Abstract
Sleep inertia is the period of impaired performance and grogginess experienced after waking. This period of impairment is of concern to workers who are on-call, or nap during work hours, and need to perform safety-critical tasks soon after waking. While several studies have investigated the best sleep timing and length to minimise sleep inertia effects, few have focused on countermeasures -especially those that can be implemented after waking (i.e. reactive countermeasures). This structured review summarises current literature on reactive countermeasures to sleep inertia such as caffeine, light, and temperature and discusses evidence for the effectiveness and operational viability of each approach. Current literature does not provide a convincing evidence-base for a reactive countermeasure. Caffeine is perhaps the best option, although it is most effective when administered prior to sleep and is therefore not strictly reactive. Investigations into light and temperature have found promising results for improving subjective alertness; further research is needed to determine whether these countermeasures can also attenuate performance impairment. Future research in this area would benefit from study design features highlighted in this review. In the meantime, it is recommended that proactive sleep inertia countermeasures are used, and that safety-critical tasks are avoided immediately after waking.
Keywords: Body temperature, Caffeine, Countermeasures, Light, Napping, Self-awakening, Shift work, Sleep inertia
Impaired performance and alertness upon waking is known as “sleep inertia”1, 2). Impairment is most severe immediately upon waking and then dissipates, generally returning to baseline levels within 15–60 min3, 4, 5, 6). Sleep inertia is a concern for industries in which workers perform safety-critical tasks soon after waking. Motorists are also at risk when driving too soon after waking, for example when following government recommendations to nap if tired during long drives7, 8). Sleep inertia has been a contributing factor in several major accidents and incidents9, 10, 11, 12). For example, an air crash involving 158 fatalities resulted from poor decisions made by the Captain who had just woken from an in-flight nap10). In another example, heavy contact between supply vessels occurred after the Chief Officer over-slept and consequently arrived on the bridge within minutes of waking11).
Factors that Influence Sleep Inertia
Sleep inertia is typically measured in laboratory settings using a combination of sleepiness scales and cognitive tests. However, the sensitivity of these cognitive tasks to sleep inertia varies13, 14). Consequently, the severity of sleep inertia observed can depend on the task used to measure it. Test batteries are often administered immediately after waking and repeated intermittently for up to one hour. Sleep inertia has been measured under a variety of conditions ranging from a full night’s sleep, to short naps at different times of day. Much of this research has focussed on establishing the best length and timing of sleep to minimise sleep inertia. Studies suggest that avoiding sleep periods ending during the circadian low5, 15), and keeping naps to less than 30 min4, 16, 17, 18) to avoid waking from deep sleep15) can minimise sleep inertia magnitude. Prior sleep loss can also exacerbate sleep inertia15, 19), which is particularly important in shiftwork where workers often experience extended wakefulness and/or sleep loss20, 21). Indeed, naps have been suggested as a potentially effective countermeasure to sleepiness following sleep loss and during the circadian low22, 23, 24, 25, 26). Generally the long-term benefits provided by the nap outweigh the short-term detriments associated with sleep inertia4, 16, 25). The challenge is to maximise the benefits of a nap while minimising and/or managing sleep inertia. In addition, there may also be individual factors that contribute to overall sleep inertia severity. For example, some sleep medications27) or sleep disorders may exacerbate sleep inertia symptoms, although there is currently very little research in this area.
Taking a Reactive Approach
Research investigating proactive strategies for optimal sleep length and timing to minimise sleep inertia and maximise alertness is important for informing industry guidelines on rest breaks and shift scheduling. In operational environments, however, it is not always feasible to plan the length and timing of a sleep period. For example, when workers are on-call (such as in the emergency services or the military), or for workers such as healthcare professionals who take naps during extended-hours or night shifts. For these workers, the need to process crucial and complex information or to engage in safety critical activities almost immediately after waking could occur at any time. Given this, it is surprising that few studies have directly sought to reduce the effects of sleep inertia through reactive countermeasures. That is, strategies implemented upon wake-up, as opposed to proactive strategies such as planning sleep timing and duration. This review examines the literature on potential reactive countermeasures to sleep inertia including caffeine, light, and temperature, and discusses possible avenues for future research.
Search Methods
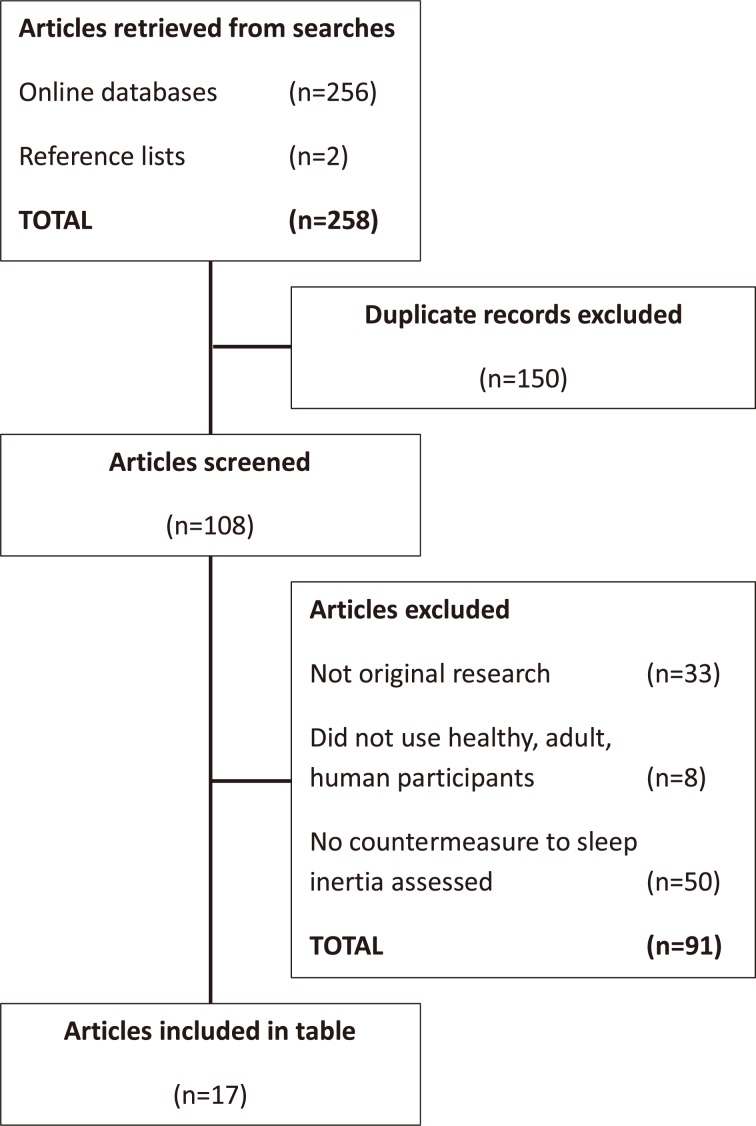
Three electronic databases (PubMed, Science Direct, and Scopus) were searched on July 8, 2015 for the following search terms paired with “sleep inertia”: adrenaline, caffeine, countermeasures, epinephrine, light, nap, noise, on-call, self-awakening, shift work, sound, and temperature. This resulted in 256 articles. Two articles were manually retrieved from reference lists. Seventeen articles remained after filtering for duplicates (n=150) and studies not meeting the inclusion criteria outlined below (n=91). Figure 1 illustrates the search and selection process. The large number of duplicate articles was a result of using multiple search terms and multiple databases which each returned similar results. The selected articles are summarised in Table 1. Note that one study is included three times as it investigated three countermeasures28).
Fig. 1.
Flowchart illustrating the structured narrative review selection process for articles populating Table 1.
