Abstract
IMPORTANCE
Early adversity is an important risk factor that relates to internalizing symptoms and altered brain structure.
OBJECTIVE
To assess the direct effects of early adversity and child internalizing symptoms (ie, depression, anxiety) on cortical gray matter (GM) volume, as well as the extent to which early adversity associates with variation in cortical GM volume indirectly via increased levels of internalizing symptoms.
DESIGN, SETTING, AND PARTICIPANTS
A prospective investigation of associations between adversity within the first 6 years of life, internalizing symptoms during childhood and early adolescence, and altered brain structure in late adolescence (age, 18-21 years) was conducted in a community-based birth cohort in England (Avon Longitudinal Study of Parents and Children). Participants from the cohort included 494 mother-son pairs monitored since the mothers were pregnant (estimated date of delivery between April 1, 1991, and December 31, 1992). Data collection for the present study was conducted between April 1, 1991, and November 30, 2010; the neuroimaging data were collected between September 1, 2010, and November 30, 2012, and data analyses for the present study occurred between January 25, 2013, and February 15, 2015. Risk factors were adversity within the first 6 years of the child’s life (including prenatal exposure) and the child’s internalizing symptoms between age 7 and 13 years.
EXPOSURES
Early childhood adversity.
MAIN OUTCOMES AND MEASURES
The main outcome was GM volume of cortical regions previously associated with major depression measured through T1-weighted magnetic resonance images collected in late adolescence.
RESULTS
Among 494 young men included in this analysis, early adversity was directly associated with lower GM volumes in the anterior cingulate cortex (β = −.18; P = .01) and higher GM volume in the precuneus (β = .18; P = .009). Childhood internalizing symptoms were associated with lower GM volume in the right superior frontal gyrus (β = −.20; P = .002). Early adversity was also associated with higher levels of internalizing symptoms (β = .37; P < .001), which, in turn, were associated with lower superior frontal gyrus volume (ie, an indirect effect) (β = −.08; 95% CI, −0.14 to −0.01; P = .02).
CONCLUSIONS AND RELEVANCE
Adversity early in life was associated with higher levels of internalizing symptoms as well as with altered brain structure. Early adversity was related to variation in brain structure both directly and via increased levels of internalizing symptoms. These findings may suggest that some of the structural variation often attributed to depression might be associated with early adversity in addition to the effect of depression.
Adversity early in life is associated with both altered brain structure and increased risk of developing internalizing symptoms (ie, depression, anxiety).1-4 Previous studies4-9 have shown that childhood adversity, including stressful life events, maltreatment, abuse, and domestic violence, are associated with structural variation in gray matter (GM) in the brain. The effect of early adversity on the brain has long been suggested to relate to neurobiological sequelae associated with excessive stress. For example, many studies10,11 have linked adversity during childhood (eg, poverty, cumulative risk exposures) to the later allostatic load (ie, “the wear and tear” of the body) associated with stress. Allostatic load is, in turn, associated with both increased risk of depression12 and stress-induced structural remodeling of the brain.13
Studies14,15 examining structural variation in GM in patients with depression vs healthy individuals have found that some of the structural variation in the depressed patients correlates with experiences of early adversity. Hence, it has been suggested14 that some of the structural brain variation usually attributed to depression may also relate to the effect of early adversity on the brain. In line with this hypothesis, a recent study9 found that early maltreatment was indirectly related to altered brain structure via the presence of psychiatric disorder during childhood (not differentiating between externalizing and internalizing symptoms). A limitation of previous adversity-brain research, however, is the use of retrospective reports of early adversity, which hinders the examination of prospective and indirect associations.
