Abstract
Cervical spondylotic myelopathy is a well-known cause of disability among older people. A significant amount of these patients is asymptomatic. Once the symptoms start, the worsening may follow a progressive manner. We should suspect of spondylotic myelopathy in any individual over 55 years presenting progressive changes in gait or losing fine motor control of the upper limbs. Despite its frequent prevalence, this condition is still neglected and many times confused with other supratentorial lesions regarding diagnostic. Here we address some of most important aspects of this disease, calling attention to pathophysiology, the natural history, presentation, differential diagnosis, clinical assessment, and treatment.
Key words: Spondylosis, Cervical spondylotic myelopathy, Spastic paraparesis
Introduction
Cervical spondylotic myelopathy (CSM) is the leading cause of myelopathy in subjects above 55 years old and the major cause of spasticity acquired in the aged population.1,2 More than 50% of middle-aged people have radiographic evidence of cervical alteration, yet only 10% have symptoms of spinal cord compression or cervical radiculopathy.3
The cervical myelopathy syndrome was first described in 1952, in some cases performed by Brain and colleagues.4 The primary pathophysiological changing is the reduction of the sagittal diameter of the spinal canal. Static and dynamic factors are responsible for the narrowed canal with spinal cord compression. This process might also occur secondary to ischemic damage, contributing to additional spine cord injury.1
Regarding the clinical onset, a large number of patients with cervical myelopathy are asymptomatic at first, but once the symptoms start, most present in a stepwise manner, with periods of stability of the symptoms, alternating with worsening.5
Clinically, the most characteristic symptoms of the CSM are the instability of gait, loss of fine motor control of the upper limbs, weakness, and neck pain with reduced range of motion in this region and urinary emergency.1 Mostly, the diagnosis of CSM is based on the signals observed in the clinical examination supported by radiological studies showing spinal cord compression. However, there is wide variation in diagnosis and symptoms presented by patients suffering criteria.6 A wide variety of clinical presentation, multiple treatments available and different backgrounds and diagnostic skills of orthopedists, neurologists, neurosurgeons, physiatrists and physical therapists, makes it difficult to approach universally. Still, despite its general prevalence, many patients with CSM are followed only by neurosurgeons or orthopedists, instead of neurologists. Thus, many neurologists are not entirely familiar with this condition in their differential diagnosis in the clinical practice. Thus, some aspects from this condition, such as pathophysiology, natural history, presentation, clinical evaluation, differential diagnosis, and treatment are mentioned here.
Pathophysiology
From the anatomic standpoint, the average normal space of the cervical spinal canal is 17 to 18 mm, ranging from 13 to 20 mm. Sizes less than 12 mm are related to increasing in determining myelopathy. Occurring as a normal process associated with the aging of the spine, disc degeneration happens initially as a consequence of disc dehydration. Due to this, several mechanisms occur, resulting in disc collapse and decrease of disc’s height.7 As a consequence, the endplates may suffer mechanical compression, determining the formation of osteophytic bars by subperiosteal bone appearance. Since it does occur disc and facet joints degeneration, such mechanism may cause spine damage in both static and dynamic situations.8 Besides, hypertrophy of flavum ligament, which occurs as a consequence of age, might determine additional spinal stenosis.9,10 As a result, any flexion or extension of cervical spine will determine worsening of spinal stenosis, establishing intermittent compression of the spinal cord. Altogether, in a long term, this mechanism will be responsible for causing additional neurological deficits.11 In addition to the previously mentioned mechanism, spinal cord ischemia might develop secondarily, leading to an additional risk of neurological disability in those patients.12,13
Interestingly, it seems to have a predilection for the spinal level insult according to the age. The spondylosis in middle-aged individuals occurs more often in C5/C6 and C6/C7 levels. Conversely, in the elderly it occurs more often at levels of C3/C4 and C4/C5. It is speculated to occur spondylosis in the elderly at lower levels when they are younger. Due to that, less mobility of these levels after aging happens, with subsequent increase of motion at upper levels, causing spondylosis at the senescence.14,15
