Abstract
The pathogenesis of severe acute pancreatitis (SAP) remains unclear. The Janus kinase and signal transducer and activator of transcription (JAK/STAT) pathway is important for various cytokines and growth factors. This study investigated the effect of the late inflammatory factor high mobility group box 1 (HMGB1) on the activation of JAK2/STAT3 in pancreatic acinar cells and the inhibitory effects of AG490 (a JAK2 inhibitor) and rapamycin (a STAT3 inhibitor) on this pathway. Rat pancreatic acinar cells were randomly divided into the control, HMGB1, AG490, and rapamycin groups. The mRNA levels of JAK2 and STAT3 at 10, 30, 60, and 120 minutes were detected using reverse transcription polymerase chain reaction (RT-PCR). The protein levels of JAK2 and STAT3 at 60 and 120 minutes were observed using Western blotting. Compared with the control group, the HMGB1 group exhibited significantly increased levels of JAK2 mRNA at each time point; STAT3 mRNA at 30, 60, and 120 minutes; and JAK2 and STAT3 proteins at 60 and 120 minutes (p < 0.01). Compared with the HMGB1 group, the AG490 and rapamycin groups both exhibited significantly decreased levels of JAK2 mRNA at each time point (p < 0.05); STAT3 mRNA at 30, 60, and 120 minutes (p < 0.01); and JAK2 and STAT3 proteins at 60 and 120 minutes (p < 0.01). HMGB1 induces the activation of the JAK2/STAT3 signaling pathway in rat pancreatic acinar cells, and this activation can be inhibited by AG490 and rapamycin. The results of this study may provide new insights for the treatment of SAP.
KEY WORDS: Rat pancreatic acinar cells, high mobility group box 1, Janus kinase 2, signal transducer and activator of transcription 3, AG490, rapamycin
INTRODUCTION
Acute pancreatitis (AP) is a multi-system disease with unknown etiology that commonly results in a clinically acute abdomen, systemic inflammatory responses, and injuries to distant organs [1,2]. The pathogenesis of AP is closely related to the intracellular events, early inflammatory reactions, and late inflammatory responses in the pancreas [3]. During AP, many inflammatory mediators are released from the inflammatory pancreatic tissue; through amplification via series of biological cascades, these mediators lead to the further release of large quantities of relevant pro-inflammatory factors. This process aggravates inflammation and can even lead to severe AP (SAP). SAP, which has mortality as high as 13% [4], develops in approximately 15% of AP cases.
A recent study has shown that inflammatory cytokines such as tumor necrosis factor-α (TNF-α), interleukin-1 (IL-1), and IL-6 released during early AP, quickly peak after AP model is successfully established. Then, the levels are rapidly lowered, whereas inflammation still continues over this time. Moreover, the clinical application of TNF-α, IL-1, and IL-6 receptor antagonists does not produce significant results, suggesting that late inflammatory mediators may be involved in the pathological reactions [5].
High mobility group box 1 (HMGB1) protein is a typical non-histone protein within the nucleus and has long been investigated due to its endonuclear capabilities. In 1999, Wang et al. Found that HMGB1 could be released into the extracellular matrix and mediate inflammation, and that it was an important late mediator [6]. After these findings, HMGB1 began to receive widespread attention as an inflammatory mediator and pro-inflammatory cytokine. Andersson et al. [7] added purified HMGB1 to macrophage cultures, which significantly stimulated the production of TNF-α, IL-1, IL-6, IL-8, and NO in a dose-dependent manner. HMGB1, as a late inflammatory mediator, caused a massive release of early inflammatory mediators. Our previous work showed that the expression of HMGB1, a late inflammatory factor, can be induced by trypsinogen activation peptide (an early pro-inflammatory cytokine) in rat pancreatic acinar cells and tissues [8,9], and the induction process may involve the Janus kinase 2 and signal transducer and activator of transcription 3 (JAK2/STAT3) signaling pathway in pancreatic acinar cells [10]. The JAK/STAT signaling pathway was found to be correlated with HMGB1-induced macrophage release of TNF-α, IL-1, and IL-6 [11-13]. Therefore, the focus of this study was to determine whether HMGB1 can activate the JAK2/STAT3 signaling pathway and expand inflammatory responses, thereby further aggravating pancreatitis in the advanced stages of SAP.
