended resources are those most likely to volunteer. Male vervet monkeys migrate repeatedly during their lives, residing in a group for a few months or a few years. Because food is not a key resource limiting male fitness, males are not expected to contribute to food defence [44]. Instead, male vervet monkeys participate in intergroup fights for one of two benefits [37]. First, males who are likely to have sired offspring react defensively when members of the opposing group are highly aggressive, such that offspring may be at risk [37]. Second, males also support females in instigating intergroup aggression; however, this primarily occurs during the mating season when doing so is associated with higher mating success [37]. As a result, females may receive little support from their larger bodied male group members for much of the year, including much of the summer season when high-quality food resources are at stake. If recruiting more active participants increases the likelihood of winning access to food resources, females potentially have a strong incentive to manipulate the participation of male group members to increase the fighting ability of their group. In social species, competitive ability is typically thought to increase with group size [29,41] but numerous studies have shown that smaller groups frequently win intergroup fights [38,45–48]. When individual participation is highly variable, larger groups can suffer defeat if defection among group members is high [46,49]. Therefore, the relative number of active participants, rather than relative group size, may determine the outcome of intergroup fights [48]. Given that only a proportion of group members typically participate in a given intergroup fight and individual participation is highly variable in vervet monkeys [17,36,37], it is very likely that the relative number of active participants determines who wins intergroup fights in this species.
Intergroup fights were comprised discrete episodes of intergroup aggression, with periods of calm in-between. Typically, aggressive episodes consisted of one or more individuals running towards the opposing group while making aggressive vocalizations, or chasing an individual from the opposing group. During calm periods, or pauses, in which the two groups were in close proximity but not interacting, we observed female actors directing social behaviours towards adult males from their own group. These social behaviours could be either affiliative (i.e. grooming) or aggressive; female–male aggression (FM-agg) typically started with female actor(s) vocalizing and making a threatening display towards a target male who was within a couple of metres. These displays often escalated into a chase, and in a couple of instances the female actors physically attacked the target male. These social behaviours typically occurred when the actor and target were near the front-line, monitoring the opposing group. Thus, FM-agg and female–male grooming (FM-gr) appear to relate directly to the context of the intergroup fight rather than an alternative context such as feeding. Here, we investigate whether these social behaviours potentially function as social incentives, used by females to manipulate the participation of male group members in future aggressive episodes.
To ascertain whether FM-agg and FM-gr function as social incentives, we first test whether females benefit from manipulating male participation in intergroup fights by examining the effect that the number of aggressive participants had on the odds of winning. Then, we investigate the spatio-temporal variability in the occurrence of these social behaviours. If females use aggression and grooming to manipulate males in defending resources that limit female fitness, females should be more likely to exhibit these behaviours during time periods, and in locations where valuable food resources are at stake. Lastly, we test if FM-agg functions as punishment for defection, and if FM-gr functions as a reward for participation. If this is the case, females who attempt to solicit male support should direct aggression towards males who did not participate in the most recent aggressive episode, but groom males who did. Furthermore, males who receive FM-agg should become more likely to participate in subsequent aggressive episodes [50], and males who receive FM-gr should maintain elevated levels of participation.
2. Material and methods
(a). Subjects and study site
Data were collected on four habituated groups of vervet monkeys at the Mawana Game Reserve (28°00′ S, 31°12′ E), South Africa, with all data collection protocols approved by the appropriate local authority, the Ezemvelo KZN Wildlife Board. Vervet monkeys live in multi-male multi-female groups, in which females are the philopatric sex and males emigrate multiple times throughout their adult lives. At this study site, groups consisted of one to seven males and five to 14 females. All animals in the four focal groups were individually recognized, as were most of the adults in the neighbouring and frequently encountered groups.
(b). Behavioural data
We conducted 1–2 days of observational data collection on each group, each week, for a total of more than 11 000 observation hours during the study period (January 2012 and February 2014). On these days we performed group scans every 30 min, and also recorded all observed social interactions (i.e. all-occurrence data). For each social interaction, we recorded the context (e.g. feeding, social), actor and recipient, and whether the actor received support from any group members.
Participation during intergroup encounters was also collected on an all-occurrence basis and encounters were deemed intergroup fights when one or more individuals from either group exhibited intergroup aggression. During aggressive episodes, participants could direct intergroup aggression towards the opposing group as a whole (e.g. run towards the group making aggressive vocalizations), or aggress specific individuals (e.g. chase, grab or bite a member of the opposing group). Throughout each intergroup fight, we recorded the time that each aggressive episode was initiated, the identity of active individuals, behaviour(s) exhibited, and the identity of the individuals intergroup aggression was directed towards. We recorded the same information when there was a social interaction within the group. One group was deemed to have won an intergroup encounter if they displaced the opposing group from the contested location. When the two groups tolerated each other until one group left the area, the encounter was categorized as having no clear winner (i.e. a draw).
We used this dataset to determine whether targeted males had participated in the last aggressive episode prior to, as well as the next aggressive episode following FM-agg or FM-gr. However, we observed both FM-agg and FM-gr before any intergroup aggression had been exhibited (n = 22), in the middle of intergroup fights (n = 39), as well as just before the opposing group retreated and the intergroup fight ended (n = 10). Additionally, there were cases where the participants of aggressive episodes, female actors or male targets, were not identified (although their age class/sex was determined). Therefore, our analyses were typically based on a subset of data in which an aggressive episode had occurred (before or after the social incentive, depending on the analysis) and the identity and behaviour of the relevant actors/targets/participants was known. We report the sample size that each analysis was based on.
(c). Statistical analyses
To examine the spatio-temporal variability in the occurrence of FM-agg and FM-gr, we used a generalized linear mixed model (GLMM), in which the dependent variable was whether or not either of these social incentives were observed in a given intergroup encounter. We set group as a random effect, a binomial error structure and a logit link function, and included four fixed effects. The three seasonal fixed effects included were the birth season (October to December), the summer season (November to May) and the mating season (April to July). The birth season was indexed by the number of small infants (less than three months old) in the group, and the summer season was indexed using monthly average normalized difference vegetation index values (NDVI), which correlates with field measurements of food availability and shelter in vervet monkeys [51] (see the electronic supplementary material for further detail). To account for the spatial variability in food resources, the last fixed effect we included in the GLMM was the relative availability of fruits in the area in which the intergroup fight took place, compared to what was available in the rest of the home range (see the electronic supplementary material for further detail).
