Abstract
MK-5046 is an orally active, potent, selective agonist of the orphan G protein–coupled receptor bombesin receptor subtype-3 (BRS-3) that is under evaluation for treatment of obesity. We report the safety, tolerability, pharmacokinetics, and pharmacodynamics of oral doses of MK-5046 (10-160 mg) in a double-blind, randomized, placebo-controlled study in healthy and obese male volunteers. MK-5046 exposure increased dose proportionally, and MK-5046 was eliminated with an apparent terminal half-life of 1.5 to 3.5 hours. Single doses transiently increased blood pressure. Patients reported adverse events (erections and feeling hot, cold, and/or jittery) that coincided with time of occurrence (Tmax) and increased with increasing dose. No changes were observed in body temperature, heart rate, plasma glucose levels, or feelings of hunger/satiety. The blood pressure and thermal experiences attenuated with a second dose 6 hours after the first. Additionally, the erections suggest a possible, unanticipated, role for BRS-3 in reproductive physiology. Oral administration of MK-5046 achieves plasma concentrations that are projected to activate BRS-3 and therefore should be suitable for exploring its biological role in humans.
Keywords: Bombesin receptor subtype-3, obesity, pharmacokinetics, erections, thermal sensation
Obesity is a significant worldwide health problem. It is associated with comorbidities including an increased risk of type 2 diabetes mellitus, hypertension, dyslipidemia, cardiovascular disease, gallstones, osteoarthritis, certain forms of cancer, and an overall reduced life expectancy.1 According to the World Health Organization, in 2005, an estimated 1.6 billion adults were overweight, and 400 million were obese. By 2015, these numbers are projected to reach 2.3 billion and 700 million, respectively.2 The current treatments for obesity are diet, exercise, and behavior modification. If these measures fail, pharmacotherapy and bariatric surgery are further options. Bariatric surgery is quite successful but is not without risks and is usually limited to more severe obesity.3,4 Currently, the only approved chronic pharmacotherapy option is orlistat, which has only modest efficacy.5
Bombesin receptor subtype-3 (BRS-3) is a member of the bombesin receptor subfamily of G protein–coupled receptors, which also includes the neuromedin B and gastrin releasing peptide receptors (NMBR and GRPR). However, despite its name and sequence similarity, BRS-3 has a low affinity for bombesin, and its natural ligand is unknown.6 Mammalian studies using bombesin are largely studies of GRPR agonism. BRS-3 is located primarily in the brain, including the hypothalamus, caudal brain stem, and several midbrain nuclei areas involved with the control of energy homeostasis.7-9 It is also present in pancreatic islets.10,11 BRS-3 has a role in regulation of energy homeostasis and body weight. Mice lacking functional BRS-3 (Brs-/y) have a reduced metabolic rate and body temperature, have slightly increased meal size (but not frequency), and develop obesity.12-14 The Brs-y mice also have reported phenotypes in assays of taste preference15 and social response/anxiety.16,17 In addition, BRS-3 mRNA has been detected in developing and injured lung18,19 and certain lung cancer cell lines,20,21 suggesting that BRS-3 may have a role in growth, development, and/or tumorigenesis.6
Recently, potent, selective BRS-3 agonist ligands have been developed,22,23 which has facilitated exploration of the biology and physiology of BRS-3.9,14 Treatment of rodents with a BRS-3 agonist increases fasting metabolic rate and body temperature and reduces food intake and body weight. Prolonged, high levels of receptor occupancy are required for maximal weight loss, suggesting that there may be a lack of tachyphylaxis for this agonist mechanism. BRS-3 agonist effectiveness was maintained in mice upon genetic ablation of the leptin, neuropeptide Y, melanocortin, or cannabinoid signaling pathways, indicating that BRS-3 has a role in energy homeostasis that complements these pathways, suggesting that BRS-3 agonists represent a new approach to the treatment of obesity.9 BRS-3 agonists augment glucose-dependent insulin secretion from established β-cell lines and pancreatic islets, suggesting that BRS-3 agonists may also have a role in the treatment of type 2 diabetes mellitus.11
MK-5046 (Supplementary Figure S1) is an orally active, potent, selective BRS-3 agonist with improved pharmacokinetics and fewer off-target activities than prior compounds.24 In preclinical experiments, MK-5046 reduced body weight in mice, rats, and dogs. With chronic dosing, effects on metabolic rate, rather than food intake, appear to be the predominant mechanism for sustained weight reduction by MK-5046.25 Here, we report the pharmacokinetics and pharmaco-dynamics of MK-5046 in a phase I study, the first administration of a BRS-3 agonist to humans.
METHODS
Drug
MK-5046, (2S)-1,1,1-trifluoro-2-[4-(1H-pyrazol-1-yl) phenyl]-3-(4-{[1-(trifluoromethyl)cyclopropyl]methyl}-1H-imidazol-2-yl)propan-2-ol (Supplementary Figure S1), was synthesized as described.24
Patients
Patients were enrolled at 2 clinical research sites, Thomas Jefferson University (TJU, Philadelphia, Pennsylvania) and Prism Research (Saint Paul, Minnesota). In panels A and B (at TJU), 17 male volunteers (13 African Americans, 2 whites, 1 Native American, 1 multiracial) were enrolled with a mean body weight of 85.3 kg (range, 65.1-101 kg) and a mean body mass index (BMI) of 26.4 kg/m2 (range, 22.5-31.2 kg/m2) (Supplementary Table S1). In panel C (n = 6 at Prism; n = 3 at TJU), 9 obese male volunteers (4 African Americans, 4 whites, 1 Hispanic) were enrolled with a mean body weight of 103.4 kg (range, 90.5-123 kg) and a mean BMI of 32.8 kg/m2 (range, 30.6-35.9 kg/m2). All patients were nonsmokers and had a Cockcroft-Gault creatinine clearance of >80 mL/min. Patients were in good general health according to routine medical history, physical examination, vital signs, and laboratory data. Patients were excluded if they had any relevant history of renal, hepatic, cardiovascular, gastrointestinal, or neurological disease or diabetes and also if they had donated blood or participated in another clinical study within 4 weeks or anticipated needing prescription or nonprescription drugs.
Every patient gave written informed consent. The protocol was approved by the RCRC and TJU Independent Review Boards. The protocol was conducted in accordance with the guidelines on good clinical practice and ethical standards for human experimentation established by the Declaration of Helsinki.
