Abstract
Aim.
To develop a CT predictor scale for the need for colectomy and to evaluate predictors of all-cause mortality within 30 days after diagnosis ofC. difficile infection (CDI).
Methods
We conducted a retrospective study of adult hospitalized patients whounderwent abdominal CT within 72 h of diagnosis of CDI.
Results
Presence of abnormal wall thickening in caecum (OR 8.0; CI 1.37–46.81; p = 0.021), transverse colon (OR 6.7; CI 1.15–35.60; p = 0.034), sigmoid colon (OR 12.6; CI 1.37–115.97; p = 0.025), pancolitis (OR 7.0; CI 1.36–36.01; p = 0.02) and bowel dilation (OR 16.5; CI 2.41–112.83; p = 0.004) predicted colectomy. With these values, a five parameter radiological scale from 0 to 24 was developed (sensitivity and NPV of 100%, cut-off of 6). Furthermore, wall thickening of caecum (OR 6.2; CI 1.06–35.57; p = 0.043), ascending colon (OR 12.0; CI 1.29–111.32; p = 0.029), descending colon (OR 17.0; CI 1.81–160.05; p = 0.013) and sigmoid (OR 10.2; CI 1.10–94.10; p = 0.041) independently predicted mortality within 30 days of CDI diagnosis.
Conclusion
We designed a CT scale to predict colectomy, able to rule out the development of fulminant colitis and the need for surgical procedure. Patients with wall thickening of the caecum, ascending, descending or sigmoid colon were more likely to die within 30 days of CDI diagnosis.
Keywords: Clostridium difficile, Colectomy, Abdominal CT scan, Nosocomial infection, Prediction score
Highlights
-
•
CT predictor scale for the need for colectomy in patients with CDI.
-
•
Abnormal wall thickening, pancolitis and bowel dilation predicted colectomy.
-
•
A five parameter radiological scale was developed with sensitivity and NPV of 100%.
-
•
Abnormal wall thickening increased likelihood of death within 30 days of CDI diagnosis.
1. Introduction
Clostridium difficile infection (CDI) is associated with severe illness, infection-related mortality of 5% and all-cause mortality of 15–20% [1]. Infection by this bacterial species is diagnosed in stool samples by either immunoassay for toxins A or B, PCR for bacterial DNA detection or anaerobic toxigenic culture. Computed tomography (CT) has shown a sensitivity of 52% and specificity of 93% for the diagnosis of CDI [2], but it is not rutinely used for diagnostic purposes. Several CT findings have been described in association with CDI including wall thickening, pancolitis, low-attenuation mural thickening, the accordion and target sign, pericolonic fat stranding, ascites and most recently, pleural effusion [3], [4]. Moreover, CT parameters have been proposed as predictors of severity and complicated C. difficile colitis. Among these, a CDI severity score that included several CT findings [5]. However, none has succeeded in adding a predictive value to CT findings on top of other clinical predictors of complicated CDI.
We sought to develop a CT predictor scale for the need for colectomy and to evaluate CT findings as predictors of all-cause mortality within 30 days of diagnosis of C. difficile infection.
2. Study and methods
2.1. Study design and population
We performed a retrospective review of hospitalized patients with a diagnosis of CDI between January 1, 2011 to September 30, 2015, who underwent abdominal CT within 72 h of diagnosis. The study was performed at the University Hospital “Dr. José Eleuterio González”, a 450-bed tertiary care hospital in Monterrey, Mexico.
Patients included were 18 years or older, and the diagnosis was determined by the Immunocard toxins A&B assay (Meridian Bioscience, Cincinnati, OH, USA), positive PCR (Cepheid XpertC. difficile/Epi) or presence of pseudomembranous colitis on colonoscopy.
Clinical data was collected from the time of diagnosis and throughout hospitalization. The primary outcome was defined as fulminant colitis with colectomy, and the secondary outcome was all-cause mortality within 30 days of diagnosis.
2.2. Radiologic evaluation
Each CT scan was retrospectively reviewed by two radiologists using a standardized form that included the following variables; colonic wall thickening, diffuse colonic involvement (pancolitis), target sign, accordion sign, pericolonic fat stranding, bowel dilation, pleural effusion, peritoneal fluid, bowel pneumatosis, pneumoperitoneum, presence of atheromatous plaques and perirenal fat stranding. Reviewers only knew the diagnosis of CDI but were blinded to the patients' clinical characteristics and outcomes. In the case of discrepancy both reviewers reevaluated the images at the same time for an agreement. All tests were performed on helical CT (GE LightSpeed VCT 64 Slice CT) and included both the abdomen and pelvis.