The aim of the present study was to examine how adverse experiences within the first 6 years of life relate prospectively to variations in cortical GM volume in adolescent males, both directly and indirectly, via increased levels of childhood internalizing symptoms. Past neuroimaging literature on depression has tended to focus on subcortical structures, such as the hippocampus and amygdala, and it has been suggested16 that this focus may have placed too much emphasis on subcortical structures in depression compared with cortical structures. Meta-analyses16-18 applying a whole-brain approach suggest that cortical regions may be implicated in depression in a more consistent manner than subcortical regions. The present study therefore focused on cortical regions, which also allowed for the explorative examination of regional thickness and surface area measures. The distinction between surface area and thickness is a relatively novel approach that has not been widely applied in depression and adversity studies. Moreover, the distinction may be important in longitudinal studies since the surface area and cortical thickness are developmentally independent19 and may vary in timing of sensitivity toward adverse environments. Studies20 of nonhuman primates have shown that the expansion of the cortical surface area occurs earlier than corresponding changes in cortical thickness. In humans, longitudinal studies21,22 have shown a substantial expansion of cortical surface area and GM volume and a more moderate increase in cortical thickness21 within the first 2 years of life. Longitudinal studies23 assessing GM development from the age of 5 years have demonstrated increases in both cortical thickness and surface areas until late childhood or early adolescence. To the best of our knowledge, no published research has examined the contribution of variation in the surface area and cortical thickness to volumetric effects of adversity before the age of 6 years. This study focused on adversity during childhood because longitudinal studies, including those cited above, have shown that cortical GM volume continues to undergo structural development throughout early childhood.21-23
Methods
Sample
The Avon Longitudinal Study of Parents and Children (ALSPAC) is an ongoing, population-based study designed to investigate the influence of various risks on the development and health of children. Pregnant women residing in the former Avon Health Authority in southwest England who had an estimated date of delivery between April 1, 1991, and December 31, 1992, were invited to participate, resulting in a cohort of 14 541 pregnancies.24,25 Approval for the study was obtained from the ALSPAC Law and Ethics Committee and the local research ethics committees. More information on ALSPAC is available (http://www.bris.ac.uk/alspac/). Data collection for the present study started on April 1, 1991, and continued until the last neuroimaging data were collected on November 30, 2012. Data analysis was performed between January 25, 2013, and February 15, 2015. All participants gave written consent to participate and received financial compensation.
A total of 507 young men (age, 18-21 years; mean [SD], 19.63 years [1.84 months]) underwent magnetic resonance imaging (MRI). This sample was restricted to male participants because the National Institutes of Health-funded project, for which the neuroimaging data were collected, examined associations between axons, testosterone, and mental health. Participants were selected based on their current domicile being within a 3-hour journey (1 way) from the scanning site and the availability of 3 blood samples obtained between ages 9 and 17 years for sex hormone assays.26 The sample included the first 507 participants who met these criteria and accepted the invitation to take part in the MRI substudy. We excluded 14 individuals owing to a failure to pass quality control of the FreeSurfer-based image-analysis pipeline (as described below), leaving 494 mother-son pairs.
Outcome Measures
Early Adversity
When the children were aged 8, 21, 33, 47, 61, and 73 months, their mothers reported on 37 family adversities, including interpersonal loss, family instability, and abuse toward the child and/or mother (full list is available in eAppendix 1 in the Supplement). At each time point, we counted the number of adversities to create a cumulative index ranging from 0 to 37.
Internalizing Symptoms
Prepubertal and early pubertal levels of internalizing symptoms (depressive and/or anxiety symptoms) were assessed via maternal reports when the boys were aged 7, 10, and 13 years using the Development and Well-being Assessment (eAppendix 1 in the Supplement).27
Sample Differences
We tested for differences between the neuroimaging subsample (N = 494) and the total sample (n = 14 541) on the study variables. The number of participants with relevant information ranged from 7278 to 10 744 in the full sample and from 429 to 462 in the brain imaging subsample. Participants in the subsample did not differ significantly from those in the full sample in terms of exposure to early adversity or level of internalizing symptoms at age 7 or 10 years, but they had higher levels of internalizing symptoms at age 13 years (odds ratio, 1.19; 95% CI, 1.04-1.36).