A Fisher's exact test was used to test if when using social incentives, females directed aggression towards males who had recently defected and grooming towards males who had recently participated. We then examined the effect that FM-agg and FM-gr subsequently had on the cooperative behaviour of males both at the population level and the individual level. At the population level, all observations of punishment (or rewards) were pooled and the identity of the target male was not considered; at the individual level, the propensity to participate before versus after receiving punishment (or rewards) was determined for each male in the population. The former was tested using a χ2 test, and the latter using a Wilcoxon signed-rank test. We further examined the effect that social incentives had on male participation by comparing the proportion of aggressive episodes in which males participated following FM-agg (or FM-gr), to their individual base-line level of participation (i.e. the proportion of episodes participated in during intergroup fights in which social incentives were observed, but they were not the male targeted). We used a χ2 test to determine whether groups with more active individuals were more likely to win intergroup fights, as well as if males were more likely to be the target of female aggression (and female grooming) during intergroup fights than in other contexts.
In order to assess the magnitude of effects for all of our analyses [52,53], we present the appropriate effect size statistics: odds ratio with χ2 tests, r with Wilcoxon signed-rank tests and R2GLMM(c) in our GLMM [54,55]. The overall significance of the GLMM model was assessed by comparing the final model to the null model (model including intercept and random effect only) using a likelihood ratio test. In all analyses, α was set at 0.05, but we also discuss non-significant trends (0.05 < p < 0.10) when they are biologically interesting. All statistical analyses were conducted in R (v. 3.0.3, [56]) and we used the lme4 package (v. 1.1–4, [57]) to fit the GLMM model.
3. Results
During more than 2 years of observation of four habituated groups of vervet monkeys, we observed more than 400 intergroup encounters, approximately half of which (n = 236) escalated into an intergroup fight. Intergroup fights were 45 min long on average, but could be extremely brief or last up to 8 h (mean ± s.d. = 45 ± 55 min, range = 1–475 min). A third of intergroup fights consisted of a single episode of intergroup aggression, but the majority of intergroup fights were prolonged, consisting of multiple aggressive episodes (mean ± s.d. = 4.6 ± 3.0 episodes; range = 0 (only the opposing group exhibited intergroup aggression) to 15 episodes) that were typically spaced 3–4 min apart. However, when neither group was able to displace the other, the two groups often gave up fighting and tolerated each other nearby. In such situations, the pause between aggressive episodes could last up to 3 h before members of either group re-initiated an intergroup fight.
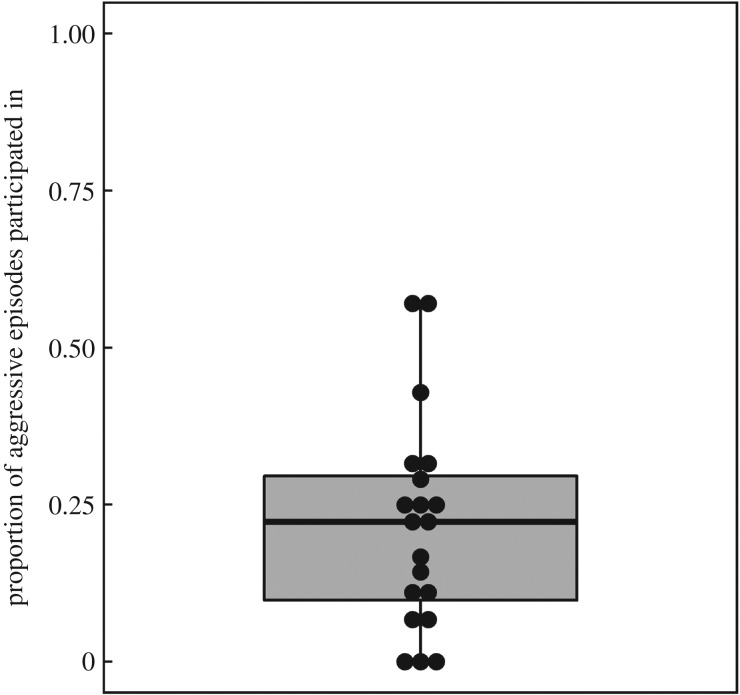
It was typically only a small proportion of group members that participated in each aggressive episode, with the average number of active males being 0.7 (s.d. = 0.7; range = 0–3), and the average number of female participants being 1.4 (s.d. = 1.5; range = 0–7). Thus, male support was absent in approximately half of the observed aggressive episodes, and it was rare that there was more than one male active at the front-line (fewer than 10% of aggressive episodes). We observed significant inter-individual variability in male participation (in intergroup fights where no FM-agg or FM-gr were observed), with some males never being observed participating, and the most active males in approximately 55% of the episodes they experienced (mean ± s.d. = 22 ± 17%; figure 1).
Figure 1.
Typical levels of participation in intergroup fights, calculated as the proportion of aggressive episodes that each male participated in during intergroup fights in which no social incentives (FM-agg or FM-gr) were observed. Each dot represents one male in the population (n = 20).
(a). Benefits of recruiting males
The number of adult participants varied greatly among intergroup fights; in some cases, no group members exhibited intergroup aggression (i.e. the group avoided or fled from a confrontation), while in other intergroup fights, up to 60% of adults were active participants. As would be expected when individual participation is so highly variable, it was the relative number of active participants throughout the intergroup fight that determined which group was able to displace the other from the contested location. The odds ratio indicates that groups which mustered more aggressive participants were 14 times more likely to win an intergroup fight than those with fewer (chi-squared test: χ21 = 26.900, p < 0.001). As a result, smaller groups were able to defeat larger groups during 41% of the intergroup fights they experienced.
