Abstract Abstract
Background
This paper describes a dataset of macrofaunal organisms associated with the sponge Sarcotragus foetidus Schmidt, 1862, collected by scuba diving from two sampling sites: one in Greece (North Aegean Sea) and one in Cyprus (Levantine Sea).
New information
This dataset includes macrofaunal taxa inhabiting the demosponge Sarcotragus foetidus and contributes to the ongoing efforts of the Ocean Biogeographic Information System (OBIS) which aims at filling the gaps in our current knowledge of the world's oceans. This is the first paper, to our knowledge, where the macrofauna associated with S. foetidus from the Levantine Basin is being recorded.
In total, 90 taxa were recorded, from which 83 were identified to the species level. Eight of these species are new records for the Levantine Basin. The dataset contains 213 occurrence records, fully annotated with all required metadata.
It is accessible at http://lifewww-00.her.hcmr.gr:8080/medobis/resource.do?r=organismic_assemblages_sarcotragus_foetidus_cyprus_greece
Keywords: Sarcotragus foetidus, Porifera , Demospongiae , macrofauna, Greece, North Aegean Sea, Cyprus, Levantine Basin, Eastern Mediterranean
Introduction
It is well known that sponges host a variety of macrobenthic organisms, providing them with shelter and constant food supply (Fishelson 1966, Koukouras et al. 1985, Koukouras et al. 1996, Koukouras et al. 1992, Çinar and Ergen 1998, Ilan et al. 1994). The relationship between sponges and their associated macrofaunal species has been investigated by several scientists (e.g. Pearse 1932, Pearse 1950, Arndt 1933, Bacescu 1971, Frith 1976, Long 1968).
The sponge Sarcotragus foetidus Schmidt, 1862 (Fig. 1) belongs to the class of Demospongiae and, more specifically, to the subclass of Keratosa, i.e. sponges with skeleton comprised of spongin fibers (order Dictyoceratida, family Irciniidae).
Figure 1.
Photo of the demosponge Sarcotragus foetidus Schmidt, 1862, collected in the course of the study.
This particular sponge species has an extensive network of small and large channels and cavities, and thus allows a variety of benthic invertebrates to inhabit them. Its surface is characterized by conules of 2–3 mm height, which are 10–15 mm apart from one another. The main skeleton is composed by a reticulate network of primary (ca. 100–200 µm in diameter) and secondary (ca. 50–100 µm in diameter) fibres (Manconi et al. 2013). Interestingly, this species was even mentioned by Aristotle, who had named it "Aplysias", meaning that it cannot be cleaned and used as a bath sponge, although its external morphology resembles to that of common bath sponges (Voultsiadou and Vafidis 2007).
The macrofaunal assemblages associated with S. foetidus have been investigated by several authors (e.g. Çinar and Ergen 1998, Çinar et al. 2002, Koukouras et al. 1985, Pansini and Daglio 1981, Rützler 1976) and in different study sites (e.g. Aegean Sea, Ligurian Sea, Tunisian coasts), although they have not been studied yet in the Levantine Sea.
General description
Purpose
This dataset includes species found associated with the demosponge S. foetidus. The sample sponges were collected from Greece (Linaraki, Sithonia, Halkidiki) and Cyprus (Milouria, Kissonerga, Pafos). Sampling in Cyprus was conducted in January of 2003 and August of 2003 and 2007, at depths between 5 and 10 meters. Sampling in Greece was conducted in February and July of 2003, in depths between 14 and 17 meters.
Project description
Personnel
Christina Pavloudi, HCMR (sample collection, taxonomic identification, data management), Michalis Mavidis, Aristotle University of Thessaloniki (sample collection, taxonomic identification), Magdalini Christodoulou, Aristotle University of Thessaloniki (sample collection, taxonomic identification), Athanasios Koukouras, Aristotle University of Thessaloniki (sample collection).
Study area description
Samples were collected from one location at the coast of Halkidiki (Greece) and one location at the coast of Cyprus (Fig. 2). The two study sites can be distinguished based on their trophic state index. North Aegean Sea can be characterized as mesotrophic to eutrophic (Kyriakidis et al. 2015), in contrast to the oligotrophic Levantine Basin (Duineveld et al. 2000). Both study sites are rocky shores dominated by different species of photohilic algae, thus no obvious differences in the sponge associated fauna can be attributed to differences in the substrate type.
Figure 2.