Study Design
This double-blind, randomized, placebo-controlled, single rising dose study was conducted at 2 study centers to assess the pharmacokinetics, pharmacodynamics, safety, and tolerability of MK-5046 in healthy male volunteers. Patients reported to the study unit 24 hours before study drug administration and remained for 24 hours after dosing. Except for a period specifically assessing the effect of food on MK-5046 pharmacokinetics, each single dose was administered while fasting. In all periods, including those in which 2 doses were administered 6 hours apart, a standard meal was given 4 hours after the first dose.
Panels A and B each had 5 treatment periods and were dosed in an alternating fashion. Panel C, comprising obese males, had 3 treatment periods. In each period for each panel, all patients received oral doses of MK-5046 or a matching placebo (6 active and 2 placebo) in a randomized, balanced manner. Patients in panel A received single MK-5046 doses of 10, 20, 40, 80, and 160 mg in a fixed sequence. Patients in panel B received single MK-5046 doses of 40, 120, and 160 mg; 40 mg after a standard, high-fat breakfast; and 2 doses of 120 mg 6 hours apart in a fixed sequence. The standard breakfast consists of 2 eggs, 2 bacon strips, 2 pieces of toast with butter, 55 to 110 g of fried potato, and 250 mL of whole milk. Patients in panel C received single MK-5046 doses of 80 and 120 mg and 2 doses of 120 mg given 6 hours apart. For each patient, there was at least a 7-day washout period between dosing days.
Pharmacokinetic Assessments
Plasma for determination of MK-5046 concentration was obtained at predose, 0.25, 0.5, 1, 1.5, 2, 3, 4, 6, 8, 10, and 12 hours after dosing. In the periods with a second dose at 6 hours, plasma samples were obtained at predose, 0.25, 0.5, 1, 1.5, 2, 3, 4, 6, 6.25, 6.5, 7, 7. 5, 8, 9, 10, 12, 14, 16, 18, and 24 hours after the first dose. A sensitive, specific, and validated high-performance liquid chromatography–tandem mass spectrometry assay was used for the determination of MK-5046 in human plasma over the concentration range of 4 to 4000 ng/mL. The lower limit of quantitation was defined as the lowest concentration on the standard curve that can be measured with a precision better than 20% coefficient of variation and accuracy within ±20% of the nominal concentrations. The lower limit of quantitation was 4 ng/mL (using 0.1 mL of plasma), the interday assay precision from standard and quality control samples for MK-5046 was <6% at all standard and quality control concentrations, and the assay accuracy ranged from 99% to 103% of nominal. The molecular mass of MK-5046 is 444 g/mol, so 100 ng/mL is 225 nM.
Plasma concentrations were used to calculate the following pharmacokinetic parameters: the area under the concentration-time curve from time x to y (AUCx-y), the maximum concentration observed in plasma (Cmax) and its time of occurrence (Tmax), and the apparent terminal half-life (t1/2) for each patient after each dose. The half-life was estimated as the quotient of ln(2) and the apparent terminal rate constant (λ), estimated by regression of the terminal log-linear portion of the plasma concentration-time profile. The AUC was calculated by use of the linear trapezoidal method (ascending concentrations) and log trapezoidal method (descending concentrations) up to the last measured concentration, and the extrapolated area was given by the quotient of the last measured concentration and the terminal rate constant (λ). The Cmax and Tmax were obtained by inspection of the concentration-time data. WinNonlin 5.0. (Pharsight, Corporation, Sunnyvale, California) was used for all calculations.
Pharmacodynamic Assessments
A 6-question visual analog scale (VAS) questionnaire for appetite/satiety26 was administered at predose, 1.5, 4, 12, and 24 hours postdose in panels A and B. Fasting insulin (panels B and C only) and glucose levels were measured at predose, 1.5, 4, and 24 hours postdose.
Safety and Tolerability
Physical examination, vital sign, 12-lead electrocar-diogram (including assessment for QTc- and PR-interval prolongation), and safety laboratory measurements comprising routine hematology, serum chemistry (including liver transaminases), and urinalysis were performed before the study, at various times after dosing, and after the study. Cuff blood pressure was measured using an automated, calibrated machine. Tympanic temperature was measured at matching times on the day before dosing and on the day of dosing. Heart rate and rhythm were monitored by telemetry from 1 hour predose through 8 hours postdose. Neurological examinations were administered at pre-dose, 1.5, 4, and 24 hours postdose (panels A, B, and C). Adverse experiences were monitored throughout the study. Investigators evaluated all clinical adverse experiences in terms of intensity (mild, moderate, or severe), duration, severity, outcome, and relationship to the study drug.
Statistical Analysis
The pharmacokinetic parameters AUC0-∞ and Cmax after single doses of MK-5046 were analyzed using a linear mixed model with panel and treatment within panel as fixed effects and patient within panel as a random effect. A natural log transformation was applied before model fitting and back transformed for reporting. Point estimates and 95% confidence intervals (CIs) for the least-squares geometric means (GMs) of the AUC0-∞ and Cmax were computed from the model. Summary statistics of median, minimum, and maximum for Tmax and harmonic mean and pseudo–standard deviation for apparent terminal t1/2 were provided. The mixed model was also used to compare 40-mg doses under fed and fasted conditions. The 90% CIs were generated from the mixed-effects model for the geometric mean ratios (GMRs) (fed vs fasted) for the AUC0-∞ and Cmax. The median difference for Tmax was calculated using the Hodges-Lehmann method of estimation. The dose proportionality of AUC0-∞ and Cmax from 10 mg to 160 mg was explored as follows. A power law model was fitted using the mixed-effects model with log-transformed dose values as fixed effect and patient within panel as a random effect on the log-transformed parameters AUC0-∞ and Cmax. Estimates of the slope and 95% CI from the power law model are reported.
Pharmacokinetic parameters for the two 120-mg doses given 6 hours apart were analyzed using a similar model as above. The GMRs (6-12 hours vs 0-6 hours) and 90% CIs were generated for the AUC and Cmax of MK-5046, and the median difference for Tmax was calculated using the Hodges-Lehmann method. All the analyses were done using SAS 9.1 (SAS Institute, Cary, North Carolina).
For pharmacodynamic parameters (heart rate, systolic and diastolic blood pressure, body temperature, fasting plasma glucose and insulin, and VAS), the within-patient changes from baseline were summarized by the mean and standard error.
