Abstract
Objective
To evaluate the association between inhalation exposure to jet propulsion fuel 8 (JP-8) and urinary metabolites among US Air Force (USAF) personnel, and investigate the role of glutathione S-transferase polymorphisms.
Methods
Personal air samples were collected from 37 full-time USAF personnel during 4 consecutive workdays and analyzed for JP-8 constituents and total hydrocarbons. Pre- and postshift urine samples were collected each day and analyzed for polycyclic aromatic hydrocarbon urinary metabolites.
Results
Work shift exposure to total hydrocarbons was significantly associated with postshift urinary 1-naphthol (β = 0.17; P = <0.0001), 2-naphthol (β = 0.09; P = 0.005), and 2-hydroxyfluorene concentrations (β = 0.08; P = 0.006), and a significant gene-environment interaction was observed with glutathione S-transferase mu-1.
Conclusions
USAF personnel experience inhalation exposure to JP-8, which is associated with absorption of JP-8 constituents while performing typical job-related tasks, and in our data the glutathione S-transferase mu-1 polymorphism was associated with differential metabolism of naphthalene.
Jet propulsion fuel 8 (JP-8), a volatile lipophilic kerosene-based fuel, is the main jet fuel used by the US Air Force (USAF). Because of its higher flash point and lower acute toxicity, JP-8 completely replaced JP-4 by 1995.1 Given that JP-8 is a complex mixture of approximately 228 hydrocarbons, it is a challenge to fully characterize personal exposure to JP-8.2 Personal and ambient air concentrations of various JP-8 constituents have been previously characterized among personnel on USAF bases, indicating a wide range of exposures, with the highest concentrations among workers who repair and inspect aircraft fuel systems and perform quality control tests on the petroleum products.3–5 Specifically, naphthalene has been suggested as a useful surrogate measure for exposure to JP-8 as it comprises 0.11% to 0.27% of the fuel on average.6
Urinary 1- and 2-naphthol concentrations have been investigated as a measure of absorbed naphthalene among USAF personnel as well as other occupations such as coke plant workers.7,8 Previous studies have reported significant associations between postshift urinary naphthol concentrations and personal work shift exposure to JP-8 and smoking.9,10 Because of the various compounds present in JP-8, several urinary metabolites may be used as biomarkers of exposure to JP-8.
Although urinary naphthol concentrations can be used to characterize exposure to naphthalene, smoking and genetic polymorphisms involved in the metabolism of naphthalene have been shown to affect urinary naphthol concentrations. Specifically, smokers with no other exposure to naphthalene with the glutathione S-transferase mu-1 (GSTM1)-null genotype have higher urinary naphthol concentrations than those with the GSTM1-present genotype.11 Nan et al12 also reported that the GSTM1-null genotype was significantly associated with higher urinary 2-naphthol concentrations among naphthalene-exposed coke oven workers and naphthalene-unexposed students after adjusting for smoking, though the effect estimate was larger among the coke oven workers (β = −3.5 vs −0.88).
We previously characterized urinary naphthols in a pilot study of 24 workers at one USAF base,7 but here we report the results from a larger follow-up study of 73 workers at three USAF bases that includes additional urinary metabolites and evaluates the potential role of GST polymorphisms. The Occupational JP8 Exposure Neuroepidemiology Study was designed to investigate the relationship between exposure to JP-8 and neurological outcomes,13 using the exposure assessment data presented here. In this article, we aimed to (1) describe pre- and postshift concentrations of urinary metabolites among Air Force personnel exposed to JP-8 at three bases, (2) assess the relationship between urinary concentrations of metabolites and breathing zone concentrations of naphthalene and total hydrocarbons (THC), and (3) investigate the potential interaction of GST polymorphisms and naphthalene air concentrations on urinary metabolite concentrations.
MATERIALS AND METHODS
Study Design
Seventy-four full-time active USAF personnel from three bases participated in this study during 1 workweek between January and April 2008. One participant did not provide biological samples, resulting in 73 participants in this analysis. All participants had worked for a minimum of 6 months at their current job and were invited to participate to ensure that both workers with routine high exposure to JP-8 and low exposure were included in the study.13 Participants were categorized a priori into high- and low-exposure groups on the basis of their typical job tasks. Specifically, participants who performed jobs with routine exposure to JP-8 such as fuel cell repair, maintenance, and fuel distribution were categorized as the high-exposure group, whereas those with minimal direct exposure to JP-8 were categorized as the low-exposure group (ie, administrative, clerical, and health care personnel). Each participant provided written informed consent before participation in the study, and all protocols were reviewed and approved by human subjects committees from the Army (US Army Research Institute of Environmental Medicine), Air Force (AF Research Laboratory at Wright Patterson AF Base), Veterans Affairs (VA Boston Healthcare System), and Boston University School of Public Health. The involvement of the Centers for Disease Control and Prevention laboratory was determined not to constitute engagement in human subjects research.
Data Collection
Each participant was monitored during 4 consecutive workdays, beginning on Monday morning after 2 days away from work and ending on Thursday afternoon. All sample collection methods have been described in detail previously.13,14 Briefly, each participant completed a self-administered baseline questionnaire on the first day of the study to collect information on demographics, behavioral factors such as smoking, alcohol, grilled food, and caffeine consumption, health history, and military work history. Additional surveys were completed at the beginning and end of each work shift to describe more detailed personal exposures during the previous period.