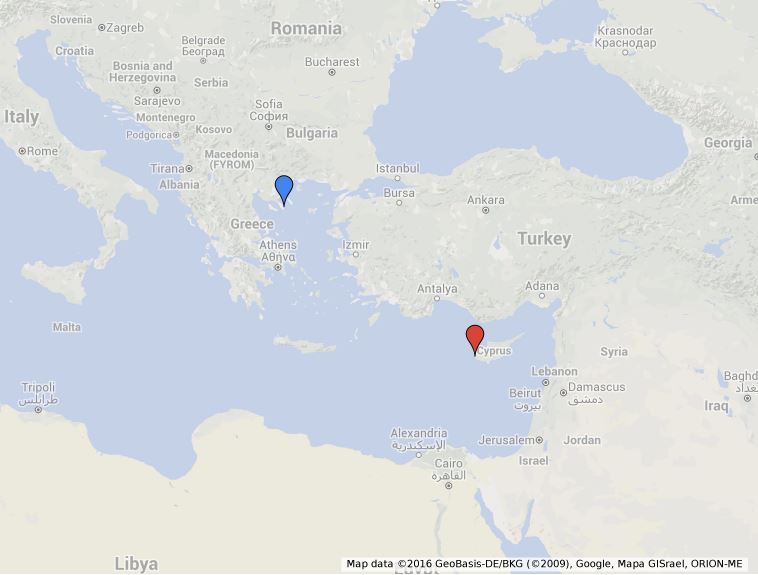
Geographical location of the study area indicating the sampling stations.
Linaraki: The sampling site is located on the peninsula of Sithonia, in the North Aegean Sea (Fig. 3a). It is a moderately exposed rocky shore with a photophilic algal assemblage dominated by Ellisolandia elongata.
Location of the sampling station in:
Figure 3a.
Greece.
Figure 3b.
Cyprus.
Milouria: The sampling site is located close to the town of Pafos (Southwest Cyprus) (Fig. 3b. It is an exposed rocky shore with a photophilic algal assemblage dominated by the the non-indigenous species Palisada perforata.
Sampling methods
Study extent
Samples were collected at single time points. Three sponges were collected in the winter and two in the summer season from Greece. In addition, three sponges were collected in the winter and four sponges (one in 2003 and three in 2007) in the summer season from Cyprus.
Sampling description
Samples were collected by scuba divers. Each sponge was first covered with a plastic bag and was subsequently detached from the substrate, manually, with a knife. Once ashore, formalin was added in every sample to a final concentration of 5% and the samples were stored in jars.
Upon return to the laboratory, the epifauna of each sponge was collected initially. The formalin solution contained in the plastic bags was filtered through a 0.5 mm mesh size sieve in order to collect epifaunal organisms that were detached from surface of the sponges. Then, the surface and volume of each sponge was measured. Sponge volume was measured by water displacement. Afterwards, sponges were cut in smaller pieces and the animals found in the sponge channels were collected.
Quality control
All scientific names were standardised against the World Register of Marine species using the Taxon Match tool. Taxon names were also kept in the dataset as they had been originally recorded, with a reference to the currently accepted name.
Geographic coverage
Description
Includes one location in Cyprus (Milouria, Kissonerga, Pafos) and one location in Greece (Linaraki, Sithonia, Halkidiki) (Christodoulou et al. 2013). More information can be found in Table 1.
Table 1.
Locality, geographical coordinates, depth (m) and physical characteristics of the sampling stations.
| Locality | Coordinates | Depth (m) | Habitat |
|---|---|---|---|
| Linaraki Beach, Sykia, Chalkikidi, Greece |
40° 2′ 15.003" N
23° 59′ 57.264" E |
14 - 17 | Rocky shore, moderately exposed. Photophilic algal assemblage dominated by Ellisolandia elongata (J.Ellis & Solander) K.R.Hind & G.W.Saunders, 2013 |
| Synthiana’s bay, Milouria, Kissonerga, Paphos, Cyprus |
34°′ 18.8148" N
32° 23′ 18.8808" E |
5 - 10 | Rocky shore, exposed. Photophilic algal assemblage dominated by Palisada perforata (Bory de Saint-Vincent) K.W. Nam, 2007 |
Coordinates
34.43 and 40.44 Latitude; 23.58 and 32.85 Longitude.
Taxonomic coverage
Description
The dataset comprises distribution information for 90 taxa, belonging in 48 families and 8 phyla. Detailed information is presented in Table 2. Of these, 8 species have been recorded for the first time in the Levantine Basin.
Table 2.
Taxa identified to the lowest taxonomic level possible and included in the dataset. *: non-indigenous species (ÖZTOPRAK et al. 2014, Pancucci-Papadopoulou et al. 2005). **: identification questionable for individuals from Cyprus (may be in fact Phascolosoma (Phascolosoma) stephensoni) (Açik 2014).
