Abstract
Climate change may soon threaten much of global biodiversity. A critical question is: can species undergo niche shifts of sufficient speed and magnitude to persist within their current geographic ranges? Here, we analyse niche shifts among populations within 56 plant and animal species using time-calibrated trees from phylogeographic studies. Across 266 phylogeographic groups analysed, rates of niche change were much slower than rates of projected climate change (mean difference > 200 000-fold for temperature variables). Furthermore, the absolute niche divergence among populations was typically lower than the magnitude of projected climate change over the next approximately 55 years for relevant variables, suggesting the amount of change needed to persist may often be too great, even if these niche shifts were instantaneous. Rates were broadly similar between plants and animals, but especially rapid in some arthropods, birds and mammals. Rates for temperature variables were lower at lower latitudes, further suggesting that tropical species may be especially vulnerable to climate change.
Keywords: animals, climate change, climatic niche, niche evolution, phylogeography, plants
1. Introduction
Rates of change in climatic niches are important to many topics, including climate change. The realized climatic niche of a species or population is the set of large-scale temperature and precipitation conditions where it occurs and can persist, and may be shaped both by tolerances to abiotic conditions and by species interactions [1–3]. Climatic niches are important because they help determine where species can occur over space and time (e.g. [2]). Climatic niche conservatism (slow rates of change) may therefore limit the geographical spread of species and populations, whereas niche shifts may facilitate expansion into new habitats and regions [4]. Thus, climatic niche shifts and conservatism are relevant to many research questions. For example, over long time scales, climatic niche shifts and conservatism may both be important to speciation (both divergence and conservatism can help drive speciation [5]) and patterns of species richness within regions (e.g. low versus high elevations [6,7]), between regions (e.g. temperate versus tropical regions [8,9]) and among clades (e.g. niche divergence drives diversification [10,11]). Over shorter time scales, niche conservatism may limit the spread of invasive species to regions climatically similar to their native range (e.g. [12]), whereas niche shifts may facilitate spread into novel habitats and regions (e.g. [13]).
Rates of niche change (or niche shifts) are especially relevant to anthropogenic climate change. Given rapid global warming, a species's climatic niche may no longer occur in its geographic range, leading to several possible outcomes (e.g. [14,15]). First, the species may remain within its original niche but track that niche over space (e.g. moving to higher latitudes or elevations as climates warm [16,17]). Second, the climatic niche may shift, either through plastic or evolutionary change, or because the new conditions are within the species's fundamental niche (e.g. if this is larger than the realized niche). Third, if neither of these two options is possible, the species may go extinct.
There is abundant evidence that many species are already shifting their geographic ranges in space in response to anthropogenic climate change (e.g. [16]). However, dispersal may not be an option for many others. For example, numerous species may be restricted to small, isolated areas, such as nature reserves, islands or mountain tops (due to human habitat modification, ecological specialization and/or natural barriers). Many species may simply disperse too slowly to keep pace with rapid climate change (e.g. [18,19]). Given that dispersal may be difficult for many species, it is critically important to know how fast climatic niches can change, and more specifically, whether they can change quickly enough to allow species to persist as climates change. One potentially important line of evidence to help address this complex problem is to estimate the rate at which climatic niches have changed in the past. Here and throughout, we refer to ‘niche shifts’ and ‘niche changes’ rather than ‘niche evolution’, because other factors could underlie individual niche shifts in addition to microevolution, such as plastic responses or changes in access to climatic regimes [20–22].
One approach for estimating past rates of change in climatic niches is to compare niches among closely related species using time-calibrated phylogenies [23,24]. For example, Quintero & Wiens [23] analysed rates of change among 540 terrestrial vertebrate species, using climatic data from their current geographic ranges. They found that climatic niche variables changed very slowly (e.g. approx. 1.34°C Myr−1 for annual mean temperature) and were slower than rates of projected climate change by approximately 10 000 to 100 000-fold. These results suggested that species may be unable to change their niches quickly enough to keep pace with anthropogenic climate change.
Nevertheless, important questions remain unanswered. First, do these results apply to other organisms besides vertebrates, such as arthropods (which include most described species) and plants (on which most species depend, including humans; for some plant results see [24])? Second, do they apply to shorter time scales? In Quintero & Wiens's study [23], species ages ranged from 0.35 to 48.12 Ma (mean = 7.77 Ma). Importantly, there was a strong inverse relationship between rates and divergence times [23]. This finding raises the possibility that over shorter time scales, climatic niche shifts may be able to keep pace with rapid environmental change. A related issue is whether rapid changes can be sustained long enough to match the absolute magnitude of projected climate change. There are also basic questions about changes in climatic niches that remain poorly explored, such as whether niche rates differ between animals and plants (or among animals), and how rates change across latitudes (e.g. [25]).
Here, we take advantage of the wealth of phylogeographic studies within plant and animal species to help address these questions. Phylogeographic studies [26] typically analyse genetic variation within species to estimate phylogenies of populations and monophyletic groups of populations (phylogroups hereafter). These phylogroups may correspond to incipient species, distinct species unrecognized by current taxonomy, or populations only partially or temporarily isolated from other conspecific populations [26]. Some phylogeographic studies have also estimated divergence times among phylogroups. These latter studies provide the opportunity to test rates of climatic niche change over shorter time scales than among species (and thus detect more rapid changes).
Here, we first survey the literature to find time-calibrated phylogenies of populations within currently recognized species. We identify 56 species with usable phylogenies, encompassing 266 phylogroups. Then, following Quintero & Wiens [23], we estimate rates of climatic niche change for these taxa, using climatic data and phylogenetic comparative methods. We then compare these rates to projected rates of climate change over the next 54 years. We also use this dataset to address general questions about rates of niche change, such as whether rates differ among clades and across latitudes.
2. Material and methods
(a). Choice of taxa
We conducted a systematic search of the literature to find potential studies, and then selected species using two main criteria: (i) a time-calibrated molecular phylogeny was available and (ii) two or more phylogroups were identified. The details of our search strategy and selection criteria are described in electronic supplementary material, appendix S1.
