Abstract
To conduct a meta-analysis of clinical trials that examined the effect of music-supported therapy on stroke-induced motor dysfunction, comprehensive literature searches of PubMed, Embase and the Cochrane Library from their inception to April 2016 were performed. A total of 10 studies (13 analyses, 358 subjects) were included; all had acceptable quality according to PEDro scale score. The baseline differences between the two groups were confirmed to be comparable. Compared with the control group, the standardized mean difference of 9-Hole Peg Test was 0.28 (−0.01, 0.57), 0.64 (0.31, 0.97) in Box and Block Test, 0.47 (0.08, 0.87) in Arm Paresis Score and 0.35 (−0.04, 0.75) in Action Research Arm Test for upper-limb motor function, 0.11 (−0.24, 0.46) in Berg Balance Scale score, 0.09 (−0.36, 0.54) in Fugl-Meyer Assessment score, 0.30 (−0.15, 0.74) in Wolf Motor Function Test, 0.30 (−0.15, 0.74) in Wolf Motor Function time, 0.65 (0.14, 1.16) in Stride length and 0.62 (0.01, 1.24) in Gait Velocity for total motor function, and 1.75 (0.94, 2.56) in Frontal Assessment Battery score for executive function. There was evidence of a positive effect of music-supported therapy, supporting its use for the treatment of stroke-induced motor dysfunction. This study was registered at PRESPERO (CRD42016037106).
Stroke is a multifaceted and complicated condition. Stroke disease is one of the major causes of long-term disability and one of the leading causes of death worldwide1,2. The time frequency and functional source analysis of the signals facilitate the quantification of the functional changes occurring in the brain in association with motor tasks after stroke and the detection of damage to neuro-motor functioning3. The personal burden of being a stroke survivor is often devastating and has major consequences for the patient’s quality of life4. Rehabilitation of upper-limb motor dysfunction and total motor dysfunction have been revealed to improve the quality of life of patients after stroke5 and are safe and effective methods for restoring social and occupational functioning.
Motor dysfunction therapy relies on both pharmacological6 and non-pharmacological treatments7. Currently, pharmacological therapy is essentially symptomatic and does not have a satisfactory impact on symptoms related to the progression of neurodegenerative diseases. Therefore, several health institutions recommend the development of non-pharmacological complementary interventions as a first-line treatment. For example, intensive motor therapy can improve important motor functions. However, the effectiveness of standard physiotherapeutic approaches in stroke rehabilitation has been found to be limited8. In the human brain, one of the most powerful sources of auditory stimulation is provided by music9. As a result, more attention has been given to the effectiveness of non-pharmacological approaches in dysfunction therapy, including a growing interest in music therapy and music-based stimulation10.
The power of music and its nonverbal nature make it an effective medium of communication when language is diminished or abolished, though the curative effect of music is still uncertain. Music easily elicits movement, stimulating interactions between perception and action systems11. Thus, music-making may be an effective way to induce plastic changes in the motor system. Music-supported therapy is a prospective new series of therapy programs, and comprehensive research suggests that it could be useful because of its promotion of relaxation and of cognitive and motor improvement in post-stroke rehabilitation12. Therefore, music-supported therapy has been developed with the aim of improving motor recovery after stroke. The definition of music-supported therapy is not only hearing the music but also singing and playing rhythm and percussion instruments and is based on four principles: (i) massive repetition and exercising of simple finger and arm movements; (ii) auditory-motor coupling and integration and reinforcement of motor effects due to immediate auditory feedback; (iii) shaping and adapting the training according to individual progress; and (iv) emotion-motivation effects due to the playfulness and emotional impact of music and the acquisition of a new skill13. Music-supported therapy may involve, for example, rhythmic auditory stimulation14, the use of a MusicGlove15 or listening to CDs16. However, the differences between these music-supported techniques have not been comprehensively considered.
Music-supported therapy has been shown to be effective in post-stroke rehabilitation of motor function in some clinical trials14,15,16,17,18,19,20,21,22,23. However, little research has focused on the potential therapeutic mechanisms by which music-supported therapy improves the motor functions of post-stroke patients. Although many researchers suggest that improvement induced by music-supported therapy is due to the combined effects of intensive repetitive practice and musical stimulation21, evidence to support these propositions has been unavailable. To explore the isolated effect of music further, we designed a systematic review on the effect of music-supported therapy on the recovery of upper-limb motor function and total motor function after stroke. No previous reviews have provided a comprehensive overview with meta-analyses.
Results
Baseline characteristics
In total, 10 eligible studies (13 analyses, 358 participants)14,15,16,17,18,19,20,21,22,23 were identified and incorporated into the systematic review and meta-analysis (Fig. 1). Of these 10 studies, 7 were randomized controlled trials (RCT), 2 of them as controlled clinical trials (CCT), and 1 was a randomized crossover trial (RCT/crossover). All included studies assessed motor function as an outcome. Summaries of the characteristics of the included trials appear in Table 1 and Table S1. The studies were conducted in a wide range of countries and continents, the publication dates ranged from 2003 to 2016, and the included studies had between 12 and 62 subjects. Table S1 summarizes the detailed characteristics of the music-supported therapy group and the control group. The statistics showed that the two groups had similar characteristics in terms of age, stroke type, time post stroke, and position (left/right). There was some evidence of a difference in gender between the two groups; however, the difference was small. In particular, baseline characteristics were comparable (Table 1).
