Abstract
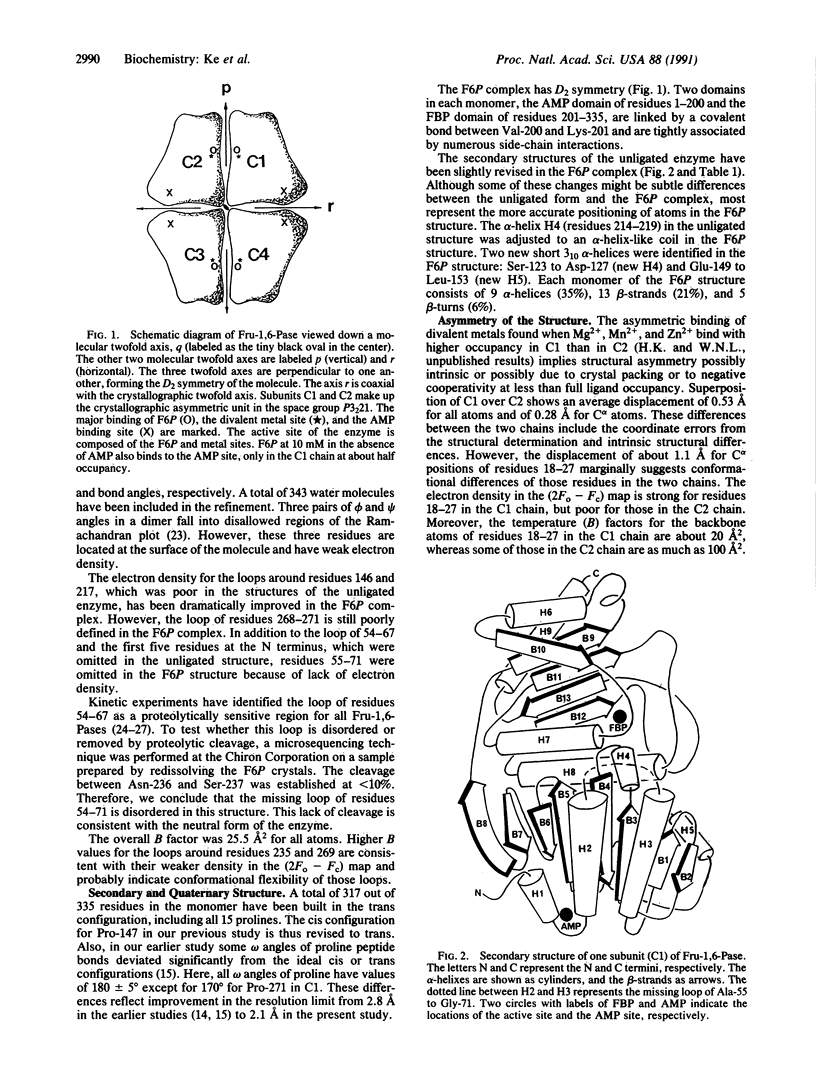
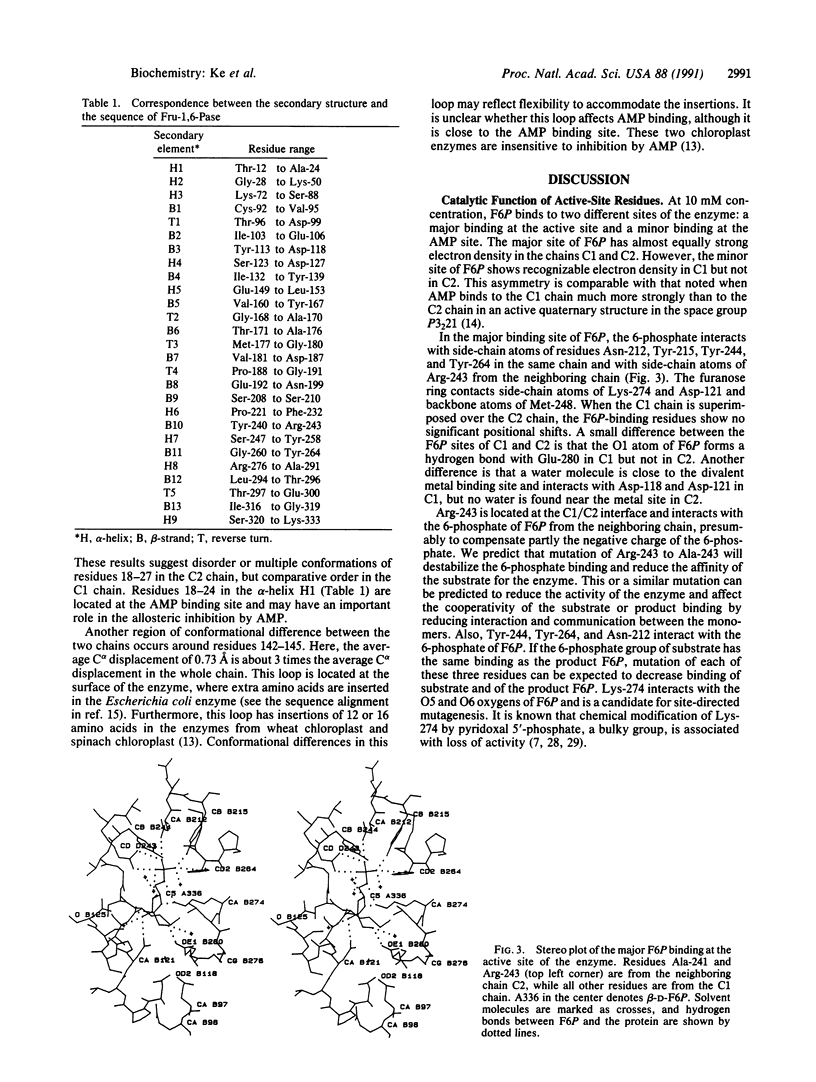
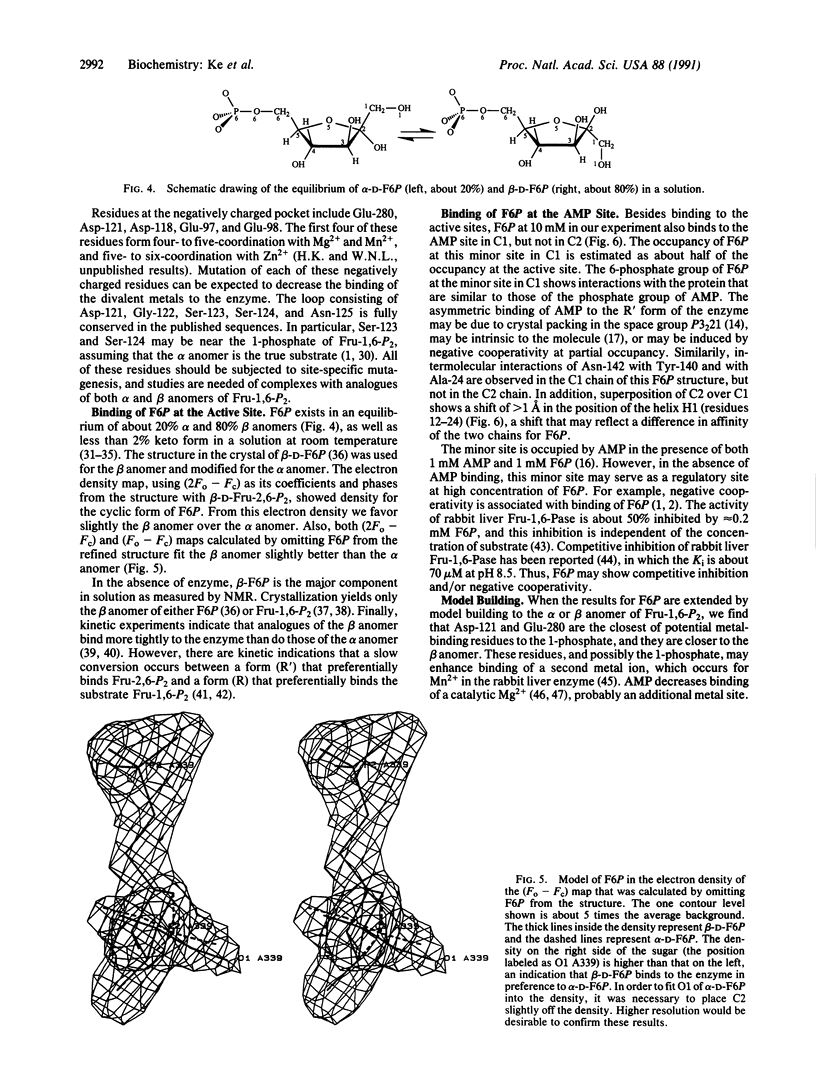
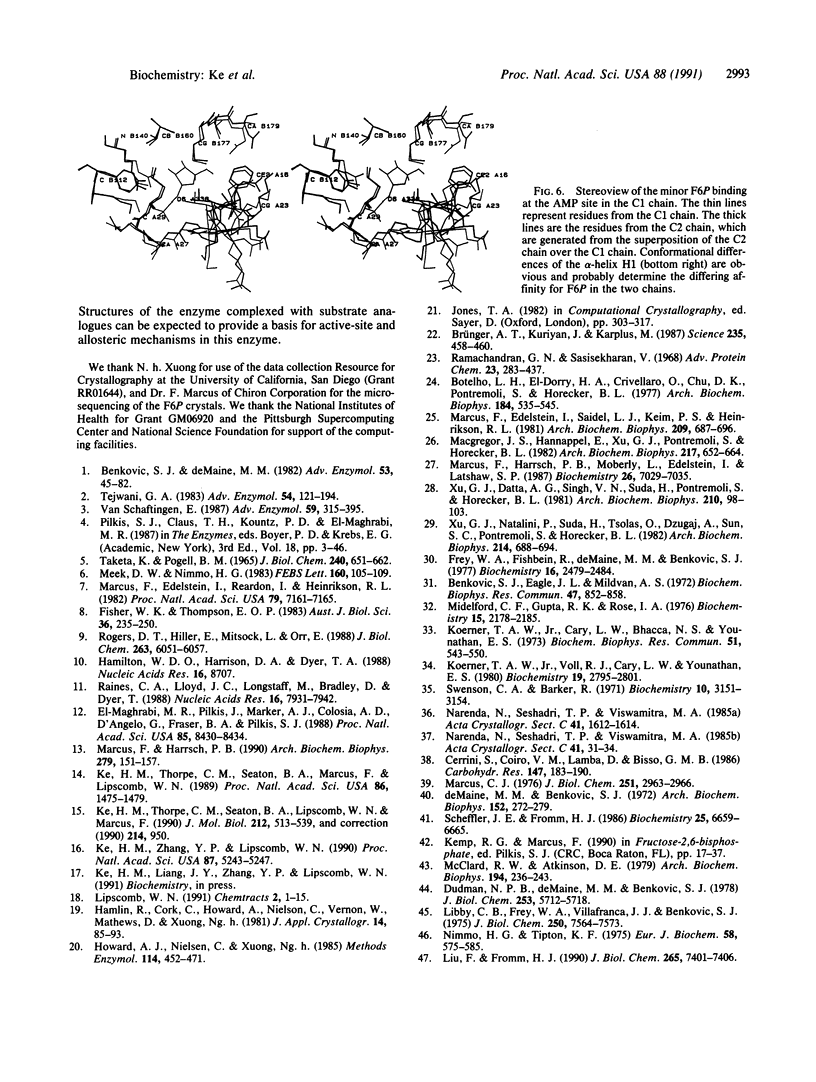
The crystal structure of fructose-1,6-bisphosphatase (EC 3.1.3.11) complexed with the product fructose 6-phosphate (F6P) has been refined at 2.1-A resolution to an R factor of 0.177 with root-mean-square deviations of 0.014 A and 2.9 degrees from the ideal geometries of bond lengths and bond angles, respectively. The secondary structures but not the trace of the unligated enzyme have been slightly revised in the F6P complex at this higher resolution. Helix H4 in the unligated structure has been refined to a helix-like coil, and two very short 3(10) helices have been found, one in H4 and one in H5. F6P at 10 mM concentration in the absence of divalent metals in our study shows major binding at the active site and minor binding at the AMP site. The major site has almost equal full occupancy in the C1 and C2 chains of the crystallographic asymmetric unit, while the minor site shows occupancy only in the C1 chain at about 50%. The electron density in both (2Fo - Fc) and (Fo - Fc) maps calculated by omitting F6P slightly favors the beta anomer of D-F6P over the alpha anomer. Possible functions of the active-site residues are discussed, and candidates are suggested for site-directed mutagenesis.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benkovic S. J., Engle J. L., Mildvan A. S. Magnetic resonance studies of the anomeric distribution and manganese binding properties of fructose phosphates. Biochem Biophys Res Commun. 1972 May 26;47(4):852–858. doi: 10.1016/0006-291x(72)90571-2. [DOI] [PubMed] [Google Scholar]
- Benkovic S. J., deMaine M. M. Mechanism of action of fructose 1,6-bisphosphatase. Adv Enzymol Relat Areas Mol Biol. 1982;53:45–82. doi: 10.1002/9780470122983.ch2. [DOI] [PubMed] [Google Scholar]