| Phylum | Class | Scientific Name | New record for the Levantine Basin |
| Mollusca | Polyplacophora | Acanthochitona fascicularis | |
| Arthropoda | Malacostraca | Alpheus dentipes | |
| Annelida | Polychaeta | Amphitrite rubra | |
| Annelida | Polychaeta | Amphitrite variabilis | |
| Annelida | Polychaeta | Arabella iricolor | |
| Sipuncula | Phascolosomatidea | Aspidosiphon (Aspidosiphon) muelleri muelleri | |
| Arthropoda | Malacostraca | Athanas nitescens | |
| Mollusca | Gastropoda | Bittium reticulatum | |
| Annelida | Polychaeta | Branchiomma bombyx | |
| Annelida | Polychaeta | Branchiosyllis exilis | |
| Porifera | Calcarea | Calcarea | |
| Mollusca | Polyplacophora | Callochiton septemvalvis | |
| Annelida | Polychaeta | Capitella capitata | |
| Arthropoda | Malacostraca | Ceradocus (Ceradocus) orchestiipes | + |
| Annelida | Polychaeta | Ceratonereis (Composetia) costae | |
| Annelida | Polychaeta | Ceratonereis (Composetia) hircinicola | |
| Arthropoda | Malacostraca | Cestopagurus timidus | |
| Arthropoda | Malacostraca | Colomastix pusilla | |
| Mollusca | Gastropoda | Columbella rustica | |
| Arthropoda | Malacostraca | Cymodoce spinosa | + |
| Arthropoda | Malacostraca | Dexamine spinosa | |
| Annelida | Polychaeta | Dipolydora armata | + |
| Annelida | Polychaeta | Dorvillea rubrovittata | |
| Arthropoda | Malacostraca | Elasmopus pocillimanus | |
| Annelida | Polychaeta | Eunice vittata | |
| Arthropoda | Malacostraca | Eurydice affinis | + |
| Arthropoda | Malacostraca | Galathea intermedia | |
| Arthropoda | Malacostraca | Gammaropsis crenulata | |
| Annelida | Polychaeta | Glycera tesselata | |
| Chordata | Actinopteri | Gobius geniporus | |
| Annelida | Polychaeta | Harmothoe spinifera | |
| Mollusca | Bivalvia | Hiatella arctica | |
| Arthropoda | Malacostraca | Hippolyte leptocerus | |
| Annelida | Polychaeta | Hydroides niger | |
| Annelida | Polychaeta | Hydroides pseudouncinatus | |
| Arthropoda | Malacostraca | Janira maculosa | + |
| Annelida | Polychaeta | Lepidasthenia elegans | |
| Arthropoda | Malacostraca | Leucothoe spinicarpa | |
| Arthropoda | Malacostraca | Liljeborgia dellavallei | |
| Mollusca | Bivalvia | Lima lima | |
| Mollusca | Bivalvia | Lithophaga lithophaga | |
| Annelida | Polychaeta | Lumbrineris coccinea | |
| Annelida | Polychaeta | Lumbrineris latreilli | |
| Annelida | Polychaeta | Lysidice collaris | |
| Annelida | Polychaeta | Lysidice ninetta | |
| Arthropoda | Malacostraca | Lysmata seticaudata | |
| Annelida | Polychaeta | Marphysa sanguinea | |
| Arthropoda | Malacostraca | Microdeutopus bifidus | |
| Mollusca | Gastropoda | Mitra cornicula | |
| Nemertea | Nemertea | Nemertea spp. | |
| Annelida | Polychaeta | Nereis pelagica | |