Figure 1. Flow of studies through the review process for systematic review and meta-analysis.
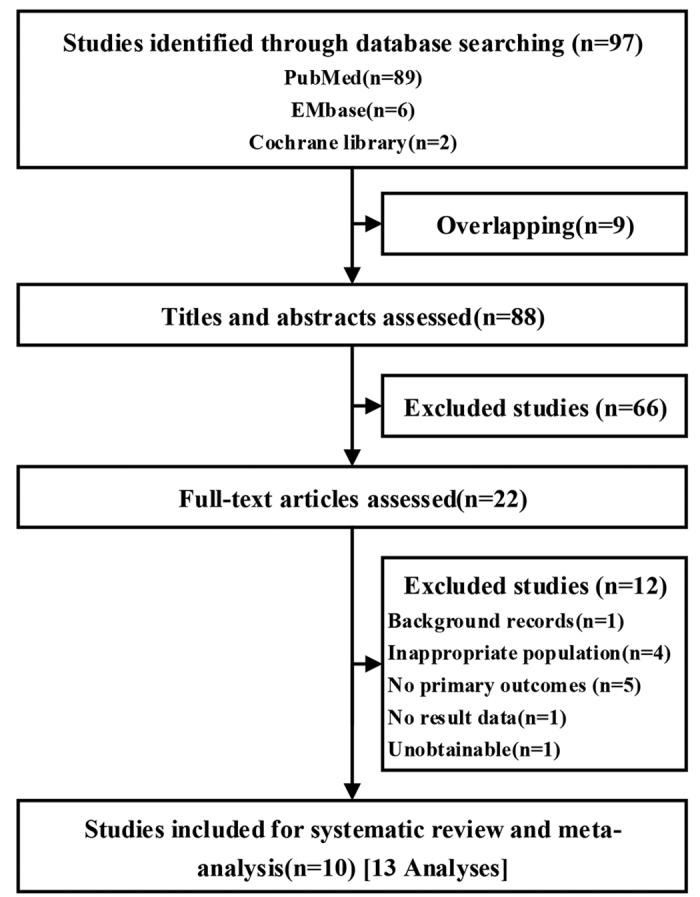
Table 1. Overall data of tests and baseline characteristics of included studies.
| Motor function tests | Studies (Analyses) | Participants (Intervention:control) | Age (SMD) | Gender (RR) | Stroke type (Hemorrhage/Ischemia,RR) | Position (Left/Right,RR) | Time post stroke (SMD) | trail design (RCT:CCT:RCT/crossover) | Delivery (Hospital:Rehabilitation centers) | Comparator (Active:usual care) | Quality (low/high) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Upper limb motor function | 9-Hole Peg Test (9HPT,Pegs-minutes)15,17,19,21 | 4 (5) | 172 (83:89) | 0.28 (−0.13,0.70) | 0.73 (0.56, 0.95)* | 0.83 (0.39, 1.8) | 1.10 (0.73,1.65) | 0.87 (−1.33, 3.07) | 1:2:1 | 4:0 | 2:3 | 3:1 |
| Box and Block Test (BBT,blocks/min)15,19,21 | 4 (4) | 138 (64:74) | 0.29 (−0.09, 0.67) | 0.69 (0.52,0.93)* | 1.30 (0.50, 3.37) | 1.06 (0.65, 1.72) | — | 0:2:1 | 3:0 | 2:2 | 2:1 | |
| Arm Paresis Score (APS)19,21 | 2 (2) | 102 (52:50) | 0.28 (−0.11, 0.67) | 0.69 (0.52,0.93)* | 1.92 (0.71, 5.23) | 1.06 (0.65, 1.72) | — | 0:2:0 | 2:0 | 0:2 | 2:0 | |
| Action Research Arm Test (ARAT)19,21 | 2 (2) | 102 (52:50) | 0.28 (−0.11, 0.67) | 0.69 (0.52,0.93)* | 1.92 (0.71, 5.23) | 1.06 (0.65, 1.72) | — | 0:2:0 | 2:0 | 0:2 | 2:0 | |
| Total motor function | Berg Balance Scale (score)14,17,22 | 2 (3) | 73 (36:37) | 0.20 (−0.26, 0.66) | 0.93 (0.63, 1.39) | 2.65 (0.97, 7.27) | 1.13 (0.79, 1.61) | −0.34 (−0.81, 0.12) | 2:0:0 | 1:1 | 2:0 | 0:2 |
| Fugl-Meyer assessment (FMA, score)15,18 | 2 (3) | 66 (27:39) | — | — | — | — | — | 1:0:1 | 1:1 | 2:1 | 0:2 | |
| Wolf motor function test (score)15,18 | 2 (3) | 66 (27:39) | — | — | — | — | — | 1:0:1 | 1:1 | 2:1 | 0:2 | |
| Wolf motor function test (time)15,18 | 2 (3) | 66 (27:39) | — | — | — | — | — | 1:0:1 | 1:1 | 2:1 | 0:2 | |
| Stride length (cm)14,23 | 2 (3) | 43 (21:22) | −0.20 (−0.84, 0.40) | — | — | 1.18 (0.82, 1.70) | 0.94 (0.14, 1.75) | 2:0:0 | 1:1 | 0:2 | 0:2 | |
| Gait Velocity (cm/s)14,23 | 2 (2) | 43 (21:22) | −0.20 (−0.84, 0.40) | — | — | 1.18 (0.82, 1.70) | 0.94 (0.14, 1.75) | 2:0:0 | 1:1 | 0:2 | 0:2 | |
| Executive functions | Frontal Assessment Battery (FAB, score)16,20 | 2 (8) | 84 (39:45) | −0.12 (−0.49, 0.25) | 0.97 (0.67, 1.38) | — | — | — | 2:0:0 | 2:0 | 2:2 | 0:2 |
*Results with significant differences.