(b). Spatio-temporal variability in the occurrence of female–male aggression and female–male grooming
We examined the spatio-temporal variability in the occurrence of FM-agg and FM-gr, and found that females were more likely to exhibit these behaviours in both the season when, and locations where high-quality food resources were available. Seasonal patterns of food availability were indexed using monthly NDVI values derived from satellite images of the study site, while the spatial distribution of food was calculated by mapping the distribution of important tree species throughout the study site, and monitoring the monthly availability of fruits on these tree species (see the electronic supplementary material). Social incentives were more commonly observed in the summer months (GLMM: b ± s.e. = 5.253 ± 1.819, z = 2.888, p = 0.004; electronic supplementary material, table S1), when tree species important in the diet of the monkeys were fruiting [51], and in areas of their home range that currently had the highest availability of fruits (b ± s.e. = 2.326 ± 0.953, z = 2.441, p = 0.015; electronic supplementary material, table S1). Thus, females were most likely to bestow social incentives in situations where and when valuable food resources were at stake.
(c). Actors and targets of female–male aggression and female–male grooming
Both putative punishment (FM-agg) and putative rewards (FM-gr) were rare events, with only 36 cases of the former and 35 cases of the latter observed throughout hundreds of intergroup encounters. Twenty-one females were observed to exhibit FM-agg (10 in Group A, three in Group B, seven in Group C and one in Group D), while 17 different females were seen using FM-gr during intergroup encounters (six in Group A, four in Group B, six in Group C and one in Group D). These actors ranged in rank from the dominant female to the lowest ranking female in their group. When putative punishment occurred during intergroup fights, and the actors were known, in 73% of cases it was the female(s) that had participated in the most recent act of intergroup aggression that exhibited FM-agg; alternatively, in 27% of cases FM-agg was exhibited by one or more bystanders. Similarly, putative rewards were typically bestowed soon after an aggressive episode (mean ± s.d. = 4.7 ± 4.6 min.) and the females that exhibited FM-gr were usually those who had participated in it (78% of cases). Although females sometimes acted alone, in 68% of cases FM-agg was exhibited by a coalition of females and/or juveniles (up to four individuals). Females who groomed male group members almost always did so alone.
We observed females directing putative punishment and rewards at a number of different males (FM-agg: five males in Group A, two in Group B, five in Group C and at least one male in Group D; FM-gr: six males in Group A, three in Group B, five in Group C and at least one male in Group D), and these targets could be either dominant or low-ranking males. When females used social incentives during intergroup fights, female actors were significantly more likely to use aggression when the target male had recently defected from participation, but use grooming with males who had recently participated (Fisher's exact test: p < 0.001; figures 2a and 3a). Males who were groomed by female group members had participated in the most recent act of intergroup aggression in 16 out of 23 (70%) of the observed cases (figure 3a). Conversely, males who received FM-agg had not participated in the most recent aggressive episode in 20 out of 24 (83%) of the observed cases (figure 2a). Furthermore, in two of the remaining 24 cases, the target male had recently participated but had begun to retreat from the front-line; thus, it is possible that females also perceived these retreating males as defecting. Notably, three males that were never observed to receive FM-gr were those that were rarely present near the front-line and were never observed to participate in intergroup fights in the absence of social incentives (figure 1). Conversely, two males that were never observed receiving FM-agg were the two males in the population who were the most active in intergroup fights (participated in approx. 55% of aggressive episodes; figure 1).
Figure 2.
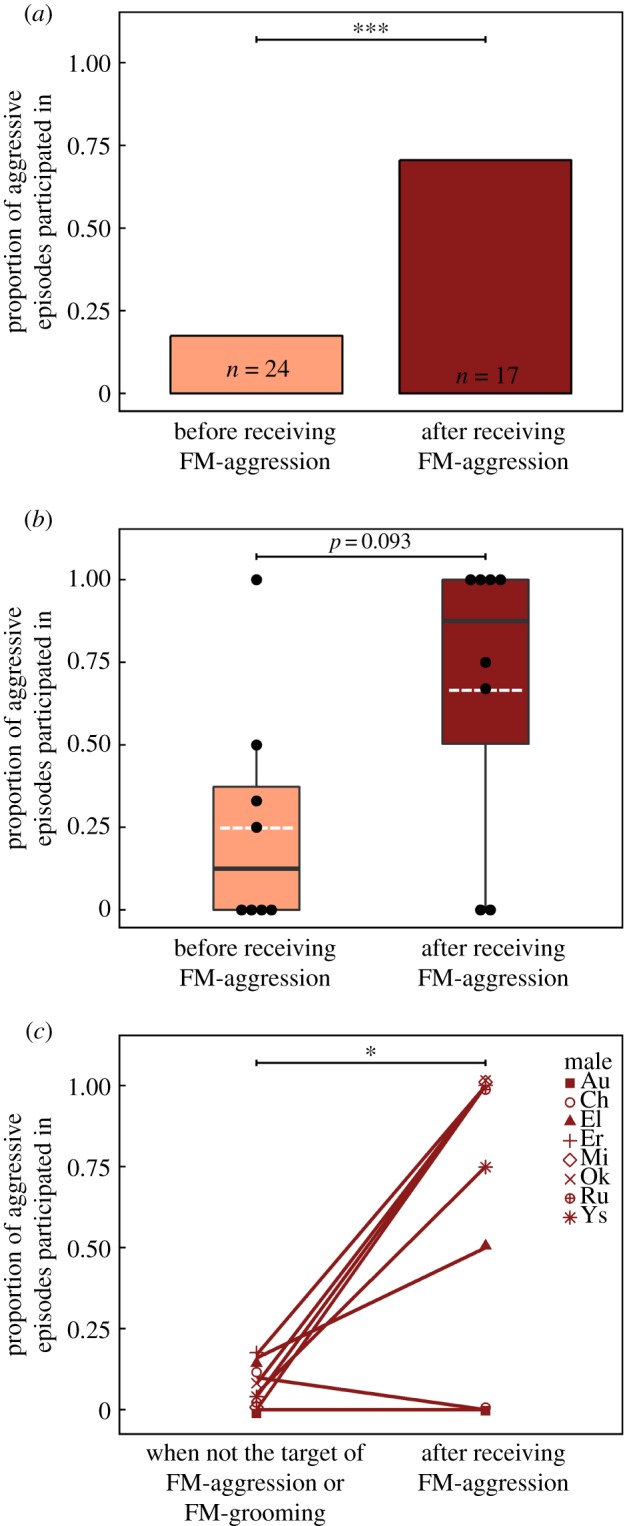
The proportion of aggressive episodes that targeted males participated in before (light) versus after (dark) receiving aggression from female group members (a) at the population level and (b) at the individual level (note: each dot represents the proportion of aggressive episodes participated in for one male in the population (n = 9 males); means are portrayed by the white dotted line and medians by the dark line). (c) The proportion of aggressive episodes targeted males participated in after being aggressed by a female group member, compared to their base-line level of participation (i.e. proportion of episodes participated in during intergroup fights where social incentives were observed, but they were not the male targeted). Significance levels denoted by *p < 0.05 and ***p < 0.001.