| Mollusca | Gastropoda | Ocinebrina aciculata | |
| Echinodermata | Ophiuroidea | Ophiactis savignyi* | |
| Arthropoda | Malacostraca | Pagurus anachoretus | |
| Annelida | Polychaeta | Palola siciliensis | |
| Arthropoda | Malacostraca | Paractaea monodi | |
| Arthropoda | Malacostraca | Paradoxapseudes intermedius | + |
| Annelida | Polychaeta | Perinereis cultrifera | |
| Sipuncula | Phascolosomatidea | Phascolosoma (Phascolosoma) granulatum** | |
| Annelida | Polychaeta | Pholoe minuta | + |
| Arthropoda | Malacostraca | Pilumnus spinifer | |
| Annelida | Polychaeta | Platynereis dumerilii | |
| Annelida | Polychaeta | Pontogenia chrysocoma | |
| Annelida | Polychaeta | Psamathe fusca | |
| Annelida | Polychaeta | Pseudopotamilla reniformis | |
| Arthropoda | Malacostraca | Quadrimaera inaequipes | |
| Annelida | Polychaeta | Serpula concharum | |
| Annelida | Polychaeta | Serpula vermicularis | |
| Annelida | Polychaeta | Spirobranchus polytrema | |
| Annelida | Polychaeta | Spirobranchus triqueter | |
| Mollusca | Bivalvia | Striarca lactea | |
| Annelida | Polychaeta | Subadyte pellucida | |
| Annelida | Polychaeta | Syllis armillaris | |
| Annelida | Polychaeta | Syllis columbretensis | |
| Annelida | Polychaeta | Syllis gerlachi | |
| Annelida | Polychaeta | Syllis gracilis | |
| Annelida | Polychaeta | Syllis hyalina | |
| Annelida | Polychaeta | Syllis krohni | |
| Annelida | Polychaeta | Syllis variegata | |
| Arthropoda | Malacostraca | Synalpheus gambarelloides | |
| Arthropoda | Malacostraca | Tanais dulongii | + |
| Annelida | Polychaeta | Trypanosyllis zebra | |
| Annelida | Polychaeta | Vermiliopsis infundibulum | |
| Annelida | Polychaeta | Vermiliopsis monodiscus | |
| Annelida | Polychaeta | Vermiliopsis striaticeps |
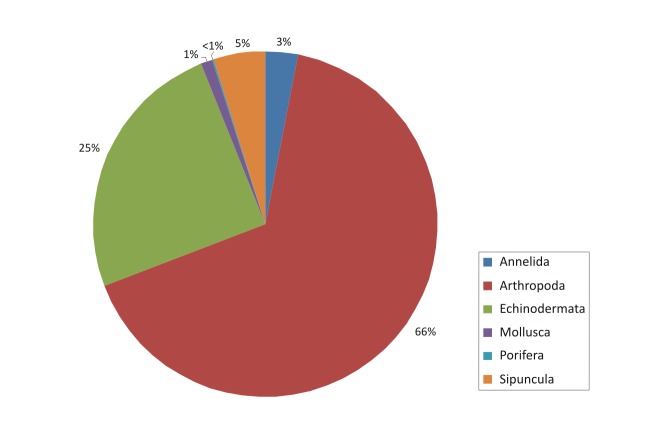
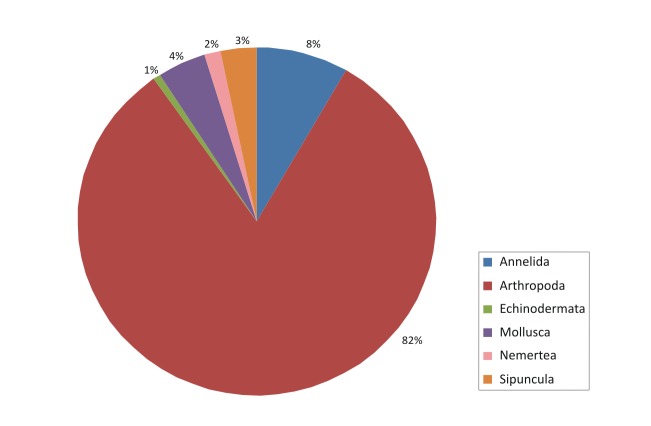
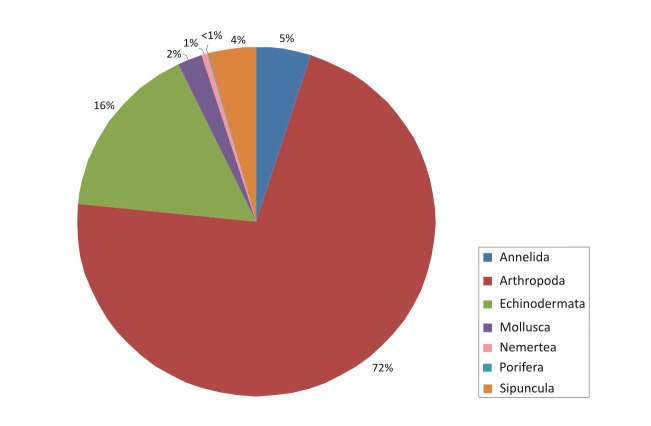
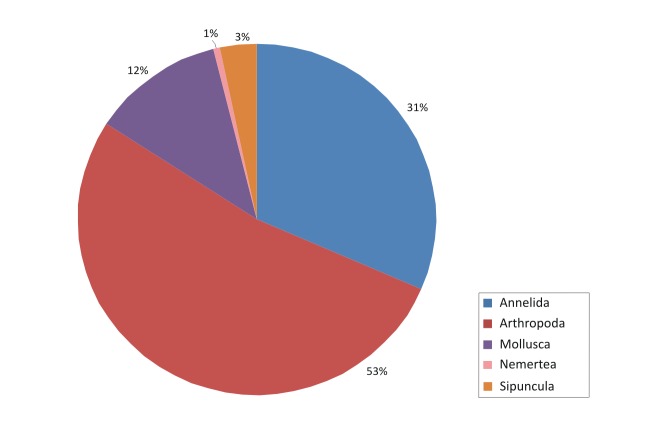
The contribution of the different phyla found in the samples is shown in the figures below (Figs 4, 5, 6, 7, 8, 9).
Figure 4.
Percentages of main phyla in the summer samples of Cyprus, as calculated based on the individual count of species per total sponge volume.
Figure 5.
Percentages of main phyla in the winter samples of Cyprus, as calculated based on the individual count of species per total sponge volume.
Figure 6.
Percentages of main phyla in all the samples of Cyprus, as calculated based on the individual count of species per total sponge volume.
Figure 7.
Percentages of main phyla in the summer samples of Greece, as calculated based on the individual count of species per total sponge volume.
Figure 8.
Percentages of main phyla in the winter samples of Greece, as calculated based on the individual count of species per total sponge volume.