Researchers generally trained the subjects with music in an interactive way, meaning that subjects not only passively listened to music on a CD player but also sang and played rhythm and percussion instruments. Comparator descriptions varied and included active control (such as tabletop exercises or audio books) and usual therapy. The duration of music-supported therapy varied between 2 weeks to 6 months.
Methodological quality
The assessments of study quality are presented in Table 1 and Table S2. The result of the PEDro scale score showed that all of our included studies had acceptable quality. Most of the studies mentioned were blinded, but a few of them were described as double-blinded (blinding to participants and therapists). Furthermore, the quality of the studies was positively correlated with the designs of the trials: RCT and RCT/crossover were superior to CCT.
Efficacy of music-supported therapy on upper-limb motor function
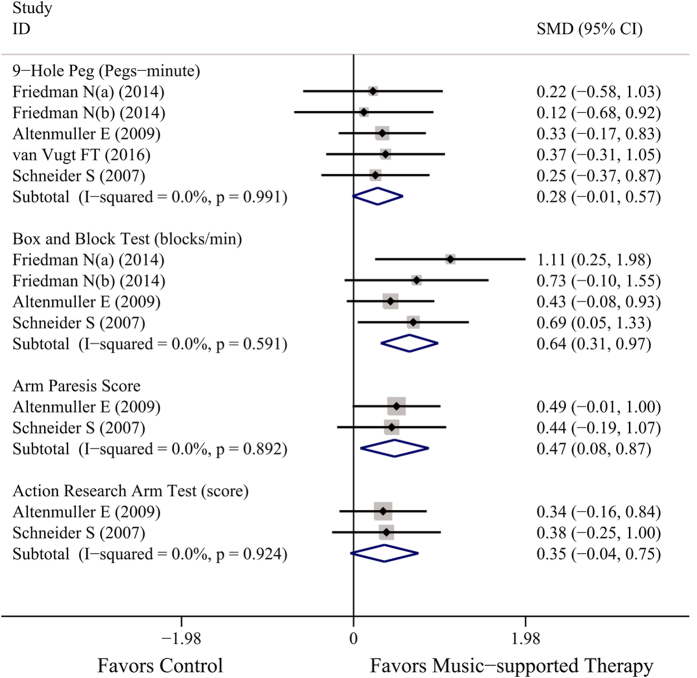
The included studies were suitable for meta-analysis of upper-limb motor function (Fig. 2), and all of the data were tested after the therapy ended. These studies contributed to four separate subanalyses, each with different types of evaluated measures.
Figure 2. Overall efficacy of music-supported therapy for upper-limb motor function.
For studies that evaluated the effect of music-supported therapy using the 9-Hole Peg Test (9HPT, Pegs-minute)15,17,19,21, the result (SMD = 0.29, 95% CI: −0.01~0.57) revealed no significant difference between the two arms with low heterogeneity (P = 0.991, I2 = 0%) among the included studies. However, the confidence interval does not exclude the possibility of a positive effect meaning that music may have a positive trend to adjust motor function.
Four analyses (3 studies)15,19,21 reported the effect of the Box and Block Test (BBT, blocks/min), and the merged result showed not only a significant positive effect of music-supported therapy (SMD = 0.64, 95% CI: 0.31~0.97) but also no heterogeneity (P = 0.591, I2 = 0%) among the included studies.
Two trials19,21 also presentedthe Arm Paresis Score (APS) and the Action Research Arm Test (ARAT) for patients; however, we were only able to find a significant difference in the ARS test (SMD = 0.47, 95% CI: 0.08~0.87) and no heterogeneity among the two types of tests (I2 = 0%).