Not surprisingly, a significant factor associated with CSM is smoking, since it is well known by its influence on vertebral bone metabolism. In an interesting paper published by Oda and colleagues, they performed an experimental assessment in a rat-smoking model, searching for the relationship between degeneration of intervertebral discs and smoking. They found that smoking increased the production and release of inflammatory cytokines, determining decomposition of chondrocyte activity and as a result, disc degeneration.16 Other factors related to CSM are the presence of Down syndrome, repeated occupational trauma, genetic predisposition and Klippel-Feil syndrome.17-20 Noteworthy is also the presence of calcification of the posterior longitudinal ligament, complete or incomplete, present in 2% of Japanese and 25% of patients with CSM and due to mutations of inorganic pirofasfatase, which controls calcium metabolism from soft tissues.5 Furthermore, flavum ligament can also calcify, providing posterior spinal cord compression.5,21
Additionally, the narrowing of the spinal canal, associated with spondylosis may lead to compression anterior spinal artery, posterior or radicular arteries, contributing to the myelopathy through both an ischemic and venous congestion mechanism. Thus, microscopic changes in pial vessels might be seen due to mechanical compression. As well known, oligodendrocytes are responsible for myelination of long intramedullary tracts. Due to its high metabolic rate, these cells would be more susceptible to apoptosis secondary to damage vascular, resulting in demyelination of the corticospinal fibers.21-23
In summary, static, dynamic and vascular mechanisms might play a role in spinal cord injury. Flexion and extension of the cervical spine with anterior and posterior compression from the spinal ligaments and bone also spurs contribute to the occurrence of myelopathy. In this case, bending or sudden extension, such as for automobile injuries whip can cause acute quadriplegia or central cord syndrome.3
Natural history
Despite the paucity of papers regarding this subject, some reports have been correlating the progressive nature of this condition and a lack of full neurological recovery after surgical procedure.13,24-26
Initially consisting in gait abnormality as the earliest manifestation, to the best of our knowledge, regression of any of symptoms was never described. Since the symptoms appear in a progressive way and many times are subtle, takes a time to be diagnosed.27
Some papers have been showing that natural history of these patients consists in an acute worsening, which is followed by a stable phase that may last years, without any worsening. Still, older patients and some patients who have moderate deficits seem to deteriorate more often justifying; though, a surgical procedure in these patients.22,28 Putting aside the disability when presented, CSM is a known risk factor for developing spinal cord lesion secondary to a minor trauma. For this reason, some authors also indicate surgery even in those patients.13,29
Despite all above mentioned, no randomized controlled studies that fulfilled rigid criteria for surgical indication were performed so far. Therefore, there is still not strong evidence that supports surgery in those patients. However, many authors do agree in perform surgery in those patients in real life, mainly in those who are declining neurologically.13,30,31
Presentation
Regarding outcome, CSM may present in three different manners. In the most common one, 70% of individuals have a stepwise deteriorating, with periods of worsening alternated with periods of stabilization. In the second type, 20% experience the progressive evolution of symptoms in a direct way, and lastly 5% of subjects have rapidly evolving progressive disease.17
It should be suspected of cervical myelopathy in any patient with difficulty for walking with an unsteady gait, many times presenting as a spastic characteristic, due to compression of the corticospinal tract.32 Concomitant presence of changes in the upper limbs, such as weakness, numbness or loss of manual skills (such as writing), associated with gait changes, should further increase the degree of suspicion of CSM.6
Clinical examination may reveal the presence of a bilateral (although asymmetric) impairment in lower limbs, with or without spastic hypertonia, Babinski’s sign, clonus, paresis and proprioceptive loss.33 Iliopsoas and quadriceps femurs are the most affected muscle concerning motor weakness, with distal muscles being affected less commonly.34
Additionally, upper limbs may be affected unilaterally or bilaterally, with atrophy of interosseous and thenar muscle along with abolition of the tendinous reflexes, if the compression occurs below C5 level. On the contrary, when the compression takes place above C5, Hoffman signal may appear, and tendinous reflexes are increased. In advanced cases, there is a loss of sphincter control, with frequency, urinary urgency, and urinary hesitancy.22,33
A valuable deep tendon reflex that helps to localize the lesion in the upper cervical spine (C2-C4) is the pectoralis muscle reflex. Once hyperactive, it might differentiate compressions occurring at this level from the lower ones.7,13 Another useful deep tendon reflex is the jaw jerk reflex, which may represent a lesion in any site above the cervical spine. Therefore, if diffuse increased reflex is present, the absence of jaw jerk reflex can rule out lesions taking place above the foramen magnum, thus confirming compression at cervical spine levels.35