The final dataset included 266 phylogroups from 56 species and 48 studies (electronic supplementary material, database S1). The 56 species encompassed diverse taxonomic groups, comprising 9 plants, 14 arthropods, 4 amphibians, 13 birds, 5 mammals and 11 squamate reptiles (lizards and snakes; ‘reptiles’ hereafter for brevity). They also spanned many geographical regions and biomes (including both arid and mesic habitats and temperate and tropical regions). Divergence times among phylogroups ranged from 29 200 years ago to 9.1 Ma (mean = 1.39 Ma), with 47% below 1 Ma and 74% below 2 Ma. See electronic supplementary material, database S1, for details on the included studies, species and phylogroups.
(b). Locality and climatic data
We obtained locality data (i.e. geographical coordinates) of sampling sites for each phylogroup either from the original study, directly from study authors or from an online museum database (VertNET). We carefully checked locality data to confirm that no localities were outside species' known ranges. The number of localities varied among species (3–216, mean = 45.5 localities/species) and phylogroups (1–99, mean = 8.79 localities/phylogroup). More widely distributed phylogroups were generally represented by more localities (electronic supplementary material, figure S1, data in database S2). Therefore, most analyses did not exclude phylogroups known from few localities (to avoid biasing results by excluding narrowly distributed phylogroups). We tested whether the number of localities per phylogroup influenced rate estimates and found that it did not. We also summarized results after excluding phylogroups with fewer than five localities, and obtained very similar results to those including all phylogroups (see Results).
We obtained climatic data for each locality from the WorldClim database v. 1.4 [27]. This database is based on average values from 1950 to 2000 from weather station data (and spatially interpolated to localities between weather stations). We focused on six climatic variables (following [23]): annual mean temperature (Bio1), hottest summer temperatures (Bio5), coldest winter temperatures (Bio6), annual mean precipitation (Bio12), and the mean precipitation of the wettest and driest quarters of the year (Bio16 and Bio17, respectively). These variables represent standard averages (Bio1, Bio12) and extreme values that may set species' range limits and reflect climatic tolerances (Bio5, Bio6, Bio16 and Bio17). We used data at the finest spatial resolution available (30 s, approx. 1 km2). We extracted climatic data from each georeferenced locality using the package raster in R v. 3.1.2 [28,29]. We calculated the mean, standard error, 10th percentile (approximating the minimum) and 90th percentile (approximating the maximum) across localities for each climatic variable for each phylogroup. Summary climatic data for each phylogroup analysed are given in electronic supplementary material, database S2.
(c). Estimating rates of climatic niche change
To estimate rates of climatic niche change, we reconstructed ancestral values of each climatic niche variable for each phylogroup (using the best-fitting model of evolution) and then estimated the rate of change as the difference between the ancestral and current values divided by the age of the phylogroup. We obtained time-calibrated trees from the original studies using TreeSnatcher Plus [30]. For each climatic niche variable and each phylogroup, we analysed the mean value across localities as well as the minimum (10th percentile) and maximum (90th percentile). Prior to ancestral reconstructions within a species or complex, we found the best-fitting likelihood model of evolution for each climatic variable. Specifically, we compared the white noise (WN; no phylogenetic signal, λ = 0), Brownian motion (BM; strong phylogenetic signal, λ = 1), Ornstein–Uhlenbeck (OU) and estimated lambda (EL; λ between 0 and 1) models. We found the maximum likelihood for each model and variable in each species, and used the associated AIC values to select the best models (detailed methodology and full results in electronic supplementary material, appendix S2 and database S3). We used both BM and OU models for ancestral reconstructions and rate estimation, but present the BM results only in the main text. Rates should depend primarily on the similarity of niche values among taxa and not reconstruction models. Indeed, the BM and OU models gave very similar rate estimates overall (see Results; electronic supplementary material, appendix S3 and tables S1–S2). The WN model would be inappropriate for ancestral reconstructions but sometimes had slightly better fit. We performed subsampling analyses with simulated and empirical data, which showed that WN tends to be selected over BM when only a few closely related taxa are included (like the intraspecific phylogroups analysed here). Importantly, this occurred even when the simulated model was BM (or, for empirical data, BM was estimated when all taxa were included). Therefore, we used the second best-fitting model in these cases (typically BM). Importantly, subsampling taxa had no significant impact on estimated rates, even when subsampling caused WN to often be favoured. These analyses are described in electronic supplementary material, appendix S2.
To reconstruct ancestral values we used the phylogenetic generalized least-squares (PGLS) approach [31]. We first transformed the tree to the selected model (BM or OU) and then performed PGLS reconstructions. For species with only two phylogroups, we used independent contrasts instead [32] because implementation of PGLS in GEIGER [33] requires at least two nodes. Ancestral reconstructions were conducted with GEIGER with customized scripts available through Dryad (doi:10.5061/dryad.7t6c8; also available as electronic supplementary material, database S8).
Climatic niche change for each phylogroup for each variable was calculated as the absolute difference between the estimated ancestral value for the most recent ancestor of that phylogroup (i.e. the node uniting that phylogroup and its sister branch) and the current value for that phylogroup (i.e. mean, minimum and maximum values among localities; see above). The niche change was then divided by the age of that ancestor (divergence time) to obtain the rate [23]. For example, a phylogroup that differed by 1°C from its reconstructed ancestor that is 1 Myr old would have a rate of 1°C Myr−1. Note that Bio12 is in units of mm yr−1, whereas Bio16 and Bio17 are in mm quarter−1 (i.e. per three months), but rates for all three are given in mm Myr−1.
We did not incorporate estimates of past climates directly into our estimates of climatic niche change. Even though estimated climatic values might be obtained for some locations for some points in the past, we do not know the detailed distributions of these molecular-based phylogroups in the past; therefore, we cannot estimate past climatic niche values for phylogroups using palaeoclimatic data. However, if populations responded to past climate change by undergoing major niche shifts, we assume that there should be some trace of these shifts in the observed niche divergence among phylogroups. By contrast, if species responded to past climate change primarily by dispersal and/or local extinction instead (or occurred in areas with limited past climate change), then there should be limited niche divergence among phylogroups. The fact that climate change occurred rapidly at some times (i.e. Pleistocene fluctuations) does not mean that species necessarily underwent rapid niche shifts to survive these changes.
