Abstract
Turtles (Testudinata) are a diverse group of amniotes that have a rich fossil record that extends back to the Late Triassic, but little is known about global patterns of disparity through time. We here investigate the cranial disparity of 172 representatives of the turtle lineage and their ancestors grouped into 20 time bins ranging from the Late Triassic until the Recent using two-dimensional geometric morphometrics. Three evolutionary phases are apparent in all three anatomical views investigated. In the first phase, disparity increases gradually from the Late Triassic to the Palaeogene with only a minor perturbation at the K/T extinct event. Although global warming may have influenced this increase, we find the Mesozoic fragmentation of Pangaea to be a more plausible factor. Following its maximum, disparity decreases strongly towards the Miocene, only to recover partially towards the Recent. The marked collapse in disparity is likely a result of habitat destruction caused by global drying, combined with the homogenization of global turtle faunas that resulted from increased transcontinental dispersal in the Tertiary. The disparity minimum in the Miocene is likely an artefact of poor sampling.
Keywords: turtles, skull evolution, geometric morphometrics, disparity through time, biogeography
1. Introduction
Turtles (Testudinata) are one of the primary lineages of living amniotes. Although the origin of the group is still somewhat controversial (see Joyce [1] for most recent summary), fossils are now available that help trace the turtle lineage back to the Palaeozoic, including the Late Triassic Odontochelys semitestacea [2], the Middle Triassic Pappochelys rosinae [3] and the Late Permian Eunotosaurus africanus [4,5]. Turtles have generally remained faithful to their protective shell ever since the lineage acquired it in the Late Triassic, but it is a common misconception that the group has remaining unchanged ever since. Important morphological innovations include multiple acquisitions of neck retraction [6], the development of jaw closure mechanisms using a trochlea [7], multiple acquisitions of shell kinesis [8–10] and the secondary reduction of the turtle shell [11]. From an ecological standpoint, turtles conquered freshwater, brackish, marine and terrestrial habitats [12], adapted to tropical to cold-temperature climates, and broadly diversified their diets, ranging from terrestrial herbivores, to piscivores, to molluscivores [13].
In recent years, a number of studies have used morphometric approaches to explore a broad set of questions pertaining to the evolution of turtles. In a pioneering study, Claude et al. [14] used three-dimensional morphometrics to investigate phylogenetic versus ecological influences on the shells of testudinoid turtles. The turtle shell has proved to be particularly fertile ground for morphometric studies and numerous publications have since followed, including, among others, the exploration of convergence in plastral shape during the acquisition of shell kinesis [15], the identification of provenance of confiscated tortoises as a way to assist in their repatriation [16], assessment of sexual dimorphism [17], the impact on habitat on symmetry [18] or the possible development of miniaturization [19]. Among other postcranial systems, geometric morphometrics have otherwise been used to investigate correlations of humeral [20] or girdle shape [21] with habitat and to explore phylogenetic versus functional correlations among cervical vertebral shape [6,22,23]. Interestingly, despite high levels of cranial variability (figure 1) and the associated emphasis on cranial morphology in turtle systematics [24,25] and apparent correlations between skull shape and feeding ecology [26], the early study of Claude et al. [27] and the more recent study of Ferreira et al. [28] are the only ones we are aware of to use this skeletal region.
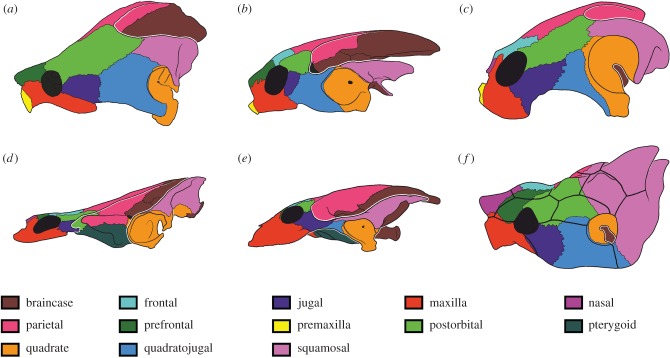
Figure 1.
Six turtle skulls in lateral view illustrating cranial disparity within the group. (a) the snapping turtle (Chelydridae) Macrochelys temminckii; (b) the pig nosed turtle (Carettochelyidae) Carettochelys insculpta; (c) the baenid (Baenidae) Palatobaena cohen; (d) the chelid turtle (Chelidae) Chelus fimbriatus; (e) the soft-shelled turtle (Trionychidae) Trionyx triunguis and (f) the cow horn turtle (Meiolaniidae) Meiolania platyceps.
The excellent fossil record of turtles should, in principle, allow for a rigorous assessment of speciation and extinction through time, but decades of neglect have resulted in a taxonomic quagmire that robs any global analysis [29] of a rigorous basis. Although significant efforts have been made in recent years to resolve the taxonomy of the primary groups of fossil turtles using modern criteria [30–33], only few, spatially and temporarily restricted studies are available that rigorously investigate diversity changes using a resolved taxonomy combined with phylogenetic methods [34,35]. As a rigorous assessment of diversity through time remains untenable until the alpha taxonomy and phylogeny of fossil turtles have been established, we here present a global analysis of turtle cranial disparity, a measure that assesses morphological diversity as opposed to taxonomic diversity [36–38]. Among tetrapods, this metric has most recently been applied, among others, to the cranial evolution in pterosaurs [39], crurotarsans [40] and theropod dinosaurs [41], but this method remains to be applied to turtles. In addition to providing a first estimate of turtle disparity through time, we here highlight the most notable disparity trends, investigate differences in disparity between the two primary clades of turtles—Pan-Cryptodira and Pan-Pleurodira—and discuss the primary factors that may have contributed to apparent differences and trends.
2. Material and methods
(a). Taxon sampling and geometric morphometrics
The primary goal of this study is to investigate temporal changes in disparity in the cranium of a broad selection of unambiguous representatives of the total clade of turtles (Pan-Testudines sensu Joyce et al. [42]). For this purpose, we sampled 172 fossil and recent species from the Late Permian to Recent in three views based on availability: 145 (66 extinct and 79 recent) species in dorsal view, 146 (61 extinct and 85 recent) species in lateral view and 158 (75 extinct and 83 recent) species in ventral view. Taxa were chosen to represent the greatest amount of tree space and morphospace. Whenever possible we attempted to capture the adult morphology of a species based on actual specimens, but we were forced to use published skull illustrations or reconstructions for the majority of fossil taxa. The full list of species and sources is provided in the electronic supplementary material, S1.
To study skull shape variation, we used two-dimensional geometric morphometrics. Skull shape was captured with 18 landmarks and 42 semi-landmarks for images taken in dorsal view, 12 landmarks and 66 semi-landmarks for images taken in lateral view, and 32 landmarks and 80 semi-landmarks for images taken in ventral view using the software tpsDig2 [43]. Landmarks were classified as either type 1 (i.e. points where three anatomical structures meet) or type 2 (i.e. points of maximum curvature) [44]. The shapes of cranial openings and of the skull outline were captured by semi-landmarks, which were plotted at equal intervals along the curves of the structures they were defining [44,45] (electronic supplementary material, S2, tables S1–S3 and figures S1–S3).
