Abstract
Objective
Although β-blockers are an established therapy in heart failure (HF) guidelines, including for patients with chronic obstructive pulmonary disease (COPD), there remain concerns regarding bronchoconstriction even with cardioselective β-blockers. We wished to assess the real-life use of β-blockers for patients with HF and comorbid COPD.
Methods
We evaluated data from the Optimum Patient Care Research Database over a period of 1 year for co-prescribing of β-blockers with either an ACE inhibitor (ACEI) or angiotensin-2 receptor blocker (ARB) in patients with HF alone versus HF+COPD. Association with inhaler therapy was also evaluated.
Results
We identified 89 861 patients with COPD, 24 237 with HF and 10 853 with both conditions. In patients with HF+COPD, the mean age was 79 years; 60% were male, and 27% had prior myocardial infarction. Of patients with HF+COPD, 22% were taking a β-blocker in conjunction with either ACEI/ARB (n=2416) compared with 41% of patients with HF only (n=10 002) (adjusted OR 0.54, 95% CI 0.51 to 0.58, p<0.001). Among HF+COPD patients taking inhaled corticosteroid (ICS) with long-acting β-agonist (LABA) and long-acting muscarinic antagonist, 27% of patients were taking an ACEI/ARB with β-blockers (n=778) versus 46% taking an ACEI/ARB without β-blockers (n=1316). Corresponding figures for those patients taking ICS/LABA were 20% (n=583) versus 48% (n=1367), respectively.
Conclusions
These data indicate a substantial unmet need for patients with COPD who should be prescribed β-blockers more often for concomitant HF.
Introduction
Chronic obstructive pulmonary disease (COPD) is one of the world's leading causes of morbidity and mortality.1 2 Cardiovascular comorbidity is common in patients with COPD, at least partly due to the common risk factor of cigarette smoking. The prevalence of COPD in patients with heart failure (HF) ranges from 11%–52% to 9%–41% in North American and European patients, respectively.3 There is a need to evaluate the drugs used in the treatment of common comorbidities in COPD, in particular the putative effects of β-blockers on the cardiovascular burden and its associated impact on mortality4 5 β-blockers are established in HF management guidelines, which reinforce their use in patients with concomitant COPD.6 However, aside from the use of long-term oxygen therapy, there are no respiratory drugs that have been shown to have a significant impact on mortality in COPD.7
COPD management guidelines also state that the benefits of selective β-1 antagonists in HF outweigh potential risks, even in patients with severe COPD.7 Because of concerns regarding potential bronchoconstriction (especially in patients with more severe COPD), primary and secondary care physicians remain somewhat reticent to prescribe β-blockers in COPD. In a retrospective study of 1603 patients with COPD and a history of myocardial infarction (MI), 22% were prescribed a beta -blocker on admission to hospital and 55% of patients were not prescribed one β-blocker.8
Initiating treatment with β-blockers in HF and COPD is not simple. It requires careful initial dose titration over a period of weeks, along with monitoring of heart rate, supine and standing blood pressure and spirometry. Moreover, β-blockers tend to be less well tolerated in older patients with comorbidities, such as diabetes, peripheral vascular disease and renal impairment, who are more prone to postural hypotension. This may further compound the reluctance to prescribe β-blockers in HF and COPD, especially in a busy outpatient setting.
Retrospective observational data have shown putative beneficial effects of β-blockade in patients with COPD.9 A meta-analysis of 15 retrospective studies including 21 596 COPD patients shows pooled estimates for mortality reduction with β-blockers of 28% and for exacerbations, 38%.10 The mortality reduction was 36% in those patients with coronary heart disease and 26% in those with HF.
Despite compelling observational data supporting the use of β-blockers in COPD, there are to date no prospective long-term randomised trials looking at either mortality or exacerbations in patients with cardiovascular disease and COPD. We wished to evaluate real-life co-prescribing of β-blockers with either ACE inhibitors (ACEI) or angiotensin-2 receptor blockers (ARB) in patients with HF and COPD and their association with inhaler therapy. We report here what we believe to be the largest study to date, investigating the use of β-blockers in patients with COPD and HF managed in primary and/or secondary care.
Methods
The Optimum Patient Care Research Database (OPCRD) is a large, bespoke electronic database comprising anonymous, longitudinal medical record data for over 2.4 million patients from over 525 primary care practices across the UK. The OPCRD has received a favourable opinion from the Health Research Authority of the UK National Health Service for clinical research use (REC reference: 15/EM/0150) and is used frequently for pharmacoepidemiological studies. The study is registered at encepp.eu, reference: ENCEPP/SDPP/11387.