Finally, we note that our phylogenetic approach does not assume any relationship between climate and speciation, nor that all niche change is evolutionary. For example, speciation may occur through climatic niche conservatism rather than niche divergence [4,5], or may be decoupled from climate entirely. We use ancestral reconstructions of realized niche variables, but some niche shifts could be caused by non-evolutionary factors (e.g. plasticity, changes in habitat availability). However, our approach will quantify niche shifts, regardless of their underlying cause. Furthermore, many previous studies have collectively shown strong phylogenetic signal and conservatism in realized climatic niches among species, suggesting an evolutionary component (e.g. [6,7,10,23]).
(d). Estimating rates of future climate change
Rates of past niche change were compared with rates of future climate change within the current geographical range of each phylogroup. To estimate future change, we obtained projected climate data for 2070 for all localities for all phylogroups, and for all six climatic variables. We used climate projections from global climate models (GCMs) available through the WorldClim database v1.4 [27,34]. We selected three projections for our analyses (for details see electronic supplementary material, appendix S4). CESM1-CAM5-1-FV2 (CE45) was the least conservative model, showing the greatest difference between the current and future climates. INMCM4 (IN45) was the most conservative model, with the smallest difference. ACCESS1-0 (AC45) was selected as intermediate.
To estimate the rate of future climate change, we took the absolute difference between ‘present-day’ climate (i.e. 1950–2000; from WorldClim) and the projected climate for each locality and climatic variable, and divided this difference by 70 years (2070 – 2000). For each phylogroup, we then calculated the mean rate of future climate change across localities for that phylogroup for each climatic variable for all three focal climatic models (electronic supplementary material, database S5). We acknowledge that 2000 is no longer the present and the time difference could be considered 95 years instead (2070 – 1975, midpoint of 1950 and 2000). However, mean values from 1950 to 2000 may be more relevant to the distribution of these phylogroups than 2015 climate (i.e. our study does not address climate-induced range shifts in the past 16 years). Furthermore, if we used 95 instead of 70, rates would only be slightly slower (by a factor of 0.74, or 70/95), and we show that our main results are robust to reasonable differences in projected rates.
(e). Other tests
In addition to comparing past and projected rates, we tested for differences in niche rates among clades and for correlations between rates of niche change and divergence times and niche change and latitude (using mean niche values across localities and BM rate estimates). First, we tested for data normality using the Shapiro–Wilk test in R. Data were not normally distributed, and we therefore used non-parametric tests (in R [28]).
We divided all species into six major clades: plants, arthropods, amphibians, birds, mammals and squamate reptiles. We then ran the Kruskal–Wallis test to evaluate whether rates differed significantly among clades overall, and used Dunn's test to compare each pair of clades.
We also used the rate estimate for each climatic variable and the age of each phylogroup to test Spearman's rank correlation between age and rate across all 266 phylogroups. Finally, we tested for correlations between rates of change for each climatic variable in each phylogroup and the absolute value of the phylogroup's mean latitude (mean across sampled localities).
Statistical analyses among species normally include a phylogenetic correction (e.g. using independent contrasts [32]). However, the branch lengths here would combine divergence times of less than 500 000 years (many phylogroups within species) with those more than 1.75 billion years (plants versus animals [35]). Therefore, we used standard statistics to avoid any potential artefacts, but with the caution that correlation strengths might be overestimated. Note that a phylogenetic correction is not relevant for our main results and conclusions (comparison of past and future rates).
3. Results
(a). Rates of climatic niche change
Rates of change in climatic niche variables were similar using BM and OU models and using mean, minimum and maximum values across localities for each phylogroup (electronic supplementary material, appendix S3, tables S1–S2 and database S4). Therefore, we present below results derived primarily from the BM model and for mean values.
Rates of change in climatic niche variables were generally slow (figure 1; electronic supplementary material, table S1). Mean rates for temperature variables (across all phylogroups) were 3.6, 2.7 and 4.7°C Myr−1 for Bio1, Bio5 and Bio6, respectively, and for precipitation variables were 342, 134 and 73 mm Myr−1 for Bio12, Bio16 and Bio17, respectively. Rates varied among phylogroups from 0 to 142°C Myr−1 across temperature variables and from 0 to 11 265 mm Myr−1 across precipitation variables. However, median values across all phylogroups were under 1°C Myr−1 for all temperature variables and below 150 mm Myr−1 for all precipitation variables (table 1).
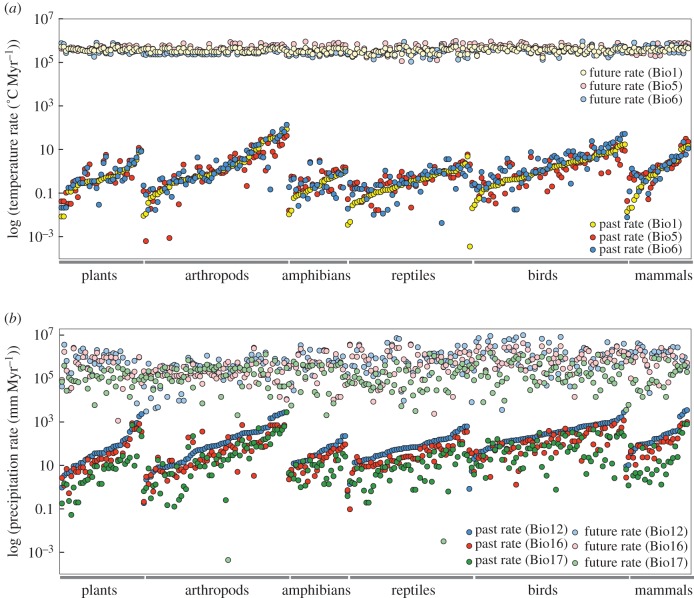
Figure 1.
Comparison of the rate of climatic niche change for 266 phylogroups with the rate of projected future climate change (within their current geographic ranges) based on the AC45 model for (a) three temperature and (b) three precipitation variables. The phylogroups are first sorted based on taxonomic group and then numerically within each group based on values for Bio1 (a) or Bio12 (b). The rates (y-axis) are on a log10 scale. Rates were estimated using the BM model and mean values of climatic niche variables among localities for each phylogroup. Comparison of rates using BM and OU models is presented in electronic supplementary material, appendix S3.
Table 1.