Figure 3.
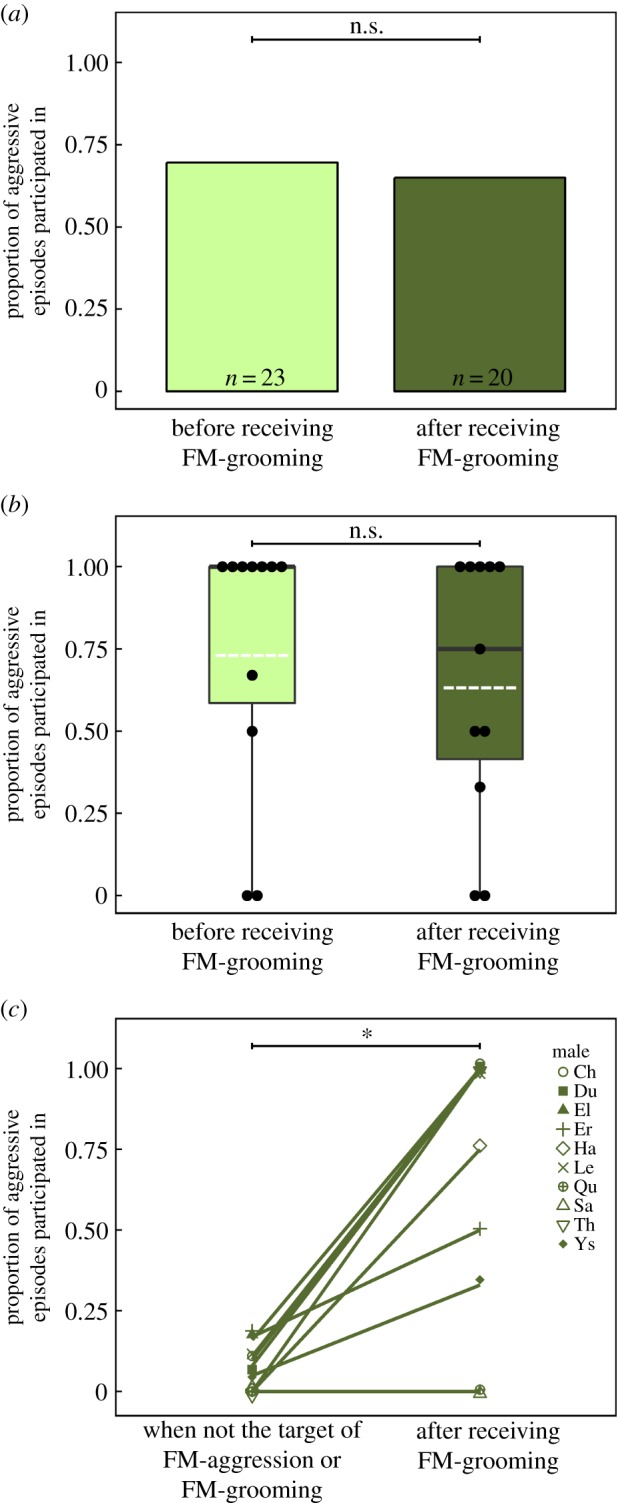
The proportion of aggressive episodes that targeted males participated in before (light) versus after (dark) receiving grooming from female group members (a) at the population level and (b) at the individual level (note: each dot represents the proportion of aggressive episodes participated in for one male in the population (n = 11 males); means are portrayed by the white dotted line and medians by the dark line). (c) The proportion of aggressive episodes targeted males participated in after being groomed by a female group member compared to their base-line level of participation (i.e. proportion of episodes participated in during intergroup fights where social incentives were observed, but they were not the male targeted). Significance levels denoted by *p < 0.05 and ***p < 0.001.
When the targets of female social behaviours are compared among contexts, we find that females were significantly more likely to target males, as opposed to females or juveniles, during intergroup fights than in other contexts. During intergroup fights, 36 out of the 41 observed cases (88%) of female aggression targeted males; conversely, in other contexts, females directed only 65 out of 360 observed acts of aggression (18%) towards male group members (chi-squared test: χ21 = 95.032, p < 0.001). During intergroup fights, 36 out of the 247 observed cases of female grooming (15%) targeted males; in other contexts, females directed 202 out of 2284 grooming events (9%) towards males (χ21 = 8.592, p = 0.003). While the odds ratio indicates that females were two times more likely to direct grooming towards male group members during intergroup fights, female aggression was almost exclusively directed towards males (odds ratio = 33 times as likely to aggress males than females or juveniles).
(d). Target behaviour following female–male aggression and female–male grooming
We analysed the effect that social incentives had on subsequent male participation, both at the population level and the individual level. At the population level, all observations of FM-agg (or FM-gr) were pooled and the identity of the target male was not considered. At this level, FM-agg had a strong impact on the subsequent behaviour of target males; the likelihood ratio indicates that targeted males were 11 times more likely to participate in the next aggressive episode following FM-agg (71% of cases, n = 17) than they were to have participated in the most recent episode before being targeted (chi-squared test: χ21 = 11.53, p < 0.001; figure 2a). Many males were only observed receiving FM-agg on one or two occasions, because there was not always an aggressive episode prior to, or following putative punishment, we were only able to perform the individual-level analysis on a subsample of seven of the nine males observed to receive FM-agg. Despite the low power associated with this limited sample size, we nevertheless detected a tendency for individual males to increase their participation following putative punishment (Wilcoxon signed-rank test: w = 2, n = 7 males, p = 0.093, r = 0.64; figure 2b). The magnitude of the effect size in the individual analysis suggests that this statistical trend is biologically meaningful, as does the finding that targets of FM-agg subsequently participated above their base-line level (i.e. the proportion of episodes participated in when they had not been the male targeted by FM-agg; Wilcoxon signed-rank test: w = 27, n = 8 males, p = 0.035, r = 0.75; figure 2c). The ‘future’ aggressive episodes that punished males participated in could be relatively soon (i.e. within 1 min.) or up to an hour after they received FM-agg (mean ± s.d. = 14.6 ± 17.7 min).