All participants wore a Casella Apex Pro IS personal air monitor (Casella USA, Amherst, New Hampshire) attached to two sorbent tubes using a Y connector, on Monday through Thursday during their work shift to collect an air sample from their breathing zone. The first sorbent tube, a two-section (100/50 mg) Anasorb coconut shell charcoal tube (Anasorb; SKC Inc, Eighty Four, Pennsylvania), was analyzed for benzene, toluene, ethylbenzene, m-/p-xylene, o-xylene, and THC using National Institute for Occupational Safety and Health method 1501.15 The second sorbent tube, a Chromosorb 106 tube (Anasorb; SKC Inc), was analyzed for naphthalene according to OSHA method 35.16 All sorbent tubes were analyzed at the Organic Chemistry Analytical Laboratory at the Harvard School of Public Health in Boston, Massachusetts.
Each participant provided a urine sample at the beginning and end of each work shift, starting with preshift on day 1 after 2 days away from work and ending with postshift on day 4, resulting in eight urine samples per individual. All urine samples were analyzed for 1- and 2-naphthol as well as 2-, 3-, and 9-hydroxyfluorene, 1-, 2-, 3-, and 4-hydroxyphenanthrene, and 1-hydroxypyrene. In addition, urine samples were analyzed for creatinine concentrations to adjust for sample concentration. All urinary analyses were conducted at the Centers for Disease Control and Prevention in Atlanta, Georgia. Briefly, after the enzymatic deconjugation of the target analytes, automatic liquid–liquid extraction into pentene was performed using the Gilson 215 Liquid Handler (Gilson Inc, Middleton, Wisconsin). The sample extracts were evaporated under a chemical fume hood, and reconstituted in toluene, and then the analytes were derivatized to yield the trimethylsiloxane derivatives. Analytical determination of the target analytes was performed by gas chromatography–isotope dilution high-resolution mass spectrometry, employing a MAT95XP (Thermo Finnigan MAT, Bremen, Germany) instrument.17 The limits of detection (LODs) were 48 ng/L for 1-naphthol, 42 ng/L for 2-naphthol, 20 ng/L for 1-hydroxypyrene, and 10 ng/L for all other urinary analytes.
A blood sample was collected from each participant post-shift on Thursday. All blood samples were analyzed for GSTM1 and glutathione S-transferase theta-1 (GSTT1) genetic polymorphisms at Brown University using polymerase chain reaction methods described by Schwartz et al.18
Statistical Analyses
All statistical analyses were performed using SAS version 9.2 (SAS Institute, Cary, North Carolina). Chi-square tests were used to compare categorical variables between the high- and low-exposure groups, and Fisher exact tests were used for categorical variables where cell sizes were small. Wilcoxon ranked sum tests were used to compare continuous variables. Geometric means were calculated for the individual creatinine-corrected urinary metabolites by exposure category (high and low) and shift (preshift and postshift) by exponentiating the mean of the natural log-transformed values. Similarly, the geometric standard deviations were calculated by exponentiating the standard deviation of the natural log-transformed values. The LOD was used for samples below the LOD. Linear mixed-effects models were used for all further analyses to account for the repeated measurements from the participants (eg, air measurements and pre-and postshift urinary samples on 4 consecutive workdays from each individual).
RESULTS
The demographic characteristics of the 73 Air Force personnel who participated in this study are presented in Table 1. For variables that varied by day (ie, the number of cigarettes smoked per work shift and the number of barbeque meals consumed per work shift), we presented data for day 2 only as a representative day. Comparisons by exposure group show that there was no statistically significant difference in age, years of service, body mass index, current smoking status, race, marital status, education level, current military rank, or genetic polymorphisms between those in the high and low-exposure groups. Nevertheless, there was a statistically significant difference in sex (P = 0.001), with a greater proportion of males in the high-exposure group than in the low-exposure group. In addition, the high-exposure group had significantly more participants who lived on the base (63% vs 31%; P = 0.007). As reported previously, the workers in the low-exposure group were exposed to significantly lower personal air concentrations of THC (0.52 mg/m3 vs 2.64 mg/m3), naphthalene (0.37 μg/m3 vs 2.25 μg/m3), and other analytes than those in the high-exposure group.3
TABLE 1.