RESULTS
Pharmacokinetics
Plasma concentration profiles and pharmacokinetic parameters after single oral doses of MK-5046 are presented in Figure 1 and Table I. Plasma MK-5046 AUC0-∞ and Cmax increased dose proportionately over the dose range studied (10-160 mg). The median Tmax was 1.0 hour, and the apparent t1/2 was 1.3 to 2.3 hours. It was noted that the plasma concentrations for the 40-mg and 160-mg doses were higher for panel B than for panel A. The reason for the apparent difference in AUC0-∞ and Cmax is unclear; the groups were studied at the same site and were of similar ages, weights, and BMIs. When 120 mg was given twice 6 hours apart, the plasma pharmacokinetic profile of the second dose was consistent with the pharmacokinetic parameters determined from the first dose. A standard, high-fat breakfast reduced the Cmax (GMR [fed vs fast] of 0.60; 90% CI, 0.45-0.79) and delayed the Tmax (median difference fed-fasted of 1.5 hours) but only modestly increased the AUC0-∞ (GMR [fed vs fast] of 1.14; 90% CI, 0.92-1.42) for a single oral 40-mg dose of MK-5046 (Figure 1B and Table I). The pharmacokinetic parameters of 80-mg and 120-mg doses in obese males in panel C (mean BMI, 32.8 kg/m2) were similar to those in panel B with a mean BMI of 26.4 kg/m2 (Figure 1C and Table I).
Figure 1.
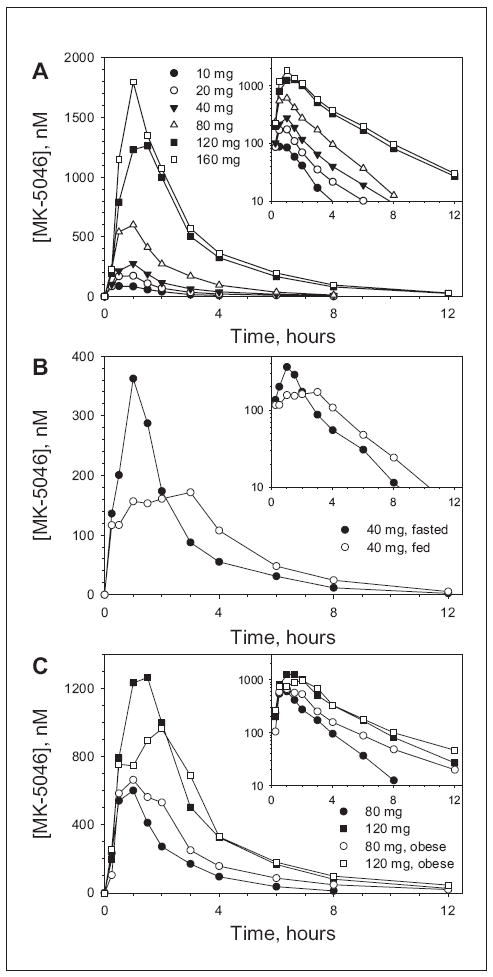
Plasma concentration-time profiles of MK-5046 after ingestion of single oral doses by male patients. Large plots are linear scale; insets are the same data using a log scale. (A) Single doses administered in the fasted state. Data are arithmetic means; n = 6 (N = 12 for 40 and 160 mg) healthy male patients/group of the indicated doses. (B) Single doses of 40 mg administered in fasted state (•) and after standard, high-fat breakfast (◦). Data are arithmetic means; n = 6 healthy male patients/group. (C) Single doses administered in the fasted state to cohorts with different mean body mass indexes (BMIs); (26.4 kg/m2, closed symbols; 32.8 kg/m2, open symbols). Data are arithmetic means; n = 6 patients/group.
Table I.
Pharmacokinetic Parameters for MK-5046 in Young Men Following Ingestion of Single Oral Doses
| Dose, mg | Panel | N | AuC0-∞, nM·ha
|
Cmax, nMa
|
Tmax, h
|
Apparent t1/2, h
|
||||
|---|---|---|---|---|---|---|---|---|---|---|
| GM | 95% CI | GM | 95% CI | Median | Minimum-Maximum | HM | Pseudo-SD | |||
| 10 | A | 6b | 147 | 96-224 | 91 | 61-135 | 0.75 | 0.50-1.50 | 1.05 | 0.63 |
| 20 | A | 6 | 244 | 162-368 | 163 | 110-241 | 0.50 | 0.25-1.00 | 1.36 | 0.93 |
| 40 | A | 6 | 396 | 263-597 | 287 | 193-426 | 0.75 | 0.50-1.00 | 1.31 | 0.53 |
| 40 | B | 6 | 716 | 465-1100 | 471 | 312-711 | 1.00 | 0.25-1.50 | 1.95 | 0.42 |
| 40, fed | B | 6 | 815 | 530-1260 | 282 | 187-426 | 2.50 | 0.25-4.00 | 2.06 | 0.56 |
| 80 | A | 6 | 1170 | 777-1760 | 738 | 497-1100 | 1.00 | 0.50-1.50 | 1.74 | 0.63 |
| 80, obese | C | 8 | 1860 | 1250-2760 | 836 | 574-1220 | 1.25 | 0.50-2.00 | 2.55 | 0.71 |
| 120 | B | 6 | 3370 | 2190-5170 | 1270 | 846-1920 | 1.25 | 0.50-2.00 | 2.28 | 0.66 |
| 120, obese | C | 6 | 3540 | 2330-5370 | 1250 | 835-1880 | 1.25 | 0.50-2.00 | 2.72 | 0.54 |
| 160 | A | 6 | 3000 | 1990-4520 | 1280 | 860-1900 | 0.75 | 0.50-1.00 | 2.21 | 0.65 |
| 160 | B | 6 | 4460 | 2900-6860 | 1830 | 1220-2770 | 1.00 | 1.00-1.50 | 2.22 | 0.57 |
GM, geometric mean; HM, harmonic mean.
Back-transformed least squares mean and 95% confidence interval (CI) from mixed-effects model performed on natural log-transformed values.
n = 5 for t1/2 and AUC0-∞ at this dose because one patient had concentrations below the limit of quantitation.