Efficacy of music-supported therapy for total motor function
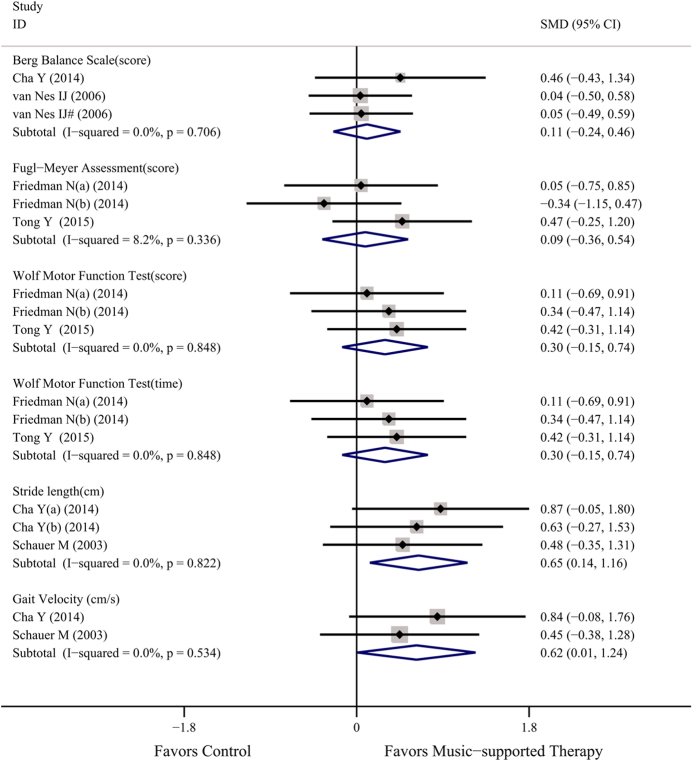
The included studies were suitable for the meta-analysis of total motor function (Fig. 3), and the results at the end of treatment and follow-up were merged for analysis. These studies contributed to six separate sub-analyses, each with different types of evaluated measures.
Figure 3. Overall efficacy of music-supported therapy for total motor function.
Two studies (3 analyses) reported the Berg Balance Scale (BBS) score14,22, each providing two results, one at the end of treatment and one at follow-up. There was no heterogeneity between the trials (P = 0.706, I2 = 0%). In a random effects meta-analysis, the SMD was 0.11 (95% CI: −0.24~0.46), suggesting that music-supported therapy might be beneficial to improve total motor function, although no significant difference was found between the two groups.
Two studies15,18 presented the Fugl-Meyer assessment score (FMA), the Wolf Motor Function Test score (WMFT) and the Wolf Motor Function Test time, with no heterogeneity found in any of the 3 evaluated measures (P > 0.05, I2 < 10%). The results of the FMA (SMD = 0.09, 95% CI: −0.36~0.54), the WMFT score (SMD = 0.30, 95% CI: −0.15~0.74) and the WMFT time (SMD = 0.30, 95% CI: −0.15~0.74) revealed that music-supported therapy group achieved better curative effects than the control group.
Total motor function could also be reported by stride length (cm) from 3 therapy-ending data14,23, and the merged results favored the music-supported therapy group (SMD = 0.65, 95% CI: 0.14~1.16) and showed no heterogeneity among studies (P = 0.822, I2 = 0%). These two studies also reported Gait Velocity (cm/s). No significant heterogeneity could be found (P = 0.534, I2 = 0%). The result (SMD = 0.62, 95% CI: 0.01~1.24) revealed that a significant difference existed between the two groups.
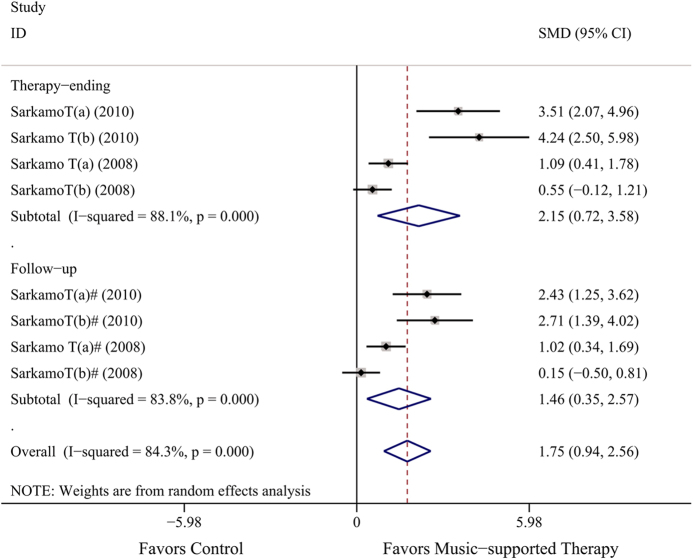
Efficacy of music-supported therapy for executive function
The overall effect on executive function was evaluated by the Frontal Assessment Battery (FAB) score, which was 1.75 (SMD, 95% CI: 0.94~2.56) from 4 therapy-ending data and 4 follow-up data15,20, revealing significant differences between the two groups. This means that music-supported therapy could improve executive function, although heterogeneity among studies was found (P = 0.000, I2 = 84.3%). Because of intensive heterogeneity, we did a subgroup analysis by time of data evaluated. In the therapy-ending group, the result of SMD was 2.35 (95% CI: 0.72~3.58; P = 0.000, I2 = 88.1%), and the SMD was 1.46 in the follow-up group (95% CI: 0.35~2.57; P = 0.000, I2 = 83.8%, Fig. 4).
Figure 4. Overall efficacy of music-supported therapy for executive function.