The primary data were loaded into MorphoJ [46] and superimposed using generalized Procrustes analyses (GPA), which reduces variation between specimens caused by scale, translation (i.e. position) and rotation, leaving only shape variation [47] (electronic supplementary material, S3). Although semi-landmarks contain less shape information than landmarks, as the positions of the former depend on those of the latter, we treated both shape coordinates as equivalent for GPA [48] because the sliding of semi-landmarks [48–50] can create considerable artificial deformation on the Procrustes shape of complex shapes [51].
Based on the Procrustes-fitted landmark coordinates, a morphospace was generated in MorphoJ using principal component analysis (PCA) on a covariance matrix. In contrast to the original shape data, the resulting principal components (PCs) describe successively smaller amounts of total variation so that a large proportion of variation can be described by a small number of variables [48,52].
(b). Disparity analyses, phylogeny and time calibration
PC scores summarize the skull shape of each taxon and provide quantitative insights into how the shape of a particular skull differs from the others included in the sample. They therefore represent shape proxies that can be used in macroevolutionary analyses to quantify major trends in skull evolution, including morphological disparity, a measure of the anatomical variation exhibited by a group of organisms [36–38]. We assessed disparity through time for turtles in general and for the two primary clades of crown Testudines in particular (i.e. Pan-Cryptodira and Pan-Pleurodira sensu Joyce et al. [42]) to test: (i) whether they exhibit temporal disparity trends and (ii) whether pan-cryptodires or pan-pleurodires developed greater amounts of disparity during certain time intervals.
To increase resolution of the temporal disparity analyses and to partially fill gaps in the fossil record where turtles are poorly sampled, we included PC scores of hypothetical ancestors into our disparity analyses [53]. The inclusion of unsampled ancestral shapes can support poorly sampled time intervals relative to later and/or better sampled bins. For the phylogenetic correction, we created an informal, time-calibrated supertree based on the recent literature (electronic supplementary material, S2, Text S1).
The time-calibrated tree was used to assign ancestors to particular time bins for later inclusion in the disparity analysis [53–55]. The stratigraphic ages of extinct terminal taxa were taken from the literature while the age of recent turtles was set to zero. A small number of taxa, such as Palatobaena cohen, are known to exist across two time bins [31]. For simplicity, we assigned these taxa to the single time bin that represents the midpoint of their known distribution. The temporal origin of select extant clades was fixed based on recent molecular calibration analyses [56]. All remaining nodes were calibrated between terminal taxa/fixed nodes by evenly distributing the available time between branches [57] (electronic supplementary material, S4).
To calculate ancestral morphologies, the PC scores of terminal taxa were mapped as continuous characters onto the time-calibrated phylogeny in Mesquite [58] using squared change parsimony [59]. The ancestral node values of the PC scores were then added to the overall dataset of PC scores of the terminal taxa.
For the temporal disparity curves, taxa were binned into time intervals of approximately equal duration spanning the Late Triassic until the Recent (electronic supplementary material, S2, table S5). For each time bin, disparity was separately calculated for the combination of all taxa and, if appropriate, separately for pan-pleurodires and pan-cryptodires.
For all disparity calculations, the sum of variances was used as the disparity metric, as it is mathematically more robust than other metrics with respect to sample size differences [37,60]. The sum of variance was estimated for each group in every bin (see below) using R [61]. Here, the minimum number of taxa for each bin was set to three. Bins with fewer taxa were not considered. All calculations are based on the first six PC scores for dorsal view (88.9% of total variation), the first seven PCs for lateral view (87.3% of total variation) and the first 11 PCs in ventral view (86.9% of total variation) from the morphometric PCA (see above), which summarize significant shape variation within the whole dataset based on the broken stick method [62] performed in PAST [63]. Differences in disparity between adjacent time bins of temporal curves (e.g. Middle Jurassic versus Upper Jurassic) and between pan-cryptodires and pan-pleurodires within a single bin (e.g. Palaeocene) were tested for significance applying a permutation test with 10 000 replicates, using a modified R script of Brusatte et al. [64], which takes sample size difference between the two units being compared into account. This script estimates whether a certain bin or group had more or less total disparity than the other by keeping sample size constant and by shuffling taxa randomly between the units being compared. To test the robustness of the results, we compared the temporal disparity curves from different views with each other, using ordinary and generalized least-squares regression analyses (electronic supplementary material, S2, Text S3 and table S23). To test for the impact of the hypothetical ancestors, all analyses were repeated with a reduced sample that only includes terminal taxa (electronic supplementary material, S2, Text S4, tables S24–S26 and figure S9).
We furthermore tested whether samples from two adjacent time bins share significantly overlapping portions of morphospace and, when applicable, whether pan-cryptodires and pan-pleurodires shared significantly overlapping portions of morphospace within the same time bin. This test differs from regular disparity analysis, which assesses whether two groups fill differently sizes areas within morphospace, regardless of their location. For the positional test, we compared different groups using non-parametric multivariate analysis of variance (npMANOVA) in PAST, which tests for significant differences in the distribution of groups in morphospace on the basis of permutation [65]. All permutations were conducted using the PC score data (see above), which were converted into a Euclidean distance matrix, permuted with 10 000 replications and compared using the Bonferroni correction. This analysis allows us to test whether one group is significantly different from another in a single time bin, and also whether a group significantly changes its morphospace occupation pattern over time. We finally tested for a correlation between disparity and climate. A simplified temperature curve was reconstructed by combined published δ18O records from the Mesozoic and Cenozoic [66,67] and compared with our disparity curve with help of Spearman's rank-order correlation test, ordinary and generalized least-square regression analyses [52]. We not only tested for correlations between the absolute values for each bin, but also for relative changes between subsequent time bins (electronic supplementary material, S2, Text S2 and tables S19–S22).