Because FM-gr largely targeted males who had participated in the most recent aggressive episode, the proportion of target males who participated in the next aggressive episode following putative rewards (13 out of 20 observed cases; 65%) was not significantly different from the proportion of target males who participated in the most recent episode (70%; chi-squared test: χ21 = 0.10, p = 0.75; figure 3a). Similarly, at the individual level, target males maintained a relatively high level of participation following FM-gr (Wilcoxon signed-rank test: w = 13.5, n = 11 males, p = 0.599, r = 0.16; figure 3b). This propensity to participate following FM-gr was biologically significant, as the targets of putative rewards subsequently participated at levels significantly higher than their base-line level (Wilcoxon signed-rank test: w = 36, n = 10 males, p = 0.014, r = 0.77; figure 3c). The future aggressive episodes in which groomed males participated could occur relatively soon (i.e. within 2 min) or up to 102 min after the reward was bestowed (mean ± s.d. = 23.8 ± 29.7 min).
4. Discussion
The aim of this study was to determine whether female vervet monkeys use the carrot (grooming) and/or the stick (aggression) to manipulate male participation in intergroup fights when the resources limiting to female fitness are at stake. We found that females were more likely to direct aggression towards males that had recently defected, but groom males that had recently participated in the intergroup fight. Given that males which received either subsequently participated at levels above their personal base-line, both FM-gr and FM-agg indeed appear to function as social incentives that effectively promote male cooperation in this context. Importantly, we observed that smaller groups were able to win intergroup fights if they mobilized a greater number of aggressive participants, indicating there was a significant benefit to recruiting male group members. We also found that females were more likely to use social incentives when the benefits were greatest. That is to say, females used the carrot and the stick in both the season when, and areas of their home range where, valuable food resources were most abundant. Together, these findings suggest that successful recruitment using social incentives may be crucial to success in intergroup fights over fitness-limiting resources, and therefore have significant effects on the fecundity of females [25,38–42].
Because of their larger body and canine size, males are probably the most valuable group members to recruit during intergroup fights. However, it is perhaps less clear why males should respond to such relatively low-cost incentives as FM-gr, or the risk of injury from FM-agg, with the relatively high-cost behaviour of participation in intergroup fights. Two possible explanations are that these low-cost incentives have consequences for male–female social relationships, and/or that receiving incentives influences the reputation of the target male with his group members [58–60]. Grooming and tolerance (i.e. the lack of aggression) are important services exchanged in the formation and maintenance of social bonds in primates [58–60], and it is possible that punishment and rewards have a disproportionate impact on male behaviour because these social interactions influence the quality of male–female social relationships. That is to say, receiving punishment could damage the target male's social relationship(s), either with the female actor(s) directly (i.e. experience based) or with other female group members who have observed the social incentive (i.e. reputation or information based). Conversely, receiving rewards could improve bond strength and potentially signal to other female group members that the target male is a valuable social partner. Thus, relatively low-cost incentives may carry higher cost consequences in the long-term, and subsequently impact male fitness (e.g. male mating success).
Although both female aggression and grooming were significantly more likely to be directed towards males (versus females and juveniles) during intergroup encounters than in other contexts, our data do not allow us to discount the possibility that males were more frequently in close proximity during these encounters. However, while proximity could potentially influence the propensity to direct grooming towards male group members, increased proximity cannot explain the overwhelming extent to which females targeted males when being aggressive. In fact, female intragroup aggression was almost exclusively directed towards males during intergroup fights, which raises the question of why females would use punishment primarily on males, rather than also with other females and juveniles. As males are the largest age-sex class, recruiting males probably has a disproportionate effect on the group's fighting ability. Not only does their larger size give them a physical advantage, but their participation in intergroup aggression appears to decrease the perceived risk of injury for smaller females, as females are more likely to participate when they have more support from their male group members [61]. Thus, recruiting males may also encourage more females to join in the fight and further increase the odds of winning. Moreover, there were also more opportunities to recruit defecting males as they frequently sat near the front-line without actively participating. Males who were investigating dispersal opportunities were often present near the front-line so that when the intergroup fight died down, they could approach and attempt to affiliate with members of the opposing group. Males who were likely to have sired offspring also often sat near the front-line, monitoring the intergroup fight, ready to respond defensively when potential offspring were perceived to be at risk [37]. Conversely, females and juveniles who were not participating in the intergroup fight typically avoided the front-line and were therefore not potential targets for punishment.
Although social incentives were typically observed during the pauses in intergroup fights, in some cases, they were bestowed when the groups were within visual range but were not interacting. Upon detecting another group nearby, it was often female group members who began to approach the opposing group while vocalizing to solicit support. When ‘enough’ group members had joined them (usually within 1 m), they instigated an escalated conflict. Thus, in gearing up for an intergroup fight, it was often females who took the initiative, and who assessed if they had gathered sufficient willing participants, or whether they should retreat from a risky confrontation. In this context, FM-agg and FM-gr may function to goad males into supporting females in instigating intergroup fights. Further work is necessary to determine how the decision to escalate versus retreat is made, and the effect that social incentives have on male behaviour in this context.