Figure 9.
Percentages of main phyla in all the samples of Greece, as calculated based on the individual count of species per total sponge volume.
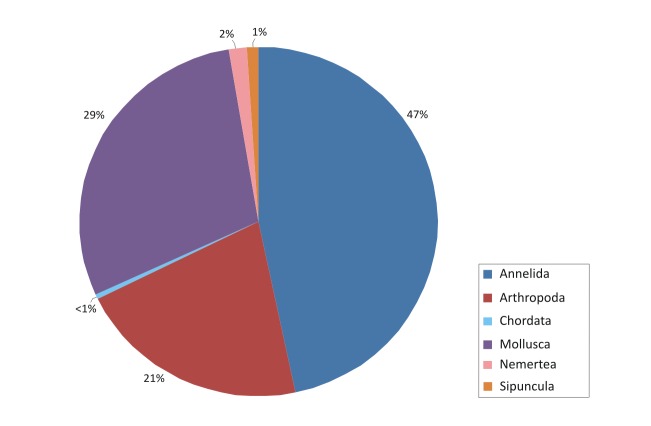
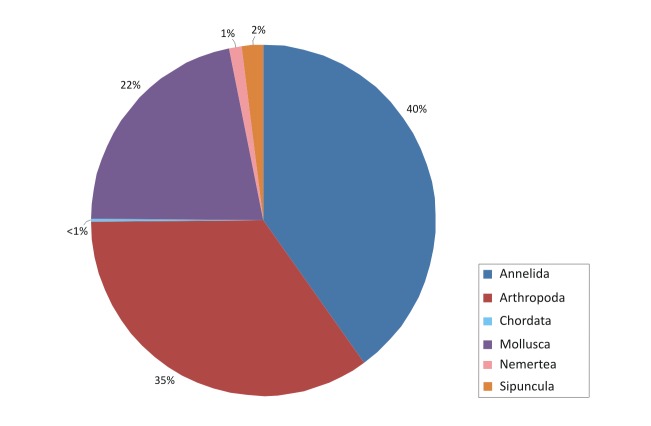
In Cyprus samples, Arthropoda was the phylum with the higher representation, both in summer (Fig. 4) and winter samples (Fig. 5). This was evident also when all the samples were included in the analysis, independent of season (Fig. 6). Interestingly, the increased abundance of Echinodermata in the summer (Fig. 4) was substantially reduced in the sponges collected during winter (Fig. 5).
However, the macrofaunal assemblage associated with the sponges collected from Greece was different; Arthropoda were still highly abundant, especially in the summer samples (Fig. 7), but other taxa such as Annelida and Mollusca showed also very high abundances and dominated the winter assemblages (Fig. 8). Overall, independently of season, the importance of the latter two phyla differentiated the sponges collected from the two locations (Fig. 9).
The aforementioned differences in the sponge associated macrofauna are also depicted on the MDS plot (Fig. 10), where it is apparent that the sponge samples cluster per country and season (ANOSIM: R = 0.811, p < 0.01).
Figure 10.
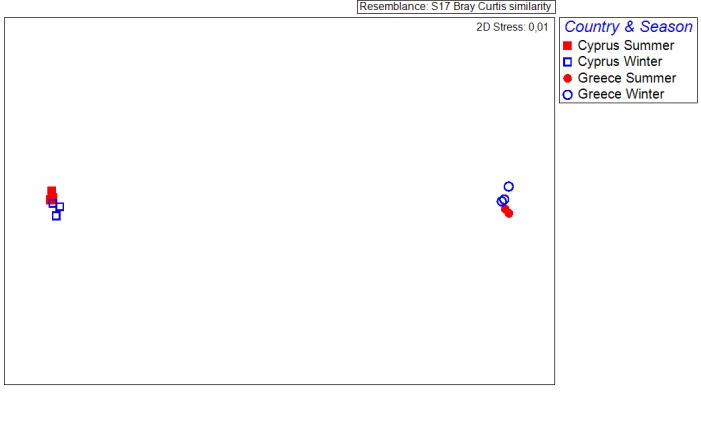
Multidimensional scaling of the sponge samples, based on the individual count of the associated species per sponge volume. Data labels according to the location of the sampling station and the sampling season.
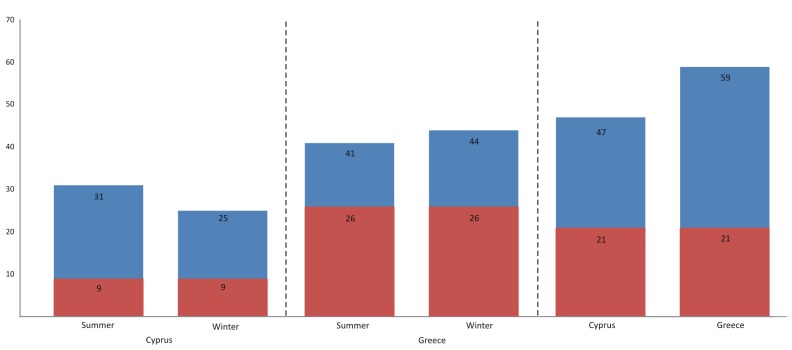
Only 9 macrofaunal species out of the 83 were found to exist in both winter and summer sponge samples from Cyprus. On the contrary, sponges from Greece had 26 species in common when the two seasons were compared. Overall, when the species lists from the sponges collected from Greece and Cyprus were compared, there were 21 species common for both locations (Fig. 11; Table 3).