Table 1. A summary of papers investigating countermeasures to sleep inertia.
| Counter-measure | Authors | Prior sleep/wake protocol | Sleep length | Wake-up timing | Inertia testing points post-sleep | Subjective alertness improved?^ | Objective performance improved?^ | Reactive? |
|---|---|---|---|---|---|---|---|---|
| Caffeine | Reyner & Horne, 199730) | Sleep restriction (5 h TIB) | 15 min | 14:40 | Drive: 0–180 min; 30-min bins KSS: every 200 s |
No obvious signs of inertia, but no nap-only comparison group. | No obvious signs of inertia, but no nap-only comparison group. | No |
| Van Dongen et al., 200131) | 88 h extended wakefulness with 2-h naps every 12 h | 2 h | 04:45 & 16:45 | 5, 75 min | Not reported | Yes (5 min) | No | |
| Hayashi et al., 200328) | Habitual sleep | 20 min | 13:00 | 0, 5, 10, 15, 20, 25, 30, 35, 40, 45, 50, 55 min | Yes (15 min) | Yes (15 min) | No | |
| Newman et al., 201332) | Habitual sleep | 1 h & ~6 h | 01:00 & 06:00 | 0, 6, 12, 18 min | Not reported | Yes (18 min) | Yes | |
| Light (post-wake) |
Hayashi et al., 200328) | Habitual sleep | 20 min | 13:00 | 0, 5, 10, 15, 20, 25, 30, 35, 40, 45, 50, 55 min | Yes (15 min) | No improvement | Yes |
| Santhi et al., 201313) | Sleep restriction (6.5 h TIB) | 6.5 h | 06:42–07:27, depending on habitual wake-up | 3, 33, 63, 93 min | No improvement | No improvement | Yes | |
| Light (pre-wake) |
Van de Werken et al., 201035) | Habitual sleep | ~8 h | ~07:00 | 1, 15, 30, 45, 60, 90 min | Yes (15 min) | No improvement | No |
| Giménez et al., 201033) | Habitual sleep | ~8 h | ~07:00 | N/A | Yes (25 min reduction time needed to feel fully awake) | Not reported | No | |
| Harrison et al., 201134) | Habitual sleep | 90 min | 15:30 | 3 min | No | No | No | |
| Thompson et al., 201436) | Habitual workday sleep | 8 h | Habitual workday timing (mean not reported) | 5, 35, 75 min | Yes (better on average over whole testing period) | Yes (better on average over whole testing period) | No | |
| Sound | Tassi et al., 199237) | Habitual sleep | 1 h | 01:00 & 04:00 | 0, 40 min. 30-min test divided into 3 min bins. |
Not reported | Yes (3 min) | Yes |
| Hayashi et al., 200438) | Habitual sleep | 20 min | 14:20 | 1, 6, 11, 16 min | Yes (1 min) | No control group. High preference music better than low preference (1 min) |
Yes | |
| Temperature | Krauchi et al., 200439) | Unknown, assume habitual | 8 h (nocturnal sleep) & 2 h (afternoon nap) | 07:00 & 18:00 | KSS at 0, 30, 60, 90, 120 min | Correlation with distal-proximal skin temperature gradient | Not reported | Yes |
| Krauchi et al., 200640) | Habitual sleep | 75 min | Multiple wake-ups across the circadian cycle | KSS at 0, 30, 60, 90, 120 min | Correlation with distal skin temperature | Not reported | Yes | |
| Self-awakening | Kaida et al., 200341) | Habitual sleep | 15–20 min | 14:20 | 5, 10, 15, 20, 25, 30 min | Yes (5 min) | No Greater P300 amplitude (15 min) |
No |
| Kaida et al., 200342) | Habitual sleep | 15–20 min | 14:20 | 5, 10, 15, 20, 25, 30 min | Yes (10 min) | No Heart rate increased (3 min before waking) |
No | |
| Ikeda & Hayashi 20106) | Habitual sleep | Habitual (mean 7.3 h) | Habitual (mean 08:06) | 1, 16, 31, 46 min | Yes (fatigue - better on average over whole testing period) No (sleepiness not improved) |
Yes (in first 15 min test session) | No | |
| Ikeda et al., 201443) | Habitual sleep | Partial restriction (5 h) | Habitual (range: 05:30–08:30) | Immediately after waking (home study) | No | Yes (only one test point) | No | |
| Face-washing | Hayashi et al., 200328) | Habitual sleep | 20 min | 13:00 | 0, 5, 10, 15, 20, 25, 30, 35, 40, 45, 50, 55 min | Yes (1 min) | No improvement | Yes |
Table notes: ^first recorded improvement; KSS: Karolinska sleepiness scale; N/A: not applicable; TIB: time in bed.
Given the diversity of methods and the exploratory nature of the many approaches to managing sleep inertia, a traditional systematic review style was not implemented. Rather than limiting the scope of the studies included in the review, a structured narrative approach was chosen to allow collation across a broad range of research in this area29). Many of the studies included in this review have methodological limitations that may have rendered them ineligible for traditional systematic review. Nevertheless, these studies highlight important research ideas, and point the way for future studies. In this way, this inclusive review is intended as a “call to arms” for future research initiatives.
Studies in Table 1 met the following inclusion criteria: original article; published in peer-reviewed journal; written in English; used healthy, human, adult participants; and directly trialled a countermeasure to sleep inertia or provided evidence for a potential countermeasure. The majority of screened articles were excluded due to not investigating a countermeasure to sleep inertia or not reporting original research (e.g. review papers) (Fig. 1). All studies in the table were laboratory-based; 15 were experimental and two were observational. Sample size ranged from n=8 to n=23 in 14 within-participant studies, and n=16 to n=44 in three between-participants studies. Both male and female participants were included in all but three studies, which only included male participants. Outcome measures, testing points, timing of awakening, and prior sleep/wake history, and length of sleep periods varied, and as such, direct comparisons between studies were not possible. Six countermeasure categories were identified: caffeine28, 30, 31, 32); light13, 28, 33, 34, 35, 36); sound37, 38); temperature39, 40); self-awakening6, 41, 42, 43); and face-washing28).
Caffeine
Caffeine is a readily available and cost effective stimulant used strategically, socially and often habitually44, 45). Caffeine promotes alertness by blocking adenosine receptors. Adenosine, a by-product of cellular energy expenditure, is a neurotransmitter inhibitor which increases with time awake and causes drowsiness46). Caffeine has been shown to improve performance and alertness under conditions of high sleep pressure such as sleep deprivation and during the night47, 48). The search yielded four studies that examined caffeine as a countermeasure to sleep inertia (Table 1). Van Dongen et al.31) found that sustained, low dose caffeine counteracted sleep inertia immediately following 2-h naps across 88 h of sleep deprivation. Notably, caffeine was administered hourly for a plasma concentration approximately 3.7 mg/L, equivalent to one-quarter cup of coffee every hour for 66 h. That is, caffeine was not administered directly after the nap, rather, at regular intervals before and after each nap. In this study, performance on a 10-min psychomotor vigilance task (PVT; a measure of behavioural alertness and vigilance, in which the participant is required to respond to stimuli presented at random intervals as quickly as possible by pressing a button) under placebo conditions deteriorated immediately post-nap, but there was no change in performance pre- to post-nap in the caffeine condition. Testing points over an hour after the nap showed no differences between the two groups. From these results the authors concluded that caffeine had eliminated the effect of sleep inertia on this task. There was evidence to suggest that caffeine affected the sleep architecture of the naps in this study. For example, it took longer for participants to fall asleep, and the amount of time spent in deep sleep was significantly reduced in some of the nap opportunities. However, this effect was not consistent across all naps, yet the reduction of sleep inertia was consistent following all naps. Therefore, the authors concluded that the action of caffeine on sleep inertia was unlikely to be due to the alteration of prior sleep bout.
More recently, Newman et al.32) trialled a caffeinated chewing gum (100 mg) administered immediately upon waking and observed improved performance on a 5-min PVT relative to placebo at 12–18 min post-nap. Caffeine administered via gum reaches peak plasma levels more quickly than via a pill49). However, there were no differences observed between conditions until at least 12–18 min post-nap, suggesting that the caffeine gum, as in the pill form46), has a delayed effect, and is therefore unable to attenuate sleep inertia during the initial, most critical period of wakefulness. The caffeine gum may, however, limit the duration of sleep inertia and therefore provide faster recovery post-nap. It is worth noting that data from the placebo condition did not consistently show clear sleep inertia effects (i.e. performance was not always worse immediately after waking). Therefore, it is difficult to determine whether caffeine had an effect on sleep inertia per se, or improved general performance under conditions of mild sleep restriction and high circadian pressure (awoken at 01:00 and 06:00 after a 1-h and 6-h sleep opportunity, respectively). As an alternative mechanism, studies have shown that chewing non-caffeinated gum can improve alertness and cognitive performance50, 51). Therefore, chewing may have acted as a countermeasure in Newman and colleagues’ study32), thus reducing sleep inertia effects in both the placebo and caffeine groups. If so, the caffeine effect would be acting on top of the chewing effect, leading to delayed improvements on background performance. Overall, the use of caffeinated gum offers promising results for truncating sleep inertia duration. This study demonstrates the importance of using a placebo gum, and ideally another placebo or control condition, so that it is possible to isolate the effects of chewing and caffeine on sleep inertia. Further study is warranted to determine whether different caffeine dosages may be more effective, or different performance tasks may be more sensitive to detect changes in sleep inertia following reactive caffeine gum administration.