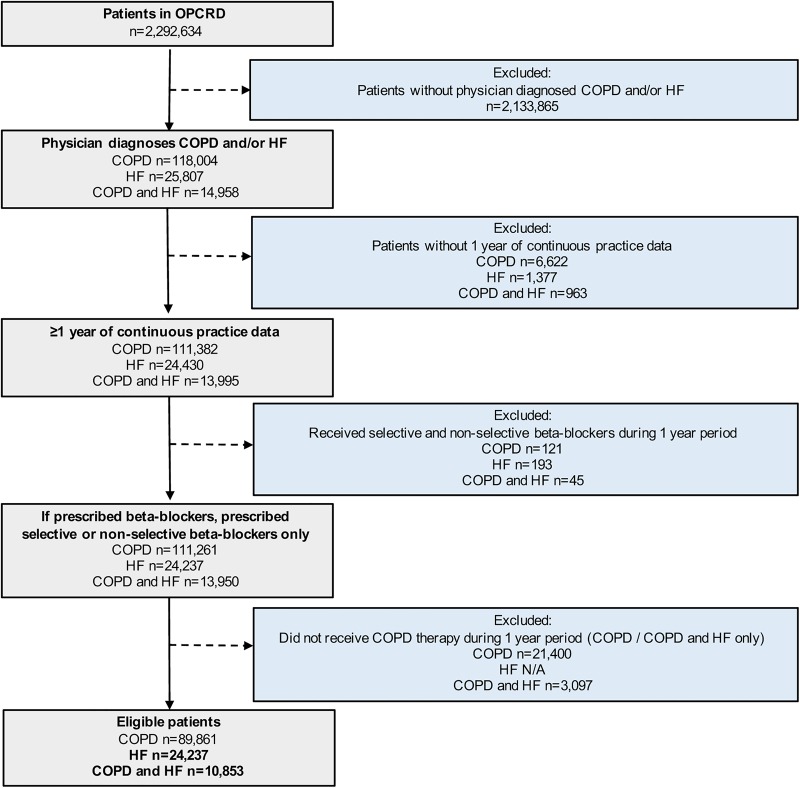
OPCRD was used to identify patients with quality outcome framework (QOF; the UK national quality improvement initiative and pay-for-performance scheme)11 diagnostic Read codes for COPD and/or HF who had at least 1 year of data available. We excluded any patients with COPD who did not receive any COPD therapy or who received both selective and non-selective β-blockers during their most recent year of data available from the database (figure 1). Patients with HF alone were compared with patients with both HF and COPD for prescriptions of ACEI/ARB and β-blockers, using the patient's most recent year of data from the database in the study period from 1 November 1988 to 1 August 2015 (1-year study period). The database was accessed on 24 September 2015. Analysis was also split by New York Heart Association (NYHA) classes 1–2 and 3–4. Furthermore, for patients with a diagnosis of both COPD and HF, the association of β-blocker use with inhaler therapy as a proxy for COPD disease severity, namely, dual therapy with inhaled corticosteroid (ICS) and long-acting β-agonist (LABA), triple therapy with the addition of long-acting muscarinic antagonist (LAMA), as well as short-acting β-agonist (SABA) or short-acting muscarinic antagonist (SAMA) were assessed. The proportion of patients using selective and non-selective β-blockers was assessed for the total population and comparative groups.
Figure 1.
Patient inclusion and exclusion criteria. COPD, chronic obstructive pulmonary disorder; HF, heart failure; OPCRD, Optimum Patient Care Research Database.
Data analysis
Age and year of study period were assessed at the end of the 1-year study period. HF and COPD treatments were assessed over the 1-year study period. Body mass index (BMI), forced expiratory volume in 1 s (FEV1), global initiative for chronic obstructive lung disease (GOLD) stage and NYHA class were assigned using the most recent value recorded in the database. Patients without a valid NYHA code were assigned a proxy NYHA class based on breathlessness coded in the database. Presence of prior MI or asthma diagnosis was assessed at any time during or prior to the period of study. Treatments for HF were compared between groups using logistic regression for unadjusted analyses and using a two-level generalised linear model with random intercepts for adjusted analyses accounting for clustering by general practice (GP). ORs, 95% CIs and p values are reported.
Analyses were conducted using IBM Statistical Package for the Social Sciences (SPSS) V.23 (SPSS Statistics; IBM, Somers, New York, USA) and Statistical Analysis System (SAS) V.9.3 (SAS Institute, Cary, North Carolina, USA).
Results
A total of 89 861 patients being treated for COPD, 24 237 with HF and 10 853 with both HF and COPD were identified. In patients who had HF and COPD, mean (SD) age was 79 (9) years; 60% were males, 27% had a prior MI and 32% had an asthma diagnosis. In those with HF alone, corresponding figures were 78 (12) years, 53% males, 30% prior MI and 26% asthma diagnosis. The median (IQR) of the study period for patients with HF and COPD was 2010 (2005–2013) and for patients with HF only was 2012 (2009–2014). Comparing patients with HF versus HF and COPD, respective mean (SD) values for BMI were 29 (6) versus 28 (6), and for FEV1% of predicted were 78% versus 57%.