3. Results
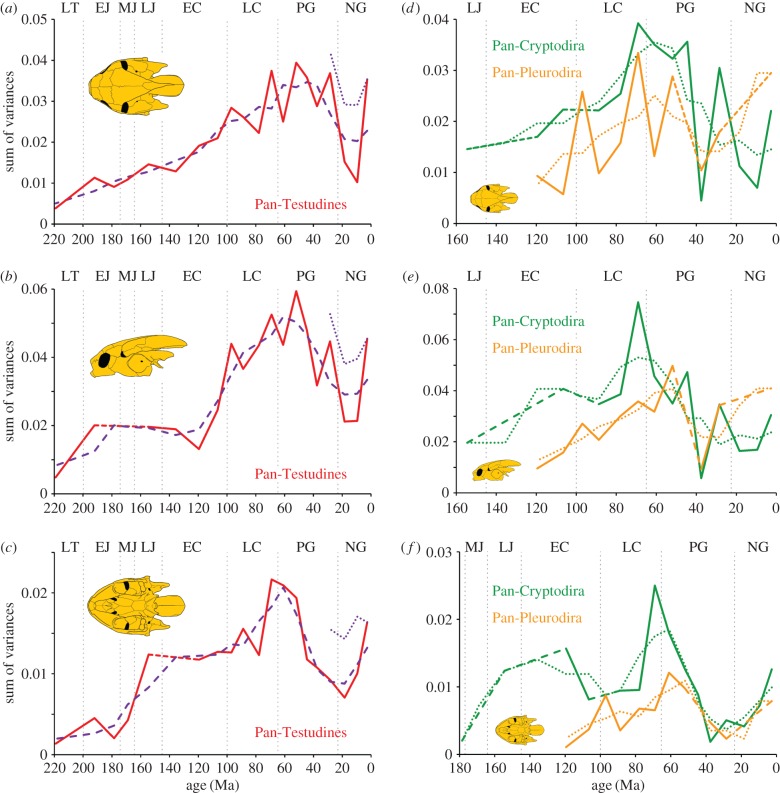
At a global scale, the greatest amount of disparity is apparent for the majority of time bins in lateral view, the least amount in ventral view, but all three views display the same general trends through time (figure 2; electronic supplementary material, S2, Text S3, tables S12 and S23 and figure S8). Although numerous other patterns could be extracted from these global disparity curves, we here highlight three trends of importance to the following discussion: (i) a general increase in disparity from the Triassic to the Early Palaeogene, (ii) a substantial collapse in disparity until the Miocene and (iii) a partial recovery until the Recent. Minor differences are apparent between the three primary views for all three trends. Most notably, the initial increase in disparity is gradual in dorsal view, but shows a prolonged Mesozoic plateau in lateral view, and a short Early Cretaceous plateau in ventral view. The disparity peak is reached in the Maastrichtian in ventral view, but only in the Early Eocene in the other two views. The point of recovery is universally located in the Miocene. For dorsal and lateral view, the cranial disparity of Recent turtles is comparable to that of those from the Oligocene and Lower Cretaceous, while Recent disparity in ventral view approximates that of the Eocene and Lower Cretaceous (electronic supplementary material, S2, figures S5–S7 and tables S6–S11). Because differences between successive time bins were not significant in most cases (electronic supplementary material, tables S7, S9 and S11), the results indicate long, steady trends in disparity. The results are essentially the same for all three views and when the analyses are run without the hypothetical ancestors (electronic supplementary material, S2, Text S3, S4, tables S23–S26 and figure S9).
Figure 2.
Temporal disparity of turtle skull shape. (a–c) Sum of variances for all representatives of the turtle stem lineage (Pan-Testudines) in dorsal, lateral and ventral view. The red solid lines illustrate temporal disparity for all time bins, whereas the red dashed lines indicate disparity differences with longer time gaps. The purple dashed lines signify the 3-point average of the disparity curve, whereas the blue-dotted lines show temporal disparity from the Oligocene to Recent after inclusion of a selection of recent taxa with long ghost lineages to these time bins. (d–f) Sum of variances for Pan-Cryptodira (green line) and Pan-Pleurodira (orange line). Dashed lines mark missing time bins owing to small sample sizes. LT, Late Triassic; EJ, Early Jurassic; MJ, Middle Jurassic; LJ, Late Jurassic; EC, Early Cretaceous; LC, Late Cretaceous; PG, Paleogene; NG, Neogene.
The cranial disparity of pan-cryptodires is generally higher than that of pan-pleurodires in all views, but these differences are only significant in the Palaeocene for dorsal view, Albian for lateral view and the Aptian for ventral view (figure 2). Pan-pleurodires only have higher cranial disparity during the Cenomanian in dorsal view, the Palaeocene in lateral view, the Late Eocene in all views and the Recent in dorsal and lateral view, but, as mentioned earlier, these differences are not significant (electronic supplementary material, S2, tables S13–S18).
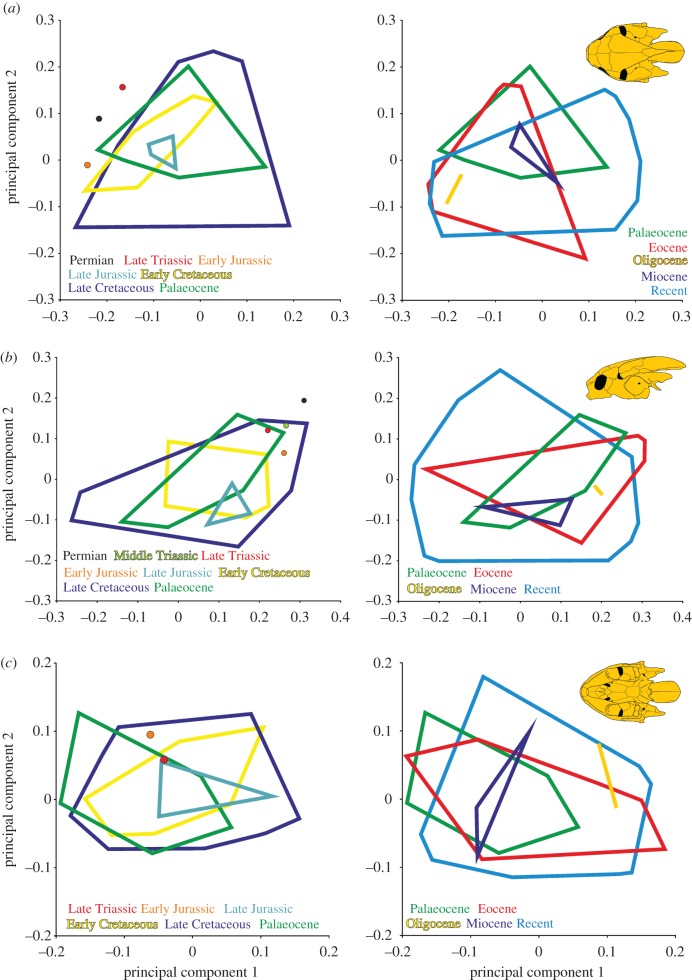
The npMANOVA reveals that neither turtles in general nor pan-cryptodires and pan-pleurodires in particular shift their position significantly in morphospace through time (figure 3). However, the latter two groups occupy significantly different areas in morphospace for each time bin when compared to each other, with exception of the lateral view during the entire Cretaceous (electronic supplementary material, S2, tables S13–S18). Finally, we find low evidence for a correlation between temporal disparity and climate change in the Cenozoic, but less so for the Mesozoic (electronic supplementary material, S2, Text S2 and tables S19–S22).
Figure 3.