Other studies have administered caffeine by adding it to decaffeinated coffee which was consumed prior to a short daytime nap28, 30). Hayashi and colleagues28) administered 200 mg of caffeine (equivalent to 2–3 cups of instant coffee52, 53)) prior to a 20-min nap. Despite pre-nap administration, significant improvements in alertness and measured performance in the caffeine condition were not observed until 15 min after the nap. However, during this 15 min period, there were no differences in alertness or performance between the nap-only and no-nap group. This indicated a lack of observable sleep inertia, which may have been due to the experimental design. Mean total sleep time during the 20-min nap opportunity ending at 13:00 was 14.8 min, with no slow wave sleep (SWS, or “deep sleep”). These conditions are not particularly conducive to sleep inertia4, 16). This study demonstrates that, when investigating sleep inertia countermeasures, it is important to use experimental circumstances that are most likely to produce sleep inertia. For example, following: scheduled awakenings at night5, 15); extended wakefulness or prior sleep loss15, 19); or longer naps4, 16, 18).
Reyner and Horne30), using 150 mg of caffeine in decaffeinated coffee (equivalent to approximately two cups of coffee52, 53)), found that the combination of caffeine and a 15-min nap was associated with better performance across a 2-h drive than following either countermeasure in isolation. The driving task commenced 5 min after the nap, within the sleep inertia period. However, as investigation of sleep inertia was not a research aim of the study, the data were reported in 30-min bins which do not allow investigation of performance changes across this peak sleep inertia period. The first 30-min bin, however, did not show any obvious signs of sleep inertia (i.e. worse performance) relative to subsequent bins. Interpretation of these results with regards to sleep inertia is further limited as performance in the caffeine-plus-nap group was not directly compared to a nap-only group, only to results from a group in a previous study54). In the previous study54), participants drove for 2 h before receiving caffeine and/or nap, whereas in the follow-up study30) they drove for 1 h. Therefore the post-treatment results of these conditions should be compared with this in mind.
There is a government-led road safety campaign, based on the above study, to promote the use of a caffeine-nap combination as a drowsy driving countermeasure55). Further, despite a lack of studies investigating sleep inertia following short naps at night, other government websites promote the use of short naps (≤30 min) with no mention of potential sleep inertia7, 8). Current policies and recommendations to counteract sleepiness while driving require a stronger evidence base with regards to sleep inertia. The studies reviewed highlight the need for experimental and observational testing which allows for the direct assessment of the impact of caffeine in combination with a nap on driving performance immediately after waking at different times of day.
There are several considerations when applying these results in the workplace. In most studies28, 30, 31), caffeine was administered before the nap. Each of these studies yielded improvements post-nap in caffeine compared to placebo groups. In unpredictable scenarios, however, such as working on-call, there may not be sufficient warning or opportunity to use caffeine proactively. Proactive use may only be effective prior to short naps. There is the potential for sleep to be disrupted if caffeine is taken beforehand, resulting in a trade-off between reduced sleep inertia and reduced total sleep or sleep quality28, 31). However, when caffeine was administered post-nap, even in a rapid acting chewing gum format, caffeine effects were not seen until 12–18 min post-nap, which, while useful in some scenarios, may not be fast enough in time-critical situations32). Therefore, when used proactively, caffeine has the potential to eliminate sleep inertia effects almost immediately after short naps. Further research into the use of caffeinated gum is needed to explore the potential benefits of this reactive countermeasure beyond the demonstrated reduction in sleep inertia duration.
Another consideration in the application of these results to operational settings is habitual use and individual caffeine sensitivity. Van Dongen and colleagues31) employed a two-week caffeine-free wash-out before the study to minimise the effects of individual differences in caffeine tolerance. This study still observed large between-subjects variability in plasma caffeine concentrations. The other studies in Table 1 did not report habitual caffeine use30), or allowed participants to drink up to four cups of coffee per day until the experimental period28, 32). This may have influenced the effects of the caffeine, with low-caffeine users more sensitive to the effects of the experimental dosage compared to high-caffeine users46). Alternatively, high-caffeine users may have experienced withdrawals in the placebo condition56). Not controlling for habitual caffeine use, however, is perhaps more ecologically valid, as participants had a range of habitual caffeine intakes, just as a work force would. Studies of caffeine use in shift workers report high levels of caffeine intake especially for: older workers, those on night and morning shifts, and following reduced sleep45, 57). Although it is important to understand caffeine effects under controlled conditions, it is also important to understand individual differences in caffeine response under natural conditions. Translating results from controlled studies into real-world scenarios is a critical step before recommending caffeine as a countermeasure to sleep inertia. Further, the long-lasting effects of caffeine may disturb subsequent sleep opportunities58), so it may not be suitable for use on some occasions, such as towards the end of a night shift.
Light
Melatonin is a hormone which helps to regulate sleep-wake cycles and is suppressed under conditions of bright light exposure59). Light also serves as a zeitgeber (time-giver) to entrain our internal body (circadian) rhythms to a 24-h cycle60, 61). Light signals are sent from the eye to a cluster of cells in the brain responsible for our circadian rhythms, the suprachiasmatic nucleus. In this way, light can be used at key times to manipulate circadian timing, leading to changes in sleep patterns62, 63). In addition to its circadian entrainment effects, bright light exposure has also been shown to directly improve alertness and cognitive performance during the day, night, and following sleep restriction64, 65, 66, 67, 68). Investigation into optimising the intensity, wavelength and duration of light exposure to manipulate both sleep and performance are ongoing. There is potential for bright light exposure to directly improve alertness and performance during the sleep inertia period.
Few studies have investigated the effect of light immediately following waking on performance and alertness during this period. The first study of this type exposed participants to bright light (2,000 lux) for 1 min after waking from a 20-min nap28). This brief exposure to bright light did not reduce sleep inertia within the first 15 min after waking. It did, however, modestly improve subjective ratings for the following 45 min. More recently, Santhi and co-authors13) investigated the effect of four light conditions (dim, blue-intermediate, blue-enhanced, and bright blue-enhanced) on a range of cognitive tasks during the sleep inertia period. Participants were woken from a 6.5-h nocturnal sleep and were exposed to light for 4 h. There were no differences between the four conditions in subjective alertness or performance measured every half-hour for the first two hours and then every hour up to 4 hours after waking. The relatively infrequent testing points during the initial sleep inertia period may have limited observations in this protocol. Participants in this study were selected based on a self-reported need for at least 60 min to feel fully alert after waking. Indeed, subjective ratings of sleepiness showed a clear sleep inertia pattern, with highest sleepiness ratings immediately after waking which dissipated across subsequent testing points. However, the performance tasks varied in their sensitivity to sleep inertia. This demonstrates the important methodological consideration of choosing an appropriate task to measure sleep inertia. Regardless of the outcome measured, however, there was no significant effect of light on performance or alertness within 4 h of waking. The improvement in working memory observed 4 h after waking was likely due to light affecting general performance rather than sleep inertia per se. These studies demonstrated that both brief and sustained light exposure after waking did not improve performance during the sleep inertia period. These results suggest that light exposure may be of limited effectiveness during the sleep inertia period. However, it may be worth investigating different light intensities and qualities.
As well as investigating light exposure after waking, some studies have looked at light exposure before waking. This approach assumes a different action pathway of light. Instead of direct alerting properties of bright light exposure during wake, exposure to light before waking applies the theory that light will act to lighten sleep before waking, therefore reducing sleep inertia35). However, Harrison and colleagues34) found no difference in measured performance or alertness following a 90-min afternoon nap during which participants were exposed to varying light intensities (0, 1, 80, 6,400 lux). Similarly, Van de Werken et al.35), despite observing improvements in subjective alertness, found no differences in post-sleep performance on a simple reaction time and addition task between a dawn simulation condition and control. In the dawn simulation, light was increased to 300 lux in the 30 min prior to wake-up; there was no light before wake-up in the control condition. There were no sleep stage differences between conditions in either the 90-min nap34) or during the 30 min of artificial dawn35).