The aim of this study was to investigate the role of HMGB1 in the activation of the JAK2/STAT3 signaling pathway in pancreatic acinar cells. To further confirm the role of HMGB1 in this process, we also explored the inhibitory effects of a JAK2 inhibitor (AG490) and a STAT3 inhibitor (rapamycin) on the pathway activation. The results of this study may shed new light on the treatment of SAP in the future.
MATERIALS AND METHODS
Animals
A total of 32 adult Sprague-Dawley (SD) male and female rats, weighing 200-250 g, were provided by the Third Military Medical University, Chongqing (certificate number Scxk (Yu) 2007-0005).
The surgical procedures were approved by the Institute of Animal Ethics of Zunyi Medical College.
Cell cultures and grouping
After the animals were euthanized by cervical dislocation, 32 SD rats were immersed in 75% ethanol for 30 minutes, and the pancreas was obtained by laparotomy in a sterile environment. The pancreatic tissue was washed and sterilized 2-3 times using 100 units/ml of dual antibiotic solution (penicillin and streptomycin and was then placed into serum-free F-12K medium for three washes to eliminate the remaining blood. Other incidental tissues, such as the mesentery, were removed, and then the pancreatic tissues were cut into approximately 1 mm3 pieces. The minced tissue fragments were placed into polypropylene culture bottles, and 10 ml of type II collagenase was added for digestion and separation of cells after removing residual cleaning solution. After tightening the cap and sealing the culture bottles, they were placed into a water bath and shaken at 37°C for 10 minutes (120 r/min). When the digestive juice became cloudy, the bottles were removed from the bath. The cloudy digestive juice was discarded, and 20 ml of new digestive juice was added. Then, digestion continued for 30 minutes under the above conditions (120 r/min). The above procedure was repeated until most of the pancreatic tissues were digested. After the large tissue pieces had settled to the bottom of the bottle, the upper cell suspension was extracted and filtered through a 200 mesh filter into F-12K culture medium. Then, digestion was terminated, and the mixture was centrifuged for 5 minutes (1000 r/min). The upper medium was discarded, and the cells were resuspended with new medium and equally mixed. The cell survival rate and count were determined using a trypan blue cell viability assay (Beyotime Biotechnology, Shanghai, China). The cell density was adjusted to approximately 107/ml, and 1.5 ml of homogenous cell suspension was pipetted into each well of a 6-well plate and then incubated in a 37°C incubator.
The final cell suspensions were divided into four major groups: Control group, HMGB1 group (HMGB1 was added to pancreatic acinar cells at a final concentration of 1 µg/mL), AG490 group (the JAK2 inhibitor AG490 was added to the cultured cells 30 minutes before HMGB1 induction at a final concentration of 25 µmol/L), and the rapamycin group (the STAT3 inhibitor rapamycin was added at a final concentration of 40 ng/ml 30 minutes before the HMGB1 induction). Each major group was subdivided into four subgroups based on HMGB1 intervention time (10, 30, 60, or 120 minutes) with 1.5 ml of cell suspension in each subgroup. After the model was successfully established, sampling was conducted at 10, 30, 60, and 120 minutes for each group to determine the expression levels of JAK2 and STAT3 mRNAs and proteins in the cells.