The averages, medians, standard deviations and ranges of absolute values of the difference in climatic niche variables between extant sister phylogroups (closest relatives). Values were based on the mean, minimum (min; 10th percentile) and maximum (max; 90th percentile) values of climatic variables among localities for each phylogroup. Values for Bio1, Bio5 and Bio6 are in °C, whereas values for Bio12, Bio16 and Bio17 are in mm yr−1).
| Bio1 | Bio5 | Bio6 | Bio12 | Bio16 | Bio17 | |
|---|---|---|---|---|---|---|
| mean | ||||||
| mean | 1.5 | 2.0 | 2.3 | 272 | 121 | 42 |
| max | 1.5 | 2.1 | 2.2 | 334 | 141 | 63 |
| min | 2.3 | 2.5 | 3.2 | 283 | 126 | 38 |
| median | ||||||
| mean | 1.0 | 1.1 | 1.5 | 187 | 69 | 22 |
| max | 0.9 | 1.2 | 1.5 | 208 | 105 | 31 |
| min | 1.7 | 1.3 | 2.1 | 176 | 74 | 15 |
| standard deviation | ||||||
| mean | 1.7 | 2.2 | 2.8 | 265 | 130 | 51 |
| max | 1.5 | 2.5 | 2.3 | 347 | 141 | 84 |
| min | 2.4 | 2.8 | 3.7 | 310 | 147 | 55 |
| range | ||||||
| mean | 0.1–8.9 | 0.0–11.8 | 0.0–5.6 | 4.1–1484 | 2.0–575 | 0.1–221 |
| max | 0.0–7.8 | 0.1–11.2 | 0.0–11.4 | 0.1–1592 | 1.0–553 | 0.6–396 |
| min | 0.1–10.2 | 0.1–12.7 | 0.0–18.7 | 2.0–1555 | 0.4–678 | 0.0–287 |
Sample sizes (localities per phylogroup) were not correlated with estimated rates (electronic supplementary material, table S3 and figure S2). Also, the mean rates of niche change using only those phylogroups with five or more localities (132 out of 266 phylogroups) were very similar to those obtained from all 266 phylogroups (electronic supplementary material, table S4). For example, rates for all phylogroups and for only those with five or more localities were within 1°C Myr−1 for all temperature variables.
The mean rates were similar for shifts towards higher versus lower climatic niche values (relative to the estimated value in the ancestor) and the number of phylogroups showing increases or decreases was similar (see electronic supplementary material, appendix S5). Maximum rates for all six variables were detected in phylogroups that shifted towards warmer temperatures and higher precipitation levels.
(b). Comparison of climate niche change with projected climate change
Rates of climatic niche change were dramatically lower than rates of projected future climate change for all phylogroups for temperature variables (figure 1a) and for most phylogroups for precipitation variables (figure 1b). For the intermediate (AC45) model, rates of projected change in Bio1 were on average 865 122 times higher than the rate of change in climatic niches (median = 65 808, range = 372–148 083 154), 732 079 times higher for Bio5 (median = 57 334; range = 512–76 010 276) and 240 193 times higher for Bio6 (median = 44 172; range = 297–13 953 632). The rates of projected change in precipitation were on average 47 696 times higher than the rate of change in precipitation niche variables for Bio12 (median = 6552; range = 0–2 642 361), 191 586 times higher for Bio16 (median = 10 037; range = 0–35 508 031) and 58 152 times higher for Bio17 (median = 11 100; range = 0–2 255 174). For precipitation variables, nine phylogroups had rates similar to that of projected anthropogenic climate change. However, in these cases, the future projected rate of climatic change was zero (i.e. the current mean precipitation values equal the predicted future mean). Overall, the differences in rates remained similar when we used the least conservative (CE45) and most conservative (IN45) future climate models (see results and summary in electronic supplementary material, database S5 and appendix S6).
In addition to the differences in rates, the absolute magnitude of differences between current and future climates was generally larger than the differences between phylogroups that have developed over thousands or even millions of years (especially for variables related to mean and high temperatures). For example, the mean absolute difference between the current climatic niches of sister phylogroups (n = 90; table 1; electronic supplementary material, database S6), regardless of the ancestral reconstruction or time of divergence, was 1.5°C (range = 0.1–8.9) for Bio1, 1.9°C (range 0.0–11.8) for Bio5 and 2.3°C (range = 0.0–15.6) for Bio6. The mean absolute difference in precipitation variables was 272 mm (range = 4–1484) for Bio12, 121 mm (range = 2–575) for Bio16 and 42 mm (range = 0–221) for Bio17. By contrast, the projected change by 2070 based on the intermediate AC45 model (mean across localities within the range of each phylogroup) across all phylogroups is on average 2.7°C (range = 1.2–4.4) for Bio1, 3.3°C (range = 0.8–7.1) for Bio5, 2.5°C (range = 0.8–6.6) for Bio6, 90 mm (range = 0–692) for Bio12, 52 mm (range = 0–294) for Bio16 and 16 mm (range = 0–107) for Bio17 (electronic supplementary material, database S5). Thus, even if the observed climatic differences among closely related phylogroups could somehow occur over only decades (instead of thousands to millions of years), they would generally not be enough to match the absolute amounts of climate change forecasted for the next 54 years for mean and hottest temperatures (Bio1, Bio5).
(c). Rates of climatic niche change among major clades
Rates of climatic niche change varied among clades for both temperature and precipitation (figure 1; electronic supplementary material, table S5). The Kruskal–Wallis test showed significant differences overall among clades for all six bioclimatic variables and Dunn's tests showed significant differences between several pairs of groups, most frequently between reptiles and other groups, and between amphibians and other groups (for full test results, see electronic supplementary material, database S7). Interestingly, there was more variation between groups of animals than between animals and plants. Specifically, mean rates were relatively low for almost all variables for amphibians, reptiles and plants, and were substantially higher in arthropods, birds and mammals. The mean rates for all six variables were lowest in amphibians and reptiles (reptiles exhibited the lowest mean rate in Bio5, amphibians in the other variables). The highest mean rates were in arthropods (for Bio 1, Bio5, Bio6 and Bio17), mammals (for Bio12) and birds (for Bio16). Exceptionally high rates were documented in some phylogroups for all variables in arthropods, as well as birds and mammals. Nevertheless, all clades were dominated by phylogroups with very low rates.