In contrast, modest performance benefits were observed both on cognitive and physical (cycling) tasks following dawn simulation, with no differences in sleep quality as measured by actigraphy36). Giménez et al.33) found that participants reported needing less time to feel fully alert after stronger artificial dawn light levels (250 lux vs 0 lux and 50 lux). Together these studies suggest that dawn light may improve subjective alertness. Further studies of the effect of dawn light on objective performance during the immediate wake-up period are necessary to determine the overall efficacy of dawn lighting to reduce subjective and objective sleep inertia.
Light may have an additional action pathway during the sleep inertia period via the cortisol awakening response (CAR). Cortisol is a hormone typically associated with stress response and follows a diurnal pattern with higher levels during the day and lower levels at night69). The CAR refers to the sharp increase in cortisol upon waking in the morning70). This response is greater in the presence of light presented immediately after waking (800 lux for 1 h)71). Less intense light presented before waking (dawn simulation: light gradually increased to 250–300 lux over 30 min before waking) has had mixed effect35, 72). Furthermore, a greater CAR has been associated with increased levels of self-reported arousal72, 73) and reduced sleepiness74).
The role of cortisol and the CAR in sleep inertia is unknown. A clue to its role comes from the discovery that SWS is associated with lower cortisol75). Deep sleep has also been associated with greater sleep inertia15, 76), although this relationship has not been consistently demonstrated18, 77). The relationship between deep sleep and sleep inertia may be mediated by cortisol, although this has not been directly investigated. If the relationship between cortisol and sleep inertia can be better established, manipulating cortisol through light interventions or exogenous administration may offer an alternative sleep inertia countermeasure. The inclusion of measures of cortisol, alertness, and performance across the sleep inertia period would be beneficial to systematically track inter-relationships between these factors during this time.
Using light after waking would be relatively straightforward to implement in the workplace. For example, strategic exposure to light boxes at work has successfully helped shift workers adjust their circadian rhythm to better match their shifts78, 79). However, a trial of bright light in a chemical plant uncovered several limitations including poor compliance with the interventions and variations in light exposure amongst workers due to different operational tasks80). The effectiveness of such a countermeasure may vary by workplace. However, a light box, or light-emitting glasses81) used immediately after waking, could feasibly be implemented as a reactive countermeasure. While using light before waking would be unsuitable for implementation in unpredictable sleep/wake environments, it may have an application for scheduled rest periods. For example, a dawn simulation light box could be used in a workplace napping room. An additional caution to be added to the use of bright light as a countermeasure to sleep inertia is the potentially unwanted effect of entrainment. For example, bright light exposure, particularly near the circadian nadir, could delay or advance circadian phase62, 63). This could lead to changes in sleep timing that are counterproductive to the work schedule and ultimately lead to sleep loss, sleepiness and poor performance.
Sound
Sound such as pink noise (random noise with more low frequency components than white noise) can be used as a sleeping aid by providing a constant, ambient auditory background thereby minimising the impact of random noises on sleep initiation and maintenance82, 83). Conversely, noise has been used to promote alertness under sleep deprivation conditions84) or as a positive stressor to improve performance85). This inconsistency in the literature may be due to variation in noise type (e.g. continuous versus intermittent). The effect of noise may also be task dependent, with significant effects more likely observed on simple compared to complex tasks86, 87, 88). The search identified two studies which used different sounds in an attempt to reduce sleep inertia.
Tassi and colleagues37) exposed participants to continuous noise during a spatial memory test performed after waking. In this study37), spatial memory after waking from a 1-h nap at 01:00 during the no noise condition was worse for up to 15 min compared to the total sleep deprivation condition (no nap, no noise). Performance in the group exposed to 75 dB of pink noise (random noise with more low frequency components than white noise) after waking, did not differ from total sleep deprivation. The authors suggest that noise had an arousing effect which reduced sleep inertia. Noise delivered after a nap ending at 04:00, however, was ineffective and may have exacerbated the effects of partial sleep deprivation under these conditions. Given the conflicting results from this study and the literature generally, more research is required to establish the effect of pink noise exposure under different sleep conditions, and on different tasks. The background noise level of the work place may also impact on the effectiveness of this countermeasure. For example, high levels of ambient noise are typically associated with elevated stress and fatigue in noisy work environments such as factories89, 90). The results of Tassi and colleagues’ study37) suggest, however, that under certain conditions, exposure to pink noise after waking could minimise sleep inertia.
Hayashi and colleagues38) took a different approach, playing excitatory music at 60 dB during a 20-min testing period after waking. Music, especially music that the participant preferred, reduced subjective sleepiness post-nap compared to a no-music control. Similarly, performance on a visual oddball task was improved with high preference music compared to low preference music. Future research in this area would benefit from the inclusion of a no nap control group to confirm that the effects observed target sleep inertia relative to general performance. Reyner and Horne91), under conditions of sleep restriction, found that playing the radio only had a brief, positive effect on alertness and simulated driving performance, and was therefore not suitable to long-distance driving. Playing music may, however, be effective in short-term sleep inertia scenarios.
From the limited literature it appears that sound (noise or music) has the potential to improve performance, at least briefly, under certain conditions. Further research in this area would help to determine the most effective sound to target sleep inertia symptoms. From an operational perspective, implementation in a work setting should be relatively easy. Indeed, background music has been used successfully in both industrial and office settings to improve vigilance and performance at work92). However, rather than background music, sound delivery would likely need to be via headphones, especially in shared sleeping environments. For example, a personal music player with a collection of songs pre-selected by shift workers (high preference music38)) could be kept in a napping room for use immediately after waking.
Temperature
Many studies have investigated the role of thermoregulation in moderating sleep onset and maintenance (for review, see Lack et al.93)). At sleep onset there is a drop in core body temperature (CBT) and an associated increase vasodilation and blood flow to the extremities in order to lose heat. This change in body temperature is thought to promote sleep onset and maintain sleep throughout the nocturnal sleep period93). Furthermore, the association between the rate of distal skin temperature increase (measured at the extremities) and sleep onset can be observed at different phases of circadian rhythm in CBT94). Manipulation of skin temperature and CBT have been found to improve sleep quality95), performance, and maintenance of wakefulness96). For example, strategies to warm the extremities (e.g. a hot water bottle at the feet) can help to promote sleep onset97). The role of thermoregulation at sleep offset, however, has received less attention98).
A tantalising preliminary study in this area has demonstrated a relationship between subjective measures of sleep inertia and changes in the distal-proximal skin temperature gradient (DPG)39). In this study, the reduction in subjective sleepiness correlated with a decrease in the DPG. Furthermore, the relationship between distal skin temperature and subjective sleepiness remained in a multiple nap protocol in which sleep and wake opportunities were rapidly alternated (75 min sleep / 150 min wake)40). The authors suggest that actively manipulating the temperature of extremities through simple cooling strategies that evoke distal vasoconstriction and reduce heat loss from the periphery may result in faster sleep inertia dissipation. Van De Werken35) also found that dawn light accelerated the DPG change after waking and that this was associated with a decrease in subjective sleepiness, but not objective performance.
From an operational perspective, if changing body temperature only changed subjective feelings, it may lead to a false sense of improved objective alertness and performance after waking99, 100, 101, 102, 103, 104). Studies further investigating the relationship between DPG and objective measures are well-warranted, however, as an intervention would be relatively easy to implement in most work places. For example, workers could place their hands and feet in a receptacle filled with cold or iced water to rapidly lose heat from their extremities immediately after waking. Such a countermeasure would also have the advantage of not negatively influencing future sleep opportunities (c.f. caffeine, light).
Self-awakening
Some studies have observed that waking from deeper stages of sleep (i.e. SWS) is associated with greater sleep inertia15, 76). This has led to studies of self-awakening to minimise the chances of waking from deep sleep. Self-awakening refers to spontaneously waking after a set period of time, without the use of an external stimulus such as an alarm. Kaida and colleagues41, 42) compared self-awakening to being woken by an experimenter after a 15-min afternoon nap. They reported that self-awakening led to lighter sleep, as scored using hypnagogic scoring105), a method which looks at the first two lighter stages of non-rapid eye movement sleep (Stage 1 and Stage 2)106) in greater resolution41). There were no differences observed across stages according to Rechtschaffen and Kales106) criteria41, 42). This finding suggests that traditional sleep scoring methods may not be sensitive enough to detect electroencephalographic changes induced by self-awakening techniques. Spectral analysis of these sleep episodes may be another useful method for understanding the mechanisms underlying self-awakening.