Detection of JAK2 and STAT3 mRNA expression
Application of the RNAiso Reagent kit, cell lysis, and mRNA extraction were performed in the culture wells according to the protocols provided by the TaKaRa Company (Dalian Biological Engineering Co., Ltd., China). The relevant primer sequences were obtained from GenBank and synthesized by the Dalian Takara company. The primers were as follows: JAK2 (with an amplification length of 101 bp): 5’-TTTGAAGACAGGGACCCTACACAG-3’ (upstream), 5’-TCATAGCGGCACATCTCCACA-3’ (downstream); STAT3 (with an amplification length of 118 bp): 5’-TTTGAGACAGAGGTGTACCACCAAG-3’ (upstream), 5’-ACCACAGGATTGATGCCCAAG-3’ (downstream); β-actin (ACTB) (with an amplification length of 150 bp): 5’-GGAGATTACTGCCCTGGCTCCTA-3’ (upstream), 5’-GACTCATCGTACTCCTGCTTGCTG-3’ (downstream). The reverse transcription reaction was conducted at 37°C for 15 minutes followed by 85°C for 5 seconds and was then maintained at 4°C. The relative expression level was calculated by the 2-ΔΔCt method based on the obtained cycle threshold (Ct) values, and the relative expression level of the target gene in each group of samples was expressed as the target gene expression level/reference gene expression level.
Western blotting
Cell culture medium from each well was lysed to extract cell proteins, and each sample was prepared as a protein solution of equal volume and equal protein concentration according to the bicinchoninic acid method. The loading sample was mixed with ×5 sodium dodecyl sulfate loading buffer at a 4:1 ratio, and the proteins were denatured in boiling water for 5 minutes. Identical amounts of sample proteins were loaded for electrophoresis and then electrically transferred to polyvinylidene fluoride membranes. Then, the membranes were blocked overnight in blocking solution and incubated with 1:200 primary antibodies (JAK2(D2E12)XP Rabbit mAb and STAT3(79D7) Rabbit mAb, purchased from Kunming NSFocus Co., Ltd.) for 2 hours. After three washes with Tris-buffered saline with tween (TBST), the membranes were incubated with 1:10,000 secondary antibody (800 CW goat anti-rabbit immunoglobulin G, provided by the Central Laboratory of Zunyi Medical College) for 2 hours. After three additional TBST washes, the membranes were subjected to chemiluminescence, and the films were exposed, developed, and fixed to visualize bands. The films were then scanned, and finally the gray value of the target band was analyzed using a UVP gel image processing system that included Labworks 4.6 software (SYNGENE, UK). The relative expression levels of the JAK2 and STAT3 proteins were expressed as the JAK2 gray value/β-actin gray value and the STAT3 gray value/β-actin gray value. The films were read by senior lab technicians from the Central Laboratory of Zunyi Medical College who were blinded to the experimental conditions.
Statistical analysis
Data were processed using SPSS 17.0 statistical software package (SPSS Inc., Chicago, IL, USA). Measurement data are presented as mean ± standard deviation (χ¯±s), and comparisons among many groups were performed using single factor analysis of variance (one-way ANOVA). A value of p < 0.05 was considered statistically significant, and a value of p < 0.01 was considered very significant.
RESULTS
Changes in cell morphology after HMGB1 stimulation
Changes in the morphology of pancreatic acinar cells were observed using electron microscopy (Figure 1). In the control group, rich rough endoplasmic reticulum with abundant ribosomes was observed near the basal region, and a large quantity of zymogen granules (ZGs) was observed on the top of cytoplasm. At 2 hours after the HMGB1 stimulation, intracytoplasmic vacuolization was observed, and most of the vacuoles had a round or oval shape with different sizes. Furthermore, the number of ZGs decreased.
FIGURE 1.
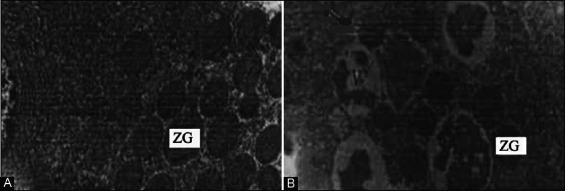
Changes in cell morphology at 2 hours after high mobility group box 1 (HMGB1) stimulation, observed by electron microscopy. (A) The control group: A large number of zymogen granules (ZGs) was observed on the top of cytoplasm (×10,000); (B) the HMGB1 group: The number of ZGs greatly decreased, vacuoles formed, and intervacuolar fusion occurred (×6000).