Data for COPD severity grading in patients with HF and COPD comprised GOLD stage A (21%), B (25%), C (19%) and D (35%). Grading according to NYHA showed: 38% class 1, 26% class 2, 27% class 3 and 10% class 4 in patients with HF and COPD, with the corresponding figures in HF alone being 54%, 26%, 15% and 5%.
Of those taking β-blockers (irrespective of ACEI and ARB use; n=24 566), there were 21 926 (89%) patients who were receiving cardioselective β-blockers, the most common being bisoprolol (77%) and atenolol (16%), and 2640 (11%) receiving non-selective β-blockers, the most common being carvedilol (45%).
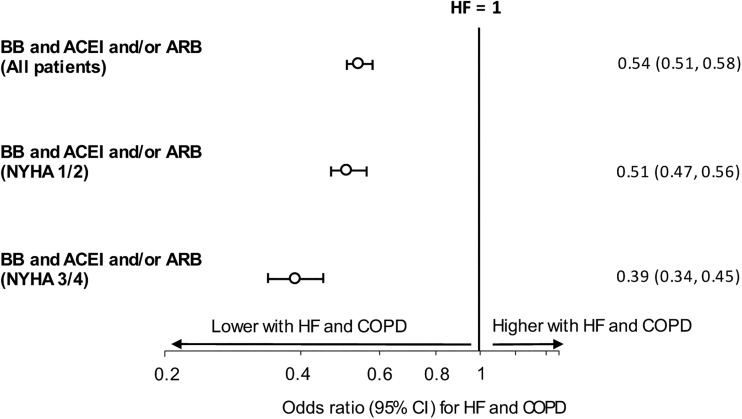
Data according to diagnosis and HF treatment are shown in table 1. Patients with HF and COPD were more likely to be taking an ACEI/ARB without a β-blocker than patients with HF alone (OR=2.18, 95% CI 2.08 to 2.29, p <0.001). After adjustment for confounders (year of study period, age, BMI, gender, prior MI, asthma diagnosis) and allowing for clustering by GP practice, patients with HF and COPD remained more likely to be taking ACEI/ARB without a β-blocker than patients with HF alone (OR=1.92, 95% CI 1.81 to 2.02, p<0.001). Those with HF and COPD were less likely to be prescribed a β-blocker in conjunction with either ACEI/ARB when compared with patients with HF alone (OR=0.41, 95% CI 0.39 to 0.43, p<0.001) (figure 2). Again, after adjustment for confounders (as above) and allowing for clustering by GP practice, patients with HF and COPD remained less likely to be prescribed a β-blocker in conjunction with either ACEI/ARB when compared with patients with HF alone (OR=0.54, 95% CI 0.51 to 0.58, p<0.001). Use of cardioselective β-blockers was similar in those with HF taking a β-blocker plus ACEI/ARB (n=9049 (90%)) and patients with HF and COPD who were taking a β-blocker plus ACEI/ARB (n=2191 (91%)).
Table 1.
HF Treatment According to Diagnosis
| HF treatment* | Total | ||||
|---|---|---|---|---|---|
| Diagnosis groups | None | ARB and/or ACEI | β-blocker only | β-blocker and ACEI and/or ARB | |
| COPD, n (%) | 55 581 (61.9) | 24 636 (27.4) | 3918 (4.4) | 5726 (6.4) | 89 861 (100) |
| HF, n (%) | 5709 (23.6) | 6576 (27.1) | 1950 (8.1) | 10 002 (41.3) | 24 237 (100) |
| COPD and HF, n (%) | 3019 (27.8) | 4864 (44.8) | 554 (5.1) | 2416 (22.3) | 10 853 (100) |
*Considering ARB, ACEI and β-blocker prescriptions only.
ACEI, ACE inhibitor; ARB, angiotensin-2 receptor blockers; COPD, chronic obstructive pulmonary disease; HF, heart failure.
Figure 2.
Prescription of β-blocker (BB) and ACEI (ACE inhibitor) or angiotensin-2 receptor blocker (ARB) for patients with heart failure (HF) alone versus patients with HF and chronic obstructive pulmonary disease (COPD)—showing data for all patients and split by New York Heart Association (NYHA) class. Data for adjusted ORs are shown with 95% CI.
At all levels of dyspnoea, patients with HF and COPD were less likely to be prescribed a β-blocker in combination with ACEI/ARB than those with HF alone, for NYHA classes 1/2 (OR=0.43, 95% CI 0.40 to 0.46, p<0.001) and for NHYA classes 3/4 (OR=0.36, 95% CI 0.32 to 0.41, p<0.001) (figure 2). After adjustment for confounders (year of study period, age, gender, prior MI, asthma diagnosis) and allowing for clustering by GP practice, patients with HF and COPD remained less likely to be prescribed a β-blocker in combination with ACEI/ARB than those with HF alone (for NYHA classes 1/2 and 3/4 respectively: OR=0.51, 95% CI 0.47 to 0.56, p<0.001; OR=0.39, 95% CI 0.34 to 0.45, p<0.001).