Kaida and colleagues41) measured physiological arousal levels by evaluating the amplitude of a specific brain response to an audible tone presented immediately after waking and repeated every 5 min for 30 min. There was a greater response after self- compared to forced-awakening at 15 min post-wake, but not at earlier or later testing points. The authors argue that this greater response following self-awakening is indicative of greater physiological arousal41). In a second study42), subjective sleepiness was improved at 10 min post-wake, but improvements in performance on an auditory oddball task were not observed until later. These results should be interpreted with caution as the experimental design (i.e. a short afternoon nap) may not have generated sleep inertia. Indeed, there was no difference in performance between the nap and no-nap group, and no immediate subjective ratings of sleep inertia. This study is another example highlighting the importance, when investigating sleep inertia countermeasures, of setting experimental conditions that are likely to produce sleep inertia. For example, Ikeda and colleagues investigated self-awakening following partial sleep restriction43, 107). In contrast to Kaida and colleagues’ studies41, 42), improvement was observed on cognitive tasks following self-awakening, but there were no significant differences in subjective sleepiness ratings.
While there is some evidence to support the use of self-awakening to reduce sleep inertia, a potential limitation in applying this technique is the inconsistent success rate of self-awakening. In Kaida and colleagues’ studies41, 42), there was a 71–82% success rate for self-awakening within ± 5 min of the 15-min target, much higher than previous studies cited by the authors which range from 18–42%41). The study, however, selected participants who were well-rested and claimed they were able to self-awaken from nocturnal sleep. Therefore this approach may be most beneficial for those who find it easier to self-awaken. It would be interesting to further this research under conditions better representing shift work scenarios, e.g. following chronic sleep restriction or extended periods of wakefulness21). In Ikeda and Hayashi’s study6), for example, participants habitually woke from an alarm, and subsequently only nine of the 30 attempted self-awakenings in the study (30%) were within 30 min of target wake time. As workers are generally required to return to shift at a specific time, it is unlikely that self-awakening would be a reliable strategy. Furthermore, this method is not a viable countermeasure for unscheduled wake-ups. For naps taken at home without time pressures, however, the self-awakening technique may offer relief from subjective feelings of sleep inertia.
Face Washing and Common Countermeasures
Anecdotally, people have wake-up routines which reduce sleep inertia. However, while commonly used countermeasures to driver fatigue such as cold air or playing the radio have been investigated91), few studies have examined their use for sleep inertia. Hayashi and colleagues’28) trialled face-washing as a practical, and commonly used, sleep inertia countermeasure following a 20-min afternoon nap. The authors describe an immediate, but short-lived, reduction in subjective sleepiness relative to nap-only, with no differences in performance on a memory search task. These results suggest that face-washing may improve subjective alertness after waking. Further research using different performance measures would be useful to establish whether the null findings for performance were specific to the memory search task used. It is possible that other tasks may be more sensitive to inertia.
In a study examining the time course of sleep inertia, Jewett et al.3) found no differences in performance on an addition task between a constant routine condition (lying in semi-recumbent position) and an ambulatory condition in which participants were able to get up, eat breakfast, and shower. Both groups were kept under the same semi-recumbent, fasting conditions until at least 35 min after waking, therefore it is not clear whether these activities would have made a difference if undertaken sooner after waking. Another study reported a positive effect of breakfast on morning mood and memory, but did not report when participants woke, so it is unclear whether it was strictly a study of sleep inertia108). More studies on common countermeasures such as showers, meals, or physical exercise are worthy of investigation. For example, a shower may act to change overall body temperature, and therefore might be more effective than face washing. Empirical research is needed to confirm effective common practices, and to de-bunk ineffective strategies.
Adrenaline and Operational Scenarios
Finally, when considering sleep inertia in real-world scenarios, adrenaline is often proposed as a natural countermeasure. Adrenaline is a stress hormone released from the adrenal glands109). Adrenaline may play a role in mitigating impairment during the sleep inertia period110). Laboratory studies of sleep inertia are typically conducted in low-stress environments. It is unknown whether a change in stress levels may affect sleep inertia, or the effectiveness of countermeasures. Further, there are no studies of adrenaline and its effect on performance and alertness during the sleep inertia period. This is likely due to constraints associated with collecting blood samples during the sleep inertia period and the short half-life of adrenaline111). Anecdotal and experimental evidence suggest that adrenaline may not be a reliable countermeasure to sleep inertia. Emergency service workers often report habituating to their high pressure roles, no longer feeling the “rush of adrenaline”. Reports are supported by a study that described changes to hormone levels following stressful field exercises and military training112). Special Forces soldiers had a different response than non-Special Forces soldiers, suggesting a degree of adaptation to stress. However, this adaptation may not be common for shift workers in less stressful occupations. Whether the hormonal response immediately upon waking in high stress scenarios changes with experience, and/or varies between individuals, is unknown. Nor do we know whether increases in hormones such as adrenaline can restore cognitive function during the sleep inertia period, or whether they may in fact further compromise clear thinking and decision-making12). Until there are studies directly investigating the role of adrenaline on cognitive performance during sleep inertia, reliance on this endogenous countermeasure is not recommended.
This area would also benefit from study in the field, which to date, are few110). A survey of emergency medical service pilots revealed that sleep inertia had occasionally compromised flight safety113) and some critical care nurses report avoiding napping on night shift due to concerns about performance impairment upon waking114). Taken together, these studies suggest that there is potential for sleep inertia-related safety risks, or at least, that the perception of sleep inertia as a safety issue exists in the field. Several incident investigation reports have cited sleep inertia as a contributing factor9, 10, 11, 12). Systematic studies of the effect of sleep inertia in real-world situations are essential for progressing our understanding of sleep inertia, and the development of effective countermeasures for practical implementation.
Conclusions
Examination of the current literature reveals a gap in the evidence-base for the implementation of a reactive countermeasure to sleep inertia which is effective within 15 min of waking. Caffeine is perhaps the closest option, although to target immediate performance and alertness, it needs to be administered prior to a short sleep bout, and in this way, is not a reactive strategy. This countermeasure may be useful, therefore, in situations where napping can be planned. Given that current investigations into light and temperature are in their infancy, further study is warranted to understand the physiological mechanisms underlying these interventions and whether performance can be improved after waking, in line with some encouraging results from subjective measures.
A challenge in identifying an effective sleep inertia countermeasure is that effectiveness may depend on sleep inertia severity. The majority of the studies reviewed assessed sleep inertia following afternoon naps, or upon waking from habitual night time sleep. There were no clear differences in the efficacy of countermeasures between these two scenarios. However, strategies that work under these conditions may not be sufficient in other scenarios. In shift work conditions, for example, the magnitude of sleep inertia may be exacerbated due to factors such as prior sleep loss, time of day, and sleep length. The impact of these factors on the effectiveness of countermeasures has yet to be investigated. Studies investigating sleep inertia countermeasures should consider directly comparing the effect of these factors, or testing countermeasures in scenarios more common to shift work (e.g. a night shift nap).
Identifying a viable countermeasure also involves assessing operational viability. Many of the countermeasures discussed could be implemented in a workplace. Developing a countermeasure that can attenuate the magnitude and/or duration of sleep inertia in both scheduled and unpredictable shift work environments warrants further investigation. In the meantime, it is recommended that proactive sleep inertia countermeasures are used, and that safety-critical tasks are avoided immediately after waking.
Acknowledgments
The authors have no conflicts of interest to declare.
References
- 1.Lubin A, Hord DJ, Tracy ML, Johnson LC (1976) Effects of exercise, bedrest and napping on performance decrement during 40 hours. Psychophysiology 13, 334–9. [DOI] [PubMed] [Google Scholar]
- 2.Tassi P, Muzet A (2000) Sleep inertia. Sleep Med Rev 4, 341–53. [DOI] [PubMed] [Google Scholar]
- 3.Jewett ME, Wyatt JK, Ritz-De Cecco A, Khalsa SB, Dijk DJ, Czeisler CA (1999) Time course of sleep inertia dissipation in human performance and alertness. J Sleep Res 8, 1–8. [DOI] [PubMed] [Google Scholar]
- 4.Tietzel AJ, Lack LC (2001) The short-term benefits of brief and long naps following nocturnal sleep restriction. Sleep 24, 293–300. [DOI] [PubMed] [Google Scholar]
- 5.Scheer FA, Shea TJ, Hilton MF, Shea SA (2008) An endogenous circadian rhythm in sleep inertia results in greatest cognitive impairment upon awakening during the biological night. J Biol Rhythms 23, 353–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ikeda H, Hayashi M (2010) The effect of self-awakening from nocturnal sleep on sleep inertia. Biol Psychol 83, 15–9. [DOI] [PubMed] [Google Scholar]
- 7.Transport Accident Commission http://www.tac.vic.gov.au/road-safety/tac-campaigns/fatigue/big-hit. Accessed May 6, 2015.