HMGB1 upregulated JAK2 and STAT3 mRNA expression
The JAK2 and STAT3 mRNA expression levels in the control, HMGB1, AG490 (JAK2 inhibitor), and rapamycin (STAT3 inhibitor) groups at 10, 30, 60, and 120 minutes were detected using reverse transcription polymerase chain reaction (RT-PCR), and the results are shown in Figures 2 and 3.
FIGURE 2.
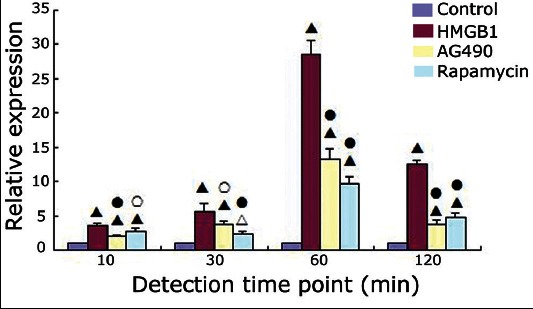
Janus kinase 2 mRNA expression in the four groups at different time points (χ̄±s). Δ: p < 0.05 versus the control group; ▲: p < 0.01 versus the control group; ○: p < 0.05 versus the high mobility group box 1 (HMGB1) group; ●: p < 0.01 versus the HMGB1 group.
FIGURE 3.
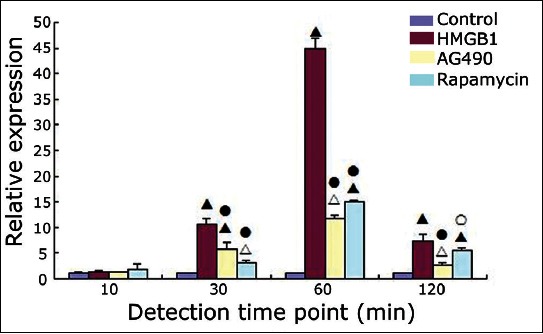
Signal transducer and activator of transcription 3 mRNA expression in the four groups at different time points (χ̄±s). Δ: p < 0.05 versus the control group; ▲: p < 0.01 versus the control group; ○: p < 0.05 versus the high mobility group box 1 (HMGB1) group; ●: p < 0.01 versus the HMGB1 group.
Compared with the control group, the HMGB1 group showed significantly increased levels of JAK2 mRNA at 10, 30, 60, and 120 minutes and STAT3 mRNA at 30, 60, and 120 minutes (p < 0.001 for all). Compared with the HMGB1 group, the relative expression levels of JAK2 mRNA at each time point and the relative expression levels of STAT3 mRNA at 30, 60, and 120 minutes were decreased in the AG490 group (JAK2 mRNA: p < 0.001, p = 0.015, p < 0.001, and p < 0.001 at 10, 30, 60, and 120 minutes, respectively; STAT3 mRNA: p < 0.001 at 30, 60, and 120 minutes) and in the rapamycin group (JAK2 mRNA: p < 0.001 at 10, 30, 60, and 120 minutes; STAT3 mRNA: p < 0.001, p < 0.001, and p = 0.026 at 30, 60, and 120 minutes, respectively). The JAK2 and STAT3 mRNA expression levels did not differ significantly between the AG490 group and the rapamycin group at any of the detected time points (p > 0.05).
HMGB1 upregulated JAK2 and STAT3 protein expression
The JAK2 and STAT3 protein expression in the four groups was observed using Western blot analysis, and the relative protein expression levels of the JAK2 and STAT3 are shown in Figures 4 and 5.
FIGURE 4.