The pharmacokinetic parameters for two 120-mg doses given 6 hours apart to patients in panels B and C are also similar (Figure 2 and Table II). In both BMI cohorts, the AUC6-12h was approximately 1.8-fold greater than the AUC0-6h. The Cmax was approximately 1.2-fold greater for the second dose, while median Tmax was 1.5 hours for both doses. The apparent elimination t1/2 was approximately 3.5 hours.
Figure 2.
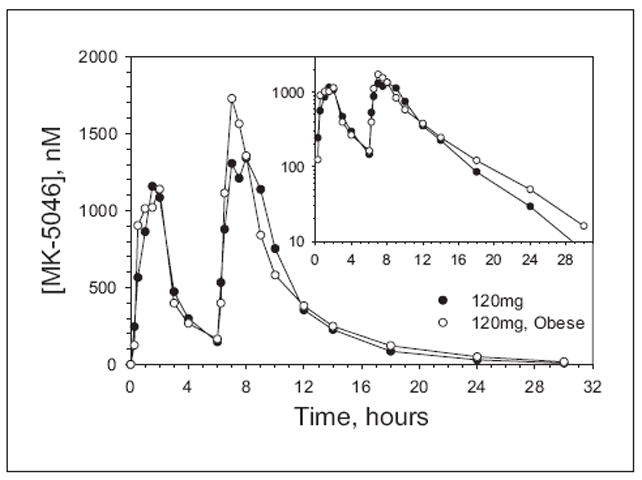
Plasma concentration-time profiles of MK-5046 in fasted state and after ingestion of two 120-mg oral doses taken 6 hours apart in healthy (•, n = 6/group) and obese (◦, n = 5/group) male patients. Data are arithmetic means. Large plot is linear scale; inset is the same data using a log scale.
Table II.
Pharmacokinetic Parameters for MK-5046 in Young Men After Ingestion of Two 120-mg Oral Doses Taken 6 Hours Apart
| Dose, mg | Panel | N | AuC, nM·ha
|
Cmax, nM
|
Tmax, h
|
Apparent t1/2, h
|
||||
|---|---|---|---|---|---|---|---|---|---|---|
| GMb | 95% CI | GMb | 95% CI | Median | Minimum-Maximum | HM | Pseudo-SD | |||
| 120, first | B | 6 | 2730 | 1800-4130 | 1470 | 1060-2060 | 1.50 | 0.25-2.00 | ||
| 120, second | 5150 | 3410-7800 | 1680 | 1200-2350 | 1.50 | 0.25-3.00 | 3.5 | 1.2 | ||
| 120, combined | 9650 | 7020-13,280 | ||||||||
| 120, first | C | 5 | 2820 | 1700-4670 | 1600 | 1140-2240 | 1.50 | 0.50-2.00 | ||
| 120, second | 4890 | 2950-8100 | 2000 | 1430-2820 | 1.50 | 0.50-2.00 | 3.7 | 0.9 | ||
| 120, combined | 9550 | 5290-17,220 | ||||||||
GM, geometric mean; HM, harmonic mean.
Second dose given 6 hours after the first dose. AUC for the first dose is AUC0-6h, for the second dose is AUC6-12h, and for the combined doses is AUC0-∞. Note that AUC6-12h includes some contribution from the first dose and therefore overestimates the specific exposure from the second dose alone.
Back-transformed least squares mean and 95% confidence interval (CI) from mixed-effects model performed on natural log-transformed values for AUC0-6h and AUC6-12h and from t distribution on natural log-transformed values for AUC0-∞.
Pharmacodynamics
Increases in systolic and diastolic blood pressure were observed and were greatest at approximately 1 hour after dosing, at approximately the Tmax (Figure 3 and Supplementary Tables S2 and S3). When two 120-mg doses were given 6 hours apart, an increase in blood pressure was observed with the first dose but attenuated with the second, despite the slightly greater Cmax levels following the second dose. These results suggest that MK-5046 increases blood pressure, but this effect is attenuated 6 hours later. To investigate the relationship between plasma exposure and blood pressure, data from the 1-hour postdose time point were used in order to minimize the effects of time-dependent attenuation. Figure 4 shows the change in blood pressure from predose to 1 hour as a function of plasma MK-5046 concentration at 1 hour, demonstrating an increase in both diastolic and systolic blood pressure with increasing exposure.
Figure 3.
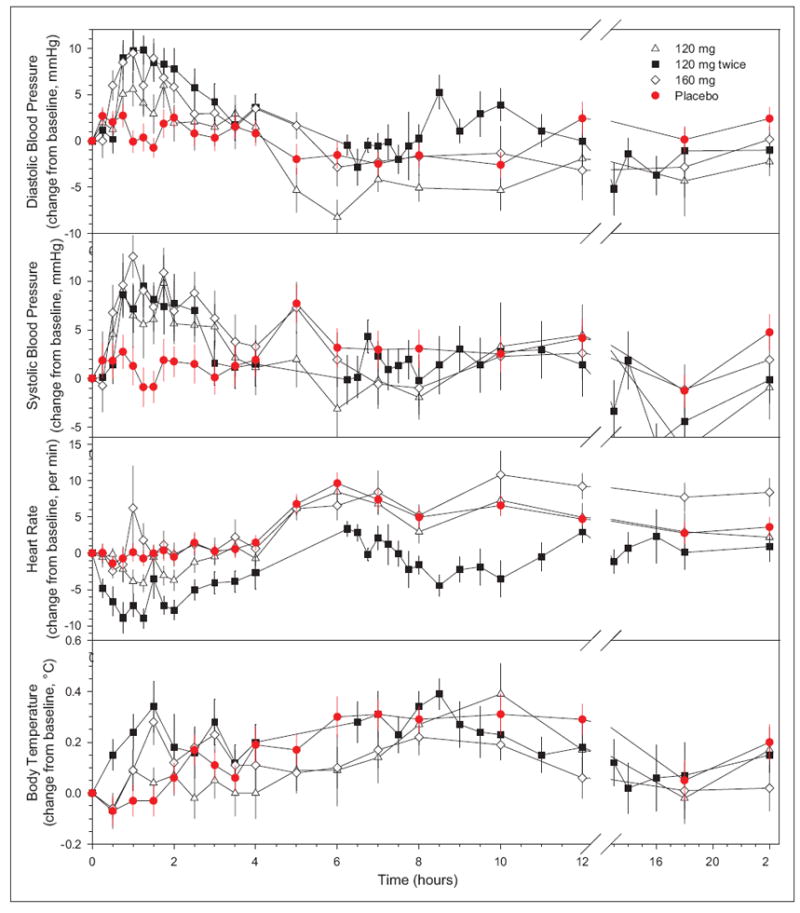
Effect of MK-5046 on blood pressure, heart rate, and body temperature. Data are change from the baseline (0 hours, which is nominally 0800) for placebo (red circles; n = 27, except n = 22 at the 5- and 6-hour points), 120 mg (open triangles; n = 12/group), 120 mg twice (black squares; second dose at 6 hours, n = 11/group), and 160 mg (open diamonds; n = 12/group) MK-5046 doses. Data are arithmetic mean ± standard error. Placebo time points sampled only with the twice-dosing cohort had an n = 5 and were omitted.