Figure 11.
Total number of species (in blue) and common number of species (in red) for both locations and both sampling seasons.
Table 3.
Species found in common between the samples. *: non-indigenous species (ÖZTOPRAK et al. 2014, Pancucci-Papadopoulou et al. 2005). **: identification questionable for individuals from Cyprus (may be in fact Phascolosoma (Phascolosoma) stephensoni) (Açik 2014).
| Cyprus | Greece | Cyprus - Greece |
| Summer - Winter | Summer - Winter | |
| Cestopagurus timidus | Alpheus dentipes | Alpheus dentipes |
| Cymodoce spinosa | Amphitrite variabilis | Aspidosiphon (Aspidosiphon) muelleri muelleri |
| Elasmopus pocillimanus | Athanas nitescens | Bittium reticulatum |
| Leucothoe spinicarpa | Bittium reticulatum | Cestopagurus timidus |
| Microdeutopus bifidus | Ceratonereis (Composetia) costae | Colomastix pusilla |
| Ophiactis savignyi* | Ceratonereis (Composetia) hircinicola | Dipolydora armata |
| Phascolosoma (Phascolosoma) granulatum** | Cestopagurus timidus | Janira maculosa |
| Quadrimaera inaequipes | Colomastix pusilla | Leucothoe spinicarpa |
| Synalpheus gambarelloides | Harmothoe spinifera | Liljeborgia dellavallei |
| Hiatella arctica | Lysidice collaris | |
| Hippolyte leptocerus | Nemertea spp. | |
| Janira maculosa | Nereis pelagica | |
| Lepidasthenia elegans | Palola siciliensis | |
| Leucothoe spinicarpa | Paradoxapseudes intermedius | |
| Liljeborgia dellavallei | Phascolosoma (Phascolosoma) granulatum** | |
| Lumbrineris coccinea | Pseudopotamilla reniformis | |
| Lumbrineris latreilli | Quadrimaera inaequipes | |
| Lysidice collaris | Serpula vermicularis | |
| Nemertea spp. | Striarca lactea | |
| Palola siciliensis | Synalpheus gambarelloides | |
| Phascolosoma (Phascolosoma) granulatum | Vermiliopsis striaticeps | |
| Spirobranchus triqueter | ||
| Subadyte pellucida | ||
| Syllis gracilis | ||
| Synalpheus gambarelloides | ||
| Vermiliopsis monodiscus |
Temporal coverage
Notes
2003-01-01 / 2003-02-282003-07-01 / 2003-08-312007-08-01 / 2007-08-31
Usage rights
Use license
Creative Commons CCZero
Data resources
Data package title
Macrofaunal assemblages associated with the sponge Sarcotragus foetidus Schmidt, 1862 (Porifera: Demospongiae) at the coasts of Cyprus and Greece
Resource link
Number of data sets
1
Data set 1.
Data set name
Macrofaunal assemblages associated with the sponge Sarcotragus foetidus Schmidt, 1862 (Porifera: Demospongiae) at the coasts of Cyprus and Greece
Data format
Darwin Core Archive
Number of columns
33
Character set
UTF-8
Download URL
Description
The dataset is available via the MedOBIS (Mediterranean node of Ocean Biogeographic Information System) Internet Publishing Toolkit (IPT) of the Hellenic Centre for Marine Research (HCMR). The data will also be harvested by and made available through the European node of the Ocean Biogeographic Information System (EurOBIS), as well as through the International OBIS database. The dataset is available as a DarwinCoreArchive, all fields are mapped to DarwinCore terms.
This publication refers to the most recent version of the dataset available through the IPT server or MedOBIS. Future changes to the dataset due to quality control activities might change its content or structure.