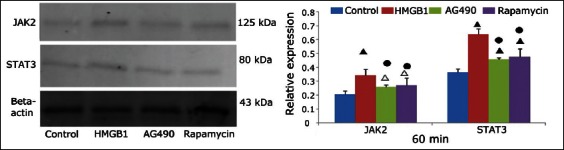
Western blot analysis of Janus kinase 2 and signal transducer and activator of transcription 3 protein expression at 60 minutes in the control, high mobility group box 1 (HMGB1), AG490, and rapamycin groups. Beta-actin was used as the internal reference. Δ: p < 0.05 versus the control group; ▲: p < 0.01 versus the control group; ●: p < 0.01 versus the HMGB1 group.
FIGURE 5.
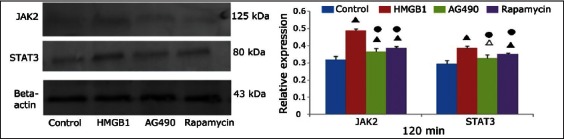
Western blot analysis of Janus kinase 2 and signal transducer and activator of transcription 3 protein expression at 120 minutes in the control, high mobility group box 1 (HMGB1), AG490, and rapamycin groups. Beta-actin was used as the internal reference. Δ: p < 0.05 versus the control group; ▲: p < 0.01 versus the control group; ●: p < 0.01 versus the HMGB1 group.
Compared with the control group, the JAK2 and STAT3 protein expression levels in the HMGB1 group at 60 and 120 minutes were significantly increased (p < 0.001 for all). Compared with the HMGB1 group, the JAK2 and STAT3 protein expression levels in the AG490 group (JAK2: p = 0.004 and p < 0.001 at 60 and 120 minutes, respectively; STAT3: p < 0.001 and p = 0.001 at 60 and 120 minutes, respectively) and the rapamycin group (JAK2: p = 0.009 and p = 0.001 at 60 and 120 minutes, respectively; STAT3: both p < 0.001) were significantly decreased. The expression levels at each time point were not significantly different between the AG490 and rapamycin groups (p > 0.05).
DISCUSSION
SAP always progresses rapidly, producing threatening conditions, and often leads to systemic inflammatory response syndrome (SIRS) and multiple organ dysfunction syndrome (MODS). SAP has become the focus of research in recent years. However, its pathogenesis has not been fully elucidated. In 1988, Rindernecht proposed the widely recognized “Leukocyte over-activation hypothesis”, which asserts that timely and effective inhibition of the inflammatory response is the key for preventing exacerbation of SAP. Recent studies of the role of cell signaling pathways in SAP have received increasing attention due to the possibility that this research can clarify the pathogenesis of SAP [14]. The RT-PCR data in this study showed that in the HMGB1 group, with a final concentration of 1 µg/ml, the level of JAK2 mRNA at each time point was increased compared to that of the control group (p < 0.05, p < 0.01), and the level of STAT3 mRNA at 30, 60, and 120 minutes was increased compared to that of the control group (p < 0.05, p < 0.01, p < 0.01). Compared to the HMGB1 group, the relative expression levels of JAK2 mRNA at each time point and the levels of STAT3 mRNA at 30, 60, and 120 minutes in the AG490 and rapamycin groups were decreased (p < 0.05 and p < 0.01, respectively).
HMGB1 could continue to mediate inflammatory responses after being released into the cell exterior [15]. HMGB1 is involved in the pathophysiology of SAP and is correlated with the severity of the disease [16,17]. JAK/STAT is an important molecular substrate for the occurrence and development of sepsis [18-20]. The JAK2/STAT3 signaling pathway exacerbates pancreatitis by inducing the release of inflammatory cytokines [21,22]. Another study showed that JAK2/STAT3 signaling pathway antagonists improved pancreatic damage in caerulein-induced pancreatitis [23]. This is consistent with our findings. In addition, the Western blot results in our study showed that after 60 and 120 minutes of HMGB1 stimulation, the JAK2 and STAT3 protein expression levels were significantly increased compared to those in the control group (p < 0.01), whereas the JAK2 and STAT3 protein expression levels at 60 and 120 minutes were significantly decreased after the treatment with inhibitors (p < 0.01). These results confirmed the results of RT-PCR. From these results, we infer that HMGB1 can induce the activation of the JAK2/STAT3 signaling pathway in rat pancreatic acinar cells, thereby aggravating pancreatitis. Inhibition of this signaling pathway can reduce the damage caused by pancreatitis.