Figure 4.
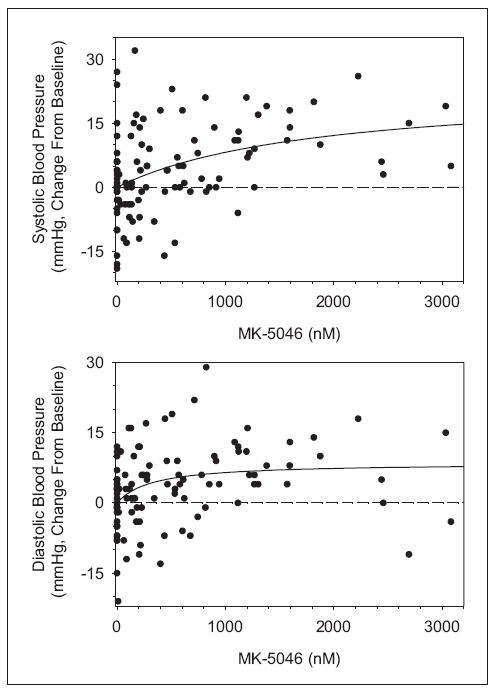
MK-5046 plasma concentration and blood pressure 1 hour after dosing. Change in blood pressure from predose to 1 hour after dosing is graphed as a function of MK-5046 plasma concentration at 1 hour after dosing. Because of attenuation with a second dose, only first dose data were included in this analysis. Emax best-fit curves (solid lines) are graphed as follows: ΔSBP = 23.33*MK-5046/(MK-5046 + 1842) and ΔDBP = 8.561*MK-5046/(MK-5046 + 315.3).
MK-5046 did not have a demonstrable effect on heart rate or on body temperature (Figure 3 and Supplementary Tables S2 and S3). To look for subtle effects on temperature, the 24-hour temperature profiles immediately preceding dosing were compared with those for the 24 hours immediately following dosing, and again, no effect on temperature was detected (Supplementary Figure S2). One patient had an asymptomatic temperature increase not correlated with drug levels (see supplementary material).
A 6-question VAS was administered to assess hunger/satiety. No clear effect of MK-5046 on VAS score was observed at 1.5, 4, 12, or 24 hours after dosing (Supplementary Figure S3 and data not shown).
MK-5046 did not affect fasting plasma glucose levels at 1.5, 4, or 24 hours after dosing (Supplementary Figure S4A). Fasting plasma insulin levels at 1.5 hours after dosing appeared increased up to 2-fold. No changes were observed at 4 or 24 hours after dosing (Supplementary Figure S4B).
Safety and Tolerability
A total of 121 adverse experiences (AEs) were reported by 22 patients: 104 events while on active drug and 17 on placebo in 105 dose periods (80 on drug, 25 on placebo). Most AEs were mild or moderate in intensity and transient in nature. There was one serious AE (ventricular tachycardia), and 2 patients were discontinued (see supplementary material).
The most common AEs associated with MK-5046 were grouped into 3 classes: feeling cold, feeling hot, and feeling jittery (see Figure 5 for the Medical Dictionary for Regulatory Activities [MedDRA] Preferred Terms constituting each of these). The feeling hot AEs were the most common. These AEs occurred with doses of 40 mg or more (Figure 5A). At 160 mg, the highest dose, 75% of patients had at least one of these AEs. The hot, cold, and jittery AEs were generally concurrent and peaked at 1 to 2 hours post-dose, approximately the Tmax (Figure 5B and Supplementary Table S4). An understanding of the simultaneous reporting of being hot and cold is aided by an example of a verbatim report: “feeling hot outside and cold inside.” For those receiving 2 doses of MK-5046 given 6 hours apart, the doses were evaluated separately, and fewer AEs were associated with the second dose (Figure 5A).
Figure 5.
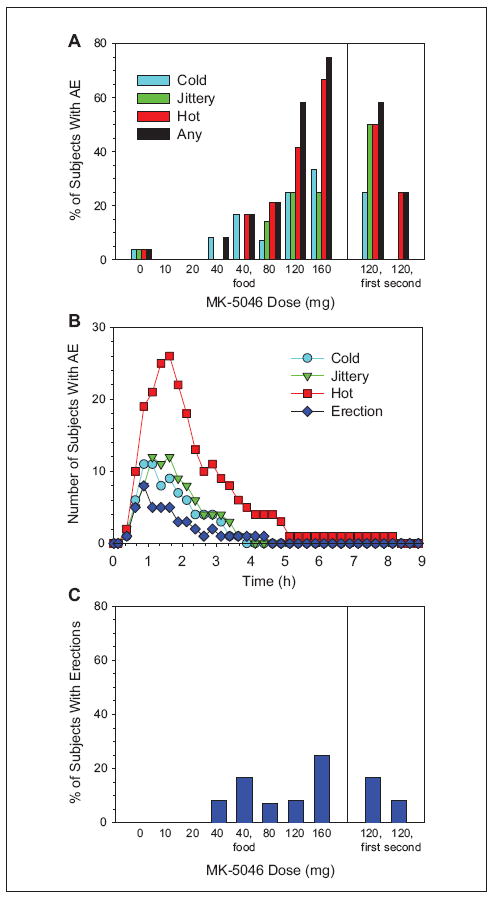
MK-5046 adverse experiences (AEs) as a function of dose administered and time. (A) The percentage of patients receiving the indicated doses who experienced one or more of the indicated AEs is indicated. The following groups of Medical Dictionary for Regulatory Activities (MedDRA) Preferred Terms were used to define the AE. Cold: chills, cold sweat, and feeling cold; Hot: feeling hot, flushing, hot flush, and hyperhidrosis; Jittery: feeling jittery, muscle tightness, muscle twitching, nervousness, and tremor. Any includes all of the cold, hot, and jittery terms. The Preferred Term “chills” includes the Lower Level Term “shivering,” but similar Lower Level Terms (eg, shaky feelings, shaking, tremor, and muscle tightness) are classified as “jittery.” (B) Time course of AE. The AE prevalence was assessed using time intervals of 15 minutes. The number of patients experiencing one or more of the indicated AEs is shown. Patients receiving placebo are omitted. When 120 mg was dosed twice, the 2 postdose periods are treated separately. (C) The percentage of patients receiving the indicated doses who experienced one or more erections is indicated.