2.3. Study definitions
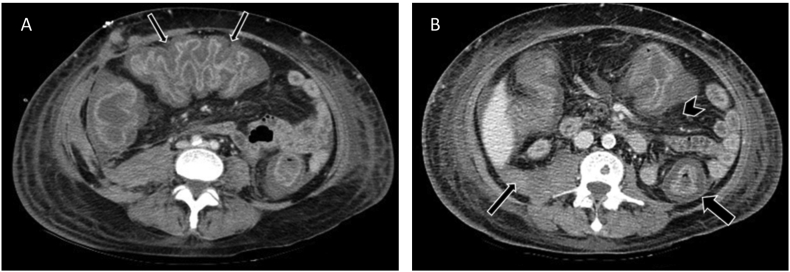
Wall thickening was measured in all segments (caecum, ascending, transverse, descending and sigmoid colon and rectum). The measurement was considered abnormal when the wall thickness was >3 mm from caecum to sigmoid colon and >4 mm in the rectum. Bowel dilation was defined as a transversal diameter measurement of >9 cm in the caecum and ascending colon, >6 cm in transverse, descending and sigmoid colon and >7 cm in the rectum. Pancolitis was described when wall thickness was present throughout caecum to the rectum. The accordion sign was reported when markedly thickened haustral folds were observed and give the appearance of alternating bands of high attenuation (mucosal hyperemia) and low attenuation (edematous haustra) (Fig. 1).The target sign was reported when there were mucosal hyperemia and submucosal edema or inflammation and recalled the appearance of a bull's-eye; it was observed at cross-section imaging of the colon. Pericolonic and perirenal fat stranding were defined as increased density of the fat striations adjacent to the colon and kidneys respectively. Peritoneal fluid was noted as any amount of free fluid in the peritoneal cavity with −20 to +20 Hounsfield units. Bowel pneumatosis was described as the presence of gas in the intestinal wall. The presence of pleural effusion, pneumoperitoneum, bowel pneumatosis and atheromatous plaques were also reported. Atheromatous plaques were reported as the presence of calcifications in the superior or inferior mesenteric artery.
Fig. 1.
Image A. Accordion sign in a patient with confirmed Clostridium difficile infection in transverse colon (thin arrows). Image B. Abdomen CT scan of a patient with confirmed Clostridium difficileinfection. Colonic wall thickness, target sign (thick arrow), peritoneal fluid (thin arrow) and pericolonic fat stranding (arrowhead) are shown.
2.4. Statistical analysis and scale
Clinical characteristics and demographic variables were described using dispersion methods. Proportions were compared with Pearson chi-squared test or the Fisher exact test. Those with a p value ≤ 0.05 from the univariate analysis served as independent variables in a subsequent logistic regression model, presented as odds ratios with their 95% confidence intervals (CIs).
A scale was elaborated with these predictor variables; points were assigned proportionally to odds ratio and then scaled from 0 to 24.A receiver operator curve (ROC) was constructed, and the area under the curve was analyzed. Sensitivity, specificity, and positive and negative predictive values were calculated. Data were analyzed with SPSS 20 software.
3. Results
Out of176 patients diagnosed with CDI during our study period, 34 underwent abdominal CT within 72 h of diagnosis. Of these34 patients, 20 were male (58.8%) and 14 female (41.2%). Patients had an average of 51.2 years (±16.9). Patients for whom a CT was performed had a mean Charlson comorbidity index of 2.09 (±2.4) and 33 out of 34 patients had previous antibiotic use (97.1%). The ATLAS [6] scoring system was used to evaluate disease severity; in the 34 patients, there was a mean score of 5.9 (range 1–9). Use of steroids or other immunosuppressants was observed in 12 subjects (35.3%). Nineteen patients (55.9%) had experienced fever and 70.6% (n = 24) abdominal pain previous to diagnosis. Mean WBC count was 19.2 ± 12.1 and mean albumin level was 2.1 ± 0.6. Fourteen out of 34 patients (41.1%) had a colonoscopy procedure, of which, 13 revealed pseudomembranous colitis.
All-cause mortality within 30 days of diagnosis was the outcome in 9 out of 34 patients (26.5%); of these, 7 (20.6%) had CDI as the primary cause of death. Ten out of 34 patients (29.4%) underwent colectomy for fulminant colitis, of these, post-colectomy mortality was reported in 3 patients (8.8%). Recurrent CDI was observed in 4 (11.8%) patients, with a mean of 2.7 ± 9.8 days for relapse.