(d). Rates of climatic niche change and time
Rates of niche change were negatively correlated with divergence times (electronic supplementary material, figure S3; Bio1: r = −0.74, p < 0.0001; Bio5: r = −0.75, p < 0.0001; Bio6: r = −0.71, p < 0.0001; Bio12: r = −0.69, p < 0.0001; Bio16: r = −0.67, p < 0.0001; Bio17: r = −0.69, p < 0.0001). For phylogroups more than 1 Myr old, temperature rates never exceeded 6°C Myr−1, and for those more than 2 Myr old, temperature rates were generally less than 1°C Myr−1. Similarly, there was no precipitation rate over 200 mm Myr−1 for any phylogroup more than 2 Myr old, whereas rates in younger phylogroups were often more than 1000 mm Myr−1.
(e). Rates of climatic niche change and latitude
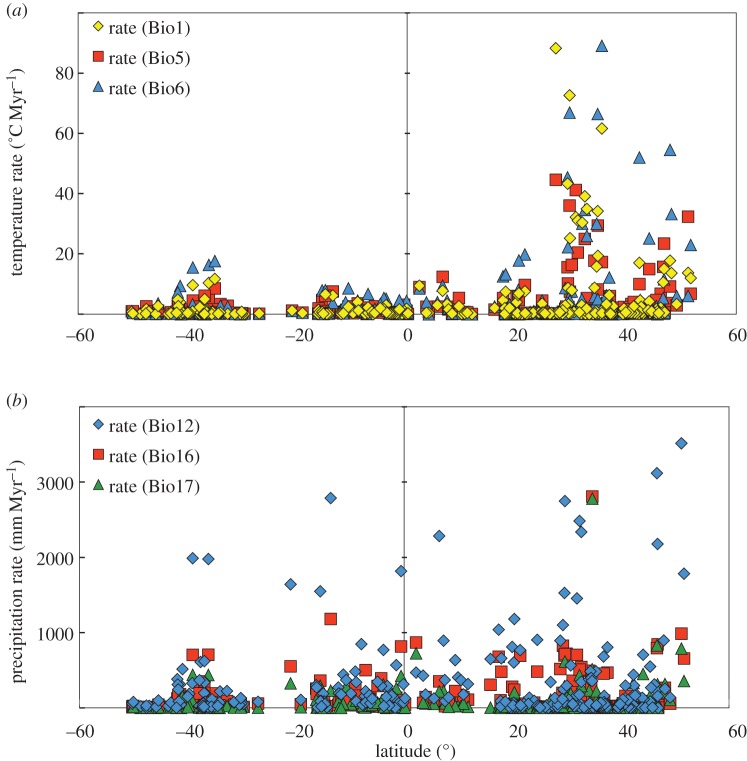
Rates of niche change were positively correlated with absolute values of latitude (mean per phylogroup) for two of the three temperature variables (figure 2a; Bio1: r = 0.11, p = 0.0068; Bio5: r = 0.12, p = 0.0058; Bio6: r = 0.02, p = 0.9750). We documented the highest temperature rates between 30 and 50° N, and elevated rates also occurred between 35 and 40° S (figure 2a). Correlations were negative for precipitation variables and significant for two of the three (figure 2b; Bio12: r = −0.18, p = 0.0041; Bio16: r = −0.20, p = 0.0013; Bio17: r = −0.07, p = 0.283). The highest precipitation rates seemed to be more evenly spread across latitudes than temperature rates (figure 2).
Figure 2.
The rates of climatic niche change for 266 phylogroups with respect to each phylogroup's mean latitude for three temperature (a) and three precipitation (b) variables. Rates were estimated using the BM model and mean values of climatic niche variables among localities for each phylogroup.
4. Discussion
In this study, we estimate rates of climatic niche change among populations from phylogeographic studies and compare these with rates of anthropogenic climate change. On average, rates of climatic niche change among populations of plants and animals were faster than rates previously estimated among vertebrate species [23]. For example, rates for annual mean temperature (Bio1) among populations here are roughly three times faster than among vertebrate species (1.3°C Myr−1 versus 3.6°C Myr−1). However, they still generally showed dramatically slower rates than projected rates of future climate change, with differences in mean rates typically more than 200 000-fold for temperature variables (median > 40 000-fold) and more than 10 000-fold for precipitation variables (median > 5000-fold), for the intermediate climate model. A few phylogroups had exceptionally fast rates, but even these were dramatically slower than rates of climate change for temperature variables (by 300-fold or more). For precipitation variables, mean rates of niche change were also substantially slower than projected climate change, and these rates overlapped only in cases with no projected change in precipitation in the next 54 years. We also found that the absolute magnitude of niche changes needed to match projected temperature changes in coming decades was (on average) smaller than niche differences that have accumulated between sister phylogroups over thousands and millions of years. Moreover, we show here that the general pattern of slow rates holds for both plants and animals, and both arthropods and vertebrates. We also find that rates are faster in arthropods, birds and mammals than in other groups, and that temperature-related variables show faster rates at higher latitudes. We discuss these topics in detail below, starting with the implications for global warming.
What do these results mean for species persistence under global warming? We acknowledge that these past rate estimates cannot tell us directly what will happen to populations and species as climates change. Nevertheless, they provide a basis for addressing what are typical (or exceptional) rates of climatic niche change among populations. There are numerous ways that particular rate estimates could be incorrect. However, none should overturn our main conclusions. First, rates could be faster at shorter time scales (e.g. [36]), and we show that the fastest rates do indeed occur between phylogroups that diverged most recently (as previously shown for climatic niche rates [23,24] and more generally for rate estimators [36]). However, the absolute differences between sister phylogroups were still relatively small, specifically for climatic variables related to mean and hottest yearly temperatures. Thus, even if populations could make changes of the magnitude that occurred over thousands or millions of years in only decades, they still might not lead to differences of the magnitude that are projected to occur in the next 54 years. Indeed, the mean projected changes by 2070 were larger than the mean differences between sister phylogroups by more than 50% for Bio1 and Bio5. Although absolute differences were smaller for precipitation, all terrestrial organisms are potentially impacted by both temperature and precipitation (not necessarily one or the other). Second, some phylogenies and divergence times within species could be incorrect. For example, many animal trees are based on mitochondrial DNA (mtDNA) data only. Mitochondrial introgression could lead to sharply underestimating divergence times but this should lead to overestimating rates of niche evolution (i.e. divergences will appear younger than they really are) rather than underestimating them. If a single interbreeding population is arbitrarily split into phylogroups by mtDNA data the impact on rate estimates is less clear, but phylogroups were generally geographically coherent. Divergence dates could also be incorrect, but we know of no plausible mechanism whereby these divergence dates would be incorrect by orders of magnitude, especially given the relatively recent time scales involved. Finally, even if the phylogeny and divergence times are correct, ancestral reconstructions could still be wrong. However, if closely related phylogroups have similar niche values, they should have low rates of change, regardless of the details of ancestral reconstructions. Indeed, sister phylogroups generally had similar values (table 1), and different reconstructions models had limited impact on rate estimates here (electronic supplementary material, appendix S3). Finally, low rates of niche change might sometimes be caused by limited geographical availability of extremes in niche space over time (e.g. on islands), which could limit how much change can occur within any phylogroup, and might help explain why rates slow over time. Importantly, this limited geographical availability of niche space that occurred naturally in the past may be amplified by human impacts, and might prevent species from dispersing to tolerable locations as climates warm.