Another AE, unprovoked erection, was reported in 4 patients (10 events) at doses of ≥40 mg (Figure 5C). These resolved spontaneously in all but lasted 3 hours 35 minutes in one patient (given 160 mg), which was classified as priapism. The erections coincided with Tmax and had similar onset and duration as the thermal AE (Figure 5 and Supplementary Table S4).
Some patients reported feeling sleepy, with a similar incidence in patients treated with placebo (7.4%) and MK-5046 (7.6%) and no clear dependence on MK-5046 dose. Other clinical AEs occurring in at least 2 patients on drug were cough (3 on drug), diarrhea (3 on drug), headache (3 on placebo, 5 on drug), nasal congestion (2 on drug), and throat irritation (1 on placebo, 2 on drug)
DISCUSSION
This study reports the first introduction of a BRS-3 agonist into humans. This study provides MK-5046 pharmacokinetic, safety, and tolerability information and identified a possible role for BRS-3 in reproductive physiology.
Single oral doses of MK-5046 were rapidly absorbed with a Tmax of approximately 1 hour and a t1/2 of 1 to 3.5 hours. While the t1/2 appears to increase with increasing dose, this is probably due to fewer data points above the assay’s limit of quantitation at lower doses; thus, the true terminal elimination t1/2 is likely closer to 3.5 hours. While the pharmacokinetics of MK-5046 are variable, the AUC and Cmax increased linearly with dose over the range studied, 10 to 160 mg. The AUC and Cmax did not vary with BMI, being similar in cohorts with mean BMIs of 26.4 and 32.8 kg/m2. A high-fat meal delayed Tmax and reduced Cmax but affected the AUC minimally. A single dose of 160 mg produced a plasma Cmax of approximately 1500 nM, which corresponds to an unbound plasma concentration of approximately 15 nM, assuming 99% binding with human plasma proteins.24 This compares favorably with the human receptor binding Ki of 3.7 nM and EC50 in a cell-based assay of 14 nM.24 Thus, the exposures achieved should be adequate to stimulate BRS-3 receptors for a number of hours, allowing study of the pharmacodynamics of BRS-3 agonism in humans.
A first dose of MK-5046 causes an increase in both systolic and diastolic blood pressure. The effect is dose dependent, correlating with plasma exposure. A persistent increase in blood pressure is unacceptable in a drug for the treatment of obesity. Interestingly, the blood pressure increase was attenuated with a second 120-mg dose given 6 hours after the first dose. At present, there is no clinical experience with doses given at other time intervals or with more than 2 doses. Blood pressure increases that attenuate with continued MK-5046 dosing were observed in rats and dogs.25
The result that MK-5046 does not appear to elevate body temperature in humans contrasts with the increases observed in mice, rats, and dogs.14,25 This difference may be due to the divergence in temperature physiology between small and large mammals. Small mammals burn a large fraction of their food intake to maintain body temperature, known as facultative thermogenesis and which largely occurs in brown adipose tissue. In mice, rats, and dogs, the BRS-3 agonist–induced increase in body temperature attenuates with continued dosing.14,25 In contrast, adult humans maintain body temperature largely by conservation of the heat from metabolic processes and by manipulation of the environment. Brown adipose tissue can be active in adult humans, but the magnitude of its contribution to body temperature is unclear.27,28 Our working hypothesis is that BRS-3 agonism causes an increase in metabolic rate in humans as well but that this does not change the body temperature detectably. The extra heat generated by the increased metabolic rate is dissipated, for example, by diaphoresis and vasodilation. The patients feel warm as this occurs. A limitation of the current study is that direct tests of this hypothesis, such as measurement of the effect of MK-5046 on metabolic rate, skin temperature, vasodilation, and heat dissipation were not performed.
MK-5046 treatment initially increased heart rate in dogs and (nonsignificantly) in rats; these effects attenuated with continued dosing.25 If the initial heart rate increase in dogs is due to an increase in metabolic rate, the lack of an observed increase in humans may be because any increase in metabolic rate is not large. For example, the correlations between heart rate and metabolic rate at low levels of exercise29,30 or with an isoprenaline infusion31 demonstrate an approximately 1-bpm increase in heart rate per 1% increase in metabolic rate. However, a contrasting observation is that a 6% increase in metabolic rate due to a cool environment (19°C vs 24°C) actually reduced heart rate by 4%.32
Evidence that BRS-3 may have a role in the nervous system in addition to its role in energy homeostasis includes the observation that Brs3 knockout mice respond differently than control mice to behavioral assays of social isolation and anxiety.9,16,17 In addition, BRS-3 affects the activity of orexin neurons.33 In the current clinical study, no obvious effect on anxiety or sleep/wake behaviors was observed.
The association of erections with MK-5046 was unexpected. No erections occurred in placebo-treated patients, and erections are an uncommon AE in phase I clinical trials. Prior to the clinical observations, there were no data explicitly identifying a role for BRS-3 in reproductive physiology. For example, male Brs3 knockout mice have not been reported to exhibit reproductive abnormalities. However, control of reproduction and energy homeostasis occurs in overlapping regions of the brain such as the hypothalamus, and BRS-3 is located in these regions. While we cannot rule out that this is an off-target effect of MK-5046, the clinical observations suggest that BRS-3 may have a role in reproductive physiology and/or sexual behavior.