Study Population Characteristics by Exposure Group
| Overall n = 73 |
Low n = 35 |
High n = 38 |
P | |
|---|---|---|---|---|
| Median age (range) | 23.7 (18–43) | 23.9 (19–43) | 23.6 (18–41) | 0.57* |
| Median years of service (range) | 4.0 (0.5–20) | 4.0 (0.5–20) | 3.3 (0.5–17) | 0.45* |
| Median BMI (range) | 25.6 (18–34) | 25.8 (22–34) | 25.6 (18–34) | 0.81* |
| Sex, n (%) | ||||
| Male | 61 (84) | 24 (69) | 37 (97) | 0.001† |
| Female | 12 (16) | 11 (31) | 1 (3) | |
| Base, n (%) | 0.02† | |||
| 1 | 20 (27.4) | 11 (31) | 9 (24) | |
| 2 | 20 (27.4) | 15 (43) | 5 (13) | |
| 3 | 33 (45.2) | 9 (26) | 24 (63) | |
| Current smoker, n (%) | 0.18† | |||
| Yes | 33 (45) | 13 (37) | 20 (53) | |
| No | 40 (55) | 22 (63) | 18 (47) | |
| Average cigarettes smoked per day, n (%) | 0.01‡ | |||
| None | 40 (55) | 22 (63) | 18 (47) | |
| 1/4 pack | 19 (26) | 8 (23) | 11 (29) | |
| 1/2 pack | 9 (12) | 1 (3) | 8 (21) | |
| 1 pack | 4 (6) | 4 (11) | 0 | |
| Missing | 1 (1) | 0 | 1 (3) | |
| Cigarettes smoked during work shift, n (%) | ||||
| Day 2 | 0.50‡ | |||
| None | 48 | 26 | 22 | |
| 1/4 pack | 18 | 7 | 11 | |
| 1/2 pack | 3 | 2 | 1 | |
| Missing | 4 | 0 | 4 | |
| Number of BBQ/grilled meals during work shift | ||||
| Day 2 | 0.34‡ | |||
| 0 | 56 | 26 | 30 | |
| 1 | 8 | 4 | 4 | |
| 2 | 2 | 2 | 0 | |
| 3 | 1 | 1 | 0 | |
| 4 | 2 | 2 | 0 | |
| Missing | 4 | 0 | 4 | |
| Race, n (%) | 0.83† | |||
| White | 53 (73) | 25 (71) | 28 (74) | |
| Nonwhite | 20 (27) | 10 (29) | 10 (26) | |
| Marital status, n (%) | 0.94‡ | |||
| Single | 28 (38) | 13 (37) | 15 (39) | |
| Married | 40 (55) | 20 (57) | 20 (53) | |
| Divorced | 5 (7) | 2 (6) | 3 (8) | |
| Live on base, n (%) | 0.007† | |||
| Yes | 35 (48) | 11 (31) | 24 (63) | |
| No | 38 (52) | 24 (69) | 14 (37) | |
| Education, n (%) | 0.64† | |||
| High school | 60 (82) | 28 (80) | 32 (84) | |
| More than high school | 13 (18) | 7 (20) | 6 (16) | |
| GSTM1, n (%) | 0.19† | |||
| Null | 35 (48) | 13 (37) | 22 (58) | |
| Present | 34 (47) | 18 (51) | 16 (42) | |
| Missing§ | 4 (5) | 4 (11) | 0 | |
| GSTT1, n (%) | 0.45† | |||
| Null | 9 (12) | 3 (9) | 6 (16) | |
| Present | 60 (82) | 28 (80) | 32 (84) | |
| Missing§ | 4 (5) | 4 (11) | 0 | |
Wilcoxon ranked sum test.
Chi-square test.
Fisher exact test
Blood samples were not collected from participants.
BBQ, barbeque; BMI, body mass index; GSTM1, glutathione S-transferase mu-1; GSTT1, glutathione S-transferase theta-1.
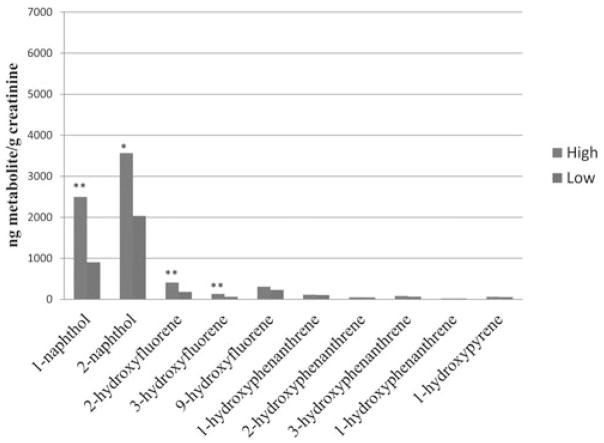
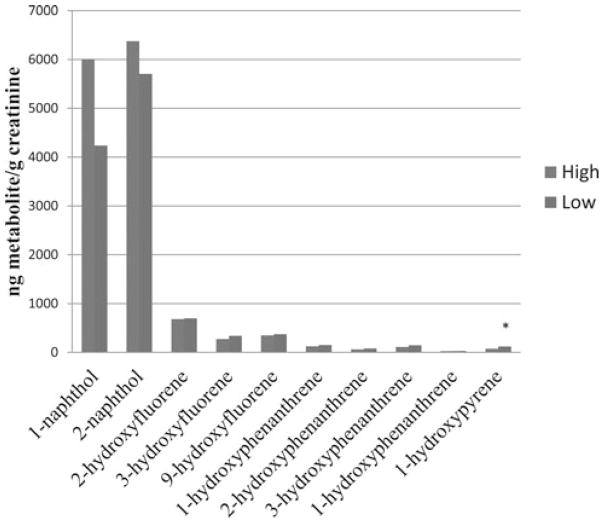
Table 2 presents the comparison of the creatinine-adjusted urinary metabolite concentrations in preshift and postshift urine samples by exposure group and smoking status. The percentage of samples below the LOD ranged from 0% to 29% in the low-exposure group and 0% to 19% in the high-exposure group, with the greatest percentage for 4-hydroxyphenanthrene concentrations. Although the greatest workday differences were observed in naphthols and hydroxyfluorenes among the high-exposure group, postshift urinary concentrations of 1-naphthol, 2- and 3-hydroxyphenanthrene were significantly higher than preshift samples in the low-exposure group among smokers, and 2-hydroxyfluorene concentrations were higher among nonsmokers. In addition, when comparing the postshift urinary concentrations between the high and low-exposure groups, significantly higher concentrations of 1-naphthol, 2-naphthol, 2-hydroxyfluorene, and 3-hydroxyfluorene were observed in the high-exposure group among nonsmokers only (Fig. 1). In contrast, higher 1-hydroxypyrene concentrations were observed in the low-exposure group among smokers (Fig. 2).
