Abstract
Objectives
Bacterial vaginosis (BV) is characterised by a change in the microbial composition of the vagina. The BV-associated organisms outnumber the health-associated Lactobacillus species and form a polymicrobial biofilm on the vaginal epithelium, possibly explaining the difficulties with antibiotic treatment. A better understanding of vaginal biofilm with emphasis on Atopobium vaginae and Gardnerella vaginalis may contribute to a better diagnosis and treatment of BV.
Methods
To this purpose, we evaluated the association between the presence of both bacteria by fluorescence in situ hybridisation (FISH) and BV by Nugent scoring in 463 vaginal slides of 120 participants participating in a clinical trial in Rwanda.
Results
A bacterial biofilm was detected in half of the samples using a universal bacterial probe. The biofilm contained A. vaginae in 54.1% and G. vaginalis in 82.0% of the samples. A. vaginae was accompanied by G. vaginalis in 99.5% of samples. The odds of having a Nugent score above 4 were increased for samples with dispersed G. vaginalis and/or A. vaginae present (OR 4.5; CI 2 to 10.3). The probability of having a high Nugent score was even higher when a combination of adherent G. vaginalis and dispersed A. vaginae was visualised (OR 75.6; CI 13.3 to 429.5) and highest when both bacteria were part of the biofilm (OR 119; CI 39.9 to 360.8).
Conclusions
Our study, although not comprehensive at studying the polymicrobial biofilm in BV, provided a strong indication towards the importance of A. vaginae and the symbiosis of A. vaginae and G. vaginalis in this biofilm.
Trial registration number
Keywords: BACTERIAL VAGINOSIS, DIAGNOSIS, GENITAL TRACT INFECT, MICROBIOLOGY, ANTIBIOTIC SENSITIVITY
Introduction
Bacterial vaginosis (BV) is the most prevalent vaginal disorder in women of reproductive age. It increases the risk of acquisition and transmission of sexually transmitted infections, including HIV, and is associated with preterm birth in pregnant women.1–3 The condition is characterised by a change in the microbial composition of the vagina: the Lactobacillus spp., associated with a healthy vaginal microbiome, are outnumbered by microaerophilic and anaerobic organisms, including Gardnerella vaginalis.3–7 The mere presence of G. vaginalis, however, is not sufficient for the diagnosis of BV using traditional diagnostic algorithms (see below) because many women without BV also have G. vaginalis in their vaginal microbiome.4 BV is, however, associated with high counts of G. vaginalis using molecular methods and/or the presence of a G. vaginalis-containing polymicrobial biofilm.4–10 Due to its strong adherence to vaginal epithelial cells and biofilm-forming capacities, it has been suggested that G. vaginalis initiates the colonisation of the vaginal epithelium and serves as a scaffolding to which other species subsequently can attach.10–12
One of the species that might attach to the biofilm initiated by G. vaginalis could be Atopobium vaginae.13–14 Several molecular studies have indicated a probable role for A. vaginae in BV,14–16 and it has also been suggested that A. vaginae plays a major part in the establishment of a biofilm, together with G. vaginalis.9 10 Considering it has been found in 80–90% of cases of relapse17 and some strains have been shown in vitro to be metronidazole resistant,18 it could be of importance in the recurrence of BV after standard treatment with metronidazole.
The current gold standard in BV research is the microscopic evaluation and scoring of vaginal slides according to Nugent.19 The diagnosis of BV is based on the absence of lactobacilli and the presence of small Gram-negative to Gram-variable rods (G. vaginalis and Bacteroides spp. morphotypes) and curved Gram-negative rods (Mobiluncus spp. morphotypes). In fact, bacterial biofilm can also be seen with this method in the form of clue cells, which are vaginal epithelial cells covered by layers of adherent Gram-negative and/or Gram-variable cells, that is, biofilms.20 Using Gram staining, it is impossible to distinguish between the different bacterial species in the biofilm. By labelling the cells with a fluorescent probe, using fluorescence in situ hybridisation (FISH), the structure and composition of the biofilm can be studied in more detail.
To study the potential role of A. vaginae and the synergy between A. vaginae and G. vaginalis in the biofilm, we used our newly developed peptide nucleic acid (PNA) A. vaginae probe11 together with an existing probe for G. vaginalis21 and a universal bacterial probe22 to investigate the composition of vaginal biofilm and its importance in BV.