Attenuation of agonist effects is common, occurring both at the receptor and downstream. BRS-3 has some constitutive activity34 and does not appear to desensitize acutely (unpublished observations). In preclinical species, the BRS-3 agonist effects on metabolic rate and body weight did not attenuate with continued dosing. In contrast, BRS-3 agonist effects on food intake, body temperature, blood pressure, and heart rate all lessened or disappeared altogether with continued dosing.9,14,25 While less pharmacodynamic data are available for the clinical observations, it appears that blood pressure and thermal AE attenuate with just a second dose in humans. There are insufficient data to determine if the erections attenuate or not.
Recent preclinical data demonstrate glucose-dependent insulin secretagogue activity for BRS-3 agonists, including functioning in human islets from nondiabetic and diabetic donors.11 While the current trial was not designed to test this aspect of BRS-3 agonism, at 1.5 hours after dosing, there was a numerical increase in fasting plasma insulin levels without a change in plasma glucose levels. Kinetic studies are needed to investigate this further, for example, to see if the glucose subsequently decreases.
The initial MK-5046 clinical data identify some challenges for its development as a treatment for obesity. An increase in blood pressure is undesirable in an obesity treatment because increased blood pressure is a cardiovascular risk factor,35 offsetting a desired benefit of treating obesity. Thus, unless an effective dose can provide clear margins over the adverse effects identified in this study, the path forward will be difficult.
In summary, MK-5046 has pharmacokinetic properties suitable for continued exploration of BRS-3 physiology in humans. Effects on blood pressure, penile erection, and thermal sensation need to be better understood and will affect whether MK-5046 (and BRS-3 agonism in general) will prove suitable for the treatment of obesity and diabetes.
Supplementary Material
Acknowledgments
This project was funded by Merck Sharp & Dohme Corp.
Footnotes
Financial disclosure: M.L.R., V.D., A.M., W.S.D., C.L., S.A.S., J.A.W., and E.L. are current or former employees of Merck Sharp & Dohme Corp and may own stock and/or have stock options in the company. W.K.K. has served as a paid consultant to Merck (<$2000/y).
Supplementary data for this article are available at http://jcp.sagepub.com/supplemental/.
References
- 1.Bray GA. Medical consequences of obesity. J Clin Endocrinol Metab. 2004;89(6):2583–2589. doi: 10.1210/jc.2004-0535. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization. [September 7, 2011];Obesity and overweight. 2006 Available at: http://www.who.int/mediacentre/factsheets/fs311/en/index.html.
- 3.Sjostrom L, Lindroos AK, Peltonen M, et al. Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N Engl J Med. 2004;351(26):2683–2693. doi: 10.1056/NEJMoa035622. [DOI] [PubMed] [Google Scholar]
- 4.Smith BR, Schauer P, Nguyen NT. Surgical approaches to the treatment of obesity: bariatric surgery. Endocrinol Metab Clin North Am. 2008;37(4):943–964. doi: 10.1016/j.ecl.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 5.Torgerson JS, Hauptman J, Boldrin MN, Sjostrom L. XENical in the prevention of diabetes in obese subjects (XENDOS) study: a randomized study of orlistat as an adjunct to lifestyle changes for the prevention of type 2 diabetes in obese patients. Diabetes Care. 2004;27(1):155–161. doi: 10.2337/diacare.27.1.155. [DOI] [PubMed] [Google Scholar]
- 6.Jensen RT, Battey JF, Spindel ER, Benya RV. International Union of Pharmacology: LXVIII. Mammalian bombesin receptors: nomenclature, distribution, pharmacology, signaling, and functions in normal and disease states. Pharmacol Rev. 2008;60(1):1–42. doi: 10.1124/pr.107.07108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ohki-Hamazaki H, Wada E, Matsui K, Wada K. Cloning and expression of the neuromedin B receptor and the third subtype of bombesin receptor genes in the mouse. Brain Res. 1997;762(1-2):165–172. doi: 10.1016/s0006-8993(97)00380-6. [DOI] [PubMed] [Google Scholar]
- 8.Liu J, Lao ZJ, Zhang J, et al. Molecular basis of the pharmacological difference between rat and human bombesin receptor subtype-3 (BRS-3) Biochemistry. 2002;41(28):8954–8960. doi: 10.1021/bi0202777. [DOI] [PubMed] [Google Scholar]
- 9.Guan XM, Chen H, Dobbelaar PH, et al. Regulation of energy homeostasis by bombesin receptor subtype-3: selective receptor agonists for the treatment of obesity. Cell Metab. 2010;11(2):101–112. doi: 10.1016/j.cmet.2009.12.008. [DOI] [PubMed] [Google Scholar]
- 10.Fleischmann A, Laderach U, Friess H, Buechler MW, Reubi JC. Bombesin receptors in distinct tissue compartments of human pancreatic diseases. Lab Invest. 2000;80(12):1807–1817. doi: 10.1038/labinvest.3780192. [DOI] [PubMed] [Google Scholar]
- 11.Feng Y, Guan XM, Li J, et al. Bombesin receptor subtype-3 (BRS-3) regulates glucose-stimulated insulin secretion in pancreatic islets across multiple species. Endocrinology. 2011;152(11):4106–4115. doi: 10.1210/en.2011-1440. [DOI] [PubMed] [Google Scholar]
- 12.Ohki-Hamazaki H, Watase K, Yamamoto K, et al. Mice lacking bombesin receptor subtype-3 develop metabolic defects and obesity. Nature. 1997;390(6656):165–169. doi: 10.1038/36568. [DOI] [PubMed] [Google Scholar]
- 13.Ladenheim EE, Hamilton NL, Behles RR, et al. Factors contributing to obesity in bombesin receptor subtype-3-deficient mice. Endocrinology. 2008;149(3):971–978. doi: 10.1210/en.2007-1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Metzger JM, Gagen K, Raustad KA, et al. Body temperature as a mouse pharmacodynamic response to bombesin receptor subtype-3 agonists and other potential obesity treatments. Am J Physiol Endocrinol Metab. 2010 Nov;299:E816–E824. doi: 10.1152/ajpendo.00404.2010. [DOI] [PubMed] [Google Scholar]