TABLE 2.
Comparison of Pre- and Postcreatinine-Adjusted Urinary Metabolites (ng/g)
| Smokers | Low (n =13)
|
High (n=20)
|
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Pre
|
n | Post
|
P | n | Pre
|
n | Post
|
P | |||||
| %<LOD | GM (GSD) | %<LOD | GM (GSD) | % <LOD | GM (GSD) | %<LOD | GM (GSD) | |||||||
| 1-Naphthol | 52 | 0 | 3344 (3.1) | 51 | 0 | 4234 (3.1) | 0.05 | 79 | 0 | 3452 (2.6) | 74 | 0 | 6006 (2.3) | <0.0001 |
| 2-Naphthol | 52 | 0 | 6284 (2.4) | 51 | 0 | 5704 (2.3) | 0.24 | 79 | 0 | 4633 (2.0) | 74 | 0 | 6376 (2.0) | 0.0002 |
| 2-Hydroxyfluorene | 52 | 0 | 627 (2.2) | 51 | 0 | 696 (2.0) | 0.18 | 79 | 0 | 437 (2.0) | 74 | 0 | 682 (2.2) | <0.0001 |
| 3-Hydroxyfluorene | 52 | 0 | 312 (2.3) | 51 | 0 | 337 (2.2) | 0.36 | 79 | 0 | 215 (2.4) | 74 | 0 | 273 (2.4) | 0.005 |
| 9-Hydroxyfluorene | 52 | 0 | 334 (2.4) | 51 | 2 | 373 (2.4) | 0.41 | 79 | 0 | 294 (2.2) | 73 | 0 | 346 (2.0) | 0.05 |
| 1-Hydroxyphenanthrene | 52 | 0 | 132 (1.8) | 51 | 0 | 149 (1.6) | 0.10 | 79 | 0 | 118 (2.1) | 73 | 0 | 124 (2.1) | 0.77 |
| 2-Hydroxyphenanthrene | 52 | 2 | 64 (2.3) | 51 | 0 | 79 (1.8) | 0.04 | 79 | 0 | 55 (1.8) | 74 | 0 | 61 (1.7) | 0.11 |
| 3-Hydroxyphenanthrene | 52 | 0 | 120 (2.1) | 51 | 0 | 147 (1.7) | 0.04 | 78 | 0 | 99 (2.1) | 71 | 0 | 109 (2.0) | 0.19 |
| 4-Hydroxyphenanthrene | 51 | 22 | 27 (2.6) | 50 | 22 | 29 (2.1) | 0.47 | 79 | 4 | 22 (2.0) | 74 | 3 | 24 (1.9) | 0.27 |
| 1-Hydroxypyrene | 50 | 0 | 122 (1.8) | 51 | 2 | 121 (1.8) | 0.89 | 78 | 1 | 87 (2.0) | 59 | 2 | 71 (2.0) | 0.04 |
|
| ||||||||||||||
| Nonsmokers | Low (n =22) | High (n = 18) | ||||||||||||
|
| ||||||||||||||
| 1-Naphthol | 87 | 0 | 813 (2.1) | 85 | 0 | 901 (2.1) | 0.25 | 72 | 0 | 1330 (2.6) | 69 | 0 | 2496 (2.8) | 0.0002 |
| 2-Naphthol | 87 | 0 | 2146 (2.1) | 85 | 0 | 2034 (1.9) | 0.44 | 72 | 0 | 2839 (2.3) | 69 | 0 | 3564 (2.7) | 0.007 |
| 2-Hydroxyfluorene | 87 | 0 | 163 (1.9) | 85 | 0 | 180 (1.8) | 0.02 | 72 | 0 | 247 (1.8) | 69 | 0 | 408 (2.2) | <0.0001 |
| 3-Hydroxyfluorene | 87 | 1 | 59 (1.8) | 85 | 7 | 62 (1.9) | 0.58 | 72 | 0 | 102 (2.1) | 69 | 0 | 134 (2.2) | 0.005 |
| 9-Hydroxyfluorene | 86 | 0 | 208 (1.9) | 85 | 0 | 228 (1.7) | 0.22 | 72 | 0 | 198 (2.0) | 69 | 0 | 305 (2.3) | 0.0004 |
| 1-Hydroxyphenanthrene | 87 | 1 | 107 (1.8) | 85 | 0 | 105 (1.8) | 0.64 | 72 | 0 | 99 (2.0) | 69 | 1 | 110 (2.2) | 0.21 |
| 2-Hydroxyphenanthrene | 87 | 7 | 42 (1.9) | 85 | 6 | 45 (1.9) | 0.23 | 71 | 0 | 43 (2.0) | 69 | 3 | 51 (2.1) | 0.14 |
| 3-Hydroxyphenanthrene | 87 | 0 | 57 (1.6) | 85 | 1 | 63 (1.6) | 0.12 | 72 | 0 | 65 (2.0) | 69 | 1 | 80 (1.9) | 0.03 |
| 4-Hydroxyphenanthrene | 86 | 23 | 20 (2.0) | 84 | 29 | 21 (2.0) | 0.61 | 72 | 19 | 17 (2.4) | 69 | 12 | 19 (2.5) | 0.28 |
| 1-Hydroxypyrene | 80 | 9 | 54 (2.0) | 85 | 19 | 54 (2.1) | 0.66 | 71 | 6 | 67 (2.1) | 62 | 6 | 61 (2.0) | 0.37 |
GM, geometric means; GSD; geometric standard deviations; LOD, limit of detection.