Both FM-agg and FM-gr were typically exhibited by females that had participated in the most recent aggressive episode (i.e. second parties); however, in a quarter of cases, social incentives were bestowed by female bystanders. Given the importance of food resources to female fitness [38–42], all female group members are likely to benefit from forcefully recruiting male group members during intergroup fights. Thus, cases where punishment and rewards were bestowed by a bystander would most accurately be described as peer punishment and rewards (as opposed to social incentives provided by a centralized authority) exhibited by self-serving third parties [34,62,63]. In primitive warfare, punishment and rewards are doled out both by other warriors (second parties) and other group members who are in many cases, likely to be self-interested third parties [16,64]; however, there is also evidence that in larger groups, third parties who do not frequently interact with the target (i.e. individuals who do not gain significant direct benefits) also use social incentives to promote warrior participation [21,65]. Communication can greatly enhance cooperation in social dilemmas [66,67], as communication allows group members to gossip about the bravery, or cowardice of warriors. As a result, individuals may behave cooperatively to improve their reputation with their group members [13] and social incentives are often bestowed by group members who were not present to observe the participation of warriors directly (e.g. women and senior group members) [16,21].
In this study, we were able to capitalize on the fact that intergroup fights in vervets consist of a number of episodes of intergroup aggression with pauses in-between. As a result, we have been able to assess if the targets of FM-agg and FM-gr had or had not participated in the most recent cooperative event, and if these social incentives promoted participation in future cooperative events. With these data, we demonstrate, to our knowledge, the first quantitative evidence that both positive and negative social incentives are used to effectively manipulate male participation in intergroup fights in a species other than our own. Furthermore, we have strived to describe the social and ecological conditions in which these social incentives occur, providing unique insight into the real-world conditions under which punishment and rewards can evolve. We urge other researchers who observe intragroup aggression and/or affiliative behaviours during (or shortly following) intergroup fights, as well as other cooperative activities, to also investigate who is the target of these behaviours, and the impact such social interactions have on future cooperative behaviour. Such investigations are critical to understanding how important social incentives are to the evolution and maintenance of cooperation in non-human animals.
Supplementary Material
Ethics
All data collection protocols were approved by the Ezemvelo KZN Wildlife Board in South Africa.
Data accessibility
The datasets supporting this article have been deposited in Dryad: http://dx.doi.org/10.5061/dryad.7q4r8 [68].
Authors' contributions
T.J.M.A.-R., C.v.S and E.P.W conceived the study; T.J.M.A.-R., E.M. and A.L.T. collected the data; T.J.M.A.-R., E.M. and A.L.T. analysed the data, and all authors wrote the paper.
Competing interests
We declare we have no competing interests.
Funding
This study was supported by Swiss National Science Foundation (Sinergia grant CRS133_133040), Claraz-Stiftung and University of Zurich Forschungskredit.
References
- 1.Maynard Smith J. 1982. Evolution and the theory of games, 234 p Cambridge, UK: Cambridge University Press. [Google Scholar]
- 2.Hardin G. 1968. The tragedy of the commons. Science 162, 1243–1248. ( 10.1126/science.162.3859.1243) [DOI] [PubMed] [Google Scholar]
- 3.Olson M. 1965. The logic of collective action: public goods and the theory of groups, 176 p Cambridge, MA: Harvard University Press. [Google Scholar]
- 4.Dugatkin L. 2002. Animal cooperation among unrelated individuals. Naturwissenschaften 89, 533–541. ( 10.1007/s00114-002-0379-y) [DOI] [PubMed] [Google Scholar]
- 5.West SA, Griffin AS, Gardner A. 2007. Evolutionary explanations for cooperation. Curr. Biol. 17, R661–R672. ( 10.1016/j.cub.2007.06.004) [DOI] [PubMed] [Google Scholar]
- 6.Clutton-Brock TH. 2009. Cooperation between non-kin in animal societies. Nature 462, 51–57. ( 10.1038/nature08366) [DOI] [PubMed] [Google Scholar]
- 7.Rand DG, Nowak MA. 2013. Human cooperation. Trends Cogn. Sci. 17, 413–425. ( 10.1016/j.tics.2013.06.003) [DOI] [PubMed] [Google Scholar]
- 8.Tucker A. 1950. A two-person dilemma. In Readings in games and information (ed. Rasmusen E.), pp. 7–8. Oxford, UK: Blackwell. [Google Scholar]
- 9.Hamburger H. 1973. N-person Prisoner's Dilemma. J. Math. Sociol. 3, 27–48. ( 10.1080/0022250X.1973.9989822) [DOI] [Google Scholar]
- 10.Diekmann A. 1985. Volunteer's dilemma. J. Conflict Resolut. 29, 605–610. ( 10.1177/0022002785029004003) [DOI] [Google Scholar]
- 11.Archetti M. 2009. Cooperation as a volunteer's dilemma and the strategy of conflict in public goods games. J. Evol. Biol. 22, 2192–2200. ( 10.1111/j.1420-9101.2009.01835.x) [DOI] [PubMed] [Google Scholar]
- 12.Kandori M. 1992. Social norms and community enforcement. Rev. Econ. Stud. 59, 63–80. ( 10.2307/2297925) [DOI] [Google Scholar]
- 13.Milinski M, Semmann D, Krambeck H-J. 2002. Reputation helps solve the ‘tragedy of the commons’. Nature 415, 424–426. ( 10.1038/415424a) [DOI] [PubMed] [Google Scholar]