Also, at the upper limbs may occur the presence of myelopathic hand with the finger escape sign. It consists in while maintaining the extended upper limbs for one minute, in the abduction and flexion of the fourth and fifth fingers. Also, may occur difficulty in holding and releasing the fingers, which consist in failure to hold and extent of fingers more than 15 times per 10 seconds.4
Sensorial symptoms are very often as well, where patients complain of awkward or numb hands.1 Tasks that require motor coordination, such as writing, button up a shirt or undo a zipper might become a challenging.36 Moreover, Lhermitte’s sign may also occur, indicating a dysfunction of posterior column. Nonetheless, this sign is not specifically related to CSM, being found in other conditions, such as multiple sclerosis.13
A well-known complication of the preexistence CSM is the central cord syndrome. It may occur in any patient who suffers a fall or trauma followed by neck hyperextension, which will determine sudden spinal cord compression due to a previous narrowed spinal canal. Clinically, those patients will present different degrees of motor deficits between the superior and inferior limbs, with the upper extremity been more severely affected than the lower one. This may be accompanied by sensory changes below the lesion, along with spasticity and neurogenic bladder.13,37
Among the clinical staging scales most commonly used in the evaluation of cervical myelopathy are myelopathy Assessment Scale Nurick (Table 1) and the modified scale of Japanese Orthopedic Association (MJOA). They are helpful regarding prognostic, and it has been used in surgical patients to assess better patient’s improvement. The Nurick scale is based mainly on gait disturbances, which may represent a shortcoming since it does not evaluate the commitment of superior limbs.8
Table 1.
NURICK scale.
| Grading | Signs and symptoms |
|---|---|
| Grade 0 | Signs and symptoms of root involvement but without evidence of spinal cord disease. |
| Grade 1 | Signs of spinal cord diseases but no difficulty walking. |
| Grade 2 | Slight difficulty in walking, which does not prevent full-time employment. |
| Grade 3 | Extreme difficulty in walking that requires assistance and prevents full-time employment and occupation. |
| Grade 4 | Able to walk only with someone else's help or with the aid of a walker. |
| Grade 5 | Chair bound or bedridden. |
Differential diagnosis
The differential diagnosis primarily involves diseases with involvement of sensory and motor pathways from the spinal cord. In this context, the observed frequency of diseases such as amyotrophic lateral sclerosis (ALS), myelopathy by vitamin B12 deficiency, and tropical spastic paraparesis associated with human T cell leukemia/lymphoma virus (HTLV) viruses in our clinic highlight the importance of keep in mind those differential diagnoses.
ALS can be clinically distinguished from CSM for the commitment of the second motor neuron in the lower limbs, with the presence of twitching, atrophy or decreased reflexes, or the presence of changes in the examination of the cranial nerves, such as dysarthria, dysphonia, dysphagia and tongue atrophy, indicating bulbar involvement. In those cases, electromyography will reveal the involvement of the second motor neuron into multiple levels.38
The subacute combined degeneration of spine cord (SCD) is caused by lack of vitamin B12 may present with a hyperintense signal on T2-weighted images on magnetic resonance imaging (MRI), simulating a CSM 20. Most often, however, coexist findings of sensory neuropathy in the lower limbs in SCD, facilitating its diagnosis. Moreover, many times cognitive and hematologic changings are also present, making the diagnosis of SDC easier.39
The HTLV-associated myelopathy or also called tropical spastic paraparesis determines spastic gait, urinary incontinence, and sphincter changes, as well as symptoms that resemble CSM.40 Besides, alterations in posterior horn may occur, simulating a posterior spinal compression. Not to mention, upper extremities might suffer an onset of weakness and paresthesias, along with deep hyperactive reflexes, which may mimic a compressive myelopathy in cervical levels above C5. The presence of a backache, changes in sensory exam committing lower extremities, and absence of cord compression on MRI indicates the diagnosis of HTLV-associated spastic paraparesis over the CSM.40
Often the presence of comorbidities in the population hinders the correct clinical evaluation of CSM. In those cases, there is spinal cord compression on imaging studies; however, insufficient to explain all the alterations observed during the neurological examination. One should mention for its high incidence, the presence of diabetic polyneuropathy, leading to instability of gait, paresthesia, atrophy, and weakness, emulating CSM symptoms.