- Botelho L. H., El-Dorry H. A., Crivellaro O., Chu D. K., Pontremoli S., Horecker B. L. Digestion of rabbit liver fructose 1,6-bisphosphatase with subtilisin: sites of cleavage and activity of the modified enzyme. Arch Biochem Biophys. 1977 Dec;184(2):535–545. doi: 10.1016/0003-9861(77)90463-5. [DOI] [PubMed] [Google Scholar]
- Brünger A. T., Kuriyan J., Karplus M. Crystallographic R factor refinement by molecular dynamics. Science. 1987 Jan 23;235(4787):458–460. doi: 10.1126/science.235.4787.458. [DOI] [PubMed] [Google Scholar]
- De Maine M. M., Benkovic S. J. On the mechanism of alkaline and neutral fructose 1, 6-diphosphatase: inhibition by substrate analogs at neutral pH. Arch Biochem Biophys. 1972 Sep;152(1):272–279. doi: 10.1016/0003-9861(72)90215-9. [DOI] [PubMed] [Google Scholar]
- Dudman N. P., deMaine M. M., Benkovic S. J. Fructose 1,6-bisphosphate. Kinetics of hydrolysis catalyzed by rabbit liver neutral fructose-1,6-bisphosphatase with Mn2+. J Biol Chem. 1978 Aug 25;253(16):5712–5718. [PubMed] [Google Scholar]
- Fisher W. K., Thompson E. O. Amino acid sequence studies on sheep liver fructose-bisphosphatase. II. The complete sequence. Aust J Biol Sci. 1983;36(3):235–250. doi: 10.1071/bi9830235. [DOI] [PubMed] [Google Scholar]
- Frey W. A., Fishbein R., de Maine M. M., Benkovic S. J. Substrate form of D-frutose 1,6-bisphosphate utilized by fructose 1,6-bisphosphatase. Biochemistry. 1977 May 31;16(11):2479–2484. doi: 10.1021/bi00630a025. [DOI] [PubMed] [Google Scholar]
- Hamilton W. D., Harrison D. A., Dyer T. A. Sequence of the Escherichia coli fructose-1,6-bisphosphatase gene. Nucleic Acids Res. 1988 Sep 12;16(17):8707–8707. doi: 10.1093/nar/16.17.8707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard A. J., Nielsen C., Xuong N. H. Software for a diffractometer with multiwire area detector. Methods Enzymol. 1985;114:452–472. doi: 10.1016/0076-6879(85)14030-9. [DOI] [PubMed] [Google Scholar]