FIGURE 1.
Postshift urinary metabolite concentrations among nonsmokers. **P = 0.0001; *P = 0.02.
FIGURE 2.
Postshift urinary metabolite concentrations among smokers. *P = 0.02.
Table 3 presents the associations between breathing zone air concentrations of THC and postshift urinary metabolites while adjusting for preshift urinary metabolite concentrations, USAF base, sex, cigarettes smoked per work shift, and postshift urinary creatinine concentrations. Breathing zone concentration of THC was a statistically significant predictor of postshift urinary concentrations of 1-naphthol (β = 0.15; P = <0.0001), 2-naphthol (β = 0.09; P = 0.005), and 2-hydroxyfluorene (β = 0.08; P = 0.006). Specifically, for each additional 10% increase in breathing zone THC, there was approximately a 1% increase in 1-naphthol, 2-naphthol, and 2-hydroxyfluorene. Similarly, breathing zone naphthalene concentrations were a statistically significant predictor of postshift urinary 1-naphthol and 2-naphthol concentrations, with slightly greater effect estimates than observed for breathing zone THC concentrations.
TABLE 3.
Effects of 8-Hour TWA THC and Naphthalene Concentrations in Air*
| Predictor | Postshift Urinary Concentration | β (SE) | P |
|---|---|---|---|
| THC, mg/m3 | 1-Naphthol | 0.17 (0.04) | <0.0001 |
| 2-Naphthol | 0.09 (0.03) | 0.005 | |
| 2-Hydroxyfluorene | 0.08 (0.03) | 0.006 | |
| 3-Hydroxyfluorene | 0.04 (0.03) | 0.23 | |
| 9-Hydroxyfluorene | 0.02 (0.03) | 0.60 | |
| 1-Hydroxyphenanthrene | −0.04 (0.03) | 0.20 | |
| 2-Hydroxyphenanthrene | 0.008 (0.03) | 0.75 | |
| 3-Hydroxyphenanthrene | −0.01 (0.03) | 0.64 | |
| 4-Hydroxyphenanthrene | −0.02 (0.03) | 0.52 | |
| 1-Hydroxypyrene | −0.03 (0.03) | 0.38 | |
| Naphthalene, μg/m3 | 1-Naphthol | 0.22 (0.04) | <0.0001 |
| 2-Naphthol | 0.11 (0.03) | 0.0006 |
Adjusted for preshift urinary concentrations, base, sex, cigarettes per shift, and postshift urinary creatinine; 8-hour TWA THC air concentrations, 8-hour TWA naphthalene air concentrations, and urinary metabolite concentrations are natural log-transformed.
SE, standard error; THC, total hydrocarbon; TWA, time-weighted average.
The final multivariate models using air THC concentrations as a predictor of postshift urinary 1-naphthol, 2-naphthol, and 2-hydroxyfluorene concentrations are presented in Table 4, showing that the models explain 85%, 79%, and 72% of the between-worker variability and 19%, 33%, and 39% of the within-worker variability, respectively. In these models, nonadjusted urinary metabolite concentrations were used, and postshift urinary creatinine concentrations were included as a predictor. Preshift urinary metabolite concentrations, postshift urinary creatinine concentrations, and current smoking status were statistically significant predictors of postshift urinary metabolites in addition to the breathing zone THC concentrations. We tested for possible interactions between smoking status and air THC concentrations but found no significant interaction when predicting any of the urinary metabolite concentrations.
TABLE 4.
Final Models of 8-Hour TWA THC in Air (μg/m3) and Postshift Urinary Metabolites
| Ln (Postshift Urinary 1-Naphthol)
|
Ln (Postshift Urinary 2-Naphthol)
|
Ln (Postshift Urinary 2-Hydroxyfluorene)
|
||||
|---|---|---|---|---|---|---|
| β (SE) | P | β (SE) | P | β (SE) | P | |
| Intercept | 4.59 (0.45) | <0.0001 | 4.83 (0.46) | <0.0001 | 3.53 (0.35) | <0.0001 |
| Ln preshift urine concentration | 0.33 (0.05) | <0.0001 | 0.34 (0.05) | <0.0001 | 0.29 (0.06) | <0.0001 |
| Base | 0.17 | 0.40 | 0.32 | |||
| 1 | −0.29 (0.17) | −0.46 (0.15) | −0.25 (0.16) | |||
| 2 | −0.23 (0.17) | −0.39 (0.15) | −0.10 (0.16) | |||
| 3 | Ref | Ref | Ref | |||
| Sex | ||||||
| Female | −0.007 (0.19) | 0.97 | 0.14 (0.17) | 0.40 | 0.01 (0.18) | 0.95 |
| Male | Ref | Ref | Ref | |||
| Cigarettes during work shift | 0.0009 | 0.006 | 0.002 | |||
| 1/2 pack | 0.92 (0.30) | 0.65 (0.24) | 0.67 (0.24) | |||
| 1/4 pack | 0.78 (0.15) | 0.49 (0.13) | 0.61 (0.13) | |||
| None | Ref | Ref | Ref | |||
| Postshift urinary creatinine | 0.005 (0.0006) | <0.0001 | 0.005 (0.0005) | <0.0001 | 0.005 (0.0004) | <0.0001 |
| Ln 8-hr TWA THC (mg/m3) | 0.17 (0.04) | <0.0001 | 0.09 (0.03) | 0.005 | 0.08 (0.03) | 0.006 |
| Random effects | ||||||
| σ2bw (intercept only) | 1.23 | 0.89 | 0.90 | |||
| σ2ww (intercept only) | 0.67 | 0.42 | 0.41 | |||
| σ2bw (full model) | 0.19 | 0.19 | 0.25 | |||
| σ2ww (full model) | 0.54 | 0.28 | 0.25 | |||
| Variability explained by model, % | ||||||
| Between-worker | 85 | 79 | 72 | |||
| Within-worker | 19 | 33 | 39 | |||
SE, standard error; THC, total hydrocarbon; TWA, time-weighted average.