In doubtful cases, the motor and somatosensory evoked potentials can be of great help to show functional impairment of spinal cord pathways at the cervical region. Although, it is assumed that motor evoked potentials are more useful in individuals with CSM and neuropathies, comparing to the somatosensory analysis.14,41,42
Noteworthy is also the occurrence of lumbar spinal stenosis associated with congenital narrowing of the cervical canal, one of the factors for the development of CSM, occurring in about 30% of cases. Frequently, patients operated for pain from lumbar spinal stenosis origin later on may develop clinical involvement of upper limbs, exposing an incipient or not previously observed CSM case.14,33
Clinical evaluation
Plain films
X-rays are still a method that has been used in many centers throughout the world. Typical finds may consist in osteophytosis, a decrease of disc space, inversion of cervical lordosis, uncovertebral joint hypertrophy, as well as spinal stenosis.43 However, these alterations may be commonly seen in age people, characterizing a non-specific method used for diagnosing cervical spine disorders, and therefore, its usage has been decreasing over the last decades.43 From the surgical standpoint, some authors have been justifying its usage by the better understanding of the sagittal balance of the cervical spine. This might be achieved by using dynamic X-rays, i.e. with flexion-extension plain films in the sagittal plane, which may reveal any potential spinal instability, which would be helpful to programming the best approach used in surgery.44
Computed tomography
Considered as an essential imaging tool in CSM cases computed tomography (CT) scan is superior to X-rays and MRI concerning bone assessment itself. Thus, CT scan evaluates bone anatomy, osteophytes, and degenerative alterations better than any other image modality. Altogether, CT scan should be part of armamentarium used in CSM patients, as well as a supplementary method to MRI, mainly in surgical patients.45,46
Magnetic resonance imaging
Regarded the mandatory study in CSM patients, MRI is the gold standard image tool in those patients (Figure 1).47 Being able to evaluate well discs, ligaments, subarachnoid space, the spinal cord itself, and any extradural compression, MRI is superior to CT scan in all these mentioned parameters.47 However, the time demanding for its realization and the fact of some patients suffer from claustrophobia, limit it in certain cases. Such effect was overcome since there has been opened MRI available in some centers over the last years.
Figure 1.
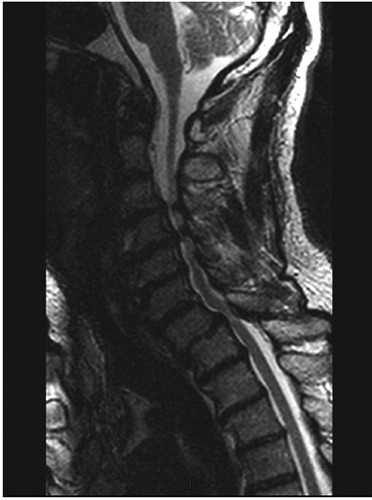
Typical cervical spine magnetic resonance imaging in cervical spondylotic myelopathy showing severe spinal cord compression in different levels.
Moreover, T1 and T2-weighted images in both sagittal and axial are the most used sequences in clinical practice. The presence of a hypointense signal on T1 sequences and hyperintense in T2 is associated with poor prognosis.8
In addition to this, its high sensitivity may create some troubles since it might diagnose some degenerative conditions in asymptomatic patients. As high as 57% of aged patients may have disk degeneration and herniation without having necessarily any symptoms.48 For this reason, the perfect match between the clinical exam and the MRI findings are mandatory for all neurologists who are dealing with those patients.