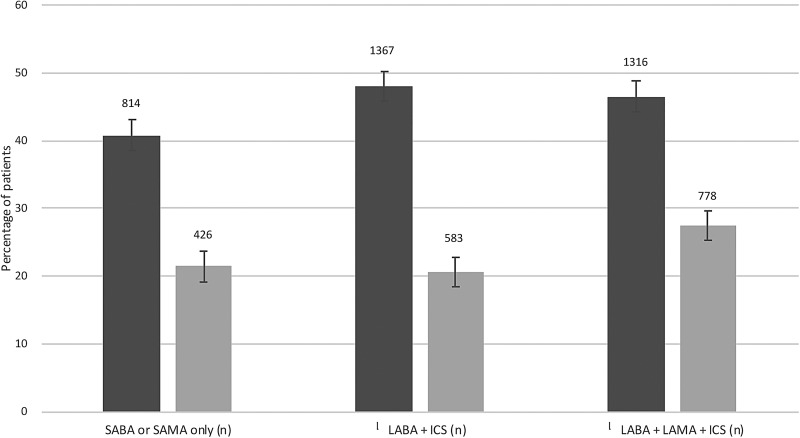
Data according to COPD inhaled therapy and HF treatment for those with diagnosis of both COPD and HF are shown in table 2 and figure 3. At all levels of COPD treatment intensity, use of ACEI/ARB with β-blocker was less likely than the use of ACEI/ARB without β-blocker in those with HF and COPD. In patients with HF and COPD on triple inhaler therapy with ICS/LABA/LAMA, there were 27% taking ACEI/ARB with β-blocker versus 46% taking ACEI/ARB without β-blocker. Corresponding figures for those patients on dual inhaler therapy with ICS/LABA were 20% versus 48%, and for those taking only SABA or SAMA, 21% versus 41%. Of patients who were taking a β-blocker plus ACEI/ARB, 696 (89%) were taking a cardioselective β-blocker and triple inhaler therapy, compared with 533 (91%) of patients on dual inhaler therapy and 395 (93%) taking only SABA or SAMA.
Table 2.
HF Treatment According to COPD Therapy for Patients With HF and COPD Diagnoses
| HF treatment | Total | ||||
|---|---|---|---|---|---|
| COPD therapy groups* for patients with COPD and HF | None | ARB and/or ACEI | β-blocker only | β-blocker and ACEI and/or ARB | |
| SABA or SAMA only, n (%) | 639 (32.1) | 814 (40.8) | 115 (5.8) | 426 (21.4) | 1994 (100) |
| LABA or LAMA, n (%) | 218 (21.0) | 407 (39.2) | 60 (5.8) | 354 (34.1) | 1039 (100) |
| LABA+ICS, n (%) | 757 (26.6) | 1367 (48.0) | 140 (4.9) | 583 (20.5) | 2847 (100) |
| LABA+LAMA, n (%) | 18 (17.1) | 43 (41.0) | 7 (6.7) | 37 (35.2) | 105 (100) |
| LAMA+ICS, n (%) | 26 (15.6) | 79 (47.3) | 6 (3.6) | 56 (33.5) | 167 (100) |
| LABA+LAMA+ICS, n (%) | 559 (19.7) | 1316 (46.5) | 180 (6.4) | 778 (27.5) | 2833 (100) |
| ICS only, n (%) | 754 (42.0) | 816 (45.5) | 45 (2.5) | 179 (10.0) | 1794 (100) |
*Considering SABA, SAMA, LABA, LAMA and ICS prescriptions only.
ACEI, ACE inhibitor; ARB, angiotensin-2 receptor blockers; COPD, chronic obstructive pulmonary disease; HF, heart failure; ICS, inhaled corticosteroid; LABA, long-acting β-agonist; LAMA, long-acting muscarinic antagonist; SABA, short-acting β-agonist; SAMA, short-acting muscarinic antagonist.
Figure 3.
Use of β-blockers in chronic obstructive pulmonary disease/heart failure according to inhaler therapy. Dark bars depict patients taking ACE inhibitor (ACEI)/ angiotensin-2 receptor blocker (ARB) without β-blockers, while lighter bars depict patients taking ACEI/ARB with β-blockers. The absolute numbers of patients are also shown. Error bars depict 95%CI. Also see table 2 for additional numerical data. ICS, inhaled corticosteroids; LABA, long-acting β-2 agonist; LAMA, long-acting muscarinic antagonist; SABA, short-acting β-2 agonist; SAMA, short-acting muscarinic antagonist.