Materials and methods
Clinical samples
Vaginal sample collection and preparation
Vaginal samples were collected from 120 women participating in a clinical trial at Rinda Ubuzima in Kigali, Rwanda, studying the safety and acceptability of a contraceptive vaginal ring (Nuvaring), including the effect of the vaginal ring on the vaginal microbiome (the Ring Plus study—Clinicaltrials.gov NCT01796613).23 Participants were between 18 and 35 years old and provided written informed consent for participation in the study. Depending on the group (continuous or intermittent ring use) to which the participant was randomised, a total of four or five samples from the same participant were taken over a period of four menstrual cycles. A total of 463 samples were analysed after Gram stain and after FISH using light microscopy and confocal laser scanning microscopy (CLSM), respectively.
Vaginal sampling was carried out by the study physician during a speculum examination in the Rinda Ubuzima research clinic. A cotton swab was brushed against the lateral walls of the vagina and was transported in its container to the Rinda Ubuzima laboratory within 20 min. Upon arrival in the laboratory, the swab was used to prepare a vaginal slide on a regular glass slide for Gram stain and a second vaginal slide on a Superfrost Plus slide (Menzel-Gläser, Braunschweig, Germany). All slides were air-dried, heat-fixed by passing through a flame twice and then stored in their appropriate boxes until Gram staining and/or shipment for FISH. The first slide was Gram stained and examined on-site in the Rinda Ubuzima laboratory in Kigali. The Superfrost Plus slides were stored and shipped at room temperature to the ITM where they were fixed for a minimum of 12 h in Carnoy solution (6:3:1, ethanol:chloroform:glacial acetic acid).11
Microbiological analysis of the vaginal samples
Peptide nucleic acid fluorescence in situ hybridisation
PNA FISH was performed as described earlier11 using species-specific probes for A. vaginae (AtoITM1) and G. vaginalis (Gard162) and the broad-range BacUni-1 probe. The hybridised samples were stored in the dark at room temperature for a maximum of 1 week before microscopic observation, using CLSM (LSM700, Zeiss, Oberkochen, Germany). Detection and identification of individual bacteria were done at 400× magnification (objective: Plan-Apochromat 40x/1.3 Oil Ph3 M27). Separate scattered bacterial cells were defined as dispersed bacteria. Aggregates of bacterial cells, sticking to the vaginal epithelial cells, were defined as adherent bacteria forming a biofilm. The species-specific signal was considered positive only if it had a positive counterpart in the 4',6-diamidino-2-phenylindole (DAPI) stain and if it displayed a positive signal simultaneously with the universal probe. Semi-quantification was done for the dispersed and adherent bacteria in three categories (absent, present in low amount, present in high amount), but for the analysis only two categories (absent or present) have been used.
Nugent score
The status of the vaginal microbiome was assessed at the Rinda Ubuzima laboratory by Nugent scoring of a Gram stained vaginal slide.19 A score of 0–3 was considered normal vaginal microbiome; a score of 4–6 intermediate microbiome and a score of 7–10 BV.
Statistical analysis
The clinical study sample size calculation was based on the primary objective to assess the pre–post changes in the vaginal microbiome and required 60 women in each group to require 95% power to detect clinically important changes in bacterial counts.23 Data analysis was done using STATA10 (StataCorp LP, Texas, USA). While the samples were collected longitudinally, they were analysed cross-sectionally, with each sample as the unit of analysis. To study the association between the presence and absence of dispersed and/or adherent A. vaginae and adherent G. vaginalis in relation to BV status, we categorised the samples into five categories (table 1) based on combinations of the presence of both bacteria in dispersed and/or adherent form as visualised by FISH. To increase the statistical power, we made the vaginal microbiome status binary: Nugent score 0–3 (reference group) versus Nugent score 4–10 (table 2). A mixed-effects logistic regression model was fitted with BV as the binary outcome (ie, Nugent 0–3 vs Nugent 4–10) and biofilm characteristics as the main dependent variable. The model was adjusted for woman, randomisation group and study visit, because multiple samples per woman at multiple study visits were included in the analysis. ORs are reported with 95% CI and the p values are from χ2 tests (table 2).
Table 1.