Two-dimensional morphospace of turtle skulls in (a) dorsal, (b) lateral and (c) ventral view based principal component axes 1 and 2, illustration the size and positional shifts of morphospace for subsequent time bins as coloured outlines. For better overview the diagram was split into two plots, showing changes in morphospace from the Permian to the Palaeocene (left) and from the Palaeocene to the Recent (right). The original scatterplots are provided in the electronic supplementary material, S2, figures S5–S7.
4. Discussion and conclusion
The study at hand represents the first disparity analyses through time for turtles using landmark-based geometric morphometrics quantifying cranial shape. All skull views show roughly the same progression, although the ventral aspect of the skull shows less disparity than the dorsal and lateral ones. These differences indicate that the skulls of turtles show more variation associated in the upper temporal emargination (dorsal and lateral views), the lower temporal emargination (lateral view), and the orbits (lateral view) than with the palate and basicranium (ventral view), a counterintuitive result given the heavy emphasis of turtle palaeontologists in retrieving phylogenetic characters from the latter anatomical region [24,25].
Although turtles explore significant amounts of morphospace, the npMANOVA reveals that turtles in general, and pan-pleurodires and pan-cryptodires in particular, hold stable positions through time (figure 3). Although this observation may lead to the tempting conclusion that turtles evolve slowly, we note that turtles apparently evolved into various ecological niches not by converging repeatedly upon the same morphotype, but rather by exploring differing solutions in nearby morphospace.
The continuous increase of cranial disparity from the Late Triassic to the Late Cretaceous (figure 2) correlates with an increase in species diversity during the Mesozoic [29] (however, see caveats regarding the applicability of this study in §1). Cranial disparity peaks late, in the Maastrichtian for ventral views and Early Eocene for dorsal and lateral views. This pattern is retained when pan-cryptodires and pan-pleurodires are investigated separately, although both groups oscillate more strongly in disparity, having local maxima in the Aptian and Cenomanian (figure 2). A slow, steady increase of disparity in concert with a slow build-up of diversity, as opposed to an explosive, early increase of either, is indicative of the concordant evolution of these two diversity measures [68]. Delayed peaks of disparity have otherwise been found for pterosaurs [39,60,69,70] and the dentition of ungulates [71] and carnivoramorphan mammals [72], but most other animal groups previously investigated reach their maximum disparity early in evolution [68,73,74].
Global climate reconstructions point towards a gradual rise in temperatures from the Jurassic to the Palaeocene [66,67], which generally correlates with our observed increase in disparity within the same time window. Given that turtles are ectothermic organisms that profit from warmer temperatures, it is plausible that global warming may have influenced this increase in disparity (see the electronic supplementary material, S2, Text S3 and table S21). However, we find two additional factors to be of interest. First, given that turtles originated in the Early Mesozoic, it is plausible that the initial increase of disparity is the result of random or adaptive exploration of the available morphospace. Given that turtles have extended generation times relative to most invertebrates or small amniotes [75,76], it is furthermore plausible that evolutionary rates are lower for the group [77,78] and that this diversification therefore took place over an unusually prolonged time period and was only stopped by climatic cooling. On the other hand, the early diversification of turtles also coincides with the break-up of Pangaea, as already noted for other reptile groups [79–82]. The increasing number of isolated landmasses, in return, may have provided turtles with a greater number of opportunities to adapt to similar environmental conditions with different morphological solutions. Although the available data do not allow us to disentangle these hypotheses rigorously, we find it notable that unrelated turtles groups that adapted to similar environmental conditions (gape-and-suction feeding trionychids, chelids or emydids; terrestrially herbivorous meiolaniids, nanhsiungchelyids and testudinids) occupy different regions in morphospace (electronic supplementary material, S2, figure S8). The fragmentation of the continents during the Mesozoic may therefore have driven diversity and disparity, as the vast majority of continental clades remain restricted to the continents upon which they originated through vicariance [83].
Turtles appear to be highly resistant to mass extinction events. We find no change in the cranial disparity of turtles at the Triassic/Jurassic extinction event, while the Cretaceous/Tertiary extinction results only in a small, but insignificant decrease in dorsal and lateral views, followed by a fast recovery. This persistence during the K/T mass extinction event is in agreement with the fossil record, as extinction across the boundary has been shown to be minimal, as least in North America, where this time interval is best documented [26,34,35,84–86]. Pan-cryptodires show a stronger decline of disparity across the K/T boundary than pan-pleurodires, primarily in lateral and ventral aspects.
After reaching a global maximum, disparity declines overall from the Eocene to the Miocene. This trend once again conspicuously mirrors global temperature estimates, which show stark cooling following the Early Eocene climatic optimum [66,67]. In contrast to the rise in disparity throughout the Mesozoic (where climate change might have played a subliminal role), we find a climatically controlled decrease in Tertiary disparity to be highly plausible (see the electronic supplementary material, S2, Text S3 and tables S20 and S22), as global cooling has otherwise been shown to have significant impact on the diversity of turtles, at least as demonstrated in North America where Palaeogene turtle faunas are well sampled and well understood [87–89]. As the principal mechanism, we suggest habitat loss resulting from the global drying that accompanied global cooling [90]. A second aspect that may have played an important role, however, is the increasing homogenization of global turtle faunas through the partial or full replacement of aboriginal turtle faunas on formerly isolated continent by modern cryptodiran faunas [83]. This process, once again, is most drastically illustrated in North America, where a fully indigenous turtle fauna consisting of helochelydrids and paracryptodires is fully replaced by cryptodires by the Palaeocene through the extinction of at least five paracryptodiran lineages filling unique portions of morphospace [34,35,84,85,88].
Although we are confident about the marked decrease in disparity in the Palaeogene, we are not convinced that the factors discussed above are responsible for the pronounced disparity minimum in the Miocene and the strong recovery towards the Recent. Instead, we believe that these trends could be partly the result of a sampling artefact caused by poor direct sampling of fossil taxa from the Oligocene to Pleistocene combined with low sampling of hypothetical ancestors, even though the ghost lineages of most extant taxa cross the Miocene time interval. As these ghost lineages positively indicate the presence of ancestral taxa, we recalculated in an exploratory analysis the disparity of the Oligocene and Miocene time bins after including a select set of Recent representatives, in particular the meiolaniid Meiolania platyceps, the chelid Pseudemydura umbrina, the pelomedusoid Pelusios sinuatus, the trionychians Apalone ferox and Carettochelys insculpta, the chelydroid Dermatemys mawii and chelonioid Dermochelys coriacea. These taxa were chosen to represent the maximum amount of morphospace with a minimum of additional taxa. Although this increase in sampling did not fully obliterate the Miocene minimum, the disparity gap between the Miocene and Recent time bin was notably reduced (see blue-dotted lines in figure 2a–c). Given that the entire sample from the Recent time bin could have legitimately been included in the Oligocene to Miocene time bins using the same rationale, we speculate that the Miocene disparity minimum is an artefact of sampling. Only a study that fully embraces ghost lineages or a study focusing on Tertiary turtle disparity using other parts of the body (e.g. the shell) or the whole skeleton will be able to test this assertion with confidence.