Discussion
The results of the present study showed that the use of β-blockers in conjunction with ACEI/ARB was significantly lower in patients with HF and COPD than in patients with HF alone. Furthermore, in those patients with HF who had more severe concomitant COPD receiving ICS/LABA/LABA, the prescribing of β-blockers in conjunction with ACEI/ARB was 41% lower in comparison with prescribing of ACEI/ARB alone. In patients with moderately severe COPD receiving ICS/LABA, the use of β-blockers with ACEI/ARB was 58% lower, while in the patients with mildest COPD taking SABA/SAMA, its use was 49% lower. These findings highlight underuse of β-blockers in COPD for concomitant treatment of HF across a wide range of COPD disease severities. This is important in view of recent retrospective data which have demonstrated that the benefits of β-blockers in reducing exacerbations occur regardless of the severity of COPD.12 In a study looking at the use of ivabradine in HF,13 post hoc analysis revealed that use of β-blockers was lower in patients with HF and concomitant COPD, and that these patients had a worse prognosis.
Underuse of β-blockers in patients with HF and COPD appears to be related to severity of dyspnoea with the ORs for β-blocker use being lower for NHYA classes 3–4 compared with classes 1–2, while COPD severity appears not to be a deterrent to prescribing β-blockers for HF.
There is a reticence to prescribe β-blockers for patients with COPD who have coronary artery disease. Egred et al14 reported that 64% of patients without COPD and acute coronary syndrome (ACS) were prescribed β-blockers, while the prescription was only to 16% of patients with COPD/ACS. A diagnosis of COPD was documented as the reason for withholding β-blockers in 33% of patients not receiving β-blockers, and non-cardiologists were 40% less likely to prescribe β-blockers. Chen et al15 reported that after an acute MI, elderly patients were 62% less likely to be prescribed β-blockers if they had a history of treated COPD or asthma.
Bronchoconstriction from β-blocker use is due to antagonism of prejunctional and postjunctional β-2 receptors,16 which uncovers cholinergic tone resulting in airway constriction. Hence, LAMAs may serendipitously prevent bronchoconstriction induced by β-blocker.17 In this study, 89% of patients were taking cardioselective β-blockers, the most common being bisoprolol. One concern among prescribers is β-1 selective antagonists potentially exhibiting a small degree of dose-related β-2 receptor blockade.17 18 In a randomised controlled trial of 27 patients with HF who had concomitant moderate-to-severe COPD, there was a 190 mL significant difference in FEV1 between bisoprolol and placebo after 4 months of treatment.18 In patients with mild-to-moderate COPD, there was a significant increase in methacholine-induced airway hyper-reactivity after treatment with metoprolol or propranolol, but not celiprolol, in comparison with placebo.19 In 2712 patients with COPD who were followed over 4.3 years, there was no adverse effect of cardioselective β-blockade on either FEV1 or forced vital capacity, even in patients with the most severe COPD who were receiving inhaled triple therapy.9 In a meta-analysis of trials with cardioselective β-blockers, there was no significant change in FEV1 compared with placebo after a single or chronic dosing, while the FEV1 response to β-2 agonists was not affected.20 The β-blockers currently licensed for HF are the β-1 selective bisoprolol, nebivolol, metoprolol and the non-selective carvedilol. Gradually titrating up the dose of β-blocker to improve cardiovascular and pulmonary tolerability is important. Bisoprolol exhibits a higher in vitro β-1/2 receptor selectivity ratio (14:1) compared with atenolol (5:1) or metoprolol (2:1).21
We found that the use of non-selective β-blockers such as carvedilol was about 10%, irrespective of the presence of concomitant COPD. This can perhaps be explained by the reticence of clinicians to use non-selective β-blockers in general, along with a lack of evidence to support any difference in efficacy in HF compared with selective antagonists. Carvedilol blocks both cardiac β-1 and β-2 receptors and causes peripheral vasodilatation due to α-receptor antagonism. The antioxidant activity of carvedilol may explain why in one trial it was found to be superior to metoprolol in patients with HF.22 A 6-week study comparing bisoprolol, metoprolol and carvedilol in patients with COPD and HF showed that central augmented pressure (a measure of cardiac afterload) and levels of N-terminal brain natriuretic peptide (a marker of left ventricular function) were both lower with carvedilol and that it also reduced FEV1.23 However, in a post hoc analysis of 2670 patients with HF, there were no differences between selective and non-selective β-blockers in terms of lower mortality or rehospitalisation in patients with and without COPD.24 A small retrospective observational study from Japan showed the use of bisoprolol was associated with fewer exacerbations than carvedilol in patients with COPD and HF.25 Ivabradine may be used as an alternative to produce heart rate reduction in HF,26 but does not confer other beneficial properties of β-blockers.