During SAP, multiple signaling pathways such as mitogen-activated protein kinase (MAPK), Ras, nuclear factor-B, receptor tyrosine kinase (RTK), phosphatidylinositol 3-kinase, JAK/STAT, and others, are interlinked with each other either directly or through a variety of transcription factors or cofactors. Among these pathways, the cascade of interactions between MAPK and JAK/STAT is quite complex, and these proteins can communicate at different levels. In addition, activated JAKs will phosphorylate the related receptor tyrosine kinase and thus provide binding targets for other signal transduction pathways that include SH2-containing adapter protein. For example, SHP2 and Sh can bind to growth factor receptor-bound protein 2 and activate Ras cascade responses [24]. The JAK2/STAT3 signaling pathway is an important pathway for cytokine signal transduction [25,26]. Thus, we speculate that during early SAP, disruption of pancreatic acinar cells causes release of pancreatic enzymes, leading to the release of local cytokines and other inflammatory mediators and activation of the JAK/STAT signaling pathway. Then, the cascade effect with other signaling pathways further leads to a greater release of inflammatory mediators, resulting in SIRS and MODS. The JAK/STAT3 pathway may be the key to cytokine cascade reactions during SAP. Inhibition of this signaling pathway might be a new option for the treatment of SAP by controlling the waterfall-like inflammatory responses that occur.
This study was conducted using cell culture experiments, and differences from the intrinsic microenvironment of the body may have biased our results. Therefore, the results obtained in this study require further confirmation using animal models.
In summary, HMGB1 can induce activation of the JAK2/STAT3 signaling pathway in the middle and late stages of SAP, whereas AG490 and rapamycin can inhibit its activation. The JAK2/STAT3 signaling pathway plays a very important role in inflammatory response cascade amplification, and the inhibition of this signaling pathway may provide a new option for the clinical treatment of SAP, but the detailed mechanism still requires further confirmation through basic and clinical research.
DECLARATION OF INTERESTS
The authors declare no conflict of interests.
REFERENCES
- [1].Zhang XH, Yuan BS, Zhu RM. Role of Fas/FasL in acute pancreatitis-associated liver injury. [Article in Chinese] World Chin J Digestol. 2008;16:1661–5. [Google Scholar]
- [2].Brisinda G, Vanella S, Crocco A, Mazzari A, Tomaiuolo P, Santullo F, et al. Severe acute pancreatitis: Advances and insights in assessment of severity and management. Eur J Gastroenterol Hepatol. 2011;23(7):541–51. doi: 10.1097/MEG.0b013e328346e21e. http://dx.doi.org/10.1097/MEG.0b013e328346e21e . [DOI] [PubMed] [Google Scholar]
- [3].Huang TT. Re-understanding of early endocellular events in acute pancreatitis. [Article in Chinese] Chin J Hepatobiliary Surg. 2006;12:75–6. [Google Scholar]
- [4].Guo Q, Li A, Xia Q, Liu X, Tian B, Mai G, et al. The role of organ failure and infection in necrotizing pancreatitis: A prospective study. Ann Surg. 2014;259(6):1201–7. doi: 10.1097/SLA.0000000000000264. http://dx.doi.org/10.1097/SLA.0000000000000264 . [DOI] [PubMed] [Google Scholar]