Gardnerella vaginalis, Atopobium vaginae and G. vaginalis with A. vaginae combinations for samples analysed with fluorescence in situ hybridisation (FISH) by absent, dispersed only and adherent ±dispersed category and stratified by Nugent scoring
| Total | Nugent 0–3 | Nugent 4–6 | Nugent 7–10 | |
|---|---|---|---|---|
| N | N (%) | N (%) | N (%) | |
| FISH all bacteria | ||||
| Absent | 0 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Dispersed only | 230 | 197 (76.0) | 19 (39.6) | 14 (9.0) |
| Adherent ± dispersed | 233 | 62 (24.0) | 29 (60.4) | 142 (91.0) |
| FISH A. vaginae (Av) | ||||
| Absent | 268 | 201 (77.6) | 24 (50.0) | 43 (27.6) |
| Dispersed only | 69 | 41 (15.8) | 10 (20.8) | 18 (11.5) |
| Adherent ± dispersed | 126 | 17 (6.6) | 14 (29.2) | 95 (60.9) |
| FISH G. vaginalis (Gv) | ||||
| Absent | 172 | 155 (59.8) | 8 (16.7) | 9 (5.8) |
| Dispersed only | 100 | 71 (27.4) | 15 (31.2) | 14 (9.0) |
| Adherent ± dispersed | 191 | 33 (12.8) | 25 (52.1) | 133 (85.2) |
| FISH Av and Gv combined | ||||
| Gv and Av absent | 170 | 153 (59.1) | 8 (16.7) | 9 (5.7) |
| Gv or Av dispersed only | 101 | 72 (27.8) | 15 (31.2) | 14 (9.0) |
| Gv adherent ± Gv dispersed and Av absent | 51 | 14 (5.4) | 8 (16.7) | 29 (18.6) |
| Gv adherent ± Gv dispersed and Av dispersed | 15 | 3 (1.1) | 3 (6.2) | 9 (5.8) |
| Gv and Av adherent ± Gv and Av dispersed | 126 | 17 (6.6) | 14 (29.2) | 95 (60.9) |
Table 2.
Association between the bacterial presence of Atopobium vaginae and Gardnerella vaginalis by fluorescence in situ hybridisation (FISH) and the vaginal microbiome defined by Nugent scoring
| G. vaginalis and A. vaginae combination | absent | G. vaginalis (Gv) or A. vaginae (Av) dispersed only | Gv adherent ± Gv dispersed and Av absent | Gv adherent ± Gv dispersed and Av dispersed only | Gv and Av adherent ± dispersed Gv and Av |
|---|---|---|---|---|---|
| Total=463 | 170 | 101 | 51 | 15 | 126 |
| Nugent 0–3 | 153 (90) | 72 (71.3) | 14 (27.5) | 3 (20) | 17 (13.5) |
| Nugent 4–10 | 17 (10) | 29 (28.7) | 37 (72.5) | 12 (80) | 109 (86.5) |
| OR (CI)* | Reference | 4.5 (2 to 10.3) | 49.2 (15.9 to 151.8) | 75.6 (13.3 to 429.5) | 119 (39.9 to 360.8) |
| p Value χ2 test* | 0.001 | <0.001 | <0.001 | <0.001 |
*The mixed-effects logistic regression model was adjusted for woman, randomisation group and visit.
Results
Characterisation of vaginal samples
In total, 463 of 527 samples from 120 women were available for FISH analysis, excluding 13 missing samples and 51 samples not readable due to the absence of epithelial cells on the slides. In all 463 samples, a positive signal was detected for the universal BacUni-1 probe. In 230 samples (49.7%), only dispersed bacteria were present, while the other 233 slides (50.3%) contained adherent bacteria as well (table 1). A. vaginae and G. vaginalis were part of this biofilm in 126 (54.1%) and 191 (82.0%) samples, respectively. Next, we visualised A. vaginae with FISH in 195 (42.1%) samples; in 69 samples (14.9% of the total 463 samples) A. vaginae was present in a dispersed state, whereas in 126 samples (27.2%) the A. vaginae bacteria were seen adherent to epithelial cells (table 1). For 122 (97.0%) of the samples with adherent A. vaginae, concurrent dispersed A. vaginae bacteria were observed. G. vaginalis was detected by FISH in 291 (62.9%) samples; it was detected as dispersed-only G. vaginalis in 100 samples (21.6% of the total 463 samples) and for the remaining 191 samples (41.3%) G. vaginalis was adherent to the epithelial cells. Furthermore, when combining the results of both bacteria and considering only the 291 G. vaginalis FISH-positive samples, A. vaginae was absent in 98 of the slides (33.7%). On the contrary, only two (0.5%) of the 195 samples showing A. vaginae (dispersed and/or adherent) with FISH were negative for G. vaginalis; this included one sample with adherent A. vaginae.