Interestingly, diffusion tensor images (DTI) tractography has become a promising tool for evaluation of CSM. Despite the lack of spatial resolution, which means that we cannot trust in the real position of the fiber after a given compression, it may provide us with a qualitative assessment. In other words, this sequence can determine whether there are fibers injured or not due to cervical compression, but are not able to establish where they are spatially. In spite of exciting results, further studies must address the role of DTI for routine use in these cases. Additionally, new algorithms should be created to improve DTI capability to determine where are the fibers geographically, which could help surgeons to plan the surgeries in a better manner.2,16
Electrodiagnostic studies
Not usually considered a tool in the diagnostic armamentarium for CSM, electromyography (EMG) may be useful when the differential diagnosis is ALS. More accurately than EMG, somatosensory evoked potentials might reveal any spinal cord dysfunction, increasing the sensibility of EMG.49 Therefore, diseases that cause superior and inferior motor neuron disease may be diagnosed in an easy fashion, ruling out some conditions such as ALS and multiple sclerosis.50
In an interesting paper published by Andrade and colleagues, they compared somatosensory evoked potentials (SEPs) and motor evoked potentials (MEP) to the presence of changings suggestive of CSM in the MRI. The image was considered as the gold standard and doing so; they were able to demonstrate alterations in both SEPs and MEP in only 61.9 and 71.4% of the cases, respectively, stressing the low sensitivity of these methods for CSM.51
Treatment
Since these patients seem to have a typical natural history, there are some particular indications for the surgical management. Thus, patients with gradual neurological deficits and those older than 60 years obtain significant benefits from the surgical treatment. These advantages are clearer in disabled patients, although patients with mild neurological deficits have greater tendency to have their deficits deteriorated when non-operative management is chosen.22
Surgical treatment
Surgical treatment is the gold standard in all cases but is mandatory in moderate or severe ones.26,27 Also, MJOA scale cases can be used for case severity assessment. Are considered mild those cases of CSM with ≥15 MJOA values; MJOA moderate 12-14 and severe if MJOA <12.28 As stressed earlier, there seems to be no difference between conservative or surgical treatment in mild cases, when follow-up is performed by three years. Therefore, this parameter may be regarded as guidance to treat these patients in a conservative manner.22
Non-surgical patients (CSM mild forms) may be treated with cervical immobilization, analgesics, anti-inflammatory and physiotherapy.4 In cases associated with cervical radiculopathies, drugs such as tricyclic antidepressants, anticonvulsants or even antagonists of drugs N-methyl-D-aspartate receptors as riluzole may be used.26
The surgical treatment involves anterior or posterior approach, depending whether kyphosis is present or not.12 However, the anterior approach has some drawbacks. The possibility of post-operative dysphagia due to surgical manipulation of the esophagus is relatively common. Still, hoarseness secondary to the recurrent laryngeal nerve may occur; besides, airway compression is a possibility when postoperative hematoma occurs. The main limitation of this approach is when more than two cervical levels are damaged, making the posterior approach mandatory.14
Therefore, when there is posterior compression or when more than two levels are involved, the posterior approach should be chosen. However, we should bear in mind the potential cervical spine instability and neck pain associated with this approach. In turn, this could lead to late deterioration of spinal cord compression and worsen of symptoms.29 Better surgical outcomes are obtained in patients below the age of 50, within a year of the onset of symptoms, and in patients without hyperintense lesion in T2 weighted MRI.30
Neuromonitoring
As an occupation area from neurologists, perioperative monitoring is becoming an indispensable tool for cervical spine surgery, tough still not regarded as a standard of care.52 Using SEPs monitoring and MEPs, motor deficits related to surgeries, as well as postoperative brachial plexopathy might be prevented.52 Moreover, the use of neuromonitoring can be prognostic since it may demonstrate increased amplitude or reduction of latency after spine cord decompression. Those parameters were proved to correlate with better motor improvements in the postoperative period, differently from those who kept with stable recordings during the intraoperative monitoring.53 Despite the above mentioned, we should mention that there is a lack of randomized trials proving the real benefit of neuromonitoring for avoiding motor deficits after cervical spine surgeries.54 However, in the author’s opinion neuromonitoring is essential when is necessary identify the level of myelopathy, and in those cases in which the patient has previously had significant spine cord compression. Although still with weak evidence for justifying its usage, the sensitiveness and specificity for identifying intraoperative neurologic damage may contribute to its widespread use among surgeons.54
Conclusions
Cervical spondylotic myelopathy is a leading cause of myelopathy among patients over 55 years. It may often represent a diagnostic challenge, especially the absence of specific findings and the high number of differential diagnoses. The exact correlation between signs and symptoms reported by patients, findings on neurological examination and the correct usage of radiological tools are essential to the therapeutic success. It should be suspected of cervical spondylotic myelopathy in anyone presenting progressive gait changes, mainly in association with sensory or motor complaints in the upper limbs. Since it is a very prevalent condition, is mandatory that all neurologists keep in mind this pathology, anticipating additional deficits during its course.