Our study only investigates the cranial disparity of turtles, but it is unclear whether the trends we discerned are unique to the cranium or representative for other anatomical systems. The two previous studies that investigated this issue for echinoid [91] and pterosaurs [39] concluded that different morphological proxies and body regions produce similar temporal disparity curves through time. However, given that the turtle shell remains highly conserved throughout turtle evolution in regards to the arrangement and number of bones and scutes and that the shape is strongly constrained by ecological factors, we speculate that the postcranial system may yield different trends in turtles [92].
This is the first study to investigate the global cranial disparity of turtles through time but some caveats diminish the results as the ancestral shapes that we included in our disparity analyses strongly depend on the phylogenetic relationships, the algorithm of time calibration [93] and the method of reconstruction [94,95]. Thus, an alternative phylogeny or the application of different calibration and reconstruction methods could potentially change the results by affecting not only the temporal binning of taxa, but also the ancestral shape itself. However, the exclusion of hypothetical ancestors produced very similar disparity and npMANOVA results, but with lower temporal resolution (see the electronic supplementary material S2, Text S4, table S24–S26 and figure S9). Finally, as shown above for the artificial Miocene disparity minimum, incomplete sampling for certain time periods can have a strong impact on the shape of the disparity curve as well, implying that a more complete fossil record would lead to a better temporal resolution by diminishing artefacts caused by long ghost lineages. With these caveats in mind, we nevertheless believe that the overall trends described in this study (i.e. an increase in disparity from the Triassic to the Early Palaeogene, a negligible impact of the K/T extinction event, and substantial decrease until today) are true signals as they are in broad agreement with other sources of data. Future studies, however, will likely be able to add significant refinement to these broad patterns and test their applicability to other body regions.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgements
We wish to thank Jeremy Jacobs and Addison Wynn for providing us with access to the herpetological collections at the USNM. We furthermore thank Steve Wang (Swarthmore College) for providing us with his R script and Eduardo Ascarrunz (University of Fribourg) and Márton Rabi (University of Tübingen) for useful discussions. Furthermore, we thank Stephen Brusatte and one anonymous reviewer for their critical comments, which helped to improve the manuscript significantly.
Data accessibility
Data forming the basis of this research and the details of analyses are available in the electronic supplementary material attached to this article.
Authors' contributions
C.F. performed all statistical analyses and created all figures. C.F. and W.G.J. otherwise conceived the project, collected data, interpreted the results and wrote the manuscript in equal parts.
Competing interests
We declare we have no competing interests.
Funding
This study was funded by a fellowship of the German Academic Exchange Service (DAAD, no. 91546784) to C.F. and by funds from the Department of Geosciences of the University of Fribourg/Switzerland to W.G.J.
References
- 1.Joyce WG. 2015. The origin of turtles: a paleontological perspective. J. Exp. Zool. 324B, 181–193. ( 10.1002/jez.b.22609) [DOI] [PubMed] [Google Scholar]
- 2.Li C, Wu X, Rieppel OC, Wang L, Zhao L. 2008. An ancestral turtle from the Late Triassic of southwestern China. Nature 456, 497–501. ( 10.1038/nature07533) [DOI] [PubMed] [Google Scholar]
- 3.Schoch RR, Sues H-D. 2015. A Middle Triassic stem-turtle and the evolution of the turtle body plan. Nature 523, 584–587. ( 10.1038/nature14472) [DOI] [PubMed] [Google Scholar]
- 4.Lyson TR, Bever GS, Bhullar B-AS, Joyce WG, Gauthier JA. 2010. Transitional fossils and the origin of turtles. Proc. R. Soc. B 6, 830–833. ( 10.1098/rsbl.2010.0371) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bever GS, Lyson TR, Field DJ, Bhullar B-AS. 2015. Evolutionary origin of the turtle skull. Nature 525, 239–242. ( 10.1038/nature14900) [DOI] [PubMed] [Google Scholar]
- 6.Werneburg I, Wilson LAB, Parr WCH, Joyce WG. 2015. Evolution of neck vertebral shape and neck retraction at the transition to modern turtles: an integrated geometric morphometric approach. Syst. Biol. 64, 187–204. ( 10.1093/sysbio/syu072) [DOI] [PubMed] [Google Scholar]
- 7.Schumacher GH. 1973. The head muscles and hyolaryngeal skeleton of turtles and crocodilians. In Biology of the reptilia (eds Gans C, Parsons TS), pp. 101–199. London, UK: Academic Press. [Google Scholar]
- 8.Bramble DM. 1974. Emydid shell kinesis: biomechanics and evolution. Copeia 1974, 707–727. ( 10.2307/1442685) [DOI] [Google Scholar]
- 9.Bramble DM, Hutchison JH. 1981. A reevaluation of the plastral kinesis in African turtles of the genus Pelusios. Herpetologica 37, 205–212. [Google Scholar]
- 10.Bramble DM, Hutchison JH, Legler JM. 1984. Kinosternid shell kinesis: structure, function and evolution. Copeia 1984, 456–475. ( 10.2307/1445203) [DOI] [Google Scholar]
- 11.Meylan PA. 1987. The phylogenetic relationships of soft-shelled turtles (Family Trionychidae). Bull. Am. Mus. Nat. Hist. 186, 1–101. [Google Scholar]
- 12.Renous S, Lapparent de Broin F, Depecker M, Davenport J, Bels V. 2007. Evolution of locomotion in aquatic turtles. In Biology of turtles (eds Wyneken J, Godfrey MH, Bels V), pp. 97–137. Boca Raton, FL: CRC Press. [Google Scholar]
- 13.Ernst CH, Barbour RW. 1989. Turtles of the world. Washington, DC: Smithsonian Institution Press. [Google Scholar]
- 14.Claude J, Paradis E, Tong H, Auffray J-C. 2003. A geometric morphometric assessment of the effects of environment and cladogenesis on the evolution of the turtle shell. Biol. J. Linn. Soc. 79, 485–501. ( 10.1046/j.1095-8312.2003.00198.x) [DOI] [Google Scholar]
- 15.Claude J. 2006. Convergence induced by plastral kinesis and phylogenetic constraints in Testudinoidea: a geometric morphometric assessment. Foss. Turt. Res. 1, 34–45. [Google Scholar]