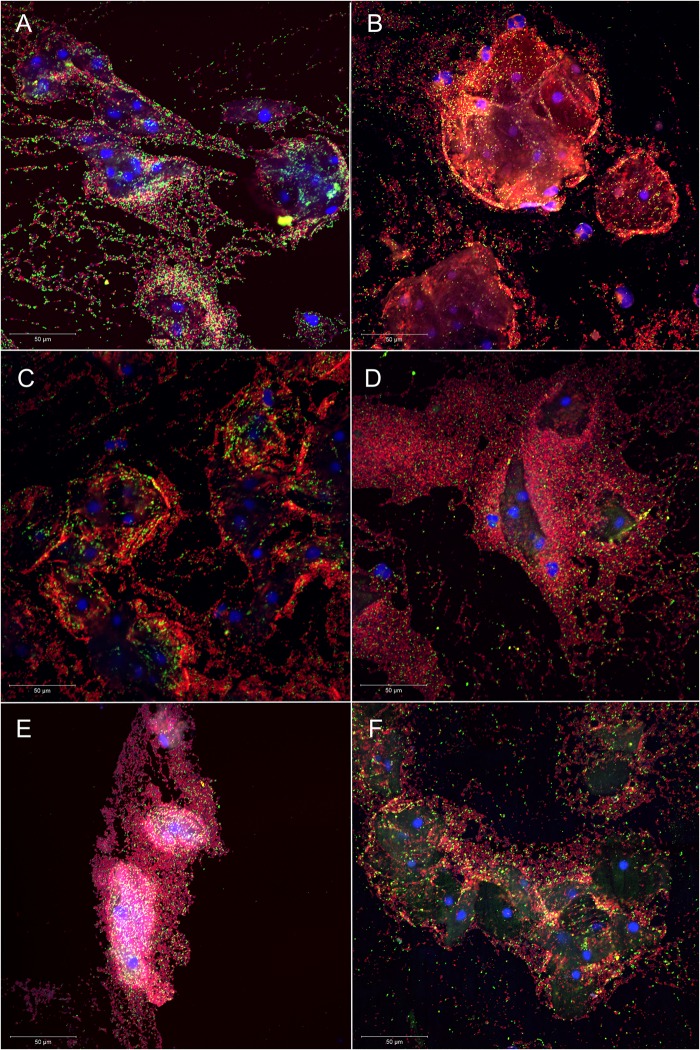
One-third of the vaginal samples (n=156; 33.7%) was classified as Nugent score 7–10, 10% as Nugent score 4–6 (n=48; 10.4%) and the remaining 259 samples (55.9%) as Nugent 0–3. The majority of the samples without A. vaginae (n=201; 75.0%) and without G. vaginalis (n=155; 90.1%) were categorised as Nugent 0–3, thus indicating a healthy microbiome. A BV microbiome, defined by a Nugent 7–10 category, was present in 75.4% of samples with adherent A. vaginae (n=95) and in 69.6% of the slides with adherent G. vaginalis (n=133). In case of absent G. vaginalis and A. vaginae by FISH (n=170, 36.7%), a healthy microbiome (Nugent 0–3) was observed for 90.0% of the 170 samples (n=153). Furthermore, when considering G. vaginalis and A. vaginae adherent samples only (n=126), 75.4% of the samples were categorised as BV (Nugent 7–10) (FISH experiments in figure 1; table 1).
Figure 1.
Superimposed confocal laser scanning images with 400× magnification of Atopobium vaginae+Gardnerella vaginalis biofilm in six vaginal samples (A–F): vaginal epithelial cells DAPI in blue, A. vaginae-specific peptide nucleic acid (PNA)-probe AtoITM1 with Alexa Fluor 488 in green and G. vaginalis-specific PNA-probe Gard162 with Alexa Fluor 647 in red. For clarity, we omitted the BacUni-1 plane, such that the bacteria that did not hybridise with Gard162 and AtoITM1 are visible in DAPI blue only.