- 8.Transport for NSW http://roadsafety.transport.nsw.gov.au/stayingsafe/fatigue/stoprevivesurvive.html. Accessed May 6, 2015.
- 9.Armentrout JJ, Holland DA, O’Toole KJ, Ercoline WR (2006) Fatigue and related human factors in the near crash of a large military aircraft. Aviat Space Environ Med 77, 963–70. [PubMed] [Google Scholar]
- 10.Government of India Ministry of Civil Aviation (2010) Report on Accident to Air India Express Boeing 737–800 Aircraft VT-AXV on 22nd May 2010 at Mangalore.
- 11.Marine Accident Investigation Branch (2011) Heavy Contact by Skandi Foula with OMS Resolution, Aberdeen Harbour 29 May 2010. UK Department for Transport: London, UK. [Google Scholar]
- 12.Transportation Safety Board of Canada (2011) Aviation Investigation Report - A11F0012.
- 13.Santhi N, Groeger JA, Archer SN, Gimenez M, Schlangen LJM, Dijk DJ (2013) Morning sleep inertia in alertness and performance: effect of cognitive domain and white light conditions. PLoS One 8, e79688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burke TM, Scheer FA, Ronda JM, Czeisler CA, Wright KP Jr (2015) Sleep inertia, sleep homeostatic and circadian influences on higher-order cognitive functions. J Sleep Res 24, 364–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dinges DF, Orne MT, Orne EC (1985) Assessing performance upon abrupt awakening from naps during quasi-continuous operations. Behav Res Methods 17, 37–45. [Google Scholar]
- 16.Brooks A, Lack L (2006) A brief afternoon nap following nocturnal sleep restriction: which nap duration is most recuperative? Sleep 29, 831–40. [DOI] [PubMed] [Google Scholar]
- 17.Lovato N, Lack L, Ferguson S, Tremaine R (2009) The effects of a 30-min nap during night shift following a prophylactic sleep in the afternoon. Sleep Biol Rhythms 7, 34–42. [Google Scholar]
- 18.Signal TL, van den Berg MJ, Mulrine HM, Gander PH (2012) Duration of sleep inertia after napping during simulated night work and in extended operations. Chronobiol Int 29, 769–79. [DOI] [PubMed] [Google Scholar]
- 19.Miccoli L, Versace F, Koterle S, Cavallero C (2008) Comparing sleep-loss sleepiness and sleep inertia: lapses make the difference. Chronobiol Int 25, 725–44. [DOI] [PubMed] [Google Scholar]
- 20.Åkerstedt T. (1988) Sleepiness as a consequence of shift work. Sleep 11, 17–34. [DOI] [PubMed] [Google Scholar]
- 21.Rajaratnam SM, Arendt J (2001) Health in a 24-h society. Lancet 358, 999–1005. [DOI] [PubMed] [Google Scholar]
- 22.Lumley M, Roehrs T, Zorick F, Lamphere J, Roth T (1986) The alerting effects of naps in sleep-deprived subjects. Psychophysiology 23, 403–8. [DOI] [PubMed] [Google Scholar]
- 23.Dinges DF, Orne MT, Whitehouse WG, Orne EC (1987) Temporal placement of a nap for alertness: contributions of circadian phase and prior wakefulness. Sleep 10, 313–29. [PubMed] [Google Scholar]
- 24.Kubo T, Takeyama H, Matsumoto S, Ebara T, Murata K, Tachi N, Itani T (2007) Impact of nap length, nap timing and sleep quality on sustaining early morning performance. Ind Health 45, 552–63. [DOI] [PubMed] [Google Scholar]
- 25.Mulrine HM, Signal TL, van den Berg MJ, Gander PH (2012) Post-sleep inertia performance benefits of longer naps in simulated nightwork and extended operations. Chronobiol Int 29, 1249–57. [DOI] [PubMed] [Google Scholar]
- 26.Ruggiero JS, Redeker NS (2014) Effects of napping on sleepiness and sleep-related performance deficits in night-shift workers: a systematic review. Biol Res Nurs 16, 134–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Frey DJ, Ortega JD, Wiseman C, Farley CT, Wright KP Jr (2011) Influence of zolpidem and sleep inertia on balance and cognition during nighttime awakening: a randomized placebo-controlled trial. J Am Geriatr Soc 59, 73–81. [DOI] [PubMed] [Google Scholar]
- 28.Hayashi M, Masuda A, Hori T (2003) The alerting effects of caffeine, bright light and face washing after a short daytime nap. Clin Neurophysiol 114, 2268–78. [DOI] [PubMed] [Google Scholar]
- 29.Collins JA, Fauser BC (2005) Balancing the strengths of systematic and narrative reviews. Hum Reprod Update 11, 103–4. [DOI] [PubMed] [Google Scholar]
- 30.Reyner LA, Horne JA (1997) Suppression of sleepiness in drivers: combination of caffeine with a short nap. Psychophysiology 34, 721–5. [DOI] [PubMed] [Google Scholar]
- 31.Van Dongen HPA, Price NJ, Mullington JM, Szuba MP, Kapoor SC, Dinges DF (2001) Caffeine eliminates psychomotor vigilance deficits from sleep inertia. Sleep 24, 813–9. [DOI] [PubMed] [Google Scholar]
- 32.Newman RA, Kamimori GH, Wesensten NJ, Picchioni D, Balkin TJ (2013) Caffeine gum minimizes sleep inertia. Percept Mot Skills 116, 280–93. [DOI] [PubMed] [Google Scholar]
- 33.Giménez MC, Hessels M, van de Werken M, de Vries B, Beersma DG, Gordijn MC (2010) Effects of artificial dawn on subjective ratings of sleep inertia and dim light melatonin onset. Chronobiol Int 27, 1219–41. [DOI] [PubMed] [Google Scholar]
- 34.Harrison EM, Gorman MR, Mednick SC (2011) The effect of narrowband 500 nm light on daytime sleep in humans. Physiol Behav 103, 197–202. [DOI] [PubMed] [Google Scholar]
- 35.Van De Werken M, Giménez MC, De Vries B, Beersma DG, Van Someren EJ, Gordijn MC (2010) Effects of artificial dawn on sleep inertia, skin temperature, and the awakening cortisol response. J Sleep Res 19, 425–35. [DOI] [PubMed] [Google Scholar]
- 36.Thompson A, Jones H, Gregson W, Atkinson G (2014) Effects of dawn simulation on markers of sleep inertia and post-waking performance in humans. Eur J Appl Physiol 114, 1049–56. [DOI] [PubMed] [Google Scholar]
- 37.Tassi P, Nicolas A, Dewasmes G, Eschenlauer R, Ehrhart J, Salame P, Muzet A, Libert JP (1992) Effects of noise on sleep inertia as a function of circadian placement of a one-hour nap. Percept Mot Skills 75, 291–302. [DOI] [PubMed] [Google Scholar]
- 38.Hayashi M, Uchida C, Shoji T, Hori T (2004) The effects of the preference for music on sleep inertia after a short daytime nap. Sleep Biol Rhythms 2, 184–91. [Google Scholar]
- 39.Kräuchi K, Cajochen C, Wirz-Justice A (2004) Waking up properly: is there a role of thermoregulation in sleep inertia? J Sleep Res 13, 121–7. [DOI] [PubMed] [Google Scholar]
- 40.Kräuchi K, Knoblauch V, Wirz-Justice A, Cajochen C (2006) Challenging the sleep homeostat does not influence the thermoregulatory system in men: evidence from a nap vs. sleep-deprivation study. Am J Physiol Regul Integr Comp Physiol 290, R1052–61. [DOI] [PubMed] [Google Scholar]
- 41.Kaida K, Nittono H, Hayashi M, Hori T (2003) Effects of self-awakening on sleep structure of a daytime short nap and on subsequent arousal levels. Percept Mot Skills 97, 1073–84. [DOI] [PubMed] [Google Scholar]
- 42.Kaida K, Nakano E, Nittono H, Hayashi M, Hori T (2003) The effects of self-awakening on heart rate activity in a short afternoon nap. Clin Neurophysiol 114, 1896–901. [DOI] [PubMed] [Google Scholar]