We further compared vital signs in patients who underwent colectomy versus no-colectomy obtained 72 h from either CDI diagnosis or preoperative, respectively. A mean heart rate of 102.4 bpm (±9.4), respiratory rate of 19 (±6.1) rpm and a mean arterial pressure of 89.1 (±12.2) for the colectomy group was reported. While a heart rate of 78.15 (±11.7) bpm, respiratory rate of 20.6 (±6.2) and mean arterial pressure of 86.6 (±10.2) was described in the no-colectomy group. However, only heart rate showed statistical significance when compared to such outcome (p ≤ 0.001).
3.1. CT scan
A CT scan was considered positive when having abnormal wall thickening, either segmental or total, colon dilation or presence of positive pseudomembranous colitis signs such as accordion and target signs. In our study, 21 out of 34 scans (61.8%) were categorized as positive. A mean of 2.60 ± 2.5 days from CT to colectomy was calculated. In our study, we did not evaluate CT's diagnostic accuracy, however, we calculated CT's ability to predict severe outcomes in CDI. When evaluating if positive CT scans were associated with increased likelihood of severe outcomes, positive CT scans were more frequent in patients who underwent colectomy (OR 1.9; CI 1.27–2.87; p = 0.005) and in patients who presented all-cause mortality within 30 days of diagnosis (OR 1.8; CI 1.21–2.54; p = 0.006). There was no difference in patients with post-colectomy mortality.
CT scans showed abnormal wall thickening in 20 out of 34 patients (58.8%), the mean maximal thickening in caecum was 6.55 mm (±5.3; range 1.3–17.8) CT characteristics are summarized in Table 1. The distribution of the thickening was pancolonic in 13 patients (38.2%). In7 patients CDI-associated segmental colitis was seen, among these, caecum sparing was seen in 4 patients (57.1%), transverse in 3 (42.9%), sigmoidal in 1 (14.3%) and rectal sparing in2 patients (28.6%) respectively. Thickening localized to a single colon segment was not identified. Some CT abnormalities were significantly present in patients with an increased colon thickness. Pericolonic fat stranding and accordion and target sign were more frequent in those with an abnormal wall thickness in the caecum, ascending, transverse, descending, sigmoid colon, rectum and pancolitis (p < 0.001). There was no correlation between other CT findings and wall thickness.
Table 1.
Abdominal CT characteristics of Patients with Clostridium difficile infection within 72 hours of diagnosis.
| Variable | Value (%) |
|---|---|
| Increased colonic wall thickness n (%) | |
| Caecum | 18 (52.9) |
| Ascending colon | 17 (50) |
| Transverse colon | 16 (47.1) |
| Descending colon | 19 (55.9) |
| Sigmoid colon | 18 (52.9) |
| Rectum | 16 (47.1) |
| Measurement of colonic wall thickness (mm) (Mean ± SD) | |
| Caecum | 6.6 ± 5.3 |
| Ascending colon | 6.2 ± 4.4 |
| Transverse colon | 6.7 ± 5.4 |
| Descending colon | 5.7 ± 4.2 |
| Sigmoid colon | 6.7 ± 4.7 |
| Rectum | 7.4 ± 5.9 |
| Pancolitis n (%) | 13 (38.2) |
| Bowel dilation n (%) | 8 (23.5) |
| Accordion sign n (%) | 8 (23.5) |
| Target sign n (%) | 14 (41.2) |
| Pericolonic fat stranding n (%) | 24 (70.6) |
| Peritoneal fluid n (%) | 25 (73.5) |
| Pneumoperitoneum n (%) | 0 (0) |
| Pleural effusion n (%) | 32 (94.1) |
| Atheromatous plaques n (%) | 16 (47.1) |
| Perirenal fat stranding n (%) | 24 (70.6) |
Several variables were significantly associated in predicting colectomy such as abnormal wall thickening in caecum (OR 8.0; CI 1.37–46.81; p = 0.021), transverse colon (OR 6.7; CI 1.15–35.60; p = 0.034), and/or in sigmoid colon (OR 12.6; CI 1.37–115.97; p = 0.025), the presence of pancolitis (OR 7.0; CI 1.36–36.01; p = 0.02) and bowel dilation (OR 16.5; CI 2.41–112.83; p = 0.004) (Table 2).Values of odds ratio were used for the development of a radiological scale to predict the need for colectomy. We named this the Monterrey CT-scale, consisting of 0–24 points (see Table 3).