- 16.Rioux Paquette S, Lapointe F-J. 2007. The use of shell morphometrics for the management of the endangered malagasy radiated tortoise (Geochelone radiata). Biol. Conserv. 134, 31–39. ( 10.1016/j.biocon.2006.08.022) [DOI] [Google Scholar]
- 17.Zuffi MAL, Plaitano A. 2007. Similarities and differences in adult tortoises: a morphological approach and its implication for reproduction and mobility between species. Acta Herpetol. 2, 79–86. [Google Scholar]
- 18.Rivera G, Claude J. 2008. Environmental media and shape asymmetry: a case study on turtle shells. Biol. J. Linn. Soc. 94, 483–489. ( 10.1111/j.1095-8312.2008.01008.x) [DOI] [Google Scholar]
- 19.Angielczyk KD, Feldman CR. 2013. Are diminutive turtles miniaturized? The ontogeny of plastron shape in emydine turtles. Biol. J. Linn. Soc. 108, 727–755. ( 10.1111/bij.12010) [DOI] [Google Scholar]
- 20.Depecker M, Renous S, Penin X, Berge C. 2006. Procrustes analysis: a tool to understand shape changes of the humerus in turtles (Chelonii). C. R. Palevol. 5, 509–518. ( 10.1016/j.crpv.2005.01.003) [DOI] [Google Scholar]
- 21.Depecker M, Berge C, Penin X, Renous S. 2006. Geometric morphometrics of the shoulder girdle in extant turtles (Chelonii). J. Anat. 208, 35–45. ( 10.1111/j.1469-7580.2006.00512.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Werneburg I. 2015. Neck motion in turtles and its relation to the shape of the temporal skull region. C. R. Palevol. 14, 527–548. ( 10.1016/j.crpv.2015.01.007) [DOI] [Google Scholar]
- 23.Werneburg I, Hinz JK, Gumpenberger M, Volpato V, Natchev N, Joyce WG. 2015. Modeling neck mobility in fossil turtles. J. Exp. Zool. 324B, 230–243. ( 10.1002/jez.b.22557) [DOI] [PubMed] [Google Scholar]
- 24.Gaffney ES, Rich TH, Vickers-Rich P, Constantine A, Vacca R, Kool L. 2007. Chubutemys, a new eucryptodiran turtle from the Early Cretaceous of Argentina, and the relationships of the Meiolaniidae. Am. Mus. Novit. 3599, 1–35. ( 10.1206/0003-0082(2007)3599%5B1:CANETF%5D2.0.CO;2) [DOI] [Google Scholar]
- 25.Joyce WG. 2007. Phylogenetic relationships of Mesozoic turtles. Bull. Peabody Mus. Nat. Hist. 48, 3–102. ( 10.3374/0079-032X(2007)48%5B3:PROMT%5D2.0.CO;2) [DOI] [Google Scholar]
- 26.Hutchison JH, Archibald JD. 1986. Diversity of turtles across the Cretaceous/Tertiary boundary in northeastern Montana. Palaeogeogr. Palaeoclimatol. Palaeoecol. 55, 1–22. ( 10.1016/0031-0182(86)90133-1) [DOI] [Google Scholar]
- 27.Claude J, Pritchard P, Tong H, Paradis E, Auffray J-C. 2004. Ecological correlates and evolutionary divergence in the skull of turtles: a geometric morphometric assessment. Syst. Biol. 53, 933–948. ( 10.1080/10635150490889498) [DOI] [PubMed] [Google Scholar]
- 28.Ferreira GS, Rincón AD, Solórzano A, Langer MC. 2015. The last marine pelomedusoids (Testudines: Pleurodira): a new species of Bairdemys and the paleoecology of Stereogenyina. PeerJ 3, e1063 ( 10.7717/peerj.1063) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nicholson DB, Holroyd PA, Benson RBJ, Barrett PM. 2015. Climate-mediated diversification of turtles in the Cretaceous. Nat. Commun. 6, 7848 ( 10.1038/ncomms8848) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gaffney ES, Tong H, Meylan PA. 2006. Evolution of the side-necked turtles: the families Bothremydidae, Euraxemydidae, and Araripemydidae. Bull. Am. Mus. Nat. Hist. 300, 1–698. ( 10.1206/0003-0090(2006)300%5B1:EOTSTT%5D2.0.CO;2) [DOI] [Google Scholar]
- 31.Joyce WG, Lyson TR. 2015. A review of the fossil record of turtles of the clade Baenidae. Bull. Peabody Mus. Nat. Hist. 56, 147–183. ( 10.3374/014.056.0203) [DOI] [Google Scholar]
- 32.Sterli J. 2015. A review of the fossil record of Gondwanan turtles of the clade Meiolaniformes. Bull. Peabody Mus. Nat. Hist. 56, 21–45. ( 10.3374/014.056.0102) [DOI] [Google Scholar]
- 33.Vitek NS, Joyce WG. 2015. A review of the fossil record of new world turtles of the clade Pan-Trionychidae. Bull. Peabody Mus. Nat. Hist. 56, 185–244. ( 10.3374/014.056.0204) [DOI] [Google Scholar]
- 34.Lyson TR, Joyce WG, Knauss GE, Pearson DA. 2011. Boremys (Testudines, Baenidae) from the latest Cretaceous and early Paleocene of North Dakota: an 11-million-year range extension and an additional K/T survivor. J. Vertebr. Paleontol. 31, 729–737. ( 10.1080/02724634.2011.576731) [DOI] [Google Scholar]
- 35.Joyce WG, Lyson TR. 2011. New material of Gilmoremys lancensis nov. comb. (Testudines: Trionychidae) from the Hell Creek Formation and the diagnosis of plastomenid turtles. J. Paleontol. 85, 442–459. ( 10.1666/10-127.1) [DOI] [Google Scholar]
- 36.Foote M. 1993. Discordance and concordance between morphological and taxonomic diversity. Paleobiology 19, 185–204. ( 10.1017/S0094837300015864) [DOI] [Google Scholar]
- 37.Wills MA, Briggs DEG, Fortey RA. 1994. Disparity as an evolutionary index: a comparison of Cambrian and recent arthropods. Paleobiology 20, 93–130. ( 10.1017/S009483730001263X) [DOI] [Google Scholar]
- 38.Ciampaglio CN, Kemp M, McShea DW. 2001. Detecting changes in morphospace occupation patterns in the fossil record: characterization and analysis of measures of disparity. Paleobiology 27, 695–715. ( 10.1666/0094-8373(2001)027%3C0695:DCIMOP%3E2.0.CO;2) [DOI] [Google Scholar]
- 39.Foth C, Brusatte SL, Butler RJ. 2012. Do different disparity proxies converge on a common signal? Insights from the cranial morphometrics and evolutionary history of Pterosauria (Diapsida: Archosauria). J. Evol. Biol. 25, 904–915. ( 10.1111/j.1420-9101.2012.02479.x) [DOI] [PubMed] [Google Scholar]
- 40.Stubbs TL, Pierce SE, Rayfield EJ, Anderson PSL. 2013. Morphological and biomechanical disparity of crocodile-line archosaurs following the end-Triassic extinction. Proc. R. Soc. B 280, 20131940 ( 10.1098/rspb.2013.1940) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brusatte SL, Montanari S, Sakamoto M, Harcourt-Smith WEH. 2012. The evolution of cranial form and function in theropod dinosaurs: insight from geometric morphometrics. J. Evol. Biol. 25, 365–377. ( 10.1111/j.1420-9101.2011.02427.x) [DOI] [PubMed] [Google Scholar]
- 42.Joyce WG, Parham JF, Gauthier JA. 2004. Developing a protocol for the conversion of rank-based taxon names to phylogenetically defined clade names, as exemplified by turtles. J. Paleontol. 78, 989–1013. ( 10.1666/0022-3360(2004)078%3C0989:DAPFTC%3E2.0.CO;2) [DOI] [Google Scholar]
- 43.Rohlf FJ. 2005. tpsDig, digitize landmarks and outlines, version 2.05. See http://life.bio.sunysb.edu/morph/soft-dataacq.html.