- 43.Ikeda H, Kubo T, Kuriyama K, Takahashi M (2014) Self-awakening improves alertness in the morning and during the day after partial sleep deprivation. J Sleep Res 23, 673–80. [DOI] [PubMed] [Google Scholar]
- 44.Nehlig A. (1999) Are we dependent upon coffee and caffeine? A review on human and animal data. Neurosci Biobehav Rev 23, 563–76. [DOI] [PubMed] [Google Scholar]
- 45.Dorrian J, Paterson J, Dawson D, Pincombe J, Grech C, Rogers AE (2011) Sleep, stress and compensatory behaviors in Australian nurses and midwives. Rev Saude Publica 45, 922–30. [DOI] [PubMed] [Google Scholar]
- 46.Fredholm BB, Bättig K, Holmén J, Nehlig A, Zvartau EE (1999) Actions of caffeine in the brain with special reference to factors that contribute to its widespread use. Pharmacol Rev 51, 83–133. [PubMed] [Google Scholar]
- 47.Wesensten NJ, Belenky G, Kautz MA, Thorne DR, Reichardt RM, Balkin TJ (2002) Maintaining alertness and performance during sleep deprivation: modafinil versus caffeine. Psychopharmacology (Berl) 159, 238–47. [DOI] [PubMed] [Google Scholar]
- 48.Wyatt JK, Cajochen C, Ritz-De Cecco A, Czeisler CA, Dijk DJ (2004) Low-dose repeated caffeine administration for circadian-phase-dependent performance degradation during extended wakefulness. Sleep 27, 374–81. [DOI] [PubMed] [Google Scholar]
- 49.Kamimori GH, Karyekar CS, Otterstetter R, Cox DS, Balkin TJ, Belenky GL, Eddington ND (2002) The rate of absorption and relative bioavailability of caffeine administered in chewing gum versus capsules to normal healthy volunteers. Int J Pharm 234, 159–67. [DOI] [PubMed] [Google Scholar]
- 50.Allen AP, Jacob TJ, Smith AP (2014) Effects and after-effects of chewing gum on vigilance, heart rate, EEG and mood. Physiol Behav 133, 244–51. [DOI] [PubMed] [Google Scholar]
- 51.Morgan K, Johnson AJ, Miles C (2014) Chewing gum moderates the vigilance decrement. Br J Psychol 105, 214–25. [DOI] [PubMed] [Google Scholar]
- 52.Brice CF, Smith AP (2002) Factors associated with caffeine consumption. Int J Food Sci Nutr 53, 55–64. [PubMed] [Google Scholar]
- 53.Mandel HG. (2002) Update on caffeine consumption, disposition and action. Food Chem Toxicol 40, 1231–4. [DOI] [PubMed] [Google Scholar]
- 54.Horne JA, Reyner LA (1996) Counteracting driver sleepiness: effects of napping, caffeine, and placebo. Psychophysiology 33, 306–9. [DOI] [PubMed] [Google Scholar]
- 55.Department for Transport http://think.direct.gov.uk/fatigue.html. Accessed May 6, 2015.
- 56.Juliano LM, Griffiths RR (2004) A critical review of caffeine withdrawal: empirical validation of symptoms and signs, incidence, severity, and associated features. Psychopharmacology (Berl) 176, 1–29. [DOI] [PubMed] [Google Scholar]
- 57.Dorrian J, Baulk SD, Dawson D (2011) Work hours, workload, sleep and fatigue in Australian Rail Industry employees. Appl Ergon 42, 202–9. [DOI] [PubMed] [Google Scholar]
- 58.McHill AW, Smith BJ, Wright KP Jr (. 2014) Effects of caffeine on skin and core temperatures, alertness, and recovery sleep during circadian misalignment. J Biol Rhythms 29, 131–43. [DOI] [PubMed] [Google Scholar]
- 59.Cagnacci A, Elliott JA, Yen SS (1992) Melatonin: a major regulator of the circadian rhythm of core temperature in humans. J Clin Endocrinol Metab 75, 447–52. [DOI] [PubMed] [Google Scholar]
- 60.Czeisler CA, Allan JS, Strogatz SH, Ronda JM, Sánchez R, Ríos CD, Freitag WO, Richardson GS, Kronauer RE (1986) Bright light resets the human circadian pacemaker independent of the timing of the sleep-wake cycle. Science 233, 667–71. [DOI] [PubMed] [Google Scholar]
- 61.Wever RA. (1989) Light effects on human circadian rhythms: a review of recent Andechs experiments. J Biol Rhythms 4, 161–85. [PubMed] [Google Scholar]
- 62.Rosenthal NE, Joseph-Vanderpool JR, Levendosky AA, Johnston SH, Allen R, Kelly KA, Souetre E, Schultz PM, Starz KE (1990) Phase-shifting effects of bright morning light as treatment for delayed sleep phase syndrome. Sleep 13, 354–61. [PubMed] [Google Scholar]
- 63.Eastman CI, Martin SK (1999) How to use light and dark to produce circadian adaptation to night shift work. Ann Med 31, 87–98. [DOI] [PubMed] [Google Scholar]
- 64.Campbell SS, Dawson D (1990) Enhancement of nighttime alertness and performance with bright ambient light. Physiol Behav 48, 317–20. [DOI] [PubMed] [Google Scholar]
- 65.French J, Hannon P, Brainard GC (1990) Effects of bright illuminance on body temperature and human performance. Ann Rev Chronopharmacol 7, 37–40. [Google Scholar]
- 66.Phipps-Nelson J, Redman JR, Dijk DJ, Rajaratnam SM (2003) Daytime exposure to bright light, as compared to dim light, decreases sleepiness and improves psychomotor vigilance performance. Sleep 26, 695–700. [DOI] [PubMed] [Google Scholar]
- 67.Vandewalle G, Balteau E, Phillips C, Degueldre C, Moreau V, Sterpenich V, Albouy G, Darsaud A, Desseilles M, Dang-Vu TT, Peigneux P, Luxen A, Dijk DJ, Maquet P (2006) Daytime light exposure dynamically enhances brain responses. Curr Biol 16, 1616–21. [DOI] [PubMed] [Google Scholar]
- 68.Cajochen C. (2007) Alerting effects of light. Sleep Med Rev 11, 453–64. [DOI] [PubMed] [Google Scholar]
- 69.Sherman B, Wysham C, Pfohl B (1985) Age-related changes in the circadian rhythm of plasma cortisol in man. J Clin Endocrinol Metab 61, 439–43. [DOI] [PubMed] [Google Scholar]
- 70.Clow A, Hucklebridge F, Stalder T, Evans P, Thorn L (2010) The cortisol awakening response: more than a measure of HPA axis function. Neurosci Biobehav Rev 35, 97–103. [DOI] [PubMed] [Google Scholar]
- 71.Scheer FA, Buijs RM (1999) Light affects morning salivary cortisol in humans. J Clin Endocrinol Metab 84, 3395–8. [DOI] [PubMed] [Google Scholar]
- 72.Thorn L, Hucklebridge F, Esgate A, Evans P, Clow A (2004) The effect of dawn simulation on the cortisol response to awakening in healthy participants. Psychoneuroendocrinology 29, 925–30. [DOI] [PubMed] [Google Scholar]
- 73.Thorn L, Hucklebridge F, Evans P, Clow A (2009) The cortisol awakening response, seasonality, stress and arousal: a study of trait and state influences. Psychoneuroendocrinology 34, 299–306. [DOI] [PubMed] [Google Scholar]
- 74.Dahlgren A, Kecklund G, Theorell T, Åkerstedt T (2009) Day-to-day variation in saliva cortisol--relation with sleep, stress and self-rated health. Biol Psychol 82, 149–55. [DOI] [PubMed] [Google Scholar]
- 75.Vgontzas AN, Mastorakos G, Bixler EO, Kales A, Gold PW, Chrousos GP (1999) Sleep deprivation effects on the activity of the hypothalamic-pituitary-adrenal and growth axes: potential clinical implications. Clin Endocrinol (Oxf) 51, 205–15. [DOI] [PubMed] [Google Scholar]
- 76.Tassi P, Bonnefond A, Engasser O, Hoeft A, Eschenlauer R, Muzet A (2006) EEG spectral power and cognitive performance during sleep inertia: the effect of normal sleep duration and partial sleep deprivation. Physiol Behav 87, 177–84. [DOI] [PubMed] [Google Scholar]
- 77.Achermann P, Werth E, Dijk DJ, Borbely AA (1995) Time course of sleep inertia after nighttime and daytime sleep episodes. Arch Ital Biol 134, 109–19. [PubMed] [Google Scholar]