Discussion
Our meta-analysis suggests that music-supported therapy has a positive effect on motor function as evaluated by the following instruments: the 9-Hole Peg Test, the Box and Block Test, the Arm Paresis Score and the Action Research Arm Test for upper limb motor function; the Berg Balance Scale, the Fugl-Meyer Assessment, the Wolf Motor Function Test, and Stride length and Gait Velocity for total motor function; and the Frontal Assessment Battery for executive function. This finding was based on a comprehensive systematic review including 10 studies (13 analyses), and nearly 400 subjects. Most trials suggested that music-supported therapy was associated with improvements in motor function. However, some outcomes did not reach statistical significance, and heterogeneity existed in others (Figs 2, 3 and 4).
For upper-limb function, four measures showed the same trend, although only two were significant (BBT and APS), and the differences in effect size were modest. The BBT (Fig. 2), which quantifies important skills—grasp and transport—and is simple and quick to administer, is more objective than the 9-HPT, APS and ARAT because it depends less on the semi-subjective rating of the evaluator. The BBT has excellent reliability and is correlated with APS and ARAT. For the six measures of total motor function, well-established clinical measures only demonstrated a positive direction of music-supported therapy, such as the BBS, while the sensitivity of the WMFT was better than that of the FMA. Moreover, significant differences appeared in stride length and gait velocity (cm/s) (Fig. 3). For executive function, both time points were significant with high heterogeneity (Fig. 4).
To determine the source of homogeneity, we used subgroup analysis on the outcome of the Frontal Assessment Battery. However, no significant differences existed between the two subgroups of the evaluation time, meaning that the leading cause of homogeneity was not found. In our meta-analysis, two of these evaluation instruments included the data at the assessment time of follow-up. The result demonstrated that, whether at the end of treatment or during follow-up after a period of time, music-supported therapy was effective. Moreover, results may be better at the end of treatment (Figs 3 and 4). Though we only used eleven instruments for assessment, more indicators in our included fundamental research are reported, such as MMNm ampliture16, SS-QoL score14, etc. Although these indicators have not been included in our meta-analysis because few analyses used them (less than two), they also showed a favorable effect of music-supported therapy. Although lacking data, the included trials were compliant with a good standard of quality, and we believe that this meta-analysis is the most comprehensive systematic review so far to investigate the use of music-supported training in stroke-induced motor dysfunction therapy. No adverse effects were reported in our included fundamental studies.
Our conclusion by meta-analysis should be verified. A published article confirmed that music-supported therapy is a viable intervention to improve motor function in chronic stroke patients13, which is consistent with the results of our meta-analysis. Moreover, the results of one study demonstrate the feasibility of rhythmic auditory stimulation to enhance gait training, which warrants further investigation of the protocol to demonstrate the effects of rhythmic auditory stimulation in stroke rehabilitation24. Furthermore, a case-report study assessed technology-aided intervention programs, which involved activating music for two post-coma men who had re-acquired consciousness, and participants enjoyed the intervention sessions with the programs and reported that the programs had beneficial effects for them25. The significance of that study is not just in supporting music-supported therapy but also in showing that technology-aided intervention programs that included music also had a positive effect. Not only our included population should be considered. Jamali S.26 and Amengual J.L.27 in their experiment used healthy subjects as a comparator, and their conclusions supported the use of music-supported therapy for chronic stroke patients. While Tan L.F.28 researched only healthy participants, the music-supported therapy group also experienced the expected effects.
The present meta-analysis has several limitations. We undertook this systematic review with a comprehensive search strategy, and although there were no data and language restrictions, it was impossible to include all published and unpublished literature, especially the unpublished literature. Furthermore, positive results are easy to publish, but negative results are not likely to leave the laboratory. Another limitation was that many of the included studies had very small sample sizes (the average sample size was less than 40), which means that many of our included studies may have lacked test powers to detect differences between the intervention group and control group. An additional limitation of many outcomes was their extensive heterogeneity, which indicated substantial variability in the outcomes of the included studies, although this was often because of the presence of baseline differences (Table 1) and anticipated differences in trial design, populations and country. For example, if the difference in gender is too large, it may lead to heterogeneity. Moreover, lack of randomization, inadequate randomization and allocation concealment were more likely to lead to heterogeneity. Subgroup analyses generally did not substantially explain and reduce the heterogeneity: we used a random effects model that takes heterogeneity into account, and the results could be explained as reflecting the average result across the group of studies. Finally, publication bias could not be excluded because the funnel plots were not able to assess publication bias in our meta-analysis due to the limited number of trials.
The beneficial effects of music-supported therapy on participants are consistent with expectations and perceptions of music. Several possible and potential mechanisms could help to explain the effects of music training on neurodegenerative symptoms. Several potential mechanisms could help to explain the effects of music-supported therapy. For example, these mechanisms may involve structural and functional neural reorganization in the brain following injury29. The understanding of the brain’s plastic properties has led to the emergence of new approaches in stroke rehabilitation30. However, the mechanisms underlying successful musical neurodegenerative dysfunction rehabilitation are not well understood. The discovery of the clinical effectiveness of rhythmic motor entertainment also brought into focus for the first time that the structural elements of music have enormous potential in clinical applications to retrain the injured brain31.