It is also important to consider the potential impact of β-2 receptor genotype on the response to β-blockers in COPD. Patients with persistent asthma who possess one or two copies of the arginine-16 β-2 receptor polymorphism are more prone to propranolol-induced bronchoconstriction,27 while the same polymorphism confers a worse outcome on survival in patients who are receiving metoprolol post ACS.28 However, in another post hoc analysis of patients with HF treated with metoprolol or carvedilol, no association with survival was seen for the arginine-16 polymorphism.29
COPD and HF often coexist in patients, and indeed, both often coexist with other comorbidities. We hypothesised that the underuse of β-blockers in patients with comorbid COPD and HF may occur because doctors perceive that they will prevent drug interactions. Further research is required into other comorbid conditions that influence underuse of β-blockers.
Study limitations
While the findings reflect real-life prescribing patterns, and the data examined are from recent years, our data should be interpreted with some caution due to the retrospective nature of the analysis. For example, some patients were receiving ICS as monotherapy or in combination with a LAMA, though this is not approved for COPD. However, these findings are consistent with the results of prior research indicating that COPD is often not treated according to GOLD or UK national guidelines.30 The QOF codes for identifying patients with COPD and HF are reliant on the accuracy of initial diagnosis. Whether patients were prescribed β-blockers in a primary or secondary care setting, or indeed whether data originated from cardiology or pulmonology specialists could not be ascertained. The dataset does not contain any information on pulmonary function outcomes; hence, long-term safety remained unassessed.
As the study period spans 27 years from 1988 to 2015, we acknowledge that coding accuracy, policy and practice will have changed over this period, and as such we have adjusted for study period in the analyses where appropriate. However, in terms of comparison between comorbid COPD/HF and HF-alone groups, we do not expect any bias as the median (IQR) of the most recent data available for the two groups was similar: HF and COPD––2010 (2005–2013), and patients with HF only––2012 (2009–2014). Additionally, QOF was introduced in 2004 and <10% of patients from either cohort had their most recent data before 2004. The QOF Read codes, which are part of the UK national quality improvement initiative and pay-for-performance scheme, ensure good reporting of the chosen diseases, which includes COPD and HF.
Future studies should link prescribing of β-blockers with ACEI/ARB in patients with HF and COPD to outcomes such as mortality, hospital admissions or oral steroid courses. Although we did not have Read codes for aldosterone antagonists, these drugs are used as add-on therapy to ACEI to obviate aldosterone escape in NHYA class 3/4.
In summary, our findings suggest that β-blockers are currently being underused in patients with HF and COPD, irrespective of the amount of inhaler therapy. Further studies are required to investigate the reasons for these prescribing patterns and whether they might differ between cardiologists and pulmonologists.
Key messages.
What is already known on this subject?
Current guidelines recommend the use of cardioselective β-blockers for heart failure (HF) in patients with chronic obstructive pulmonary disease (COPD). However, primary and secondary care physicians still remain reticent to prescribe β-blockers because of concerns regarding potential bronchoconstriction, especially in more severe patients.
What might this study add?
The results of this study showed that the use of β-blockers in conjunction with ACE inhibitor (ACEI)/angiotensin-2 receptor blocker (ARB) was significantly lower in patients with HF and COPD than in patients with HF alone, irrespective of the severity of COPD.
How might this impact on clinical practice?
There is an unmet need in terms of underuse of β-blockers in patients with HF and COPD, in turn suggesting that prospective randomised controlled trials are required to determine the benefit–risk ratio of β-blockers in such patients.
Acknowledgments
The data in this article have been partly presented in preliminary abstract form as a poster at the winter meeting of the British Thoracic Society, London, 2 December 2015 (10.1136/thoraxjnl-2015-207770.161).
Footnotes
Contributors: BL had the original idea and wrote the initial draft of the manuscript. DBP had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. DS, GD, VT, JL, JM and VC contributed substantially to the study design, data analysis and interpretation and the writing of the manuscript. The data were provided through the Respiratory Effectiveness Group.