The association between naphthalene in personal air and urinary naphthols was significantly different by GST polymorphisms (Table 5). Specifically, the naphthalene concentrations in personal air had a significantly larger effect on urinary naphthols among workers with the GSTM1-present genotype than among those with the GSTM1-null genotype. A significant gene-environment interaction was not observed for GSTT1 genotypes, and we did not observe a main effect of either GSTM1 or GSTT1. No other genetic association was observed with the other urinary metabolites.
TABLE 5.
The Effect of GSTM1 and GSTT1 on Postshift Urinary 1-Naphthol and 2-Naphthol Concentrations
| Predictor Variables | Ln (post 1-naphthol)
|
Ln (Post 2-Naphthol)
|
||
|---|---|---|---|---|
| β (SE) | P | β (SE) | P | |
| GSTM1 | ||||
| Intercept | 4.31 (0.4) | <0.0001 | 3.82 (0.5) | <0.0001 |
| Ln (pre 1- or 2-naphthol) | 0.49 (0.1) | <0.0001 | 0.56 (0.1) | <0.0001 |
| Ln (air naphthalene) | 0.38 (0.1) | <0.0001 | 0.26 (0.1) | <0.0001 |
| GSTM1 (present vs null) | −0.07 (0.2) | 0.68 | 0.04 (0.1) | 0.77 |
| GSTM1 × ln (air naphthalene) | 0.19 (0.1) | 0.03 | 0.15 (0.1) | 0.05 |
| GSTT1 | ||||
| Intercept | 4.11 (0.4) | <0.0001 | 3.71 (0.5) | <0.0001 |
| Ln (pre 1- or 2-naphthol) | 0.51 (0.1) | <0.0001 | 0.57 (0.1) | <0.0001 |
| Ln (air naphthalene) | 0.27 (0.05) | <0.0001 | 0.18 (0.04) | <0.0001 |
| GSTT1 (present vs null) | −0.15 (0.2) | 0.51 | −0.15 (0.2) | 0.48 |
| GSTT1 × ln (air naphthalene) | 0.04 (0.1) | 0.73 | 0.05 (0.1) | 0.64 |
GSTM1, glutathione S-transferase mu-1; GSTT1, glutathione S-transferase theta-1.
DISCUSSION
We investigated the effects of inhalation exposure to JP-8 constituents and the role of genetic polymorphisms on the concentrations of several urinary metabolites among Air Force personnel. Workers who were categorized a priori as having high exposure to JP-8 had higher urinary concentrations of 1-naphthol, 2-naphthol, and 2-hydroxyfluorene, suggesting that the a priori categorization on the basis of workers’ typical job tasks and routine exposure to JP-8 can serve as a useful surrogate exposure metric in the absence of actual measurements. Correspondingly, we observed significant increases in urinary metabolites from preshift to postshift and significant associations with personal work shift air THC measurements, suggesting that these urinary metabolites serve as good biomarkers of occupational exposures. In addition, a significant gene–environment interaction was observed, indicating that the GSTM1 polymorphism has an effect on the relationship between personal exposure to naphthalene in air and urinary naphthol concentrations.
Although postshift urinary samples had significantly higher concentrations of some metabolites compared with preshift samples among individuals in the low-exposure group, further analyses among those in the low-exposure group indicated that these differences were mainly observed among smokers (1-naphthol, 2-hydroxyphenanthrene, and 3-hydroxyphenanthrene). Similar urinary concentrations of 2-, 3-hydroxyphenanthrene among the low-and high-exposure groups further support that these concentrations resulted from smoking rather than exposure to JP-8. In contrast, post-shift urinary concentrations of 2-hydroxyfluorene were significantly higher than preshift concentrations among nonsmokers, though the magnitude of the change was comparable and slightly higher for smokers. This significance of the difference in 2-hydroxyfluorene may be due to a greater sample size (22 nonsmokers vs 13 smokers). Others have shown that urinary 2-hydroxyfluorene is a sensitive and specific biomarker of exposure to polycyclic aromatic hydrocarbons, with smokers having significantly higher concentrations than nonsmokers19,20 and roofers having higher concentrations postshift compared than preshift.21 Urinary naphthol and 2-hydroxyfluorene concentrations may be influenced by exposure to JP-8 because higher urinary concentrations observed among those in the high-exposure group. Further analyses are warranted to determine the effect of smoking on these urinary biomarkers, possibly using more precise measures of smoking such cotinine concentrations.