Previous narrative reviews of non-pharmacological therapy have reported positive results. Generally speaking, a previous meta-analysis32 based on stroke patients had similar trends as the results we obtained, meaning that non-pharmacological therapy will be important to pursue in future clinical practice. Music is a non-pharmacological, non-invasive, non-adverse reaction and inexpensive intervention training that can be delivered easily and successfully. Further clinical trials of music-supported therapy should include large sample sizes, be robust, and be randomized to confirm the effect of music-supported therapy, particularly on patient-relevant or disease-specific outcomes. Further studies should ensure that the appropriate methods are used for randomization, blinding and intent-to-treat. Further trials should assess outcomes using standardized or prescribed measures at similar time points. Analyses of individual data would be valuable for further exploration. More normative studies will be used for further meta-analysis.
In summary, there was evidence of a positive effect of music-supported therapy, supporting its use for the treatment of motor dysfunction. On a local scale, patients with stroke-induced motor dysfunction could be encouraged to undertake music-supported therapy.
Methods
We followed the standards set by the systematic review and meta-analyses (PRISMA)33 statement, and our study was conducted according to the protocol registered with PROSPERO (Number CRD42016037106)34.
Eligibility criteria
We included randomized controlled trials, controlled clinical trials, and randomized crossover trials that compared music-supported therapy to no music therapy or to usual care of patients with stroke-induced motor dysfunction. We considered trials including motor function tests except those that did not use motor function as the leading indicator. Patients diagnosed with any type of stroke by each individual study were accepted.
We searched PubMed, Embase and Cochrane Library for clinical trials published up to April 2016. There were no language restrictions for the search. We combined both MeSH and free text for identifying relevant literature.
Data collection, extraction and quality assessment
Two investigators (ZYS and CJY) examined study eligibility. Both independently extracted and tabulated data from the studies on a standardized data extraction form, and disagreements were resolved through consensus or referral to a third reviewer (ZMY or ZQC). Discrepancies and unobtainable data were resolved by group discussion of at least three investigators. Randomized controlled trails (RCT), controlled clinical trials (CCT) and randomized crossover trials (RCT/crossover, before-after studies without control groups) were eligible for this meta-analysis.
We extracted baseline information on publication, year, country, study design, participants (n, age, male%), stroke type, position (left/right), delivery, etc. from each study. The design of every individual study was also used as baseline information, including intervention method, intervention frequency and duration, and outcome assessment time.
We assessed the quality of the included studies with the Physiotherapy Evidence Database (PEDro) scale score35. The PEDro is an 11-item scale to assess the quality of clinical trials. If the answer of the first item was “NO”, the study was excluded from meta-analysis. When the PEDro score is >4 (max score was 10), the study is considered high quality. Different trial designs result in a different score (RCT, CCT, other and unclear), thus affecting the final scores. Data extraction and quality assessment were performed independently by two investigators and adjudication by the third when required.
Outcome measures
The outcome was motor function, which included upper limb motor function and total motor function; meta-analysis was suitable for this outcome, although we used various tests to obtain it. The 9-Hole Peg Text (9HPT, Pegs-minute)21, Box and Block Test (BBT)36, Arm Paresis Score (APS) and Action Research Arm Test (ARAT)37 are used for upper-limb motor function assessment. Furthermore, the Berg Balance Scale (BBS)38, Fugl—Meyer assessment (FMA)39, Wolf motor function test (WMFT)40, and Stride length (SL) and gait velocity (cm/s)41 are used for total motor function assessment. Moreover, the Frontal Assessment Battery (FAB)42 was used to evaluate executive function, which involves performing a set of short mental and motor tasks.
Statistical analysis
We tabulated the characteristics and results of all included studies. The statistical heterogeneity was also tested by I2, when I2 < 25% was identified as low heterogeneity43. We used a random effects model for heterogeneity because we assumed that there would be heterogeneity between studies using the P value (P < 0.05) and I2 statistic (I2 > 50%). All instruments were continuous variables, and we analyzed the SMD in the change from baseline and the 95% confidence interval (CI) for analysis. For studies that reported multiple interventions and comparators, we defined them as substudies to avoid double-counting and data neglect, and results from crossover studies were included in additional analyses (defined as sub-studies). We used StataMP statistical software (version 14, Stata Co. College Station, TX, United States) for meta-analysis.
Additional Information
How to cite this article: Zhang, Y. et al. Improvement in Stroke-induced Motor Dysfunction by Music-supported Therapy: A Systematic Review and Meta-analysis. Sci. Rep. 6, 38521; doi: 10.1038/srep38521 (2016).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Material
Footnotes
The authors declare no competing financial interests.
Author Contributions Z.Y.S. and C.J.Y. conceived and designed the experiment; Z.Y.S., Z.Y.Q., R.T.S. and Z.Q.C. performed the experiments; Z.Y.S., C.J.Y. and Z.M.Y. analyzed the data; C.J.Y., Z.M.Y. and Z.Q.C. contributed materials/analysis tools; Z.Y.S. and C.J.Y. wrote the paper; all authors reviewed and approved the final manuscript.