- Ke H. M., Thorpe C. M., Seaton B. a., Lipscomb W. N., Marcus F. Structure refinement of fructose-1,6-bisphosphatase and its fructose 2,6-bisphosphate complex at 2.8 A resolution. J Mol Biol. 1990 Apr 5;212(3):513–539. doi: 10.1016/0022-2836(90)90329-k. [DOI] [PubMed] [Google Scholar]
- Ke H. M., Zhang Y. P., Lipscomb W. N. Crystal structure of fructose-1,6-bisphosphatase complexed with fructose 6-phosphate, AMP, and magnesium. Proc Natl Acad Sci U S A. 1990 Jul;87(14):5243–5247. doi: 10.1073/pnas.87.14.5243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ke H., Thorpe C. M., Seaton B. A., Marcus F., Lipscomb W. N. Molecular structure of fructose-1,6-bisphosphatase at 2.8-A resolution. Proc Natl Acad Sci U S A. 1989 Mar;86(5):1475–1479. doi: 10.1073/pnas.86.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koerner T. A., Jr, Cary L. W., Bhacca N. S., Younathan E. S. Tautomeric composition of D-fructose phosphates in solution by Fourier transform carbon-13 nuclear magnetic resonance. Biochem Biophys Res Commun. 1973 Apr 2;51(3):543–550. doi: 10.1016/0006-291x(73)91348-x. [DOI] [PubMed] [Google Scholar]
- Koerner T. A., Jr, Voll R. J., Cary L. W., Younathan E. S. Carbon-13 nuclear magnetic resonance studies and anomeric composition of ketohexose phosphates in solution. Biochemistry. 1980 Jun 10;19(12):2795–2801. doi: 10.1021/bi00553a040. [DOI] [PubMed] [Google Scholar]
- Libby C. B., Frey W. A., Villafranca J. J., Benkovic S. J. Kinetic and binding studies of Mn (II) and fructose 1,6-bisphosphate with rabbit liver hexosebisphosphatase. J Biol Chem. 1975 Oct 10;250(19):7564–7573. [PubMed] [Google Scholar]