- 44.Bookstein FL. 1991. Morphometric tools for landmark data. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 45.Bookstein FL, et al. 1999. Comparing frontal cranial profiles in archaic and modern Homo by morphometric analysis. Anat. Rec. 257, 217–224. ( 10.1002/(SICI)1097-0185(19991215)257:6%3C217::AID-AR7%3E3.0.CO;2-W) [DOI] [PubMed] [Google Scholar]
- 46.Klingenberg CP. 2011. MorphoJ: an integrated software package for geometric morphometrics. Mol. Ecol. Resour. 11, 353–357. ( 10.1111/j.1755-0998.2010.02924.x) [DOI] [PubMed] [Google Scholar]
- 47.Rohlf FJ, Slice DE. 1990. Extensions of the Procrustes method for the optimal superimposition of landmarks. Syst. Zool. 39, 40–59. ( 10.2307/2992207) [DOI] [Google Scholar]
- 48.Zelditch ML, Swiderski DL, Sheets HD. 2012. Geometric morphometrics for biologists: a primer. Amsterdam, The Netherlands: Elsevier Academic Press. [Google Scholar]
- 49.Bookstein FL. 1997. Landmark methods for forms without landmarks: morphometrics of group differences in outline shape. Med. Image Anal. 1, 225–243. ( 10.1016/S1361-8415(97)85012-8) [DOI] [PubMed] [Google Scholar]
- 50.Gunz P, Mitteroecker P, Bookstein FL. 2004. Semilandmarks in three dimensions. In Modern morphometrics in physical anthropology (ed. Slice DE.), pp. 73–98. New York, NY: Plenum Publishers. [Google Scholar]
- 51.Foth C, Hedrick BP, Ezcurra MD. 2016. Cranial ontogenetic variation in early saurischians and the role of heterochrony in the diversification of predatory dinosaurs. PeerJ 4, e1589 ( 10.7717/peerj.1589) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hammer O, Harper DAT. 2006. Paleontological data analysis. Malden, MA: Blackwell Publishing. [Google Scholar]
- 53.Brusatte SL, Montanari S, Yi H, Norell MA. 2011. Phylogenetic corrections for morphological disparity analysis: new methodology and case studies. Paleobiology 37, 1–22. ( 10.1666/09057.1) [DOI] [Google Scholar]
- 54.Bapst DW. 2013. A stochastic rate-calibrated method for time-scaling phylogenies of fossil taxa. Methods Ecol. Evol. 4, 724–733. ( 10.1111/2041-210X.12081) [DOI] [Google Scholar]
- 55.Foth C, Ezcurra MD, Sookias RB, Brusatte SL, Butler RJ. 2016. Unappreciated diversification of stem archosaurs during the Middle Triassic predated the dominance of dinosaurs. BMC Evol. Biol. 16, 188 ( 10.1186/s12862-016-0761-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Joyce WG, Parham JF, Lyson TR, Warnock RCM, Donoghue PCJ. 2013. A divergence dating analysis of turtles using fossil calibrations: an example of best practices. J. Paleontol. 87, 612–634. ( 10.1666/12-149) [DOI] [Google Scholar]
- 57.Brusatte SL, Benton MJ, Ruta M, Lloyd GT. 2008. Superiority, competition, and opportunism in the evolutionary radiation of dinosaurs. Science 321, 1485–1488. ( 10.1126/science.1161833) [DOI] [PubMed] [Google Scholar]
- 58.Maddison WP, Maddison DR.2015. Mesquite: a modular system of evolutionary analysis. Version 3.04. See http://mesquiteproject.org .
- 59.Maddison WP. 1991. Squared-change parsimony reconstructions of ancestral states for continuous-valued characters on a phylogenetic tree. Syst. Zool. 40, 304–314. ( 10.2307/2992324) [DOI] [Google Scholar]
- 60.Butler RJ, Brusatte SL, Andres B, Benson RBJ. 2012. How do geological sampling biases affect studies of morphological evolution in deep time? A case study of pterosaur (Reptilia: Archosauria) disparity. Evolution 66, 147–162. ( 10.1111/j.1558-5646.2011.01415.x) [DOI] [PubMed] [Google Scholar]
- 61.R-Development-Core-Team. 2011. R: a language and environment for statistical computing. See http://www.r-project.org.