References
- Koski L., Mernar T. J. & Dobkin B. H. Immediate and long-term changes in corticomotor output in response to rehabilitation: correlation with functional improvements in chronic stroke. Neurorehabilitation and Neural Repair 18(4), 230–249 (2004). [DOI] [PubMed] [Google Scholar]
- Cramer S. C. & Nudo R. J. (Eds.) Brain repair after stroke Cambridge University Press (2010). [Google Scholar]
- Comani S. et al. Assessing neuro-motor recovery in a stroke survivor with high-resolution EEG, robotics and Virtual Reality. In Engineering in Medicine and Biology Society (EMBC), 2015 37th Annual International Conference of the IEEE (pp. 3925–3928). IEEE (2015, August). [DOI] [PubMed]
- Dowswell G., Lawler J., Dowswell T., Young J., Forster A. & Hearn J. Investigating recovery from stroke: a qualitative study. Journal of clinical nursing 9(4), 507–515 (2000). [DOI] [PubMed] [Google Scholar]
- Hamzat T. K. & Peters G. O. Motor function recovery and quality of life among stroke survivors in Ibadan, Nigeria. A 6-month follow-up study. European journal of physical and rehabilitation medicine 45(2), 179–183 (2009). [PubMed] [Google Scholar]
- Chollet F. et al. Fluoxetine for motor recovery after acute ischaemic stroke (FLAME): a randomised placebo-controlled trial. The Lancet Neurology 10(2), 123–130 (2011). [DOI] [PubMed] [Google Scholar]
- Samuelkamaleshkumar S., Reethajanetsureka S., Pauljebaraj P., Benshamir B., Padankatti S. M. & David J. A. Mirror therapy enhances motor performance in the paretic upper limb after stroke: a pilot randomized controlled trial. Archives of physical medicine and rehabilitation 95(11), 2000–2005 (2014). [DOI] [PubMed] [Google Scholar]
- Langhammer B. & Stanghelle J. K. Bobath or motor relearning programme? A comparison of two different approaches of physiotherapy in stroke rehabilitation: a randomized controlled study. Clinical rehabilitation 14(4), 361–369 (2000). [DOI] [PubMed] [Google Scholar]
- Sacks O. The power of music. Brain 129(10), 2528–2532 (2006). [DOI] [PubMed] [Google Scholar]
- Baran M. et al. Interdisciplinary concepts for design and implementation of mixed reality interactive neurorehabilitation systems for stroke. Physical therapy 95(3), 449–460 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zatorre R. J., Chen J. L. & Penhune V. B. When the brain plays music: auditory–motor interactions in music perception and production. Nature reviews neuroscience 8(7), 547–558 (2007). [DOI] [PubMed] [Google Scholar]
- Thaut M. H., Peterson D. A. & McIntosh G. C. Temporal entrainment of cognitive functions. Annals of the New York Academy of Sciences 1060(1), 243–254 (2005). [DOI] [PubMed] [Google Scholar]
- Ripollés P. et al. Music supported therapy promotes motor plasticity in individuals with chronic stroke. Brain imaging and behavior 1–19 (2015). [DOI] [PubMed] [Google Scholar]
- Cha Y., Kim Y., Hwang S. & Chung Y. Intensive gait training with rhythmic auditory stimulation in individuals with chronic hemiparetic stroke: A pilot randomized controlled study. NeuroRehabilitation 35(4), 681–688 (2014). [DOI] [PubMed] [Google Scholar]
- Friedman N. et al. Retraining and assessing hand movement after stroke using the MusicGlove: comparison with conventional hand therapy and isometric grip training. Journal of neuroengineering and rehabilitation 11(1), 1 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Särkämö T. et al. Auditory and cognitive deficits associated with acquired amusia after stroke: a magnetoencephalography and neuropsychological follow-up study. PLoS One 5(12), e15157 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Vugt F. T., Kafczyk T., Kuhn W., Rollnik J. D., Tillmann B. & Altenmüller E. The role of auditory feedback in music-supported stroke rehabilitation: A single-blinded randomised controlled intervention. Restorative neurology and neuroscience (Preprint), 1–16 (2016). [DOI] [PubMed] [Google Scholar]
- Tong Y. et al. Music-supported therapy (MST) in improving post-stroke patients’ upper-limb motor function: a randomised controlled pilot study. Neurological research 37(5), 434–440 (2015). [DOI] [PubMed] [Google Scholar]
- Altenmüller E., Marco-Pallares J., Münte T. F. & Schneider S. Neural Reorganization Underlies Improvement in Stroke-induced Motor Dysfunction by Music-supported Therapy. Annals of the New York Academy of Sciences 1169(1), 395–405 (2009). [DOI] [PubMed] [Google Scholar]
- Särkämö T. et al. Music listening enhances cognitive recovery and mood after middle cerebral artery stroke. Brain 131(3), 866–876 (2008). [DOI] [PubMed] [Google Scholar]
- Schneider S., Schönle P. W., Altenmüller E. & Münte T. F. Using musical instruments to improve motor skill recovery following a stroke. Journal of neurology 254(10), 1339–1346 (2007). [DOI] [PubMed] [Google Scholar]