- 14.Sherratt TN, Roberts G, Kassen R. 2009. Evolutionary stable investment in products that confer both an individual benefit and a public good. Front. Biosci. 14, 4557–4564. ( 10.2741/3548) [DOI] [PubMed] [Google Scholar]
- 15.Archetti M, Scheuring I. 2011. Coexistence of cooperation and defection in public goods games. Evolution 65, 1140–1148. ( 10.1111/j.1558-5646.2010.01185.x) [DOI] [PubMed] [Google Scholar]
- 16.Glowacki L, Wrangham RW. 2013. The role of rewards in motivating participation in simple warfare. Hum. Nat. 24, 444–460. ( 10.1007/s12110-013-9178-8) [DOI] [PubMed] [Google Scholar]
- 17.Willems EP, Arseneau TJM, Schleuning X, van Schaik CP. 2015. Communal range defence in primates as a public goods dilemma. Phil. Trans. R. Soc. B 370, 2015003 ( 10.1098/rstb.2015.0003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fehr E, Gächter S. 2000. Cooperation and punishment in public goods experiments. Am. Econ. Rev. 90, 980–994. ( 10.1257/aer.90.4.980) [DOI] [Google Scholar]
- 19.Sefton M, Shupp R, Walker JM. 2007. The effect of rewards and sanctions in provision of public goods. Econ. Inq. 45, 671–690. ( 10.1111/j.1465-7295.2007.00051.x) [DOI] [Google Scholar]
- 20.Boyd R, Gintis H, Bowles S. 2010. Coordinated punishment of defectors sustains cooperation and can proliferate when rare. Science 328, 617–620. ( 10.1126/science.1183665) [DOI] [PubMed] [Google Scholar]
- 21.Mathew S, Boyd R. 2011. Punishment sustains large-scale cooperation in prestate warfare. Proc. Natl Acad. Sci. USA 108, 11 375–11 380. ( 10.1073/pnas.1105604108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goodall J. 1986. The chimpanzees of Gombe: patterns of behavior, 673 p Cambridge, MA: Harvard University Press. [Google Scholar]
- 23.Aureli F, Schaffner CM, Verpooten J, Slater K, Ramos-Fernandez G. 2006. Raiding parties of male spider monkeys: insights into human warfare? Am. J. Phys. Anthropol. 131, 486–497. ( 10.1002/ajpa.20451) [DOI] [PubMed] [Google Scholar]
- 24.Kruuk H. 1972. The spotted hyena: a study of predation and social behavior, 335 p Chicago, IL: Chicago University Press. [Google Scholar]
- 25.Cheney DL, Seyfarth RM. 1987. The influence of intergroup competition on the survival and reproduction of female vervet monkeys. Behav. Ecol. Sociobiol. 21, 375–386. ( 10.1007/BF00299932) [DOI] [Google Scholar]
- 26.Mech LD. 1994. Buffer zones of territories of gray wolves as regions of intraspecific strife. J. Mammal. 75, 199–202. ( 10.2307/1382251) [DOI] [Google Scholar]
- 27.Cant MA, Otali E, Mwanguhya F. 2002. Fighting and mating between groups in a cooperatively breeding mammal, the banded mongoose. Ethology 108, 541–555. ( 10.1046/j.1439-0310.2002.00795.x) [DOI] [Google Scholar]
- 28.Gros-Louis J, Perry S, Manson J. 2003. Violent coalitionary attacks and intraspecific killing in wild white-faced capuchin monkeys (Cebus capucinus). Primates 44, 341–346. ( 10.1007/s10329-003-0050-z) [DOI] [PubMed] [Google Scholar]
- 29.Mosser A, Packer C. 2009. Group territoriality and the benefits of sociality in the African lion, Panthera leo. Anim. Behav. 78, 359–370. ( 10.1016/j.anbehav.2009.04.024) [DOI] [Google Scholar]
- 30.Nunn CL, Lewis RJ. 2001. Cooperation and collective action. In Economics in nature (eds Noe R, van Hooff JARAM, Hammerstein P), pp. 42–66. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 31.Nunn CL, Deaner RO. 2004. Patterns of participation and free riding in territorial conflicts among ringtailed lemurs (Lemur catta). Behav. Ecol. Sociobiol. 57, 50–61. ( 10.1007/s00265-004-0830-5) [DOI] [Google Scholar]
- 32.Willems EP, Hellriegel B, van Schaik CP. 2013. The collective action problem in primate territory economics. Proc. R. Soc. B 280, 20130081 ( 10.1098/rspb.2013.0081) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Willems EP, van Schaik CP. 2015. Collective action and the intensity of between-group competition in nonhuman primates. Behav. Ecol. 26, 625–631. ( 10.1093/beheco/arv001) [DOI] [Google Scholar]
- 34.Raihani NJ, Grutter AS, Bshary R. 2010. Punishers benefit from third-party punishment in fish. Science 327, 171 ( 10.1126/science.1183068) [DOI] [PubMed] [Google Scholar]
- 35.Raihani NJ, Pinto AI, Grutter AS, Wismer S, Bshary R. 2012. Male cleaner wrasses adjust punishment of female partners according to the stakes. Proc. R. Soc. B 279, 365–370. ( 10.1098/rspb.2011.0690) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cheney DL. 1981. Intergroup encounters among free-ranging vervet monkeys. Folia Primatol. 35, 124–146. ( 10.1159/000155970) [DOI] [PubMed] [Google Scholar]
- 37.Arseneau TJM, Taucher A, Van Schaik CP, Willems EP. 2015. Male monkeys fight in between-group conflicts as protective parents and reluctant recruits. Anim. Behav. 110, 39–50. ( 10.1016/j.anbehav.2015.09.006) [DOI] [Google Scholar]
- 38.Robinson JG. 1988. Group size in wedge-capped capuchin monkeys Cebus olivaceus and the reproductive success of males and females. Behav. Ecol. Sociobiol. 23, 187–197. ( 10.1007/BF00300353) [DOI] [Google Scholar]
- 39.Lee PC, Hauser MD. 1998. Long-term consequences of changes in territory quality on feeding and reproductive strategies of vervet monkeys. J. Anim. Ecol. 67, 347–358. [Google Scholar]
- 40.Takahata Y, et al. 1998. Does troop size of wild Japanese macaques influence birth rate and infant mortality in the absence of predators? Primates 39, 245–251. ( 10.1007/bf02557737) [DOI] [Google Scholar]
- 41.Williams JM, Oehlert GW, Carlis JV, Pusey AE. 2004. Why do male chimpanzees defend a group range? Anim. Behav. 68, 523–532. ( 10.1126/science.327542) [DOI] [Google Scholar]