The presence of A. vaginae, G. vaginalis and combinations of both bacteria in dispersed and adherent forms in relation to BV status
The group of FISH samples without A. vaginae and G. vaginalis was used as the reference group (table 2). Compared with this reference group, the odds of having a Nugent score of 4–10 were increased when one or both bacteria were present in the dispersed state without adhering to the vaginal epithelium (OR 4.5 (CI 2 to 10.3)); it was increased further when G. vaginalis was part of an adherent biofilm on the epithelium (OR 49.2 (CI 15.9 to 151.8)) and even more when dispersed A. vaginae accompanied this G. vaginalis biofilm (OR 75.6 (CI 13.3 to 429.5)); ultimately the OR was highest when A. vaginae was part of the G. vaginalis biofilm as well (OR 119 (CI 39.9 to 360.8)).
Discussion
We set out to study the potential role of A. vaginae in BV and the synergy between A. vaginae and G. vaginalis in the BV-associated biofilm.
Our study confirms that both A. vaginae and G. vaginalis are important constituents of the vaginal epithelial biofilm.9 11 Adherent A. vaginae and G. vaginalis were visualised in, respectively, 54.1% and 82.0% of samples with bacterial biofilm (detected using the universal BacUni-1 probe), suggesting an important role for both bacteria in this polymicrobial biofilm. Using FISH, we only found two samples containing A. vaginae (dispersed in both, adherent in one) in the absence of G. vaginalis, while more than one-third of the G. vaginalis-positive samples was negative for A. vaginae. This is in accordance with prior reports on the association of A. vaginae with G. vaginalis.9 11 15 16 24 We showed that the presence of both bacteria in the samples, regardless of their existence in a biofilm, was associated with an elevated or high Nugent score indicative for vaginal dysbiosis and BV. The highest probability of having a Nugent score higher than 3 was seen when both A. vaginae and G. vaginalis were part of a biofilm attaching to the vaginal epithelial cells.
The association of G. vaginalis with BV was originally described in 1954 by Gardner and Dukes.25 The involvement of A. vaginae in BV, however, has only been established 10 years ago.13–15 Swidsinski et al9 found vaginal biopsies with vaginal biofilm to be positive for G. vaginalis and A. vaginae when using fluorescent probes, although in our hands this A. vaginae probe cross-reacted with other vaginal species as well.11
The presence of A. vaginae in the BV-associated biofilm could have a major impact on treatment. Susceptibility to metronidazole, the standard treatment for BV, varied significantly across various A. vaginae strains in vitro.18 In vivo data are scarce, but Bradshaw et al17 found that rates of recurrence of BV were higher when A. vaginae was present in the vaginal microbiome in addition to G. vaginalis. In another study with topical metronidazole gel by Ferris et al,13 it was shown that a high concentration of A. vaginae before treatment was associated with complete or partial failure of treatment for BV. In the above studies, no distinction was made between dispersed and biofilm-associated bacteria. Nevertheless, as bacteria in a biofilm are less sensitive to antibiotic treatment26 and considering the evidence from our study that the formation of a bacterial biofilm is more likely to occur when A. vaginae is present in the vaginal microbiome, future design of studies may want to take this distinction into account when treating BV.
Our study has shed new light on the significance of A. vaginae and the synergy between A. vaginae and G. vaginalis in vaginal dysbiosis, using highly specific PNA probes for both bacteria. However, a limitation was that we used multiple samples from the 120 women of the Ring Plus study. Ideally, we should repeat the study in a larger group of women. Furthermore, although we assessed the association between bacterial biofilm and vaginal dysbiosis, more research is needed to unravel the exact mechanisms of biofilm formation in BV, including the role and the importance of both bacteria studied, to finally define improved regimens for treatment of BV. Moreover, since BV is a polymicrobial condition, new research should study the involvement of other bacteria related to BV.
In conclusion, the presented study uncovered a key piece of the BV puzzle confirming first, the importance of A. vaginae in BV-associated biofilm and second, showing the joint presence of A. vaginae and G. vaginalis in a biofilm. Future studies covering a wide array of BV-associated bacteria may help to further delineate biofilm mechanisms in BV.
Key messages.
This study shows that Atopobium vaginae is an important constituent of the vaginal biofilm, and is of relevance in the context of bacterial vaginosis (BV).
We show that A. vaginae is almost always accompanied by Gardnerella vaginalis in BV, but that G. vaginalis can be found without A. vaginae in the vaginal microbiome.