- [5].Hyvönen MT, Herzig KH, Sinervirta R, Albrecht E, Nordback I, Sand J, et al. Activated polyamine catabolism in acute pancreatitis: Alpha-methylated polyamine analogues prevent trypsinogen activation and pancreatitis-associated mortality. Am J Pathol. 2006;168(1):115–22. doi: 10.2353/ajpath.2006.050518. http://dx.doi.org/10.2353/ajpath.2006.050518 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Wang H, Bloom O, Zhang M, Vishnubhakat JM, Ombrellino M, Che J, et al. HMG-1 as a late mediator of endotoxin lethality in mice. Science. 1999;285(5425):248–51. doi: 10.1126/science.285.5425.248. http://dx.doi.org/10.1126/science.285.5425.248 . [DOI] [PubMed] [Google Scholar]
- [7].Andersson U, Wang H, Palmblad K, Aveberger AC, Bloom O, Erlandsson-Harris H, et al. High mobility group 1 protein (HMG-1) stimulates proinflammatory cytokine synthesis in human monocytes. J Exp Med. 2000;192(4):565–70. doi: 10.1084/jem.192.4.565. http://dx.doi.org/10.1084/jem.192.4.565 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Wang GL, Dui DH, Bai L, Liu Y, Tian F, Wei W. Research of TAP induction the pancreatic acinar cells to release HMGB1 in rat. [Article in Chinese] Chin J Bases Clin Gen Surg. 2012;19(10):1074–8. [Google Scholar]
- [9].Liu Y, Dui DH, Wang GL. Expression and significance of high mobility group box 1 in rats with acute pancreatitis. Chin J Exp Surg. 2012;29:2041–4. [Google Scholar]
- [10].Ren HY, Dui DH, Liu Y. Effect of Janus kinase 2/signal transducer and activators of transcription 3 signaling pathway on the release of high mobility group box 1 in trypsinogen activation peptide-induced rat pancreatic acinar cells. [Article in Chinese] Chin J Exp Surg. 2014;29:1511–3. [Google Scholar]
- [11].Liu H, Yao YM. Activation of Janus kinase/signal transducer and activator of transcription pathway in macrophages induced by high mobility group box 1. [Article in Chinese] Chin Crit Care Med. 2004;16:221–31. [PubMed] [Google Scholar]
- [12].Fiuza C, Bustin M, Talwar S, Tropea M, Gerstenberger E, Shelhamer JH, et al. Inflammation-promoting activity of HMGB1 on human microvascular endothelial cells. Blood. 2003;101(7):2652–60. doi: 10.1182/blood-2002-05-1300. http://dx.doi.org/10.1182/blood-2002-05-1300 . [DOI] [PubMed] [Google Scholar]
- [13].Rendon-Mitchell B, Ochani M, Li J, Han J, Wang H, Yang H, et al. IFN-gamma induces high mobility group box 1 protein release partly through a TNF-dependent mechanism. J Immunol. 2003;170(7):3890–7. doi: 10.4049/jimmunol.170.7.3890. http://dx.doi.org/10.4049/jimmunol.170.7.3890 . [DOI] [PubMed] [Google Scholar]
- [14].Bhatia M, Brady M, Shokuhi S, Christmas S, Neoptolemos JP, Slavin J. Inflammatory mediators in acute pancreatitis. J Pathol. 2000;190(2):117–25. doi: 10.1002/(SICI)1096-9896(200002)190:2<117::AID-PATH494>3.0.CO;2-K. http://dx.doi.org/10.1002/(SICI)1096-9896(200002)190:2<117:: AID-PATH494>3.3.CO;2-B . [DOI] [PubMed] [Google Scholar]
- [15].Bianchi ME, Manfredi AA. Immunology. Dangers in and out. Science. 2009;323(5922):1683–4. doi: 10.1126/science.1172794. http://dx.doi.org/10.1126/science.1172794 . [DOI] [PubMed] [Google Scholar]