- van Nes I. J. et al. Long-term effects of 6-week whole-body vibration on balance recovery and activities of daily living in the postacute phase of stroke a randomized, controlled trial. Stroke 37(9), 2331–2335 (2006). [DOI] [PubMed] [Google Scholar]
- Schauer M. & Mauritz K. H. Musical motor feedback (MMF) in walking hemiparetic stroke patients: randomized trials of gait improvement. Clinical rehabilitation 17(7), 713–722 (2003). [DOI] [PubMed] [Google Scholar]
- Hayden R., Clair A. A., Johnson G. & Otto D. The effect of rhythmic auditory stimulation (RAS) on physical therapy outcomes for patients in gait training following stroke: a feasibility study. International Journal of Neuroscience 119(12), 2183–2195 (2009). [DOI] [PubMed] [Google Scholar]
- Lancioni G. E. et al. Technology-aided recreation and communication opportunities for post-coma persons affected by lack of speech and extensive motor impairment. Research in developmental disabilities 34(9), 2959–2966 (2013). [DOI] [PubMed] [Google Scholar]
- Jamali S., Fujioka T. & Ross B. Neuromagnetic beta and gamma oscillations in the somatosensory cortex after music training in healthy older adults and a chronic stroke patient. Clinical Neurophysiology 125(6), 1213–1222 (2014). [DOI] [PubMed] [Google Scholar]
- Amengual J. L. et al. Sensorimotor plasticity after music-supported therapy in chronic stroke patients revealed by transcranial magnetic stimulation. PloS One 8(4), e61883 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan L. F., Dienes Z., Jansari A. & Goh S. Y. Effect of mindfulness meditation on brain–computer interface performance. Consciousness and cognition 23, 12–21 (2014). [DOI] [PubMed] [Google Scholar]
- Chopp M., Zhang Z. G. & Jiang Q. Neurogenesis, angiogenesis, and MRI indices of functional recovery from stroke. Stroke 38(2), 827–831 (2007). [DOI] [PubMed] [Google Scholar]
- Käll L. B., Lundgren-Nilsson Å., Blomstrand C., Pekna M., Pekny M. & Nilsson M. The effects of a rhythm and music-based therapy program and therapeutic riding in late recovery phase following stroke: a study protocol for a three-armed randomized controlled trial. BMC neurology 12(1), 1 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thaut M. H. The discovery of human auditory–motor entrainment and its role in the development of neurologic music therapy. Progress in brain research 217, 253–266 (2015). [DOI] [PubMed] [Google Scholar]
- Thieme H., Mehrholz J., Pohl M., Behrens J. & Dohle C. Mirror therapy for improving motor function after stroke. Stroke 44(1), e1–e2 (2013). [DOI] [PubMed] [Google Scholar]
- Moher D., Liberati A., Tetzlaff J. & Altman D. G. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Annals of internal medicine 151(4), 264–269 (2009). [DOI] [PubMed] [Google Scholar]
- Centre for Reviews and Dissemination. Systematic Reviews: CRD’s Guidance for Undertaking Reviews in Health Care (Internet). York, England: University of York; http://www.crd.york.ac.uk/prospero/register_new_review.asp?RecordID=37106&UserID=16909 (2009).
- Maher C. G., Sherrington C., Herbert R. D., Moseley A. M. & Elkins M. Reliability of the PEDro scale for rating quality of randomized controlled trials. Physical therapy 83(8), 713–721 (2003). [PubMed] [Google Scholar]
- Wade D. T., Langton-Hewer R., Wood V. A., Skilbeck C. E. & Ismail H. M. The hemiplegic arm after stroke: measurement and recovery. Journal of Neurology, Neurosurgery & Psychiatry 46(6), 521–524 (1983). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyle R. C. A performance test for assessment of upper limb function in physical rehabilitation treatment and research. International Journal of Rehabilitation Research 4(4), 483–492 (1981). [DOI] [PubMed] [Google Scholar]
- Berg K., Wood-Dauphinee S. & Williams J. I. The Balance Scale: reliability assessment with elderly residents and patients with an acute stroke. Scandinavian journal of rehabilitation medicine 27(1), 27–36 (1995). [PubMed] [Google Scholar]
- Fugl-Meyer A. R., Jääskö L., Leyman I., Olsson S. & Steglind S. The post-stroke hemiplegic patient. 1. a method for evaluation of physical performance. Scandinavian journal of rehabilitation medicine 7(1), 13–31 (1974). [PubMed] [Google Scholar]
- Wolf S. L., Lecraw D. E., Barton L. A. & Jann B. B. Forced use of hemiplegic upper extremities to reverse the effect of learned nonuse among chronic stroke and head-injured patients. Experimental neurology 104(2), 125–132 (1989). [DOI] [PubMed] [Google Scholar]
- Perry J. & Burnfield J. M. Gait analysis. Normal and pathological function 2nd ed. SLACK Incorporated Publishing, Thorofare, NJ (2010). [Google Scholar]
- Dubois B., Slachevsky A., Litvan I. & Pillon B. F. A. B. The FAB A frontal assessment battery at bedside. Neurology 55(11), 1621–1626 (2000). [DOI] [PubMed] [Google Scholar]
- Higgins J. & Thompson S. G. Quantifying heterogeneity in a meta-analysis. Statistics in medicin 21(11), 1539–1558 (2002). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.