Selection of Regions of Interest
To identify relevant brain regions, we used a meta-analytic technique called activation likelihood estimation (ALE) computed in GingerALE, version 2.1.28 GingerALE was used to identify, in a systematic manner, regions of interest (ROIs) that have shown consistently (across multiple studies29) lower glucose metabolism (hypometabolism) in patients with depression compared with individuals serving as healthy controls. An ROI-based approach has several advantages: (1) it limits the analyses to brain regions that are thought to be relevant to depression, thereby reducing the risk of false-positive and false-negative results, and (2) extraction of ROI data permits our complex longitudinal modeling approach.
We examined studies of variation in glucose metabolism at rest, rather than studies of brain structure because we believe these can provide an unbiased identification of functionally relevant neural substrates of depression. Glucose metabolism at rest can reflect altered neural processing tendencies in patients with depression in the absence of a task. Such altered neural processing tendencies may, over developmental time or progression of depression, lead to altered brain structure in the same way that functional recruitment associated with the practice of a new skill (eg, juggling) leads to altered brain structure.30 Indeed, previous studies of depressed patients have found that altered glucose metabolism often overlaps with structural variation in the same brain regions.29
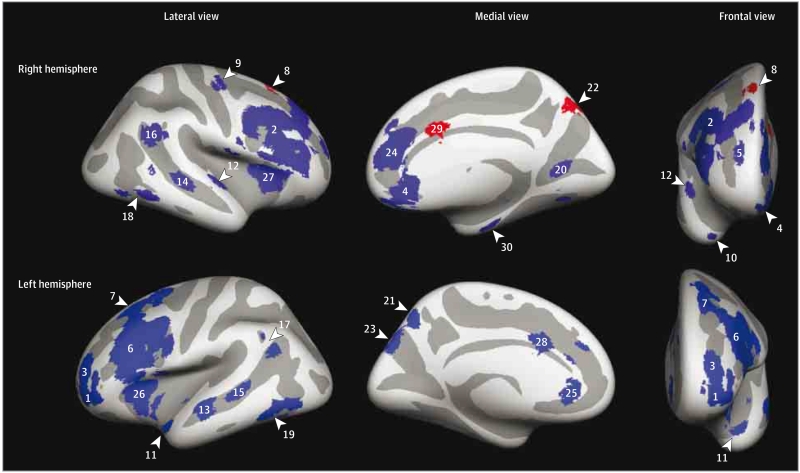
The ALE was based on data obtained from 14 studies (eReferences in the Supplement) using fluorodeoxyglucose F18 positron emission tomography performed at rest. More information on the ALE and inclusion criteria as well as included studies is available in eAppendix 2 and eTable 1 in the Supplement. Thirteen studies (eReferences in the Supplement) met the inclusion criteria, resulting in a sample size of 271 depressive patients and 193 controls. The ALE probability map was thresholded using a minimum cluster of 500 mm3 and a false discovery rate of q = 0.5, which resulted in 17 clusters. Large clusters encompassing multiple local maxima were divided into smaller regions, and medial clusters were divided into separate regions for each hemisphere. This left 30 cortical ROIs (Table 1, Figure 1, and the eFigure in the Supplement).
Table 1. Cortical ROIs Derived From the ALE of Hypometabolism in Depressed vs Control Participants.