Even if our niche rate estimates are largely accurate, species might still be able to persist in the face of dramatic climate change. For example, species' realized climatic niches might greatly underestimate their actual climatic tolerances. However, our analyses do not directly assume that realized and fundamental niches are identical. The geographical and climatic distributions of these phylogroups are presumably shaped by a combination of biotic and abiotic factors. There is also evidence that as climate warms, changes in species interactions will be the most common proximate cause of local extinctions and declines from climate change, rather than limited physiological tolerances (e.g. [37,38]). Therefore, the fundamental niche may be less relevant to predicting the impacts of climate change on species than the realized niche. Finally, despite the precedent of slow past rates, populations might still be able to shift their niches at a much faster rate as climate warms. Although this might be possible in theory, there is mounting evidence that this is often failing to occur. Specifically, the geographical ranges of many species are shifting towards higher latitudes and elevations (e.g. [16]), including many contractions at the warm edges of species ranges (e.g. lower latitudes and elevations). If rapid niche shifts were possible, then these warm-edge populations should still persist. Yet these local extinctions are already widespread [39–43], despite a global increase in mean annual temperature (so far) of less than 1°C [34]. A new study [44] showed that climate-related local extinctions have already occurred in 47% of 976 species surveyed, including diverse regions, climatic zones, habitats and clades (animals, plants).
Our results also show exceptionally fast rates of change in some populations. These seem to be related to our overall finding that rates are higher in temperature-related variables at higher latitudes, and are highest in some arthropods, mammals and birds. We discuss the latitudinal pattern first, and then the taxonomic pattern. We find higher rates in temperature-related variables in populations at higher latitudes, a pattern previously found in birds [25]. One potential explanation is that at higher latitudes, stronger long-term climatic fluctuations may have driven niche divergence (e.g. [25]), a hypothesis supported by theory on climate and speciation [5]. At higher latitudes, species may also have broader temperature-related niche breadths (e.g. [45,46]), and physiological tolerances for temperature (e.g. [47,48]), which might facilitate niche divergence across the species range. The higher rates in some precipitation variables at lower latitudes show an interesting parallel with this pattern (given greater precipitation seasonality and precipitation niche breadths at lower latitudes [45,46]). Regardless of its causes, the pattern of slower temperature-related rates in tropical species is important because it provides another line of evidence suggesting that tropical species may be particularly vulnerable to global warming (e.g. [47,49]). Indeed, local extinctions from climate change so far appear to be more common in tropical species [44]. Unfortunately, the majority of the world's species are thought to occur in the tropics (e.g. [50]).
We also find that exceptionally fast rates of niche divergence are concentrated in arthropods, birds and mammals. Intriguingly, plants, amphibians and reptiles have consistently low rates for temperature-related variables, with faster rates for plants only in precipitation-related variables in a few phylogroups. What explains these differences in rates? We have some speculative ideas, but these will require further analysis. Higher rates in some arthropods might be explained by their faster generation times relative to vertebrates (e.g. [51]), which might facilitate faster rates of niche change (although some plants should also have faster rates by this argument). In birds and mammals, faster rates might be explained by their shared endothermic metabolism, such that they can regulate their own internal body temperatures [52]. This might allow their conspecific populations to occur under very different climatic conditions. In summary, our results suggest that amphibians, reptiles and plants might be especially susceptible to climate change. Intriguingly, extensive local extinctions related to climate change have been suggested for both amphibians (e.g. [53]) and reptiles [43], although the relationship between climate change and amphibian declines has been controversial (e.g. [54]).
Overall, our results show that rates of climatic niche change among populations of plants and animals are dramatically slower than projected rates of future climate change. Thus, our results support a growing body of evidence suggesting that populations may not be able to change their climatic niches rapidly enough to keep pace with changing conditions as global climate warms (although many species might avoid extinction through dispersal or other processes). More broadly, our results suggest that rates of change in climatic niches are broadly similar across plants and animals, but with exceptionally fast rates in a few cases, particularly in some arthropods, birds and mammals. Our results are also consistent with the idea that tropical species might be at higher risk from climate change (e.g. [44,47,49]), given our results showing their slower rates of change in temperature-related variables.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgements
We thank many undergraduate students at the University of Arizona for assistance compiling locality data, including N. Carpenter, K. Croneigh, G. Mathews, J. Myrand and J. Wyman. We thank two anonymous reviewers for helpful comments.
Data accessibility
All data are available in the electronic supplementary material.
Authors' contributions
T.J. and J.J.W. designed the study, T.J. performed analyses, and T.J. and J.J.W. wrote the paper.
Competing interests
We declare we have no competing interests.
Funding
T.J. was supported by an NIH IRACDA PERT fellowship through the Center for Insect Science (K12 GM000708) at the University of Arizona.