Competing interests: BL reports grants and personal fees from Chiesi, personal fees from Boerhingher Ingelheim, grants and personal fees from Meda, grants and personal fees from Teva, grants from Janssen, grants from AstraZeneca, grants from Roche, outside the submitted work; VT reports other from Cambridge Research Support, outside the submitted work; JL reports other from Observational and Pragmatic Research Institute, during the conduct of the study; JM reports other from Research in Real Life, outside the submitted work; DBP has board membership with Aerocrine, Almirall, Amgen, AstraZeneca plc, Boehringer Ingelheim, Chiesi, Meda, Mundipharma, Napp, Novartis International AG and Teva. Consultancy: A Almirall, Amgen, AstraZeneca plc, Boehringer Ingelheim, Chiesi, GlaxoSmithKline plc, Meda, Mundipharma, Napp, Novartis International AG, Pfizer and Teva; Grants and unrestricted funding for investigator-initiated studies from UK National Health Service, British Lung Foundation, Aerocrine, AKL, Almirall, AstraZeneca plc, Boehringer Ingelheim, Chiesi, Eli Lilly, GlaxoSmithKline plc, Meda, Merck & Co., Mundipharma, Napp, Novartis International AG, Orion, Pfizer, Respiratory Effectiveness Group, Takeda, Teva and Zentiva; Payments for lectures/speaking: Almirall, AstraZeneca plc, Boehringer Ingelheim, Chiesi, Cipla, GlaxoSmithKline plc, Kyorin, Meda, Merck & Co., Mundipharma, Novartis International AG, Pfizer, SkyePharma, Takeda and Teva; Payment for manuscript preparation: Mundipharma and Teva; Patents (planned, pending or issued): AKL; Payment for the development of educational materials: GlaxoSmithKline plc, Novartis International AG; Stock/Stock options: Shares in AKL which produces phytopharmaceuticals and owns 80% of Research in Real Life and its subsidiary social enterprise Optimum Patient Care; received payment for travel/accommodations/meeting expenses from Aerocrine, Boehringer Ingelheim, Mundipharma, Napp, Novartis International AG and Teva; Funding for patient enrolment or completion of research: Almirral, Chiesi, Teva and Zentiva; Peer reviewer for grant committees: Medical Research Council (2014), Efficacy and Mechanism Evaluation programme (2012), HTA (2014).
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Lozano R, Naghavi M, Foreman K, et al. . Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012;380:2095–128. 10.1016/S0140-6736(12)61728-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.López-Campos JL, Ruiz-Ramos M, Soriano JB. Mortality trends in chronic obstructive pulmonary disease in Europe, 1994–2010: a joinpoint regression analysis. Lancet Respir Med 2014;2:54–62. 10.1016/S2213-2600(13)70232-7 [DOI] [PubMed] [Google Scholar]
- 3.Hawkins NM, Petrie MC, Jhund PS, et al. . Heart failure and chronic obstructive pulmonary disease: diagnostic pitfalls and epidemiology. Eur J Heart Fail 2009;11:130–9. 10.1093/eurjhf/hfn013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Celli BR, Decramer M, Wedzicha JA, et al. . An official American thoracic society/european respiratory society statement: research questions in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2015;191:e4–27. 10.1164/rccm.201501-0044ST [DOI] [PubMed] [Google Scholar]
- 5.Bhatt SP, Dransfield MT. Chronic obstructive pulmonary disease and cardiovascular disease. Transl Res 2013;162:237–51. 10.1016/j.trsl.2013.05.001 [DOI] [PubMed] [Google Scholar]
- 6.McMurray JJ, Adamopoulos S, Anker SD, et al. . ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail 2012;14:803–69. [DOI] [PubMed] [Google Scholar]
- 7.Vestbo J, Hurd SS, Agustí AG, et al. . Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med 2013;187:347–65. 10.1164/rccm.201204-0596PP [DOI] [PubMed] [Google Scholar]
- 8.Quint JK, Herrett E, Bhaskaran K, et al. . Effect of β blockers on mortality after myocardial infarction in adults with COPD: population based cohort study of UK electronic healthcare records. BMJ 2013;347:f6650 10.1136/bmj.f6650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Short PM, Lipworth SI, Elder DH, et al. . Effect of beta blockers in treatment of chronic obstructive pulmonary disease: a retrospective cohort study. BMJ 2011;342:d2549 10.1136/bmj.d2549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Du Q, Sun Y, Ding N, et al. . Beta-blockers reduced the risk of mortality and exacerbation in patients with COPD: a meta-analysis of observational studies. PLoS One 2014;9:e113048 10.1371/journal.pone.0113048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.British Medical Association, NHS Employers. Quality and Outcomes Framework (QOF) guidance 2015-2016 (8th revision) 2015.