By tackling constituents of the biofilm, the above knowledge can contribute to a more effective and goal-oriented treatment and improve women's reproductive health.
Supplementary Material
Acknowledgments
We wish to thank the participants of this study, the study staff of Rinda Ubuzima and the STI reference laboratory team of ITM, Antwerp.
Footnotes
Handling editor: Jackie A Cassell
Contributors: All authors were involved in the main study that generated the data. For the present study, LH wrote the first draft of the manuscript. VJ, TC, MV and JvdW revised and edited the text. IDB, TC, LH, VJ and JvdW created the experimental design. SA, LM, VM and LH performed the testing and VJ and LH performed the data analysis. All authors revised and approved the present version of the manuscript.
Funding: This work has been funded by The European & Developing Countries Clinical Trials Partnership (EDCTP) through a project entitled ‘Preparing for clinical trials with vaginal rings that protect women from HIV and unintended pregnancy’ (grant code SP.2011.41304.043); the University of Liverpool and the ITM.
Competing interests: None declared.
Patient consent: Obtained.
Ethics approval: The Ring Plus study was approved by the Rwanda National Ethics Committee, Rwanda (Approval number 481/RNEC/2013); the ethics committees of the Institute of Tropical Medicine (ITM), Belgium (Approval number 864/13); the Antwerp University Hospital, Belgium (Approval number 13/7/85) and the University of Liverpool, UK (Approval number RETG000639IREC).
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: The database relevant to the study has been made available to all collaborators.
References
- 1.Eschenbach DA. History and review of bacterial vaginosis. Am J Obstet Gynecol 1993;169:441–5. 10.1016/0002-9378(93)90337-I [DOI] [PubMed] [Google Scholar]
- 2.Hillier SL, Marrazzo JM, Holmes KK. Bacterial vaginosis. In: Holmes KK, Sparling PF, Mardh PA, et al., eds Sexually transmitted diseases. New York: McGraw-Hill, 2008;737–68. [Google Scholar]
- 3.Martin HL, Richardson BA, Nyange PM, et al. Vaginal lactobacilli, microbial flora, and risk of human immunodeficiency virus type 1 and sexually transmitted disease acquisition. J Infect Dis 1999;180:1863–8. 10.1086/315127 [DOI] [PubMed] [Google Scholar]
- 4.van de Wijgert JH, Borgdorff H, Verhelst R, et al. The vaginal microbiota: what have we learned after a decade of molecular characterization? PLoS ONE 2014;9:e105998 10.1371/journal.pone.0105998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Srinivasan S, Hoffman NG, Morgan MT, et al. Bacterial communities in women with bacterial vaginosis: high resolution phylogenetic analyses reveal relationships of microbiota to clinical criteria. PLoS ONE 2012;7:e37818 10.1371/journal.pone.0037818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Patterson JL, Stull-Lane A, Girerd PH, et al. Analysis of adherence, biofilm formation and cytotoxicity suggests a greater virulence potential of Gardnerella vaginalis relative to other bacterial-vaginosis-associated anaerobes. Microbiology 2010;156:392–9. 10.1099/mic.0.034280-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jespers V, van de Wijgert J, Cools P, et al. The significance of Lactobacillus crispatus and L. vaginalis for vaginal health and the negative effect of recent sex : a cross-sectional descriptive study across groups of African women. BMC Infect Dis 2015;15:1–14. 10.1186/s12879-014-0722-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jespers V, Crucitti T, van de Wijgert JH, et al. A DNA tool for early detection of vaginal dysbiosis in African women. Res Microbiol 2016;167:133–41. 10.1016/j.resmic.2015.10.006 [DOI] [PubMed] [Google Scholar]
- 9.Swidsinski A, Mendling W, Loening-Baucke V, et al. Adherent biofilms in bacterial vaginosis. Obstet Gynecol 2005;106:1013–23. 10.1097/01.AOG.0000183594.45524.d2 [DOI] [PubMed] [Google Scholar]
- 10.Swidsinski A, Loening-Baucke V, Mendling W, et al. Infection through structured polymicrobial Gardnerella biofilms (StPM-GB). Histol Histopathol 2014;29:567–87. [DOI] [PubMed] [Google Scholar]