- Liu F., Fromm H. J. Kinetic studies on the mechanism and regulation of rabbit liver fructose-1,6-bisphosphatase. J Biol Chem. 1990 May 5;265(13):7401–7406. [PubMed] [Google Scholar]
- MacGregor J. S., Hannappel E., Xu G. J., Pontremoli S., Horecker B. L. Conservation of primary structure at the proteinase-sensitive site of fructose 1,6-bisphosphatases. Arch Biochem Biophys. 1982 Sep;217(2):652–664. doi: 10.1016/0003-9861(82)90547-1. [DOI] [PubMed] [Google Scholar]
- Marcus C. J. Inhibition of bovine hepatic fructose-1,6-diphosphatase by substrate analogs. J Biol Chem. 1976 May 25;251(10):2963–2966. [PubMed] [Google Scholar]
- Marcus F., Edelstein I., Reardon I., Heinrikson R. L. Complete amino acid sequence of pig kidney fructose-1,6-bisphosphatase. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7161–7165. doi: 10.1073/pnas.79.23.7161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus F., Edelstein I., Saidel L. J., Keim P. S., Heinrikson R. L. The covalent structure of pig kidney fructose 1,6-bisphosphatase: sequence of the 60-residue NH2-terminal peptide produced by digestion with subtilisin. Arch Biochem Biophys. 1981 Jul;209(2):687–696. doi: 10.1016/0003-9861(81)90330-1. [DOI] [PubMed] [Google Scholar]
- Marcus F., Harrsch P. B. Amino acid sequence of spinach chloroplast fructose-1,6-bisphosphatase. Arch Biochem Biophys. 1990 May 15;279(1):151–157. doi: 10.1016/0003-9861(90)90475-e. [DOI] [PubMed] [Google Scholar]
- Marcus F., Harrsch P. B., Moberly L., Edelstein I., Latshaw S. P. Spinach chloroplast fructose-1,6-bisphosphatase: identification of the subtilisin-sensitive region and of conserved histidines. Biochemistry. 1987 Nov 3;26(22):7029–7035. doi: 10.1021/bi00396a026. [DOI] [PubMed] [Google Scholar]
