Abstract
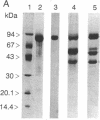
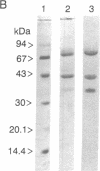
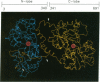
Two forms of lactoferrin, an intact lactoferrin and a "nicked" but apparently intact (i.e., 78-kDa) form, have been isolated from the urine of preterm infants fed human milk. These two forms of lactoferrin, demonstrated to be entirely of maternal origin, were copurified using affinity columns of immobilized single-stranded DNA-agarose. The relative concentrations of the intact lactoferrin and the "nicked" lactoferrin were determined after denaturation and separation by reverse-phase HPLC. N-terminal sequence analyses showed that the intact 78-kDa form had lost two residues from its N terminus. The nicked 78-kDa form was composed of only two fragments; one fragment was identified as the N terminus of the N-lobe (residues 3-283). The other fragment started with Ser-284 and included the alpha-helical structures at the C terminus of the N-lobe, as well as the entire C-lobe. Although no disulfide bonds connect these two fragments, they were tightly associated in vivo and were not separated in vitro except under denaturing conditions. Limited in vitro digestion of human milk lactoferrin with trypsin produced a nicked, but stable (78-kDa), form of DNA-binding lactoferrin nearly indistinguishable from the isolated urinary lactoferrin, except for the absence of one additional arginine residue at the N terminus of the N-lobe. Residues involved in the stable molecular interaction between fragments were evaluated using data obtained from the high-resolution crystal structure of hololactoferrin. Two features, entirely within the N-lobe, account for the lack of fragment dissociation after cleavage at residue 283 in vivo: an extensive interface at the hinge region behind the iron-binding cleft and an "anchor" sequence traversing the remainder of the N-lobe at 90 degrees relative to the fragment interface. These results document the remarkably limited degradation of absorbed lactoferrin in vivo and suggest that iron-binding activity, receptor-binding properties, and postulated immune cell regulatory functions remain intact.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson B. F., Baker H. M., Norris G. E., Rice D. W., Baker E. N. Structure of human lactoferrin: crystallographic structure analysis and refinement at 2.8 A resolution. J Mol Biol. 1989 Oct 20;209(4):711–734. doi: 10.1016/0022-2836(89)90602-5. [DOI] [PubMed] [Google Scholar]
- Anderson B. F., Baker H. M., Norris G. E., Rumball S. V., Baker E. N. Apolactoferrin structure demonstrates ligand-induced conformational change in transferrins. Nature. 1990 Apr 19;344(6268):784–787. doi: 10.1038/344784a0. [DOI] [PubMed] [Google Scholar]
- Bagby G. C., Jr, Rigas V. D., Bennett R. M., Vandenbark A. A., Garewal H. S. Interaction of lactoferrin, monocytes, and T lymphocyte subsets in the regulation of steady-state granulopoiesis in vitro. J Clin Invest. 1981 Jul;68(1):56–63. doi: 10.1172/JCI110254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beletskaya L. V., Gnezditskaya E. V. Hetero-organic thymus antigens. Thymus. 1985;7(6):377–385. [PubMed] [Google Scholar]
- Bennett R. M., Kokocinski T. Lactoferrin content of peripheral blood cells. Br J Haematol. 1978 Aug;39(4):509–521. doi: 10.1111/j.1365-2141.1978.tb03620.x. [DOI] [PubMed] [Google Scholar]
- Cox T. M., Mazurier J., Spik G., Montreuil J., Peters T. J. Iron binding proteins and influx of iron across the duodenal brush border. Evidence for specific lactotransferrin receptors in the human intestine. Biochim Biophys Acta. 1979 Nov 15;588(1):120–128. doi: 10.1016/0304-4165(79)90377-5. [DOI] [PubMed] [Google Scholar]
- Davidson L. A., Lönnerdal B. Persistence of human milk proteins in the breast-fed infant. Acta Paediatr Scand. 1987 Sep;76(5):733–740. doi: 10.1111/j.1651-2227.1987.tb10557.x. [DOI] [PubMed] [Google Scholar]
- Gnezditskaia E. V., Bukhova V. P., Zakharova N. A., Malkina L. A. Stimuliatsiia ékspressii retseptorov dlia IgM i IgG na limfotsitakh timusa cheloveka pri deistvii laktoferrina in vitro. Biull Eksp Biol Med. 1987 Apr;103(4):447–449. [PubMed] [Google Scholar]
- Goldblum R. M., Schanler R. J., Garza C., Goldman A. S. Human milk feeding enhances the urinary excretion of immunologic factors in low birth weight infants. Pediatr Res. 1989 Feb;25(2):184–188. doi: 10.1203/00006450-198902000-00021. [DOI] [PubMed] [Google Scholar]
- Goldman A. S., Garza C., Schanler R. J., Goldblum R. M. Molecular forms of lactoferrin in stool and urine from infants fed human milk. Pediatr Res. 1990 Mar;27(3):252–255. doi: 10.1203/00006450-199003000-00009. [DOI] [PubMed] [Google Scholar]