Table 2.
Univariate Logistic regression of CT findings with colectomy and all-cause mortality with odds ratio and their 95% confidence interval (CI).
| Variable | Colectomy OR (95% CI) |
P | All-cause mortality OR (95% CI) |
P |
|---|---|---|---|---|
| Wall thickness | ||||
| Increased caecum | 8.0 (1.37–46.81) | 0.021 | 6.2 (1.06–35.57) | 0.043 |
| Increased ascending colon | 5.6 (0.97–32.20) | 0.054 | 12.0 (1.29–111.32) | 0.029 |
| Increased transverse colon | 6.7 (1.15–35.60) | 0.034 | 00 (00–00) | NS |
| Increased descending colon | 3.9 (0.80–18.98) | 0.093 | 17.0 (1.81–160.05) | 0.013 |
| Increased sigmoid colon | 12.6 (1.37–115.97) | 0.025 | 10.2 (1.1–94.1) | 0.041 |
| Increased rectum | 5.6 (0.97–32.20) | 0.054 | 4.5 (0.77–26.86) | 0.096 |
| Pancolitis | 7.0 (1.36–36.01) | 0.020 | 11.1 (1.80–68.4) | 0.010 |
| Bowel dilation | 16.5 (2.41–112.83) | 0.004 | 2.0 (0.37–10.92) | NS |
| Accordion sign | 1.6 (0.31–8.68) | NS | 2.0 (0.37–10.92) | NS |
| Target sign | 3.0 (0.65–13.76) | NS | 4.3 (0.84–21.5) | 0.080 |
| Pericolonic fat stranding | 2.0 (0.34–11.70) | NS | 4.5 (0.48–41.99) | NS |
| Peritoneal fluid | 4.5 (0.48–41.99) | NS | 3.8 (0.40–35.44) | NS |
| Atheromatous plaques | 0.4 (0.08–1.75) | NS | 3.0 (0.61–14.86) | NS |
| Perirenal fat stranding | 0.3 (0.05–1.28) | 0.098 | 4.5 (0.48–41.99) | NS |
Table 3.
Monterrey CT scale to predict fulminant colitis and colectomy in patients with Clostridium difficile infection.
| Parameter | Assigned score |
|---|---|
| Increased caecum wall thickness >3 mm | 4 |
| Increased transverse colon wall thickness >3 mm | 3 |
| Increased sigmoid colon wall thickness >3 mm | 6 |
| Pancolitis | 3 |
| Bowel dilation | 8 |
| Totala | 24 |
Positive score is greater or equal to 6.
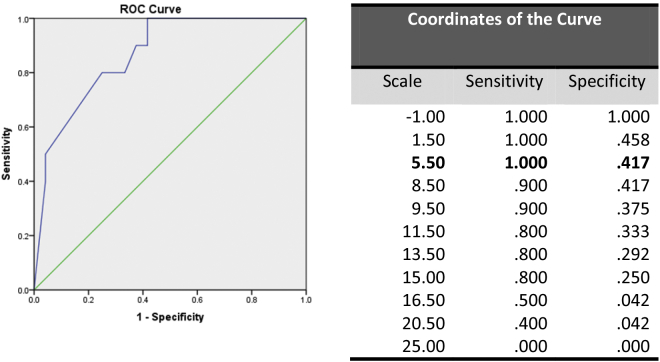
An area under the receiver operator curve (AROC) was generated, obtaining an area under the curve of 0.867 (CI0.74–0.99; p = 0.001) (Fig. 2). The cut-off defined for the scale was ≥6. We internally validated our scale model and calculated a sensitivity of 100%, specificity of 58.3%, and positive predictive value of 50% and negative predictive value of 100%. Patients with 0–6 points did not undergo colectomy and survived for more than 30 days.
Fig. 2.
Area under the curve under the receiver operator curve (AROC) was generated to determine the cut-off, obtaining an area under the curve of 0.867 (CI 0.744–0.990; p = 0.001). The cut-off defined for the scale was ≥6.
A secondary outcome of all-cause mortality within 30 days of diagnosis was also evaluated by univariate binary logistic regression. Wall thickening of caecum (OR 6.2; CI 1.06–35.57; p = 0.043), ascending colon (OR 12.0; CI 1.29–111.32; p = 0.029), descending colon (OR 17.0; CI 1.81–160.05; p = 0.013) and sigmoid (OR 10.2; CI 1.10–94.10; p = 0.041) independently predicted mortality within 30 days of CDI diagnosis. In addition, the presence of pancolitis increased the likelihood of mortality by 11.08% (OR 11.1; CI 1.80–68.40; p = 0.010). (Table 2).