- 78.Stewart KT, Hayes BC, Eastman CI (1995) Light treatment for NASA shiftworkers. Chronobiol Int 12, 141–51. [DOI] [PubMed] [Google Scholar]
- 79.Boivin DB, James FO (2002) Circadian adaptation to night-shift work by judicious light and darkness exposure. J Biol Rhythms 17, 556–67. [DOI] [PubMed] [Google Scholar]
- 80.Budnick LD, Lerman SE, Nicolich MJ (1995) An evaluation of scheduled bright light and darkness on rotating shiftworkers: trial and limitations. Am J Ind Med 27, 771–82. [DOI] [PubMed] [Google Scholar]
- 81.Wright HR, Lack LC, Partridge KJ (2001) Light emitting diodes can be used to phase delay the melatonin rhythm. J Pineal Res 31, 350–5. [DOI] [PubMed] [Google Scholar]
- 82.Kawada T, Suzuki S (1993) Sleep induction effects of steady 60 dB (A) pink noise. Ind Health 31, 35–8. [DOI] [PubMed] [Google Scholar]
- 83.Zhou J, Liu D, Li X, Ma J, Zhang J, Fang J (2012) Pink noise: effect on complexity synchronization of brain activity and sleep consolidation. J Theor Biol 306, 68–72. [DOI] [PubMed] [Google Scholar]
- 84.Wilkinson RT. (1963) Interaction of noise with knowledge of results and sleep deprivation. J Exp Psychol 66, 332–7. [DOI] [PubMed] [Google Scholar]
- 85.Poulton EC. (1976) Arousing environmental stresses can improve performance, whatever people say. Aviat Space Environ Med 47, 1193–204. [Google Scholar]
- 86.Koelega HS, Brinkman JA (1986) Noise and vigilance: an evaluative review. Hum Factors 28, 465–81. [DOI] [PubMed] [Google Scholar]
- 87.Hockey GRJ. (1970) Effect of loud noise on attentional selectivity. Q J Exp Psychol 22, 28–36. [Google Scholar]
- 88.Hancock PA. (1984) Environmental stressors. In: Sustained attention in human performance, Warm JS (Ed.), Wiley, New York. [Google Scholar]
- 89.Melamed S, Bruhis S (1996) The effects of chronic industrial noise exposure on urinary cortisol, fatigue and irritability: a controlled field experiment. J Occup Environ Med 38, 252–6. [DOI] [PubMed] [Google Scholar]
- 90.Kjellberg A, Muhr P, Sköldström B (1998) Fatigue after work in noise—an epidemiological survey study and three quasi-experimental field studies. Noise Health 1, 47–55. [PubMed] [Google Scholar]
- 91.Reyner LA, Horne JA (1998) Evaluation “in-car” countermeasures to sleepiness: cold air and radio. Sleep 21, 46–50. [PubMed] [Google Scholar]
- 92.Penn PE, Bootzin RR (1990) Behavioural techniques for enhancing alertness and performance in shift work. Work Stress 4, 213–26. [Google Scholar]
- 93.Lack LC, Gradisar M, Van Someren EJ, Wright HR, Lushington K (2008) The relationship between insomnia and body temperatures. Sleep Med Rev 12, 307–17. [DOI] [PubMed] [Google Scholar]
- 94.Lack L, Gradisar M (2002) Acute finger temperature changes preceding sleep onsets over a 45-h period. J Sleep Res 11, 275–82. [DOI] [PubMed] [Google Scholar]
- 95.Raymann RJ, Swaab DF, Van Someren EJ (2008) Skin deep: enhanced sleep depth by cutaneous temperature manipulation. Brain 131, 500–13. [DOI] [PubMed] [Google Scholar]
- 96.Fronczek R, Raymann RJ, Romeijn N, Overeem S, Fischer M, van Dijk JG, Lammers GJ, Van Someren EJ (2008) Manipulation of core body and skin temperature improves vigilance and maintenance of wakefulness in narcolepsy. Sleep 31, 233–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kräuchi K, Cajochen C, Werth E, Wirz-Justice A (1999) Warm feet promote the rapid onset of sleep. Nature 401, 36–7. [DOI] [PubMed] [Google Scholar]
- 98.Kräuchi K, Cajochen C, Wirz-Justice A (2005) Thermophysiologic aspects of the three-process-model of sleepiness regulation. Clin Sports Med 24, 287–300, ix. [DOI] [PubMed] [Google Scholar]
- 99.Bruck D, Pisani DL (1999) The effects of sleep inertia on decision-making performance. J Sleep Res 8, 95–103. [DOI] [PubMed] [Google Scholar]
- 100.Dorrian J, Lamond N, Dawson D (2000) The ability to self-monitor performance when fatigued. J Sleep Res 9, 137–44. [DOI] [PubMed] [Google Scholar]
- 101.Dorrian J, Lamond N, Holmes AL, Burgess HJ, Roach GD, Fletcher A, Dawson D (2003) The ability to self-monitor performance during a week of simulated night shifts. Sleep 26, 871–7. [DOI] [PubMed] [Google Scholar]
- 102.Biggs SN, Smith A, Dorrian J, Reid K, Dawson D, van den Heuvel C, Baulk S (2007) Perception of simulated driving performance after sleep restriction and caffeine. J Psychosom Res 63, 573–7. [DOI] [PubMed] [Google Scholar]
- 103.Tremaine R, Dorrian J, Lack L, Lovato N, Ferguson S, Zhou X, Roach G (2010) The relationship between subjective and objective sleepiness and performance during a simulated night-shift with a nap countermeasure. Appl Ergon 42, 52–61. [DOI] [PubMed] [Google Scholar]
- 104.Hilditch CJ, Dorrian J, Centofanti SA, Van Dongen HP, Banks S (2015) Sleep inertia associated with a 10-min nap before the commute home following a night shift: A laboratory simulation study. Accid Anal Prev S0001-4575(15)30126-3; Epub ahead of print (In press). [DOI] [PubMed] [Google Scholar]
- 105.Hori T, Hayashi M, Morikawa T (1994) Topographical EEG changes and the hypnagogic experience. In: Sleep onset: Normal and abnormal processes, Ogilvie R and Harsh JR (Eds.), 237–53, American Psychological Association, Washington, DC. [Google Scholar]
- 106.Rechtschaffen A, Kales A (1968) A manual of standardized terminology, techniques and scoring system for sleep stages of human subjects. Los Angeles: Brain Information Service/Brain Research Institute, University of California. [Google Scholar]
- 107.Ikeda H, Hayashi M (2010) The effect of self-awakening from nocturnal sleep on sleep inertia. Biol Psychol 83, 15–9. [DOI] [PubMed] [Google Scholar]
- 108.Smith AP, Clark R, Gallagher J (1999) Breakfast cereal and caffeinated coffee: effects on working memory, attention, mood, and cardiovascular function. Physiol Behav 67, 9–17. [DOI] [PubMed] [Google Scholar]
- 109.Matter R, Carroll J, Dyer C (2000) Neuroendocrine responses to stress. In: The biology of animal stress: basic principles and implications for animal welfare, Moberg GP and Mench JA (Eds.), 43–76, CABI Publishing, New York. [Google Scholar]
- 110.Rosekind MR, Solutions A (2008) Managing fatigue in EMS flight operations: challenges and opportunities. Alertness Solutions White Paper.
- 111.Ferreira SH, Vane JR (1967) Half-lives of peptides and amines in the circulation. Nature 215, 1237–40. [DOI] [PubMed] [Google Scholar]
- 112.Weeks SR, McAuliffe CL, Durussel D, Pasquina PF (2010) Physiological and psychological fatigue in extreme conditions: the military example. PM R 2, 438–41. [DOI] [PubMed] [Google Scholar]
- 113.Gregory KB, Winn W, Johnson K, Rosekind MR (2010) Pilot fatigue survey: exploring fatigue factors in air medical operations. Air Med J 29, 309–19. [DOI] [PubMed] [Google Scholar]
- 114.Fallis WM, McMillan DE, Edwards MP (2011) Napping during night shift: practices, preferences, and perceptions of critical care and emergency department nurses. Crit Care Nurse 31, e1–11. [DOI] [PubMed] [Google Scholar]