References
- 1.McCormick WE, Steinmetz MP, Benzel EC. Cervical spondylotic myelopathy: make the difficult diagnosis, then refer for surgery. Cleve Clin J Med 2003;70:899-904. [DOI] [PubMed] [Google Scholar]
- 2.Wang SQ, Li X, Cui JL, et al. Prediction of myelopathic level in cervical spondylotic myelopathy using diffusion tensor imaging. J Magn Reson Imaging 2015;41:1682-8. [DOI] [PubMed] [Google Scholar]
- 3.Klineberg E. Cervical spondylotic myelopathy: a review of the evidence. Orthop Clin North Am 2010;41:193-202. [DOI] [PubMed] [Google Scholar]
- 4.Lavelle WF, Bell GR. Cervical myelopathy: history and physical examination. Spine Surg 2007;19:6-11. [Google Scholar]
- 5.Etheridge J, Kalantar SB. The pathophysiology and biological mechanisms of cervical spondylotic myelopathy. Spine Surg 2014;26:62-7. [Google Scholar]
- 6.Amenta PS, Ghobrial GM, Krespan K, et al. Cervical spondylotic myelopathy in the young adult: A review of the literature and clinical diagnostic criteria in an uncommon demographic. Clin Neurol Neurosurg 2014;120:68-72. [DOI] [PubMed] [Google Scholar]
- 7.Watson JC, Broaddus WC, Smith MM, Kubal WS. Hyperactive pectoralis reflex as an indicator of upper cervical spinal cord compression: Report of 15 cases. J Neurosurg 1997;86:159-61. [DOI] [PubMed] [Google Scholar]
- 8.Edwards CC, Riew KD, Anderson PA, et al. Cervical myelopathy: current diagnostic and treatment strategies. Spine J 2003;3:68-81. [DOI] [PubMed] [Google Scholar]
- 9.Fehlings MG, Skaf G. A review of the pathophysiology of cervical spondylotic myelopathy with insights for potential novel mechanisms drawn from traumatic spinal cord injury. Spine 1998;23:2730-6. [DOI] [PubMed] [Google Scholar]
- 10.Young WF. Cervical spondylotic myelopathy: a common cause of spinal cord dysfunction in older persons. Am Fam Physic 2000;62:1064-70, 1073. [PubMed] [Google Scholar]
- 11.Ichihara K, Taguchi T, Sakuramoto I, et al. Mechanism of the spinal cord injury and the cervical spondylotic myelopathy: new approach based on the mechanical features of the spinal cord white and gray matter. J Neurosurg Spine 2003;99:278-85. [DOI] [PubMed] [Google Scholar]
- 12.Kumaresan S, Yoganandan N, Pintar FA, et al. Contribution of disc degeneration to osteophyte formation in the cervical spine: a biomechanical investigation. J Orthop Res 2001;19:977-84. [DOI] [PubMed] [Google Scholar]
- 13.Baron EM, Young WF. Cervical spondylotic myelopathy: a brief review of its pathophysiology, clinical course, and diagnosis. Neurosurgery 2007;60:S1-35. [DOI] [PubMed] [Google Scholar]
- 14.Salvi FJ, Jones JC, Weigert BJ. The assessment of cervical myelopathy. Spine J 2006;6:S182-S9. [DOI] [PubMed] [Google Scholar]
- 15.Chen J, Liu Z, Zhong G, et al. Surgical treatment for cervical spondylotic myelopathy in elderly patients: A retrospective study. Clin Neurol Neurosurg 2015;132:47-51. [DOI] [PubMed] [Google Scholar]
- 16.Rajasekaran S, Yerramshetty JS, Chittode VS, et al. The assessment of neuronal status in normal and cervical spondylotic myelopathy using diffusion tensor imaging. Spine (Phila Pa 1976) 2014;39:1183-9. [DOI] [PubMed] [Google Scholar]
- 17.Clair SS, Bell GR. Natural history of cervical spondylotic myelopathy. Spine Surg 2007;19:2-5. [Google Scholar]