- 42.Takahata Y, Koyama N, Ichino S, Miyamoto N, Nakamichi M. 2006. Influence of group size on reproductive success of female ring-tailed lemurs: distinguishing between IGFC and PFC hypotheses. Primates 47, 383–387. ( 10.1007/s10329-006-0185-9) [DOI] [PubMed] [Google Scholar]
- 43.Isbell LA, Cheney DL, Seyfarth RM. 1990. Costs and benefits of home range shifts among vervet monkeys (Cercopithecus aethiops) in Amboseli National Park, Kenya. Behav. Ecol. Sociobiol. 27, 351–358. ( 10.1007/BF00164006) [DOI] [Google Scholar]
- 44.Trivers RL. 1972. Parental investment and sexual selection. In Sexual selection and the descent of man (ed. Campbell B.), pp. 136–179. Chicago, IL: Aldine Publishing Company. [Google Scholar]
- 45.Sugiura H, Saito C, Sato S, Agetsuma N, Takahashi H, Tanaka T, Furuichi T, Takahata Y. 2000. Variation in intergroup encounters in two populations of Japanese macaques. Int. J. Primatol. 21, 519–535. ( 10.1023/a:1005448120967) [DOI] [Google Scholar]
- 46.Crofoot MC, Gilby IC, Wikelski MC, Kays RW. 2008. Interaction location outweighs the competitive advantage of numerical superiority in Cebus capucinus intergroup contests. Proc. Natl Acad. Sci. USA 105, 577–581. ( 10.1073/pnas.0707749105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bonanni R, Valsecchi P, Natoli E. 2010. Pattern of individual participation and cheating in conflicts between groups of free-ranging dogs. Anim. Behav. 79, 957–968. ( 10.1016/j.anbehav.2010.01.016) [DOI] [Google Scholar]
- 48.Zhao Q, Tan CL. 2010. Inter-unit contests within a provisioned troop of Sichuan snub-nosed monkeys (Rhinopithecus roxellana) in the Qinling Mountains, China. Am. J. Primatol. 73, 262–269. ( 10.1002/ajp.20892) [DOI] [PubMed] [Google Scholar]
- 49.Crofoot MC, Gilby IC. 2012. Cheating monkeys undermine group strength in enemy territory. Proc. Natl Acad. Sci. USA 109, 501–505. ( 10.1073/pnas.1115937109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Clutton-Brock TH, Parker GA. 1995. Punishment in animal societies. Nature 373, 209–216. ( 10.1038/373209a0) [DOI] [PubMed] [Google Scholar]
- 51.Willems EP, Barton RA, Hill RA. 2009. Remotely sensed productivity, regional home range selection, and local range use by an omnivorous primate. Behav. Ecol. 20, 985–992. ( 10.1093/beheco/arp087) [DOI] [Google Scholar]
- 52.Garamszegi LZ, et al. 2009. Changing philosophies and tools for statistical inferences in behavioral ecology. Behav. Ecol. 20, 1363–1375. ( 10.1093/beheco/arp137) [DOI] [Google Scholar]
- 53.Nuzzo R. 2014. Statistical errors. Nature 506, 150–152. ( 10.1038/506150a) [DOI] [PubMed] [Google Scholar]
- 54.Field A, Miles J, Field Z. 2012. Discovering statistics using R, 958 p London, UK: SAGE Publications. [Google Scholar]
- 55.Nakagawa S, Schielzeth H. 2013. A general and simple method for obtaining R2 from generalized linear mixed-effects models. Methods Ecol. Evol. 4, 133–142. ( 10.1111/j.2041-210x.2012.00261.x) [DOI] [Google Scholar]
- 56.R Core Team. 2014. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- 57.Bates D, Maechler M, Bolker B, Walker S. 2015. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67, 1–48. ( 10.18637/jss.v067.i01) [DOI] [Google Scholar]
- 58.Henzi SP, Barrett L. 1999. The value of grooming to female primates. Primates 40, 47–59. ( 10.1007/bf02557701) [DOI] [PubMed] [Google Scholar]
- 59.Silk JB, Alberts SC, Altmann J. 2006. Social relationships among adult female baboons (Papio cynocephalus). II. Variation in the quality and stability of social bonds. Behav. Ecol. Sociobiol. 61, 197–204. ( 10.1007/s00265-006-0250-9) [DOI] [Google Scholar]
- 60.Silk JB, et al. 2010. Female chacma baboons form strong, equitable, and enduring social bonds. Behav. Ecol. Sociobiol. 64, 1733–1747. ( 10.1007/s00265-010-0986-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Arseneau-Robar TJM, Taucher AL, Schnider AB, van Schaik CP, Willems EP. In press Intra- and interindividual differences in the costs and benefits of intergroup aggression in female vervet monkeys. Anim. Behav. [Google Scholar]
- 62.Baldassarri D, Grossman G. 2011. Centralized sanctioning and legitimate authority promote cooperation in humans. Proc. Natl Acad. Sci. USA 108, 11 023–11 027. ( 10.1073/pnas.1105456108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Raihani NJ, Thornton A, Bshary R. 2012. Punishment and cooperation in nature. Trends Ecol. Evol. 27, 288–295. ( 10.1016/j.tree.2011.12.004) [DOI] [PubMed] [Google Scholar]
- 64.Chagnon NA. 1988. Life histories, blood revenge, and warfare in a tribal population. Science 239, 985–992. ( 10.1126/science.239.4843.985) [DOI] [PubMed] [Google Scholar]
- 65.Mathew S, Boyd R. 2014. The cost of cowardice: punitive sentiments towards free riders in Turkana raids. Evol. Hum. Behav. 35, 58–64. ( 10.1016/j.evolhumbehav.2013.10.001) [DOI] [Google Scholar]
- 66.Deutsch M. 1958. Trust and suspicion. J. Conflict Resolut. 2, 265–279. ( 10.1177/002200275800200401) [DOI] [Google Scholar]
- 67.Balliet D. 2009. Communication and cooperation in social dilemmas: a meta-analytic review. J. Conflict Resolut. 54, 39–57. ( 10.1177/0022002709352443) [DOI] [Google Scholar]
- 68.Arseneau-Robar TJM, Taucher AL, Müller E, van Schaik C, Bshary R, Willems EP. 2016. Data from: Female monkeys use both the carrot and the stick to promote male participation in intergroup fights. Dryad Digital Respository. ( 10.5061/dryad.7q4r8) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets supporting this article have been deposited in Dryad: http://dx.doi.org/10.5061/dryad.7q4r8 [68].