Data set 1.
| Column label | Column description |
|---|---|
| recordNumber | A unique identifier for the record within the data set or collection |
| institutionCode | The name (or acronym) in use by the institution having custody of the object(s) or information referred to in the record |
| basisOfRecord | The specific nature of the data record, as described in http://terms.tdwg.org/wiki/dwc:basisOfRecord |
| individualCount | The number of individuals in a replicate sample unit |
| year | The sampling year |
| month | The sampling month |
| sampletrackcode | Denotes the code of each sample |
| fieldNumber | Denotes the code of each replicate unit |
| continent | The name of the continent in which the sampling location occurs |
| country | The name of the country in which the sampling location occurs |
| countryCode | The standard code of the country in which the sampling location occurs |
| locality | The specific location where the sample was taken |
| waterBody | The name of the water body in which the sampling location occurs |
| higherGeographyID | The id of the higher geography of the sampling location according to marineregions.org |
| minimumDepthInMeters | The lesser depth of a range of depth below the local surface, in meters |
| maximumDepthInMeters | The greater depth of a range of depth below the local surface, in meters |
| decimalLatitude | The geographic latitude (in decimal degrees, using the spatial reference system given in geodeticDatum) of the geographic center of a Location. Positive values are north of the Equator, negative values are south of it. Legal values lie between -90 and 90, inclusive |
| decimalLongitude | The geographic longitude (in decimal degrees, using the spatial reference system given in geodeticDatum) of the geographic center of a Location. Positive values are east of the Greenwich Meridian, negative values are west of it. Legal values lie between -180 and 180, inclusive |
| coordinateUncertaintyInMeters | The horizontal distance (in meters) from the given decimalLatitude and decimalLongitude describing the smallest circle containing the whole of the sampling location |
| samplingProtocol | The description of the method or protocol used for sample collection |
| taxonNameAsInFile | The scientific name of the taxon, as given by the data provider |
| scientificNameID | A unique identifier for each scientific name |
| scientificName | The accepted scientific name of the taxon, not including authorship |
| kingdom | The full scientific name of the kingdom in which the taxon is classified |
| phylum | The full scientific name of the phylum in which the taxon is classified |
| class | The full scientific name of the class in which the taxon is classified |
| order | The full scientific name of the order in which the taxon is classified |
| family | The full scientific name of the family in which the taxon is classified |
| genus | The full scientific name of the genus in which the taxon is classified |
| subgenus | The full scientific name of the subgenus in which the taxon is classified |
| specificEpithet | The species epithet of the scientificName |
| scientificNameAuthorship | The authorship information for the scientificName formatted according to the conventions of the applicable nomenclaturalCode |
| taxonID | Aphia ID for the accepted scientific names (Unique Identifier for the taxon within the World Register of Marine Species - www.marinespecies.org) |
Acknowledgements
This work was supported by the LifeWatchGreece infrastructure (MIS 384676), funded by the Greek Government under the General Secretariat of Research and Technology (GSRT), ESFRI Projects, National Strategic Reference Framework (NSRF). The authors would like to thank Professor Athanasios Koukouras for his supervision and comments during the Bachelor Thesis of Ms Christina Pavloudi. In addition, the authors would like to thank members of the Editorial Team of the LifeWatchGreece collection, and specifically Dr. Christos Arvanitidis, Dr. Eva Chatzinikolaou and Dr. Vasilis Gerovasileiou, for their valuable comments and suggestions.
References
- Açik Şermin. Checklist of Sipuncula from the coasts of Turkey. https://doi.org/10.3906/zoo-1405-74. Turkish Journal of Zoology. 2014;38:723–733. doi: 10.3906/zoo-1405-74. [DOI] [Google Scholar]
- Arndt W. Die Biologischen Beziehungen zwischen Schwämmen und Krebsen. Mitteilungen aus dem Zoologischen Museum in Berlin. 1933;19:221–305. [Google Scholar]
- Bacescu M. Les spongiaires; un des plus interessants biotopes benthiques marins. Rapport Commission international Mer Méditerranée. 1971;20(3):239–241. [Google Scholar]
- Christodoulou Magdalini, Paraskevopoulou Sofia, Syranidou Evdokia, Koukouras Athanasios. The amphipod (Crustacea: Peracarida) fauna of the Aegean Sea, and comparison with those of the neighbouring seas. https://doi.org/10.1017/s002531541200183x. Journal of the Marine Biological Association of the United Kingdom. 2013;93(5):1303–1327. doi: 10.1017/s002531541200183x. [DOI] [Google Scholar]