4. Discussion
The role of CT to predict severity outcomes in patients with CDI has not been fully established. In this study, we aimed to identify predictors for the need for colectomy in patients with CDI, develop a CT scale for colectomy and to evaluate if CT findings could predict all-cause mortality within 30 days of diagnosis. The most important results of our study are the identification of 5 abdominal CT findings that independently predict the likelihood of colectomy forCDI: increased colonic wall thickness in the caecum, transverse colon, sigmoid colon, pancolitis and bowel dilation. Moreover, the elaboration of a radiological scale that can rule out the development of fulminant colitis and the need for colectomy with a sensitivity and negative predictive value of 100%. To our knowledge, this is the first study that has succeeded in evaluating CT findings as predictors of the necessity of surgical treatment.
The selected sample of patients who developed CDI had a high WBC count, hypoalbuminemia, developed fever and abdominal pain. Of the patients who underwent CT,35.3% had received immunosuppression treatment and had a mean ATLAS score of 5.9. The majority of patients (73.5%) had a moderate disease (score4-7), and 23.5% had severe disease (score 8–10). We have used this scale for the routine evaluation of patients with CDI in our hospital and found out that patients with score 4–7 had a greater probability of colectomy and that patients with a score ≥8 were more likely to die. [7] In the present study, of the nine patients that presented all-cause mortality, seven had an ATLAS score of 4–7, and two had a score 8–10. Thus, the results of the present study did not confirm the association of ATLAS score and all-cause mortality.
Diverse studies have evaluated CT findings as predictors of surgical treatment to determine whether patients with C. difficile colitis had radiographic features distinct from patients successfully treated with standard therapy [8], [9]. Nonetheless, both failed to identify variables that were associated with this outcome.
Furthermore, several clinical scores have been developed to predict complicated CDI. One of these scores, the Hines VA index [5]incorporates clinical and CT findings: temperature >38 °C, ileus, systolic blood pressure <100 mmHg, leukocytosis, thickened colonic wall, colonic dilation, and ascites. When tested by Fujitani et al. the Hines VA index had the highest kappa correlation compared with other scoring systems but particularly no statistical significance for abnormal CT findings was observed as an independent risk factor in their population [10].
Previous studies have reviewed CT abnormalities in patients with confirmed CDI. It has been reported that the presence and degree of submucosal edema, depicted in the accordion and target sign, abnormal wall thickening, degree of dilation, nodular contour and ascites do not correlate with clinical outcomes in patients with toxic megacolon. In this population, CT was helpful to detect diffuse colitis and life-threatening abdominal complications in patients with pseudomembranous colitis [11]. In our population the absence of some of these findings correlated with a better clinical outcome.
Our findings are similar to those described in previous studies with some exceptions. Valiquette et al. [4] found the accordion sign in 56% of their population compared with 4% in the study by Kirkpatrick et al. [2] and 23.5% in our study, making this finding inconstant. Peritoneal fluid and pleural effusion were seen in a significantly higher number of our patients (73.5% and 94.1%) than those in the study by Valiquette et al. (56% and 40%, respectively) [4]. We speculate that these differences are due to higher comorbidities in our patients and prolonged hospital stay prior to CDI diagnosis. Similar results were found in these both studies for the presence of target sign (51% vs. 41.2%).
All-cause mortality within 30 days was also analyzed as a secondary outcome and five CT parameters independently predicted mortality within 30 days of CDI diagnosis. These parameters were wall thickening of the caecum, ascending, descending, sigmoid colon and pancolitis. Descending colon wall thickening increased the likelihood of death by 17%, followed by ascending colon wall thickness with 12% and pancolitis with 11.08%. According to these results, CT findings can also give additive value as important predictive tools for all-cause mortality and should be considered in the development of novel severity scores or in updating current scores.
We acknowledge some limitations of our study; i.e.,not all of the patients with confirmed C. difficile colitis underwent abdominal CT. Of the totality of patients diagnosed with CDI during the study period, a total of 18 had severe disease (ATLAS higher than 7 points), of these 10 did not undergo CT scan, thus leaving the possibility that other severely ill patients were left out of our analysis. In addition, abdominal CT was most likely to be performed for patients with the more severe disease and by only examining those patients who had a CT ordered, there was already a medical decision involved to order the CT scan, thus creating a selection bias.