- 18.Jumah K, Nyame P. Relationship between load carrying on the head and cervical spondylosis in Ghanaians. West Afr J Med 1993;13:181-2. [PubMed] [Google Scholar]
- 19.Olive PM, Whitecloud TS, 3rd, Bennett JT. Lower cervical spondylosis and myelopathy in adults with Down’s syndrome. Spine 1988;13:781-4. [DOI] [PubMed] [Google Scholar]
- 20.Yoo K, Origitano TC. Familial cervical spondylosis: case report. J Neurosurg 1998;89:139-41. [DOI] [PubMed] [Google Scholar]
- 21.Baptiste DC, Fehlings MG. Pathophysiology of cervical myelopathy. Spine J 2006;6:S190-S7. [DOI] [PubMed] [Google Scholar]
- 22.Lees F, Turner JA. Natural history and prognosis of cervical spondylosis. Br Med J 1963;2:1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gledhill R, Harrison B, McDonald W. Demyelination and remyelination after acute spinal cord compression. Exp Neurol 1973;38:472-87. [DOI] [PubMed] [Google Scholar]
- 24.Clarke E, Robinson PK. Cervical myelopathy: a complication of cervical spondylosis. Brain 1956;79:483-510. [DOI] [PubMed] [Google Scholar]
- 25.Rao R. Neck pain, cervical radiculopathy, and cervical myelopathy. J Bone Joint Surg 2002;84:1872-81. [DOI] [PubMed] [Google Scholar]
- 26.Spillane JD, Lloyd GH. The diagnosis of lesions of the spinal cord in association with “osteoarthritic” disease of the cervical spine. Age 1952;68:57. [DOI] [PubMed] [Google Scholar]
- 27.Sadasivan KK, Reddy RP, Albright JA. The natural history of cervical spondylotic myelopathy. Yale J Biol Med 1993;66:235. [PMC free article] [PubMed] [Google Scholar]
- 28.Nurick S. The natural history and the results of surgical treatment of the spinal cord disorder associated with cervical spondylosis. Brain 1972;95:101-8. [DOI] [PubMed] [Google Scholar]
- 29.Firooznia H, Ahn JH, Rafii M, Ragnarsson KT. Sudden quadriplegia after a minor trauma. The role of preexisting spinal stenosis. Surg Neurol 1985;23:165-8. [DOI] [PubMed] [Google Scholar]
- 30.Clifton A, Stevens J, Whitear P, Kendall B. Identifiable causes for poor outcome in surgery for cervical spondylosis. Neuroradiology 1990;32:450-5. [DOI] [PubMed] [Google Scholar]
- 31.Sampath P, Bendebba M, Davis JD, Ducker TB. Outcome of patients treated for cervical myelopathy: a prospective, multicenter study with independent clinical review. Spine 2000;25:670-6. [DOI] [PubMed] [Google Scholar]
- 32.Karadimas SK, Gatzounis G, Fehlings MG. Pathobiology of cervical spondylotic myelopathy. Eur Spine J 2014;24:132-8. [DOI] [PubMed] [Google Scholar]
- 33.Wang L, Hee HT, Wong HK. (iv) Cervical spondylotic myelopathy: a brief review of its pathophysiology, presentation, assessment, natural history and management. Orthop Trauma 2011;25:181-9. [Google Scholar]
- 34.Chiles BW, 3rd, Leonard MA, Choudhri HF, Cooper PR. Cervical spondylotic myelopathy: patterns of neurological deficit and recovery after anterior cervical decompression. Neurosurgery 1999;44:762-9. [DOI] [PubMed] [Google Scholar]
- 35.Heller J. The syndromes of degenerative cervical disease. J South Orthop Assoc 1996;5:207-12. [PubMed] [Google Scholar]
- 36.Emery SE. Cervical spondylotic myelopathy: diagnosis and treatment. J Am Acad Orthop Surg 2001;9:376-88. [DOI] [PubMed] [Google Scholar]
- 37.Schneider RC, Cherry G, Pantek H. The syndrome of acute central cervical spinal cord injury: with special reference to the mechanisms involved in hyperextension injuries of cervical spine. J Neurosurg 1954;11:546-77. [DOI] [PubMed] [Google Scholar]