- Hutchens T. W., Henry J. F., Yip T. T., Hachey D. L., Schanler R. J., Motil K. J., Garza C. Origin of intact lactoferrin and its DNA-binding fragments found in the urine of human milk-fed preterm infants. Evaluation by stable isotopic enrichment. Pediatr Res. 1991 Mar;29(3):243–250. doi: 10.1203/00006450-199103000-00005. [DOI] [PubMed] [Google Scholar]
- Hutchens T. W., Henry J. F., Yip T. T. Purification and characterization of intact lactoferrin found in the urine of human milk-fed preterm infants. Clin Chem. 1989 Sep;35(9):1928–1933. [PubMed] [Google Scholar]
- Hutchens T. W., Magnuson J. S., Yip T. T. Interaction of human lactoferrin with DNA: one-step purification by affinity chromatography on single-stranded DNA-agarose. Pediatr Res. 1989 Dec;26(6):618–622. doi: 10.1203/00006450-198912000-00021. [DOI] [PubMed] [Google Scholar]
- Hutchens T. W., Yip T. T. Metal ligand-induced alterations in the surface structures of lactoferrin and transferrin probed by interaction with immobilized copper(II) ions. J Chromatogr. 1991 Jan 4;536(1-2):1–15. doi: 10.1016/s0021-9673(01)89232-3. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Magnuson J. S., Henry J. F., Yip T. T., Hutchens T. W. Structural homology of human, bovine, and porcine milk lactoferrins: evidence for shared antigenic determinants. Pediatr Res. 1990 Aug;28(2):176–181. doi: 10.1203/00006450-199008000-00019. [DOI] [PubMed] [Google Scholar]
- Metz-Boutigue M. H., Jollès J., Mazurier J., Schoentgen F., Legrand D., Spik G., Montreuil J., Jollès P. Human lactotransferrin: amino acid sequence and structural comparisons with other transferrins. Eur J Biochem. 1984 Dec 17;145(3):659–676. doi: 10.1111/j.1432-1033.1984.tb08607.x. [DOI] [PubMed] [Google Scholar]
- Morrissey J. H. Silver stain for proteins in polyacrylamide gels: a modified procedure with enhanced uniform sensitivity. Anal Biochem. 1981 Nov 1;117(2):307–310. doi: 10.1016/0003-2697(81)90783-1. [DOI] [PubMed] [Google Scholar]
- Nichols B. L., McKee K. S., Henry J. F., Putman M. Human lactoferrin stimulates thymidine incorporation into DNA of rat crypt cells. Pediatr Res. 1987 Jun;21(6):563–567. doi: 10.1203/00006450-198706000-00011. [DOI] [PubMed] [Google Scholar]
- Powell M. J., Ogden J. E. Nucleotide sequence of human lactoferrin cDNA. Nucleic Acids Res. 1990 Jul 11;18(13):4013–4013. doi: 10.1093/nar/18.13.4013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prentice A., Ewing G., Roberts S. B., Lucas A., MacCarthy A., Jarjou L. M., Whitehead R. G. The nutritional role of breast-milk IgA and lactoferrin. Acta Paediatr Scand. 1987 Jul;76(4):592–598. doi: 10.1111/j.1651-2227.1987.tb10526.x. [DOI] [PubMed] [Google Scholar]
- Prentice A., MacCarthy A., Stirling D. M., Vasquez-Velasquez L., Ceesay S. M. Breast-milk IgA and lactoferrin survival in the gastrointestinal tract--a study in rural Gambian children. Acta Paediatr Scand. 1989 Jul;78(4):505–512. doi: 10.1111/j.1651-2227.1989.tb17928.x. [DOI] [PubMed] [Google Scholar]
- Raha S., Dosquet C., Abgrall J. F., Jolles P., Fiat A. M., Caen J. P. KRDS--a tetrapeptide derived from lactotransferrin--inhibits binding of monoclonal antibody against glycoprotein IIb-IIIa on ADP-stimulated platelets and megakaryocytes. Blood. 1988 Jul;72(1):172–178. [PubMed] [Google Scholar]
- Rochard E., Legrand D., Mazurier J., Montreuil J., Spik G. The N-terminal domain I of human lactotransferrin binds specifically to phytohemagglutinin-stimulated peripheral blood human lymphocyte receptors. FEBS Lett. 1989 Sep 11;255(1):201–204. doi: 10.1016/0014-5793(89)81091-9. [DOI] [PubMed] [Google Scholar]
- Schanler R. J., Goldblum R. M., Garza C., Goldman A. S. Enhanced fecal excretion of selected immune factors in very low birth weight infants fed fortified human milk. Pediatr Res. 1986 Aug;20(8):711–715. doi: 10.1203/00006450-198608000-00002. [DOI] [PubMed] [Google Scholar]
- Spik G., Brunet B., Mazurier-Dehaine C., Fontaine G., Montreuil J. Characterization and properties of the human and bovine lactotransferrins extracted from the faeces of newborn infants. Acta Paediatr Scand. 1982 Nov;71(6):979–985. doi: 10.1111/j.1651-2227.1982.tb09560.x. [DOI] [PubMed] [Google Scholar]
- Yip T. T., Nakagawa Y., Porath J. Evaluation of the interaction of peptides with Cu(II), Ni(II), and Zn(II) by high-performance immobilized metal ion affinity chromatography. Anal Biochem. 1989 Nov 15;183(1):159–171. doi: 10.1016/0003-2697(89)90184-x. [DOI] [PubMed] [Google Scholar]