5. Conclusion
We designed a CT scale able to rule out the development of fulminant colitis and the need for colectomy when applied to patients with CDI diagnosis. Furthermore, patients with wall thickening of the caecum, ascending, descending or sigmoid colon were more likely to die within 30 days of CDI diagnosis.
Conflicts of interest
None.
Sources of funding
None.
Ethical approval
Research was approved by ethics committee of Universidad Autonoma de Nuevo Leon with reference number IF14-500.
Trial registry number – ISRCTN
NA.
Author contribution
Laura Palau-Davila: study concept or design, data collection, data analysis or interpretation, writing the paper.
Reynaldo Lara-Medrano: study concept or design, data interpretation.
Adrián Negreros-Osuna: study concept or design, data collection.
Matías Salinas-Chapa: study concept or design, data collection.
Elvira Garza-González: study concept or design, writing the paper.
Eva Marìa Gutierrez-Delgado: data collection.
Adrián Camacho-Ortiz: study concept or design, data analysis or interpretation, writing the paper.
Guarantor
Adrian Camacho-Ortiz, M.D. Ph.D.
Acknowledgements
None.
References
- 1.Longo D.L., Leffler D.A., Lamont J.T. Clostridium Difficile infection. N. Engl. J. Med. 2015;372:1539–1548. doi: 10.1056/NEJMra1403772. [DOI] [PubMed] [Google Scholar]
- 2.Kirkpatrick I.D., Greenberg M. Evaluating the CT diagnosis of Clostridium Difficile colitis: should CT guide therapy? AJR. Am. J. Roentgenol. 2001;176:635–639. doi: 10.2214/ajr.176.3.1760635. [DOI] [PubMed] [Google Scholar]
- 3.Kawamoto S., Horton K.M., Fishman E.K. Pseudomembranous colitis: spectrum of imaging findings with clinical and pathologic correlation. Radiographics. 1999;19:887–897. doi: 10.1148/radiographics.19.4.g99jl07887. [DOI] [PubMed] [Google Scholar]
- 4.Valiquette L., Pépin J., Do X.-V. Prediction of complicated Clostridium Difficile infection by pleural effusion and increased wall thickness on computed tomography. Clin. Infect. Dis. 2009;49:554–560. doi: 10.1086/600879. [DOI] [PubMed] [Google Scholar]
- 5.Belmares J., Gerding D.N., Parada J.P., Miskevics S., Weaver F., Johnson S. Outcome of metronidazole therapy for Clostridium Difficile disease and correlation with a scoring system. J. Infect. 2007;55:495–501. doi: 10.1016/j.jinf.2007.09.015. [DOI] [PubMed] [Google Scholar]
- 6.Miller M.A., Louie T., Mullane K., Weiss K., Lentnek A., Golan Y., Kean Y., Sears P. Derivation and validation of a simple clinical bedside score (ATLAS) for Clostridium difficile infection which predicts response to therapy. BMC Infect. Dis. 2013;13:148. doi: 10.1186/1471-2334-13-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hernández-García R, Garza-González E, Miller M, Arteaga-Muller G, Galván-de los Santos AM, Camacho-Ortiz A. Application of the ATLAS Score for Evaluating the Severity of Clostridium difficile Infection in Teaching Hospitals in Mexico. [DOI] [PMC free article] [PubMed]
- 8.Kawamoto S., Horton K.M., Fishman E.K. Pseudomembranous colitis: can CT predict which patients will need surgical intervention? J. Comput. Assist. Tomogr. 1999;23:79–85. doi: 10.1097/00004728-199901000-00017. [DOI] [PubMed] [Google Scholar]
- 9.Ash L., Baker M.E., O'Malley C.M., Gordon S.M., Delaney C.P., Obuchowski N.A. Colonic abnormalities on CT in adult hospitalized patients with Clostridium Difficile colitis: prevalence and significance of findings. AJR. Am. J. Roentgenol. 2006;186:1393–1400. doi: 10.2214/AJR.04.1697. [DOI] [PubMed] [Google Scholar]
- 10.Fujitani S., George, difficile L., Murthy A.R. Comparison of clinical severity score indices for Clostridium Difficile infection. Infect. Control Hosp. Epidemiol. 2011;32:220–228. doi: 10.1086/658336. [DOI] [PubMed] [Google Scholar]
- 11.Imbriaco M., Balthazar E.J. Toxic megacolon: role of CT in evaluation and detection of complications. Clin. Imaging. 2001;25:349–354. doi: 10.1016/s0899-7071(01)00330-8. [DOI] [PubMed] [Google Scholar]