| Cortical Label | ROI No. | GM Volume, No. of Voxels |
Weighted Center |
||
|---|---|---|---|---|---|
| x | y | z | |||
| L frontopolar cortex (lateral) | 1 | 10032 | −38 | 53 | −2 |
|
| |||||
| R middorsolateral frontal cortex | 2 | 33 208 | 43 | 27 | 23 |
|
| |||||
| L frontopolar cortex (lateral) | 3 | 3064 | −26 | 50 | 13 |
|
| |||||
| R ventromedial prefrontal cortex | 4 | 5712 | 7 | 37 | −17 |
|
| |||||
| R frontal medial cortex | 5 | 6560 | 16 | 41 | 39 |
|
| |||||
| L middorsolateral frontal cortex | 6 | 28 760 | −44 | 19 | 26 |
|
| |||||
| L superior frontal sulcus | 7 | 7448 | −24 | 16 | 52 |
|
| |||||
| R superior frontal gyrus | 8 | 2736 | 16 | 20 | 54 |
|
| |||||
| R frontal precentral sulcus | 9 | 2400 | 36 | −17 | 60 |
|
| |||||
| R temporal pole | 10 | 2248 | 29 | 19 | −37 |
|
| |||||
| L temporal pole | 11 | 1952 | −43 | 9 | −20 |
|
| |||||
| R superior temporal gyrus | 12 | 2728 | 56 | −8 | 0 |
|
| |||||
| L superior temporal sulcus (anterior) | 13 | 3040 | −55 | −16 | −8 |
|
| |||||
| R superior temporal sulcus (posterior) | 14 | 2864 | 47 | −35 | −7 |
|
| |||||
| L superior temporal sulcus (posterior) | 15 | 2896 | −47 | −39 | 0 |
|
| |||||
| R supramarginal/angular gyrus | 16 | 5056 | 50 | −51 | 26 |
|
| |||||
| L supramarginal/angular gyrus | 17 | 2864 | −42 | −53 | 28 |
|
| |||||
| R fusiform gyrus | 18 | 3040 | 55 | −62 | −12 |
|
| |||||
| L fusiform gyrus | 19 | 3424 | −49 | −62 | −12 |
|
| |||||
| R lingual gyrus | 20 | 2896 | 26 | −66 | 1 |
|
| |||||
| L precuneus | 21 | 1712 | −5 | −68 | 46 |
|
| |||||
| R precuneus | 22 | 1472 | 6 | −72 | 45 |
|
| |||||
| L parieto-occipital sulcus | 23 | 3096 | −12 | −80 | 33 |
|
| |||||
| R anterior cingulate sulcus (rostral) | 24 | 6900 | 10 | 40 | 16 |
|
| |||||
| L anterior cingulate sulcus (rostral) | 25 | 1248 | −2 | 37 | 23 |
|
| |||||
| L insula (anterior) | 26 | 5840 | −34 | 17 | 1 |
|
| |||||
| R insula (anterior) | 27 | 2656 | 36 | 22 | 3 |
|
| |||||
| L anterior cingulate cortex (caudal) | 28 | 2728 | −2 | 16 | 34 |
|
| |||||
| R anterior cingulate cortex (caudal) | 29 | 2352 | 2 | 16 | 34 |
|
| |||||
| R parahippocampal gyrus | 30 | 2016 | 27 | −18 | −33 |
Abbreviations: ALE, activation likelihood estimation; GM,gray matter; L, left, R, right; ROI, region of interest.
Figure 1. Image Showing the 30 Cortical Regions of Interest (ROIs) on an Inflated Brain After Projection Into FreeSurfer.
The 3 significant ROIs are highlighted in red and nonsignificant ROIs are shown in blue. Numbers refer to the ROI numbers presented in Table 1.
MRI Acquisition
The MRIs were acquired on a 3-T magnet (GE Healthcare), using an 8-channel, receiver-only head coil. The T1-weighted images were obtained using a 3-dimensional fast-spoiled gradient-echo sequence with the following factors: oblique-axial orientation (plane passing through the anterior-posterior commissures); 1-mm isotropic field of view, 256 × 192 × 210 mm; repetition time, 7.9 milliseconds; echo time, 3.0 milliseconds; inversion time, 450 milliseconds; and flip angle, 20°.
MRI Analysis
For each ROI, we obtained 3 measures: GM volume, cortical thickness, and surface area. The latter 2 measures were considered to dissect their relative contribution to cortical GM volume, which served as the primary measure of interest (GM volume = thickness × surface). All measures were generated using FreeSurfer, version 5.3.0.31 More information about the calculation and extraction of measures of GM volume, cortical thickness, and surface area is available in eAppendix 3 in the Supplement.
Control Variables
Analyses examining variation in GM volume, surface area, or thickness controlled for prenatal and adolescent adversity (from age 12 to 16 years) and duration of breastfeeding since these factors may affect neural development. We controlled for total brain volume and total surface area in the analyses examining GM volume or surface areas to ensure that observed effects were not attributable to individual differences in brain size. Because brain size does not correlate with cortical thickness, no correction was necessary.