- 12.Bhatt SP, Wells JM, Kinney GL, et al. . β-Blockers are associated with a reduction in COPD exacerbations. Thorax 2016;71:8–14. 10.1136/thoraxjnl-2015-207251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tavazzi L, Swedberg K, Komajda M, et al. . Clinical profiles and outcomes in patients with chronic heart failure and chronic obstructive pulmonary disease: an efficacy and safety analysis of SHIFT study. Int J Cardiol 2013;170:182–8. 10.1016/j.ijcard.2013.10.068 [DOI] [PubMed] [Google Scholar]
- 14.Egred M, Shaw S, Mohammad B, et al. . Under-use of beta-blockers in patients with ischaemic heart disease and concomitant chronic obstructive pulmonary disease. QJM 2005;98:493–7. 10.1093/qjmed/hci080 [DOI] [PubMed] [Google Scholar]
- 15.Chen J, Radford MJ, Wang Y, et al. . Effectiveness of beta-blocker therapy after acute myocardial infarction in elderly patients with chronic obstructive pulmonary disease or asthma. J Am Coll Cardiol 2001;37:1950–6. 10.1016/S0735-1097(01)01225-6 [DOI] [PubMed] [Google Scholar]
- 16.Lipworth BJ, Williamson PA. Think the impossible: beta-blockers for treating asthma. Clin Sci (Lond) 2010;118:115–20. 10.1042/CS20090398 [DOI] [PubMed] [Google Scholar]
- 17.Short PM, Anderson WJ, Williamson PA, et al. . Effects of intravenous and oral β-blockade in persistent asthmatics controlled on inhaled corticosteroids. Heart 2014;100:219–23. 10.1136/heartjnl-2013-304769 [DOI] [PubMed] [Google Scholar]
- 18.Hawkins NM, MacDonald MR, Petrie MC, et al. . Bisoprolol in patients with heart failure and moderate to severe chronic obstructive pulmonary disease: a randomized controlled trial. Eur J Heart Fail 2009;11:684–90. 10.1093/eurjhf/hfp066 [DOI] [PubMed] [Google Scholar]
- 19.van der Woude HJ, Zaagsma J, Postma DS, et al. . Detrimental effects of beta-blockers in COPD: a concern for nonselective beta-blockers. Chest 2005;127:818–24. 10.1016/S0954-6111(03)00168-9 [DOI] [PubMed] [Google Scholar]
- 20.Salpeter SR, Ormiston TM, Salpeter EE, et al. . Cardioselective beta-blockers for chronic obstructive pulmonary disease: a meta-analysis. Respir Med 2003;97: 1094–101. 10.1016/S0954-6111(03)00168-9 [DOI] [PubMed] [Google Scholar]
- 21.Baker JG. The selectivity of beta-adrenoceptor antagonists at the human beta1, beta2 and beta3 adrenoceptors. Br J Pharmacol 2005;144:317–22. 10.1038/sj.bjp.0706048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.DiNicolantonio JJ, Fares H, Niazi AK, et al. . β-Blockers in hypertension, diabetes, heart failure and acute myocardial infarction: a review of the literature. Open Heart 2015;2:e000230 10.1136/openhrt-2014-000230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jabbour A, Macdonald PS, Keogh AM, et al. . Differences between beta-blockers in patients with chronic heart failure and chronic obstructive pulmonary disease: a randomized crossover trial. J Am Coll Cardiol 2010;55:1780–7. 10.1016/j.jacc.2010.01.024 [DOI] [PubMed] [Google Scholar]
- 24.Mentz RJ, Wojdyla D, Fiuzat M, et al. . Association of beta-blocker use and selectivity with outcomes in patients with heart failure and chronic obstructive pulmonary disease (from OPTIMIZE-HF). Am J Cardiol 2013;111:582–7. 10.1016/j.amjcard.2012.10.041 [DOI] [PubMed] [Google Scholar]
- 25.Kubota Y, Asai K, Furuse E, et al. . Impact of beta-blocker selectivity on long-term outcomes in congestive heart failure patients with chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis 2015:515–23. 10.1016/S0140-6736(10)61198-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Swedberg K, Komajda M, Böhm M, et al. . Ivabradine and outcomes in chronic heart failure (SHIFT): a randomised placebo-controlled study. Lancet 2010;376:875–85. 10.1016/S0140-6736(10)61198-1 [DOI] [PubMed] [Google Scholar]
- 27.Anderson WJ, Short PM, Manoharan A, et al. . Influence of β2-adrenoceptor 16 genotype on propranolol-induced bronchoconstriction in patients with persistent asthma. Ann Allergy Asthma Immunol 2014;112:475–6. 10.1016/j.anai.2014.02.016 [DOI] [PubMed] [Google Scholar]
- 28.Lanfear DE, Jones PG, Marsh S, et al. . Beta2-adrenergic receptor genotype and survival among patients receiving beta-blocker therapy after an acute coronary syndrome. JAMA 2005;294:1526–33. 10.1001/jama.294.12.1526 [DOI] [PubMed] [Google Scholar]
- 29.Sehnert AJ, Daniels SE, Elashoff M, et al. . Lack of association between adrenergic receptor genotypes and survival in heart failure patients treated with carvedilol or metoprolol. J Am Coll Cardiol 2008;52:644–51. 10.1016/j.jacc.2008.05.022 [DOI] [PubMed] [Google Scholar]
- 30.Price D, West D, Brusselle G, et al. . Management of COPD in the UK primary-care setting: an analysis of real-life prescribing patterns. Int J Chron Obstruct Pulmon Dis 2014;9:889–904. 10.2147/COPD.S62750 [DOI] [PMC free article] [PubMed] [Google Scholar]