- 38.Hernández AH. Rol de los estudios neurofisiológicos en el diagnóstico diferencial entre la esclerosis lateral amiotrófica y la mielopatía espondilótica cervical. Acta Neurol Colomb 2013;29. [Google Scholar]
- 39.Saperstein DS, Barohn RJ. Peripheral neuropathy due to cobalamin deficiency. Curr Treat Options Neurol 2002;4:197-201. [DOI] [PubMed] [Google Scholar]
- 40.Nakamura T. HTLV-I-associated myelopathy/tropical spastic paraparesis (HAM/TSP): the role of HTLV-I-infected Th1 cells in the pathogenesis, and therapeutic strategy. FoliaNeuropathol 2009;47:182-94. [PubMed] [Google Scholar]
- 41.Chistyakov AV, Soustiel JF, Hafner H, et al. The value of motor and somatosensory evoked potentials in evaluation of cervical myelopathy in the presence of peripheral neuropathy. Spine 2004;29:E239-E47. [DOI] [PubMed] [Google Scholar]
- 42.Toledano M, Bartleson J. Cervical spondylotic myelopathy. Neurol Clin 2013;31:287-305. [DOI] [PubMed] [Google Scholar]
- 43.Rahim K, Stambough J. Radiographic evaluation of the degenerative cervical spine. Orthop Clin North Am 1992;23:395-403. [PubMed] [Google Scholar]
- 44.Epstein JA. The surgical management of cervical spinal stenosis, spondylosis, and myeloradiculopathy by means of the posterior approach. Spine 1988;13:864-9. [DOI] [PubMed] [Google Scholar]
- 45.Freeman T, Martinez C. Radiological evaluation of cervical spondylotic disease: limitation of magnetic resonance imaging for diagnosis and preoperative assessment. Perspect Neurol Surg 1992;3:34-54. [Google Scholar]
- 46.Jahnke R, Hart B. Cervical stenosis, spondylosis, and herniated disc disease. Radiol Clin North Am 1991;29:777-91. [PubMed] [Google Scholar]
- 47.Al-Mefty O, Harkey LH, Middleton TH, et al. Myelopathic cervical spondylotic lesions demonstrated by magnetic resonance imaging. J Neurosurg 1988;68:217-22. [DOI] [PubMed] [Google Scholar]
- 48.Teresi LM, Lufkin RB, Reicher MA, et al. Asymptomatic degenerative disk disease and spondylosis of the cervical spine: MR imaging. Radiology 1987;164:83-8. [DOI] [PubMed] [Google Scholar]
- 49.Restuccia D, Di Lazzaro V, Lo Monaco M, et al. Somatosensory evoked potentials in the diagnosis of cervical spondylotic myelopathy. Electromyogr Clin Neurophysiol 1992;32:389-95. [PubMed] [Google Scholar]
- 50.Andrade DO. [Somatosensory and motor evoked potentials in patients with cervical spondylosis]. Arq Neuropsiquiatr 2005;63:843-6. [Article in Portuguese] [DOI] [PubMed] [Google Scholar]
- 51.Andrade DO. Potencial evocado somatossensitivo e motor na espodilose cervical. Arq Neuropsiquiatr 2005;63:843-6. [DOI] [PubMed] [Google Scholar]
- 52.Roh MS, Wilson-Holden TJ, Padberg AM, Pet al. The utility of somatosensory evoked potential monitoring during cervical spine surgery: how often does it prompt intervention and affect outcome? Asian Spine J 2007;1:43-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bouchard JA, Bohlman HH, Biro C. Intraoperative improvements of somatosensory evoked potentials: correlation to clinical outcome in surgery for cervical spondylitic myelopathy. Spine 1996;21:589-94. [DOI] [PubMed] [Google Scholar]
- 54.Matsumoto M, Ishida K. [Intraoperative neurophysiological monitoring of the spinal cord]. Masui 2012;61:16-24. [Article in Japanese] [PubMed] [Google Scholar]