- 11.Hardy L, Jespers V, Dahchour N, et al. Unravelling the bacterial vaginosis-associated biofilm: a multiplex Gardnerella vaginalis and Atopobium vaginae fluorescence in situ hybridization assay using peptide nucleic acid probes. PLoS ONE 2015;10:e0136658 10.1371/journal.pone.0136658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Machado A, Cerca N. The influence of biofilm formation by Gardnerella vaginalis and other anaerobes on bacterial vaginosis. J Infect Dis 2015;2015;212:1856–61. 10.1093/infdis/jiv338 [DOI] [PubMed] [Google Scholar]
- 13.Ferris MJ, Masztal A, Aldridge KE, et al. Association of Atopobium vaginae, a recently described metronidazole resistant anaerobe, with bacterial vaginosis. BMC Infect Dis 2004;4:5 10.1186/1471-2334-4-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burton JP, Devillard E, Cadieux PA, et al. Detection of Atopobium vaginae in postmenopausal women by cultivation-independent methods warrants further investigation. J Clin Microbiol 2004;42:1829–31. 10.1128/JCM.42.4.1829-1831.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Verstraelen H, Verhelst R, Claeys G, et al. Culture-independent analysis of vaginal microflora: the unrecognized association of Atopobium vaginae with bacterial vaginosis. Am J Obstet Gynecol 2004;191:1130–2. 10.1016/j.ajog.2004.04.013 [DOI] [PubMed] [Google Scholar]
- 16.Menard JP, Fenollar F, Henry M, et al. Molecular quantification of Gardnerella vaginalis and Atopobium vaginae loads to predict bacterial vaginosis. Clin Infect Dis 2008;47:33–43. 10.1086/588661 [DOI] [PubMed] [Google Scholar]
- 17.Bradshaw CS, Tabrizi SN, Fairley CK, et al. The association of Atopobium vaginae and Gardnerella vaginalis with bacterial vaginosis and recurrence after oral metronidazole therapy. J Infect Dis 2006;194:828–36. 10.1086/506621 [DOI] [PubMed] [Google Scholar]
- 18.De Backer E, Verhelst R, Verstraelen H, et al. Antibiotic susceptibility of Atopobium vaginae. BMC Infect Dis 2006;6:51 10.1186/1471-2334-6-51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nugent RP, Krohn MA, Hillier SL. Reliability of diagnosing bacterial vaginosis is improved by a standardized method of gram stain interpretation. J Clin Microbiol 1991;29:297–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cook RL, Reid G, Pond DG, et al. Clue cells in bacterial vaginosis: immunofluorescent identification of the adherent gram-negative bacteria as Gardnerella vaginalis. J Infect Dis 1989;160:490–6. 10.1093/infdis/160.3.490 [DOI] [PubMed] [Google Scholar]
- 21.Machado A, Almeida C, Salgueiro D, et al. Fluorescence in situ hybridization method using peptide nucleic acid probes for rapid detection of Lactobacillus and Gardnerella spp. BMC Microbiol 2013;13:82 10.1186/1471-2180-13-82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Perry-O'Keefe H, Stender H, Broomer A, et al. Filter-based PNA in situ hybridization for rapid detection, identification and enumeration of specific micro-organisms. J Appl Microbiol 2001;90:180–9. 10.1046/j.1365-2672.2001.01230.x [DOI] [PubMed] [Google Scholar]
- 23.Schurmans C, De Baetselier I, Kestelyn E, et al. The ring plus project: safety and acceptability of vaginal rings that protect women from unintented pregnancy. BMC Public Health 2015;15:348 10.1186/s12889-015-1680-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Verhelst R, Verstraelen H, Claeys G, et al. Cloning of 16S rRNA genes amplified from normal and disturbed vaginal microflora suggests a strong association between Atopobium vaginae, Gardnerella vaginalis and bacterial vaginosis. BMC Microbiol 2004;4:16 10.1186/1471-2180-4-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gardner HL, Dukes CD. New etiologic agent in nonspecific bacterial vaginitis. Science 1954;120:853 10.1126/science.120.3125.853 [DOI] [PubMed] [Google Scholar]
- 26.Høiby N, Bjarnsholt T, Moser C, et al. ESCMID guideline for the diagnosis and treatment of biofilm infections 2014. Clin Microbiol Infect 2015;21(Suppl 1):S1–25. 10.1016/j.cmi.2014.10.024 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.