- McClard R. W., Atkinson D. E. Effects of fructose 6-phosphate and adenylates on the activities of rabbit liver fructose bisphosphatase and phosphofructokinase. Arch Biochem Biophys. 1979 Apr 15;194(1):236–243. doi: 10.1016/0003-9861(79)90614-3. [DOI] [PubMed] [Google Scholar]
- Meek D. W., Nimmo H. G. The interaction of fructose 2,6-bisphosphate with an allosteric site of rat liver fructose 1,6-bisphosphatase. FEBS Lett. 1983 Aug 22;160(1-2):105–109. doi: 10.1016/0014-5793(83)80946-6. [DOI] [PubMed] [Google Scholar]
- Midelfort C. F., Gupta R. K., Rose I. A. Fructose 1,6-bisphosphate: isomeric composition, kinetics, and substrate specificity for the aldolases. Biochemistry. 1976 May 18;15(10):2178–2185. doi: 10.1021/bi00655a023. [DOI] [PubMed] [Google Scholar]
- Nimmo H. G., Tipton K. F. The allosteric properties of beef-liver fructose bisphosphatase. Eur J Biochem. 1975 Oct 15;58(2):575–585. doi: 10.1111/j.1432-1033.1975.tb02408.x. [DOI] [PubMed] [Google Scholar]
- Raines C. A., Lloyd J. C., Longstaff M., Bradley D., Dyer T. Chloroplast fructose-1,6-bisphosphatase: the product of a mosaic gene. Nucleic Acids Res. 1988 Aug 25;16(16):7931–7942. doi: 10.1093/nar/16.16.7931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandran G. N., Sasisekharan V. Conformation of polypeptides and proteins. Adv Protein Chem. 1968;23:283–438. doi: 10.1016/s0065-3233(08)60402-7. [DOI] [PubMed] [Google Scholar]
- Rogers D. T., Hiller E., Mitsock L., Orr E. Characterization of the gene for fructose-1,6-bisphosphatase from Saccharomyces cerevisiae and Schizosaccharomyces pombe. Sequence, protein homology, and expression during growth on glucose. J Biol Chem. 1988 May 5;263(13):6051–6057. [PubMed] [Google Scholar]
- Scheffler J. E., Fromm H. J. Regulation of rabbit liver fructose-1,6-bisphosphatase by metals, nucleotides, and fructose 2,6-bisphosphate as determined from fluorescence studies. Biochemistry. 1986 Oct 21;25(21):6659–6665. doi: 10.1021/bi00369a050. [DOI] [PubMed] [Google Scholar]