In contrast to Serdar et al,9,10 who observed a decrease in variability explained by the model after adjusting naphthol concentrations for urinary creatinine, postshift urinary creatinine concentration was included as a significant predictor in our final regression models (Table 4) because it explained some of the within-worker variability and there was no decrease in the between-worker variability explained. On the other hand, we observed a significant interaction between naphthalene air concentrations and GSTM1 polymorphism only when urinary metabolite concentrations were not adjusted for creatinine, similar to findings reported by Yang et al.11
Although some have shown a significant negative main effect of the GSTM1-present genotype on urinary naphthol concentrations,11,12 and others have shown a significant positive effect,22 we did not observe a significant main effect of GSTM1, but we are the first to report a significant gene–environment interaction with air naphthalene concentrations. In addition, effect modification of GSTM1 by smoking status has been shown,11 but we are unable to present our results by smoking status because of small sample size and limited power. Another limitation of our study is the lack of other genetic polymorphisms associated with naphthalene metabolism, such as CYP2E1.23 Similar to other findings, we observed no significant association between GSTT1 polymorphism and urinary metabolite concentrations.12,22
Because of the considerable variability in the composition of JP-8, results of the models using THC concentrations in air may not be generalizable to other occupational settings or even all Air Force bases over time.24 In fact, the average percent naphthalene was 0.10% of THC in air among nonsmokers in this study, but the range was 0.002% to 0.64%, showing some variability in the composition of their exposure.
We collected only one urinary spot sample preshift and post-shift from each participant, but we were able to collect samples over 4 consecutive workdays. Spot samples have greater variability than first-void or 24-hour urine samples, but we were able to reduce variability by adjusting for creatinine concentrations. In addition, the collection of repeated samples reduced the number of participants needed to observe statistically differences in metabolite concentrations.25
CONCLUSIONS
Urinary 1- and 2-naphthol concentrations, as well as 2-hydroxyfluorene, reflect occupational exposures to JP-8 during the workday among Air Force personnel and may be used as biomarkers of exposure to jet fuel. It is also important to consider smoking status and GSTM1 polymorphisms. Other routes of exposure, such as dermal, should also be investigated as urinary metabolite concentrations may also reflect exposure through the skin.
Learning Objectives.
Discuss previous research on individuals exposed to jet propulsion fuel 8 (JP-8), including genetic and environmental factors that may affect urinary naphthol levels.
Identify the new findings on JP-8 constituents associated with post-shift increases in levels of specific metabolites.
Outline the new evidence for a significant gene-environment interaction affecting urinary metabolite concentrations after air naphthalene exposure.
Acknowledgments
This research project was funded by the US Army Medical Research and Materiel Command Award (W81XWH-06-1-0105; PI: SP Proctor) to the Henry M. Jackson Foundation for the Advancement of Military Medicine, Inc.
We sincerely thank the USAF personnel for their participation in this research study. We also thank Kristin Heaton, Elisa Kryskow, Nichole Longcore, Kian Merchant-Borna, and Deborah Watkins for their assistance.
Footnotes
The opinions and assertions contained herein are the private views and opinions of the authors and are not to be construed as official or as reflecting the views of the Army, the Department of Defense, or the Centers for Disease Control and Prevention.
Authors Rodrigues, Smith, Maule, Sjodin, Li, Romanoff, Kelsey, Proctor, and McClean have no relationships/conditions/circumstances that present potential conflict of interest.
The JOEM editorial board and planners have no financial interest related to this research.
References
- 1.Agosta A. Development of a Chemical Surrogate for JP-8 Aviation Fuel Using a Pressurized Flow Reactor. Philadelphia, PA: Drexel University; 2002. [Google Scholar]
- 2.Ritchie G, Still K, Rossi J, 3rd, Bekkedal M, Bobb A, Arfsten D. Biological and health effects of exposure to kerosene-based jet fuels and performance additives. J Toxicol Environ Health B Crit Rev. 2003;6:357–451. doi: 10.1080/10937400306473. [DOI] [PubMed] [Google Scholar]
- 3.Merchant-Borna K, Rodrigues EG, Smith KW, Proctor SP, McClean MD. Characterization of inhalation exposure to jet fuel among U.S. Air Force personnel. Ann Occup Hyg. 2012;56:736–745. doi: 10.1093/annhyg/mes014. [DOI] [PubMed] [Google Scholar]