- 62.Jackson DA. 1993. Stopping rules in principal components analysis: a comparison of heuristical and statistical approaches. Ecology 74, 2204–2214. ( 10.2307/1939574) [DOI] [Google Scholar]
- 63.Hammer O, Harper DAT, Ryan PD. 2001. PAST: paleontological statistics software package for education and data analysis. Palaeontol. Electronica 4, 1–9. [Google Scholar]
- 64.Brusatte SL, Lloyd GT, Wang SC, Norell MA. 2014. Gradual assembly of avian body plan culminated in rapid rates of evolution across the dinosaur–bird transition. Curr. Biol. 24, 2386–2392. ( 10.1016/j.cub.2014.08.034) [DOI] [PubMed] [Google Scholar]
- 65.Anderson MJ. 2001. A new method for non-parametric multivariate analysis of variance. Austral Ecol. 26, 32–46. [Google Scholar]
- 66.Veizer J, Godderis Y, François LM. 2000. Evidence for decoupling of atmospheric CO2 and global climate during the Phanerozoic eon. Nature 408, 698–701. ( 10.1038/35047044) [DOI] [PubMed] [Google Scholar]
- 67.Zachos J, Pagani M, Sloan L, Thomas E, Billups K. 2001. Trends, rhythms, and aberrations in global climate 65 Ma to present. Science 292, 686–693. ( 10.1126/science.1059412) [DOI] [PubMed] [Google Scholar]
- 68.Erwin DH. 2007. Disparity: morphological pattern and developmental context. Palaeontology 50, 57–73. ( 10.1111/j.1475-4983.2006.00614.x) [DOI] [Google Scholar]
- 69.Dyke GJ, McGowan AJ, Nudds RL, Smith D. 2009. The shape of pterosaur evolution: evidence from the fossil record. J. Evol. Biol. 22, 890–898. ( 10.1111/j.1420-9101.2008.01682.x) [DOI] [PubMed] [Google Scholar]
- 70.Prentice KC, Ruta M, Benton MJ. 2011. Evolution of morphological disparity in pterosaurs. J. Syst. Palaeontol. 9, 337–353. ( 10.1080/14772019.2011.565081) [DOI] [Google Scholar]
- 71.Jernvall J, Hunter JP, Fortelius M. 2000. Trends in the evolution of molar crown types in ungulate mammals: evidence from the northern hemisphere. In Development, function and evolution of teeth (eds Teaford MF, Smith MM, Ferguson MWJ), pp. 269–281. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 72.Wesley-Hunt GD. 2005. The morphological diversification of carnivores in North America. Paleobiology 31, 35–55. ( 10.1666/0094-8373(2005)031%3C0035:TMDOCI%3E2.0.CO;2) [DOI] [Google Scholar]
- 73.Briggs DEG, Fortey RA, Wills MA. 1992. Morphological disparity in the Cambrian. Science 256, 1670–1673. ( 10.1126/science.256.5064.1670) [DOI] [PubMed] [Google Scholar]
- 74.Hughes M, Gerber S, Wills MA. 2013. Clades reach highest morphological disparity early in their evolution. Proc. Natl Acad. Sci. USA 110, 13 875–13 879. ( 10.1073/pnas.1302642110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Congdon JD, Dunham AE, van Loben Sels RC. 1993. Delayed sexual maturity and demographics of Blanding's Turtles (Emydoidea blandingii): implications for conservation and management of long-lived organisms. Conserv. Biol. 7, 826–833. ( 10.1046/j.1523-1739.1993.740826.x) [DOI] [Google Scholar]
- 76.Marsack K, Swanson BJ. 2009. A genetic analysis of the impact of generation time and road-based habitat fragmentation on eastern box turtles (Terrapene c. carolina). Copeia 2009, 647–652. ( 10.1643/CE-08-233) [DOI] [Google Scholar]
- 77.Eo SH, DeWoody JA. 2010. Evolutionary rates of mitochondrial genomes correspond to diversification rates and to contemporary species richness in birds and reptiles. Proc. R. Soc. B 277, 3587–3592. ( 10.1098/rspb.2010.0965) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Green RE, et al. 2014. Three crocodilian genomes reveal ancestral patterns of evolution among archosaurs. Science 346, 1254449 ( 10.1126/science.1254449) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Upchurch P, Hunn CA, Norman DB. 2002. An analysis of dinosaurian biogeography: evidence for the existence of vicariance and dispersal patterns caused by geological events. Proc. R. Soc. Lond. B 269, 613–621. ( 10.1098/rspb.2001.1921) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Turner AH. 2004. Crocodyliform biogeography during the Cretaceous: evidence of Gondwanan vicariance from biogeographical analysis. Proc. R. Soc. Lond. B 271, 2003–2009. ( 10.1098/rspb.2004.2840) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Holtz TRJ, Chapman RE, Lamanna MC. 2004. Mesozoic biogeography of Dinosauria. In The Dinosauria (eds Weishampel DB, Dodson P, Osmólska H), pp. 627–642. Berkeley, CA: University of California Press. [Google Scholar]
- 82.Rauhut OWM, López-Arbarello A. 2008. Archosaur evolution during the Jurassic: a southern perspective. Rev. Asoc. Geol. Argent. 63, 557–585. [Google Scholar]
- 83.Joyce WG, Rabi M, Clark JM, Xu X. 2016. A toothed turtle from the Late Jurassic of China and the global biogeographic history of turtles. BMC Biol. 16, 236 ( 10.1186/s12862-016-0762-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lyson TR, Joyce WG. 2009. A revision of Plesiobaena (Testudines: Baenidae) and an assessment of baenid ecology across the K/T boundary. J. Paleontol. 83, 833–853. ( 10.1666/09-035.1) [DOI] [Google Scholar]
- 85.Lyson TR, Joyce WG. 2011. Cranial anatomy and phylogenetic placement of the enigmatic turtle Compsemys victa Leidy, 1856. J. Paleontol. 85, 789–801. ( 10.1666/10-081.1) [DOI] [Google Scholar]
- 86.Holroyd PA, Wilson GP, Hutchison JH. 2014. Temporal changes within the latest Cretaceous and early Paleogene turtle faunas of northeastern Montana. Geol. Soc. Am. Spec. Pap. 503, 299–312. ( 10.1130/2014.2503(11)) [DOI] [Google Scholar]
- 87.Hutchison JH. 1996. Testudines. In The terrestrial Eocene-Oligocene transition in North America (eds Prothero DR, Emry RJ), pp. 337–353. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 88.Hutchison JH. 1998. Turtles across the Paleocene/Eocene epoch boundary in west-central North America. In Late Paleocene–early Eocene climatic and biotic events in the marine and terrestrial records (eds Aubrey MP, Lucas SG, Berggren WA). New York, NY: Columbia University Press. [Google Scholar]
- 89.Rödder D, et al. 2013. Evaluating the significance of paleophylogeographic species distribution models in reconstructing Quaternary range-shifts of nearctic chelonians. PLoS ONE 8, e72855 ( 10.1371/journal.pone.0072855) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hutchison JH. 1992. Western North American reptile and amphibian record across the Eocene/Oligocene boundary and its climatic implications. In Eocene–Oligocene climate and biotic evolution (eds Prothero DR, Berggren WA), pp. 451–467. Princeton, NJ: Princeton University Press. [Google Scholar]
- 91.Villier L, Eble GJ. 2004. Assessing the robustness of disparity estimates: the impact of morphometric scheme, temporal scale, and taxonomic level in spatangoid echinoids. Paleobiology 30, 652–665. ( 10.1666/0094-8373(2004)030%3C0652:ATRODE%3E2.0.CO;2) [DOI] [Google Scholar]
- 92.Hopkins MJ, Lidgard S. 2012. Evolutionary mode routinely varies among morphological traits within fossil species lineages. Proc. Natl Acad. Sci. USA 109, 20 520–20 525. ( 10.1073/pnas.1209901109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bapst DW. 2014. Assessing the effect of time-scaling methods on phylogeny-based analyses in the fossil record. Paleobiology 40, 331–351. ( 10.1666/13033) [DOI] [Google Scholar]
- 94.Martins EP. 1999. Estimation of ancestral states of continuous characters: a computer simulation study. Syst. Biol. 48, 642–650. ( 10.1080/106351599260210) [DOI] [Google Scholar]
- 95.Webster AJ, Purvis A. 2002. Testing the accuracy of methods for reconstructing ancestral states of continuous characters. Proc. R. Soc. Lond. B 269, 143–149. ( 10.1098/rspb.2001.1873) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data forming the basis of this research and the details of analyses are available in the electronic supplementary material attached to this article.