- [16].Yasuda T, Ueda T, Takeyama Y, Shinzeki M, Sawa H, Nakajima T, et al. Significant increase of serum high-mobility group box chromosomal protein 1 levels in patients with severe acute pancreatitis. Pancreas. 2006;33(4):359–63. doi: 10.1097/01.mpa.0000236741.15477.8b. http://dx.doi.org/10.1097/01.mpa.0000236741.15477.8b . [DOI] [PubMed] [Google Scholar]
- [17].Long K, Liu XQ, Sun M. Relation between high mobility group protein B1 and the severity of acute pancreatitis in rats. [Article in Chinese] J Kunming Med Univ. 2012;10(33):23–5. [Google Scholar]
- [18].Liu H, Yao YM, Dong YQ, Yan YU. The role of Janus kinase-signal transducer and transcription activator pathway in the regulation of synthesis and release of lipopolysaccharide-induced high mobility group box-1 protein. [Article in Chinese] Chin J Burns. 2005;21:414–7. [PubMed] [Google Scholar]
- [19].Cai B, Cai JP, Luo YL, Chen C, Zhang S. The specific roles of JAK/STAT signaling pathway in sepsis. Inflammation. 2015;38(4):1599–608. doi: 10.1007/s10753-015-0135-z. http://dx.doi.org/10.1007/s10753-015-0135-z . [DOI] [PubMed] [Google Scholar]
- [20].Paracha RZ, Ahmad J, Ali A, Hussain R, Niazi U, Tareen SH, et al. Formal modelling of toll like receptor 4 and JAK/STAT signaling pathways: Insight into the roles of SOCS-1, interferon-ß and proinflammatory cytokines in sepsis. PLoS One. 2014;9(9):e108466. doi: 10.1371/journal.pone.0108466. http://dx.doi.org/10.1371/journal.pone.0108466 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Li ML, Zhu RM, Zhang XH, Guo JY, Yang MF, Wu XW, et al. The role of JAK2/STAT3 signaling pathway in the lung injury rat with severe acute pancreatitis. [Article in Chinese] Med J Chin PLA. 2011;36:611–3. [Google Scholar]
- [22].Li ML, Xu XB, Wang B, Jin XX, Guo MX, Zhang XH. Influence of proinflammatory factors on the JAK2/STAT3 signaling pathway in early severe acute pancreatitis. [Article in Chinese] Acta Univ Med Anhui. 2014;49(10):1392–5. [Google Scholar]
- [23].Chen P, Huang L, Zhang Y, Qiao M, Yao W, Yuan Y. The antagonist of the JAK-1/STAT-1 signaling pathway improves the severity of cerulein-stimulated pancreatic injury via inhibition of NF-kB activity. Int J Mol Med. 2011;27(5):731–8. doi: 10.3892/ijmm.2011.632. DOI: 10.3892/ijmm.2011.632. [DOI] [PubMed] [Google Scholar]
- [24].Rawlings JS, Rosler KM, Harrison DA. The JAK/STAT signaling pathway. J Cell Sci. 2004;117(8):1281–3. doi: 10.1242/jcs.00963. http://dx.doi.org/10.1242/jcs.00963 . [DOI] [PubMed] [Google Scholar]
- [25].Szulawska A, Arkusinska J, Czyz M. Accumulation of gamma-globin mRNA and induction of irreversible erythroid differentiation after treatment of CML cell line K562 with new doxorubicin derivatives. Biochem Pharmacol. 2007;73(2):175–84. doi: 10.1016/j.bcp.2006.09.028. http://dx.doi.org/10.1016/j.bcp.2006.09.028 . [DOI] [PubMed] [Google Scholar]
- [26].Lee C, Lim HK, Sakong J, Lee YS, Kim JR, Baek SH. Janus kinase-signal transducer and activator of transcription mediates phosphatidic acid-induced interleukin (IL)-1beta and IL-6 production. Mol Pharmacol. 2006;69(3):1041–7. doi: 10.1124/mol.105.018481. [DOI] [PubMed] [Google Scholar]