References
- 1.Holt RD. 2009. Bringing the Hutchinsonian niche into the 21st century: ecological and evolutionary perspectives. Proc. Natl Acad. Sci. USA 106, 19 659–19 665. ( 10.1073/pnas.0905137106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Soberón J. 2007. Grinnellian and Eltonian niches and geographic distributions of species. Ecol. Lett. 10, 1115–1123. ( 10.1111/j.1461-0248.2007.01107.x) [DOI] [PubMed] [Google Scholar]
- 3.Hutchinson GE. 1957. A treatise on limnology. New York, NY: Wiley and Sons. [Google Scholar]
- 4.Wiens JJ, Graham CH. 2005. Niche conservatism: integrating evolution, ecology, and conservation biology. Annu. Rev. Ecol. Evol. Syst. 36, 519–539. ( 10.1146/annurev.ecolsys.36.102803.095431) [DOI] [Google Scholar]
- 5.Hua X, Wiens JJ. 2013. How does climate influence speciation? Am. Nat. 182, 1–12. ( 10.1086/670690) [DOI] [PubMed] [Google Scholar]
- 6.Hutter CR, Guayasamin JM, Wiens JJ. 2013. Explaining Andean megadiversity: the evolutionary and ecological causes of glassfrog elevational richness patterns. Ecol. Lett. 16, 1135–1144. ( 10.1111/ele.12148) [DOI] [PubMed] [Google Scholar]
- 7.Kozak KH, Wiens JJ. 2010. Niche conservatism drives elevational diversity patterns in Appalachian salamanders. Am. Nat. 176, 40–54. ( 10.1086/653031) [DOI] [PubMed] [Google Scholar]
- 8.Pyron RA, Wiens JJ. 2013. Large-scale phylogenetic analyses reveal the causes of high tropical amphibian diversity. Proc. R. Soc. B 280, 20131622 ( 10.1098/rspb.2013.1622) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smith BT, Bryson RW Jr, Houston DD, Klicka J. 2012. An asymmetry in niche conservatism contributes to the latitudinal species diversity gradient in New World vertebrates. Ecol. Lett. 15, 1318–1325. ( 10.1111/j.1461-0248.2012.01855.x) [DOI] [PubMed] [Google Scholar]
- 10.Kozak KH, Wiens JJ. 2010. Accelerated rates of climatic-niche evolution underlie rapid species diversification. Ecol. Lett. 13, 1378–1389. ( 10.1111/j.1461-0248.2010.01530.x) [DOI] [PubMed] [Google Scholar]
- 11.Gomez-Rodriguez C, Baselga A, Wiens JJ. 2015. Is diversification rate related to climatic niche width? Glob. Ecol. Biogeogr. 24, 383–395. ( 10.1111/geb.12229) [DOI] [Google Scholar]
- 12.Petitpierre B, Kueffer C, Broennimann O, Randin C, Daehler C, Guisan A. 2012. Climatic niche shifts are rare among terrestrial plant invaders. Science 335, 1344–1348. ( 10.1126/science.1215933) [DOI] [PubMed] [Google Scholar]
- 13.Broennimann O, Treier UA, Mueller-Schaerer H, Thuiller W, Peterson AT, Guisan A. 2007. Evidence of climatic niche shift during biological invasion. Ecol. Lett. 10, 701–709. ( 10.1111/j.1461-0248.2007.01060.x) [DOI] [PubMed] [Google Scholar]
- 14.Holt RD. 1990. The microevolutionary consequences of climate change. Trends Ecol. Evol. 5, 311–315. ( 10.1016/0169-5347(90)90088-U) [DOI] [PubMed] [Google Scholar]
- 15.Visser ME. 2008. Keeping up with a warming world; assessing the rate of adaptation to climate change. Proc. R. Soc. B 275, 649–659. ( 10.1098/rspb.2007.0997) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen IC, Hill JK, Ohlemueller R, Roy DB, Thomas CD. 2011. Rapid range shifts of species associated with high levels of climate warming. Science 333, 1024–1026. ( 10.1126/science.1206432) [DOI] [PubMed] [Google Scholar]
- 17.Parmesan C, Yohe G. 2003. A globally coherent fingerprint of climate change impacts across natural systems. Nature 421, 37–42. ( 10.1038/nature01286) [DOI] [PubMed] [Google Scholar]
- 18.Loarie SR, Duffy PB, Hamilton H, Asner GP, Field CB, Ackerly DD. 2009. The velocity of climate change. Nature 462, 1052–1055. ( 10.1038/nature08649) [DOI] [PubMed] [Google Scholar]
- 19.Sandel B, Arge L, Dalsgaard B, Davies RG, Gaston KJ, Sutherland WJ, Svenning JC. 2011. The influence of Late Quaternary climate-change velocity on species endemism. Science 334, 660–664. ( 10.1126/science.1210173) [DOI] [PubMed] [Google Scholar]
- 20.Jackson ST, Overpeck JT. 2000. Responses of plant populations and communities to environmental changes of the late Quaternary. Paleobiology 26, 194–220. ( 10.1666/0094-8373(2000)26%5B194:ROPPAC%5D2.0.CO;2) [DOI] [Google Scholar]
- 21.Rödder D, Lötters S. 2009. Niche shift versus niche conservatism? Climatic characteristics of the native and invasive ranges of the Mediterranean house gecko (Hemidactylus turcicus). Glob. Ecol. Biogeogr. 18, 674–687. ( 10.1111/j.1466-8238.2009.00477.x) [DOI] [Google Scholar]
- 22.Jezkova T, Olah-Hemmings V, Riddle BR. 2011. Niche shifting in response to warming climate after the last glacial maximum: inference from genetic data and niche assessments in the chisel-toothed kangaroo rat (Dipodomys microps). Glob. Change Biol. 17, 3486–3502. ( 10.1111/j.1365-2486.2011.02508.x) [DOI] [Google Scholar]
- 23.Quintero I, Wiens JJ. 2013. Rates of projected climate change dramatically exceed past rates of climatic niche evolution among vertebrate species. Ecol. Lett. 16, 1095–1103. ( 10.1111/ele.12144) [DOI] [PubMed] [Google Scholar]
- 24.Cang FA, Wilson AA, Wiens JJ. 2016. Climate change is projected to outpace rates of niche change in grasses. Biol. Lett. 12, 20160368 ( 10.1098/rsbl.2016.0368) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lawson AM, Weir JT. 2014. Latitudinal gradients in climatic-niche evolution accelerate trait evolution at high latitudes. Ecol. Lett. 17, 1427–1436. ( 10.1111/ele.12346) [DOI] [PubMed] [Google Scholar]
- 26.Avise JC. 2000. Phylogeography: the history and formation of species. Cambridge, MA: Harvard University Press. [Google Scholar]
- 27.Hijmans RJ, Cameron SE, Parra JL, Jones PG, Jarvis A. 2005. Very high resolution interpolated climate surfaces for global land areas. Int. J. Climatol. 25, 1965–1978. ( 10.1002/joc.1276) [DOI] [Google Scholar]
- 28.R Development Core Team. 2015. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- 29.Hijmans RJ, van Etten J. 2012. raster: Geographic analysis and modeling with raster data. R package version 2.0-12. See http://CRAN.R-project.org/package=raster.