- Swenson C. A., Barker R. Proportion of keto and aldehydo forms in solutions of sugars and sugar phosphates. Biochemistry. 1971 Aug 3;10(16):3151–3154. doi: 10.1021/bi00792a026. [DOI] [PubMed] [Google Scholar]
- TAKETA K., POGELL B. M. ALLOSTERIC INHIBITION OF RAT LIVER FRUCTOSE 1,6-DIPHOSPHATASE BY ADENOSINE 5'-MONOPHOSPHATE. J Biol Chem. 1965 Feb;240:651–662. [PubMed] [Google Scholar]
- Tejwani G. A. Regulation of fructose-bisphosphatase activity. Adv Enzymol Relat Areas Mol Biol. 1983;54:121–194. doi: 10.1002/9780470122990.ch3. [DOI] [PubMed] [Google Scholar]
- Van Schaftingen E. Fructose 2,6-bisphosphate. Adv Enzymol Relat Areas Mol Biol. 1987;59:315–395. doi: 10.1002/9780470123058.ch7. [DOI] [PubMed] [Google Scholar]
- Xu G. J., Datta A. G., Singh V. N., Suda H., Pontremoli S., Horecker B. L. Rabbit liver fructose 1,6-bisphosphatase: labeling of the active and allosteric sites with pyridoxal 5-phosphate and sequence of a nonapeptide from the active site. Arch Biochem Biophys. 1981 Aug;210(1):98–103. doi: 10.1016/0003-9861(81)90168-5. [DOI] [PubMed] [Google Scholar]
- Xu G. J., Natalini P., Suda H., Tsolas O., Dzugaj A., Sun S. C., Pontremoli S., Horecker B. L. Rabbit liver fructose-1,6-bisphosphatase: location of an active site lysyl residue in the COOH-terminal fragment generated by a lysosomal proteinase. Arch Biochem Biophys. 1982 Apr 1;214(2):688–694. doi: 10.1016/0003-9861(82)90075-3. [DOI] [PubMed] [Google Scholar]
- el-Maghrabi M. R., Pilkis J., Marker A. J., Colosia A. D., D'Angelo G., Fraser B. A., Pilkis S. J. cDNA sequence of rat liver fructose-1,6-bisphosphatase and evidence for down-regulation of its mRNA by insulin. Proc Natl Acad Sci U S A. 1988 Nov;85(22):8430–8434. doi: 10.1073/pnas.85.22.8430. [DOI] [PMC free article] [PubMed] [Google Scholar]