- Çinar Melih Ertan, Ergen Zeki. Polychaetes associated with the sponge Sarcotragus muscarum Schmidt, 1864 from the Turkish Aegean coast. https://doi.org/10.1080/00785236.1998.10426964. Ophelia. 1998;48(3):167–183. doi: 10.1080/00785236.1998.10426964. [DOI] [Google Scholar]
- Çinar Melih Ertan, Katağan Tuncer, Ergen Zeki, Sezgin Murat. Zoobenthos-inhabiting Sarcotragus muscarum (Porifera: Demospongiae) from the Aegean Sea. http://link.springer.com/article/10.1023%2FA%3A1021260314414. Hydrobiologia. 2002;482(1):107–117. doi: 10.1023/A:1021260314414. [DOI] [Google Scholar]
- Duineveld G. C.A, Tselepides A, Witbaard R, Bak R. P.M, Berghuis E. M, Nieuwland G, van der Weele J, Kok A. Benthic–pelagic coupling in the oligotrophic Cretan Sea. https://doi.org/10.1016/s0079-6611(00)00029-x. Progress in Oceanography. 2000;46:457–481. doi: 10.1016/s0079-6611(00)00029-x. [DOI] [Google Scholar]
- Fishelson L. Israel South Red Sea expedition, 1962, reports no. 20: Spirastrella inconstans Dendy (Porifera) as an ecological niche in the littoral zone of the Dahlak archipelago (Eritrea) Bulletin of the Sea Fisheries Research Station, Israel. 1966;41:17–25. [Google Scholar]
- Frith Dawn W. Animals associated with sponges at North Hayling. https://doi.org/10.1111/j.1096-3642.1976.tb01005.x. Zoological Journal of the Linnean Society. 1976;58(4):353–362. doi: 10.1111/j.1096-3642.1976.tb01005.x. [DOI] [Google Scholar]
- Ilan Micha, Ben-Eliahu M. Nechama, Galil Bella. Three deep water sponges from the Eastern Mediterranean and their associated fauna. https://doi.org/10.1080/00785326.1994.10429901. Ophelia. 1994;39(1):45–54. doi: 10.1080/00785326.1994.10429901. [DOI] [Google Scholar]
- Koukouras A, Voultsiadou-Koukoura E, Chintiroglou H, Dounas C. Benthic bionomy of the North Aegean Sea III. A comparison of the macrobenthic animal assemblages associated with seven sponge species. Cahiers de Biologie Marine. 1985;26:301–319. [Google Scholar]
- Koukouras A., Russo A., Voultsiadou-Koukoura E., Arvanitidis C., Stefanidou D. Macrofauna associated with sponge species of different morphology. https://doi.org/10.1111/j.1439-0485.1996.tb00418.x. Marine Ecology. 1996;17(4):569–582. doi: 10.1111/j.1439-0485.1996.tb00418.x. [DOI] [Google Scholar]
- Koukouras A., Russo A., Voultsiadou-Koukoura E., Dounas C., Chintiroglou C. Relationship of sponge macrofauna with the morphology of their hosts in the North Aegean Sea. https://doi.org/10.1002/iroh.19920770406. Internationale Revue der gesamten Hydrobiologie und Hydrographie. 1992;77(4):609–619. doi: 10.1002/iroh.19920770406. [DOI] [Google Scholar]
- Kyriakidis Phaedon C., Vasios George K., Kitsiou Dimitra. Proc. SPIE 9535; Third International Conference on Remote Sensing and Geoinformation of the Environment (RSCy2015), 95351W; 2015. 7. [DOI] [Google Scholar]
- Long E. R. The associates of four species of marine sponges of Oregon and Washington. Pacific Science. 1968;22:347–351. [Google Scholar]
- Manconi Renata, Cadeddu Barbara, Ledda Fabio, Pronzato Roberto. An overview of the Mediterranean cave-dwelling horny sponges (Porifera, Demospongiae) https://doi.org/10.3897/zookeys.281.4171. ZooKeys. 2013;281:1–68. doi: 10.3897/zookeys.281.4171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ÖZTOPRAK Başak, DOĞAN Alper, DAĞLI Ertan. Checklist of Echinodermata from the coasts of Turkey. https://doi.org/10.3906/zoo-1405-82. TURKISH JOURNAL OF ZOOLOGY. 2014;38:892–900. doi: 10.3906/zoo-1405-82. [DOI] [Google Scholar]
- Pancucci-Papadopoulou M. A., Zenetos A., Corsini-Foka M., Politou C. Y. Update Of Marine Alien Species In Hellenic Waters. https://doi.org/10.12681/mms.188. Mediterranean Marine Science. 2005;6(2):147–158. doi: 10.12681/mms.188. [DOI] [Google Scholar]
- Pansini M, Daglio S. Osservazioni sull’ inquilinismo di policheti erranti in alcune demospongie del litorale Ligure. Bollettino dei Musei e degli Istituti Biologici dell'Università di Genova. 1981;48-49:55–60. [Google Scholar]
- Pearse A. S. Inhabitants of certain sponges at Dry Tortugas. Papers from the Tortugas Laboratory of the Carnegie Institution of Washington. 1932;28:117–124. [Google Scholar]
- Pearse A. S. Notes on the inhabitants of certain sponges at Bimini. http://www.jstor.org/stable/1931369. Ecology. 1950;31(1):149–159. doi: 10.2307/1931369. [DOI] [Google Scholar]
- Rützler Klaus. Ecology of Tunisian commercial sponges. https://repository.si.edu/bitstream/handle/10088/7850/iz_Ruetzler_1976.pdf?sequence=1&isAllowed=y Tethys. 1976;7:249–264. [Google Scholar]
- Voultsiadou Eleni, Vafidis Dimitris. Marine invertebrate diversity in Aristotle’s zoology. http://www.repository.naturalis.nl/document/49637 Contributions to Zoology. 2007;76(2):103–120. [Google Scholar]