- 4.Pleil JD, Smith LB, Zelnick SD. Personal exposure to JP-8 jet fuel vapors and exhaust at air force bases. Environ Health Perspect. 2000;108:183–192. doi: 10.1289/ehp.00108183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smith KW, Proctor SP, Ozonoff A, McClean MD. Inhalation exposure to jet fuel (JP8) among U.S. Air Force personnel. J Occup Environ Hyg. 2010;7:563–572. doi: 10.1080/15459624.2010.503755. [DOI] [PubMed] [Google Scholar]
- 6.Egeghy PP, Hauf-Cabalo L, Gibson R, Rappaport SM. Benzene and naphthalene in air and breath as indicators of exposure to jet fuel. Occup Environ Med. 2003;60:969–976. doi: 10.1136/oem.60.12.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smith KW, Proctor SP, Ozonoff AL, McClean MD. Urinary biomarkers of occupational jet fuel exposure among air force personnel. J Expo Sci Environ Epidemiol. 2012;22:35–45. doi: 10.1038/jes.2011.38. [DOI] [PubMed] [Google Scholar]
- 8.Bieniek G. Urinary naphthols as an indicator of exposure to naphthalene. Scand J Work Environ Health. 1997;23:414–420. doi: 10.5271/sjweh.263. [DOI] [PubMed] [Google Scholar]
- 9.Serdar B, Egeghy PP, Waidyanatha S, Gibson R, Rappaport SM. Urinary biomarkers of exposure to jet fuel (JP-8) Environ Health Perspect. 2003;111:1760–1764. doi: 10.1289/ehp.6275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Serdar B, Egeghy PP, Gibson R, Rappaport SM. Dose-dependent production of urinary naphthols among workers exposed to jet fuel (JP-8) Am J Ind Med. 2004;46:234–244. doi: 10.1002/ajim.20049. [DOI] [PubMed] [Google Scholar]
- 11.Yang M, Koga M, Katoh T, Kawamoto T. A study for the proper application of urinary naphthols, new biomarkers for airborne polycyclic aromatic hydrocarbons. Arch Environ Contam Toxicol. 1999;36:99–108. doi: 10.1007/s002449900447. [DOI] [PubMed] [Google Scholar]
- 12.Nan HM, Kim H, Lim HS, et al. Effects of occupation, lifestyle and genetic polymorphisms of CYP1A1, CYP2E1, GSTM1 and GSTT1 on urinary 1-hydroxypyrene and 2-naphthol concentrations. Carcinogenesis. 2001;22:787–793. doi: 10.1093/carcin/22.5.787. [DOI] [PubMed] [Google Scholar]
- 13.Proctor SP, Heaton KJ, Smith KW, et al. The Occupational JP8 Exposure Neuroepidemiology Study (OJENES): repeated workday exposure and central nervous system functioning among US Air Force personnel. Neurotoxicology. 2011;32:799–808. doi: 10.1016/j.neuro.2011.06.010. [DOI] [PubMed] [Google Scholar]
- 14.Merchant-Borna K, Rodrigues EG, Smith KW, Proctor SP, McClean MD. Characterization of Inhalation Exposure to Jet Fuel among U.S. Air Force Personnel. Ann Occup Hyg. 2012;56:736–745. doi: 10.1093/annhyg/mes014. [DOI] [PubMed] [Google Scholar]
- 15.NIOSH. Aromatic Hydrocarbons; Method 1501. NIOSH Manual of Analytical Methods. 4. Cincinnati, OH: National Institute for Occupational Safety and Health; 2003. [Google Scholar]
- 16.OSHA. Analytical Methods Manual. Salt Lake City, UT: OSHA Organic Methods Evaluation Branch; 1982. [Google Scholar]
- 17.Li Z, Romanoff LC, Trinidad DA, et al. Measurement of urinary monohydroxy polycyclic aromatic hydrocarbons using automated liquid-liquid extraction and gas chromatography/isotope dilution high-resolution mass spectrometry. Anal Chem. 2006;78:5744–5751. doi: 10.1021/ac0606094. [DOI] [PubMed] [Google Scholar]
- 18.Schwartz J, Park SK, O’Neill MS, et al. Glutathione-S-transferase M1, obesity, statins, and autonomic effects of particles: gene-by-drug-by-environment interaction. Am J Respir Crit Care Med. 2005;172:1529–1533. doi: 10.1164/rccm.200412-1698OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Toriba A, Chetiyanukornkul T, Kizu R, Hayakawa K. Quantification of 2-hydroxyfluorene in human urine by column-switching high performance liquid chromatography with fluorescence detection. Analyst. 2003;128:605–610. doi: 10.1039/b212738e. [DOI] [PubMed] [Google Scholar]
- 20.Chetiyanukornkul T, Toriba A, Kizu R, Hayakawab K. Urinary 2-hydroxyfluorene and 1-hydroxypyrene in smokers and nonsmokers in Japan and Thailand. Polycyclic Aromatic Compounds. 2004;24:464–474. [Google Scholar]
- 21.Serdar B, Lee D, Dou Z. Biomarkers of exposure to polycyclic aromatic hydrocarbons (PAHs) and DNA damage: a cross-sectional pilot study among roofers in South Florida. BMJ Open. 2013;2 doi: 10.1136/bmjopen-2012-001318. pii: e001318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee CY, Lee JY, Kang JW, Kim H. Effects of genetic polymorphisms of CYP1A1, CYP2E1, GSTM1, and GSTT1 on the urinary levels of 1-hydroxypyrene and 2-naphthol in aircraft maintenance workers. Toxicol Lett. 2001;123:115–124. doi: 10.1016/s0378-4274(01)00374-5. [DOI] [PubMed] [Google Scholar]
- 23.Wilson AS, Davis CD, Williams DP, Buckpitt AR, Pirmohamed M, Park BK. Characterisation of the toxic metabolite(s) of naphthalene. Toxicology. 1996;114:233–42. doi: 10.1016/s0300-483x(96)03515-9. [DOI] [PubMed] [Google Scholar]
- 24.Mayfield HT. JP-8 Composition and Variability. Tyndall Air Force Base, FL: Armstrong Laboratory; 1996. Contract No.: AL/EQ-TR-1996–0006. [Google Scholar]
- 25.Li Z, Romanoff LC, Lewin MD, et al. Variability of urinary concentrations of polycyclic aromatic hydrocarbon metabolite in general population and comparison of spot, first-morning, and 24-h void sampling. J Expo Sci Environ Epidemiol. 2010;20:526–535. doi: 10.1038/jes.2009.41. [DOI] [PMC free article] [PubMed] [Google Scholar]