- 30.Laubach T, von Haeseler A, Lercher MJ. 2012. TreeSnatcher Plus: capturing phylogenetic trees from images. BMC Bioinform. 13, 110 ( 10.1186/1471-2105-13-110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Martins EP, Hansen TF. 1997. Phylogenies and the comparative method: a general approach to incorporating phylogenetic information into the analysis of interspecific data. Am. Nat. 149, 646–667. ( 10.1086/286013) [DOI] [Google Scholar]
- 32.Felsenstein J. 1985. Phylogenies and the comparative method. Am. Nat. 125, 1–15. ( 10.1086/284325) [DOI] [Google Scholar]
- 33.Harmon LJ, Weir JT, Brock CD, Glor RE, Challenger W. 2008. GEIGER: investigating evolutionary radiations. Bioinformatics 24, 129–131. ( 10.1093/bioinformatics/btm538) [DOI] [PubMed] [Google Scholar]
- 34.IPCC. 2014. Climate change 2014: impacts, adaptation, and vulnerability. Part A: global and sectoral aspects. Contribution of Working Group II to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 35.Parfrey LW, Lahr DJG, Knoll AH, Katz LA. 2011. Estimating the timing of early eukaryotic diversification with multigene molecular clocks. Proc. Natl Acad. Sci. USA 108, 13 624–13 629. ( 10.1073/pnas.1110633108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hunt G. 2012. Measuring rates of phenotypic evolution and the inseparability of tempo and mode. Paleobiology 38, 351–373. ( 10.1666/11047.1) [DOI] [Google Scholar]
- 37.Cahill AE, et al. 2013. How does climate change cause extinction? Proc. R. Soc. B 280, 1–9. ( 10.1098/rspb.2012.1890) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ockendon N, et al. 2014. Mechanisms underpinning climatic impacts on natural populations: altered species interactions are more important than direct effects. Glob. Change Biol. 20, 2221–2229. ( 10.1111/gcb.12559) [DOI] [PubMed] [Google Scholar]
- 39.Hickling R, Roy DB, Hill JK, Thomas CD. 2005. A northward shift of range margins in British Odonata. Glob. Change Biol. 11, 502–506. ( 10.1111/j.1365-2486.2005.00904.x) [DOI] [Google Scholar]
- 40.Jones SJ, Lima FP, Wethey DS. 2010. Rising environmental temperatures and biogeography: poleward range contraction of the blue mussel, Mytilus edulis L., in the western Atlantic. J. Biogeogr. 37, 2243–2259. ( 10.1111/j.1365-2699.2010.02386.x) [DOI] [Google Scholar]
- 41.Moritz C, Patton JL, Conroy CJ, Parra JL, White GC, Beissinger SR. 2008. Impact of a century of climate change on small-mammal communities in Yosemite National Park, USA. Science 322, 261–264. ( 10.1126/science.1163428) [DOI] [PubMed] [Google Scholar]
- 42.Wilson RJ, Gutierrez D, Gutierrez J, Martinez D, Agudo R, Monserrat VJ. 2005. Changes to the elevational limits and extent of species ranges associated with climate change. Ecol. Lett. 8, 1138–1146. ( 10.1111/j.1461-0248.2005.00824.x) [DOI] [PubMed] [Google Scholar]
- 43.Sinervo B, et al. 2010. Erosion of lizard diversity by climate change and altered thermal niches. Science 328, 894–899. ( 10.1126/science.1184695) [DOI] [PubMed] [Google Scholar]
- 44.Wiens JJ. 2016. Climate-related local extinctions are already widespread among plant and animal species. PLoS Biol. 11, e2001104 ( 10.1371/journal.pbio.e2001104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Janzen DH. 1967. Why mountain passes are higher in the tropics. Am. Nat. 101, 233–249. ( 10.1086/282487) [DOI] [Google Scholar]
- 46.Vazquez DP, Stevens RD. 2004. The latitudinal gradient in niche breadth: concepts and evidence. Am. Nat. 164, E1–E19. ( 10.1086/421445) [DOI] [PubMed] [Google Scholar]
- 47.Deutsch CA, Tewksbury JJ, Huey RB, Sheldon KS, Ghalambor CK, Haak DC, Martin PR. 2008. Impacts of climate warming on terrestrial ectotherms across latitude. Proc. Natl Acad. Sci. USA 105, 6668–6672. ( 10.1073/pnas.0709472105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sunday JM, Bates AE, Dulvy NK. 2011. Global analysis of thermal tolerance and latitude in ectotherms. Proc. R. Soc. B 278, 1823–1830. ( 10.1098/rspb.2010.1295) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Urban MC. 2015. Accelerating extinction risk from climate change. Science 348, 571–573. ( 10.1126/science.aaa4984) [DOI] [PubMed] [Google Scholar]
- 50.Hillebrand H. 2004. On the generality of the latitudinal diversity gradient. Am. Nat. 163, 192–211. ( 10.1086/381004) [DOI] [PubMed] [Google Scholar]
- 51.Thomas JA, Welch JJ, Lanfear R, Bromham L. 2010. A generation time effect on the rate of molecular evolution in invertebrates. Mol. Biol. Evol. 27, 1173–1180. ( 10.1093/molbev/msq009) [DOI] [PubMed] [Google Scholar]
- 52.Pough FH, Heiser JB, Janis CM. 2009. Vertebrate life, 8th edn San Francisco, CA: Pearson. [Google Scholar]
- 53.Pounds JA, et al. 2006. Widespread amphibian extinctions from epidemic disease driven by global warming. Nature 439, 161–167. ( 10.1038/nature04246) [DOI] [PubMed] [Google Scholar]
- 54.Rohr JR, Raffel TR. 2010. Linking global climate and temperature variability to widespread amphibian declines putatively caused by disease. Proc. Natl Acad. Sci. USA 107, 8269–8274. ( 10.1073/pnas.0912883107) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are available in the electronic supplementary material.