- 15.Yamada K, Wada E, Imaki J, Ohki-Hamazaki H, Wada K. Hyperresponsiveness to palatable and aversive taste stimuli in genetically obese (bombesin receptor subtype-3-deficient) mice. Physiol Behav. 1999;66(5):863–867. doi: 10.1016/s0031-9384(99)00032-3. [DOI] [PubMed] [Google Scholar]
- 16.Yamada K, Ohki-Hamazaki H, Wada K. Differential effects of social isolation upon body weight, food consumption, and responsiveness to novel and social environment in bombesin receptor subtype-3 (BRS-3) deficient mice. Physiol Behav. 2000;68(4):555–561. doi: 10.1016/s0031-9384(99)00214-0. [DOI] [PubMed] [Google Scholar]
- 17.Yamada K, Santo-Yamada Y, Wada E, Wada K. Role of bombesin (BN)-like peptides/receptors in emotional behavior by comparison of three strains of BN-like peptide receptor knockout mice. Mol Psychiatry. 2002;7(1):113–117. doi: 10.1038/sj.mp.4000974. [DOI] [PubMed] [Google Scholar]
- 18.Shan L, Emanuel RL, Dewald D, et al. Bombesin-like peptide receptor gene expression, regulation, and function in fetal murine lung. Am J Physiol Lung Cell Mol Physiol. 2004;286(1):L165–L173. doi: 10.1152/ajplung.00436.2002. [DOI] [PubMed] [Google Scholar]
- 19.Tan YR, Qi MM, Qin XQ, et al. Wound repair and proliferation of bronchial epithelial cells enhanced by bombesin receptor subtype 3 activation. Peptides. 2006;27(7):1852–1858. doi: 10.1016/j.peptides.2005.12.012. [DOI] [PubMed] [Google Scholar]
- 20.Ryan RR, Weber HC, Mantey SA, et al. Pharmacology and intracellular signaling mechanisms of the native human orphan receptor BRS-3 in lung cancer cells. J Pharmacol Exp Ther. 1998;287(1):366–380. [PubMed] [Google Scholar]
- 21.Fathi Z, Corjay MH, Shapira H, et al. BRS-3: a novel bombesin receptor subtype selectively expressed in testis and lung carcinoma cells. J Biol Chem. 1993;268(8):5979–5984. [PubMed] [Google Scholar]
- 22.He S, Dobbelaar PH, Liu J, et al. Discovery of substituted biphenyl imidazoles as potent, bioavailable bombesin receptor subtype-3 agonists. Bioorg Med Chem Lett. 2010;206:1913–1917. doi: 10.1016/j.bmcl.2010.01.154. [DOI] [PubMed] [Google Scholar]
- 23.Liu J, He S, Jian T, et al. Synthesis and SAR of derivatives based on 2-biarylethylimidazole as bombesin receptor subtype-3 (BRS-3) agonists for the treatment of obesity. Bioorg Med Chem Lett. 2010;20(7):2074–2077. doi: 10.1016/j.bmcl.2010.02.076. [DOI] [PubMed] [Google Scholar]
- 24.Sebhat IK, Franklin C, Lo M-C, et al. Discovery of MK-5046, a potent, selective bombesin receptor subtype-3 agonist for the treatment of obesity. ACS Med Chem Lett. 2011;2:43–47. doi: 10.1021/ml100196d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guan XM, Metzger JM, Yang L, et al. Antiobesity effect of MK-5046, a novel bombesin receptor subtype-3 agonist. J Pharmacol Exp Ther. 2011;336(2):356–364. doi: 10.1124/jpet.110.174763. [DOI] [PubMed] [Google Scholar]
- 26.Flint A, Raben A, Blundell JE, Astrup A. Reproducibility, power and validity of visual analogue scales in assessment of appetite sensations in single test meal studies. Int J Obes Relat Metab Disord. 2000;24(1):38–48. doi: 10.1038/sj.ijo.0801083. [DOI] [PubMed] [Google Scholar]
- 27.Nedergaard J, Bengtsson T, Cannon B. Unexpected evidence for active brown adipose tissue in adult humans. Am J Physiol Endocrinol Metab. 2007;293(2):E444–E452. doi: 10.1152/ajpendo.00691.2006. [DOI] [PubMed] [Google Scholar]
- 28.Virtanen KA, Lidell ME, Orava J, et al. Functional brown adipose tissue in healthy adults. N Engl J Med. 2009;360(15):1518–1525. doi: 10.1056/NEJMoa0808949. [DOI] [PubMed] [Google Scholar]
- 29.Spurr GB, Prentice AM, Murgatroyd PR, Goldberg GR, Reina JC, Christman NT. Energy expenditure from minute-by-minute heart-rate recording: comparison with indirect calorimetry. Am J Clin Nutr. 1988;48(3):552–559. doi: 10.1093/ajcn/48.3.552. [DOI] [PubMed] [Google Scholar]
- 30.Schulz S, Westerterp KR, Bruck K. Comparison of energy expenditure by the doubly labeled water technique with energy intake, heart rate, and activity recording in man. Am J Clin Nutr. 1989;49(6):1146–1154. doi: 10.1093/ajcn/49.6.1146. [DOI] [PubMed] [Google Scholar]
- 31.Mansell PI, Fellows IW, Birmingham AT, Macdonald IA. Metabolic and cardiovascular effects of infusions of low doses of isoprenaline in man. Clin Sci (Lond) 1988;75(3):285–291. doi: 10.1042/cs0750285. [DOI] [PubMed] [Google Scholar]
- 32.Celi FS, Brychta RJ, Linderman JD, et al. Minimal changes in environmental temperature result in a significant increase in energy expenditure and changes in the hormonal homeostasis in healthy adults. Eur J Endocrinol. 2010;163(6):863–872. doi: 10.1530/EJE-10-0627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Furutani N, Hondo M, Tsujino N, Sakurai T. Activation of bombesin receptor subtype-3 influences activity of orexin neurons by both direct and indirect pathways. J Mol Neurosci. 2010;42(1):106–111. doi: 10.1007/s12031-010-9382-5. [DOI] [PubMed] [Google Scholar]
- 34.Gbahou F, Holst B, Schwartz TW. Molecular basis for agonism in the BB3 receptor: an epitope located on the interface of transmembrane-III, -VI, and -VII. J Pharmacol Exp Ther. 2010;333(1):51–59. doi: 10.1124/jpet.109.162131. [DOI] [PubMed] [Google Scholar]
- 35.Vasan RS, Larson MG, Leip EP, et al. Impact of high-normal blood pressure on the risk of cardiovascular disease. N Engl J Med. 2001;345(18):1291–1297. doi: 10.1056/NEJMoa003417. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