Statistical Analysis
Prospective associations were estimated in a latent path model. We used latent variables because they maximize the common variance between the indicators and minimize the inclusion of error variance.32 The latent factor for early adversity was created using factor scores for adversity from birth until the child’s third birthday and then from age 3 to 6 years as indicators. These two factors were highly correlated, preventing examination of the association of effects related to a specific period (age 0-3 or 3-6 years). The latent factor for child internalizing symptoms was created using symptom counts at ages 7, 10, and 13 years.
Statistical analyses were carried out in Mplus, version 7,33 using full-information maximum likelihood estimation. Mplus includes respondents with missing data because list-wise deletion of cases with incomplete data can increase sample bias.34 Model fit was assessed using the χ2 statistic, which tests the difference between observed and expected covariance matrices and produces a nonsignificant value if this difference is close to zero.35 In the event of a significant χ2 value, we examined the relative fit indices, including the comparative fit index and the Tucker-Lewis index,36 as well as the root mean square error of approximation.37
As a model-building strategy, we first ran independent models with each ROI as a single brain outcome. Regions of interest that were associated with either early adversity or childhood internalizing symptoms were added to a multivariate model. We applied false discovery rate correction38 to the multivariate model to correct for multiple comparisons. Indirect effects were modeled using the MODEL INDIRECT command in Mplus, bootstrapping 10 000 times with bias-corrected 95% CIs to account for potential nonnormality in the SEs of indirect pathways.
Results
Descriptive Statistics
Seven ROIs showed univariate associations with early adversity or childhood internalizing symptoms and were added to a multivariate model (eResults and eTable 2 in the Supplement). Only the superior frontal gyrus (ROI No. 8 in Table 1 and Figure 1), the precuneus (ROI No. 22), and the caudal anterior cingulate cortex (ROI No. 29) survived false discovery rate correction and were included in the subsequent analyses.
Correlations among the study variables are reported in Table 2. Early adversity was positively correlated with childhood internalizing symptoms and with precuneus GM volume but negatively correlated with ACC GM volume. Childhood internalizing symptoms were negatively correlated with superior frontal gyrus GM volume.
Table 2. Correlations Among Study Variables.
| Variable | β Correlation Between ROI GM Volumes and Risk Factorsa | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | |
| Main | |||||||||
| 1. Early adversity | 1.00b | NA | NA | NA | NA | NA | NA | NA | NA |
| 2. Childhood internalizing | .37b | 1.00b | NA | NA | NA | NA | NA | NA | NA |
| 3. GM volume, superior frontal gyrus | .03 | −0.16b | 1.00b | NA | NA | NA | NA | NA | NA |
| 4. GM volume, precuneus | .11b | 0.004 | 0.17b | 1.00b | NA | NA | NA | NA | NA |
| 5. GM volume, anterior cingulate cortex | −.10b | 0.04 | 0.19b | 0.14b | 1.00b | NA | NA | NA | NA |
| Control | |||||||||
| 6. Prenatal adversity | .58b | 0.18b | 0.07 | 0.005 | −0.03 | 1.00b | NA | NA | NA |
| 7. Adolescent adversity | .25b | 0.17b | 0.02 | 0.01 | −0.04 | 0.22b | 1.00b | NA | NA |
| 8. Duration of breastfeeding | −.12a | −0.02 | −0.08 | −0.02 | −0.09b | −0.04 | 0.04 | 1.00b | NA |
| 9. Total GM brain volume | .04 | 0.03 | 0.34b | 0.38b | 0.27b | 0.05 | −0.06 | −0.10b | 1.00b |
Abbreviations: GM, gray matter; NA, not applicable; ROI, region of interest.
Numbers refer to the numbered variables.
Significant 2-tailed probability values at P < .05.
Multivariate Path Model
The model showed good fit to the data as indicated by a nonsignificant χ2 test result ( = 26.46; P > .82). All estimates and their significance values are available in the eResults and eTable 3 in the Supplement.
Direct Effects
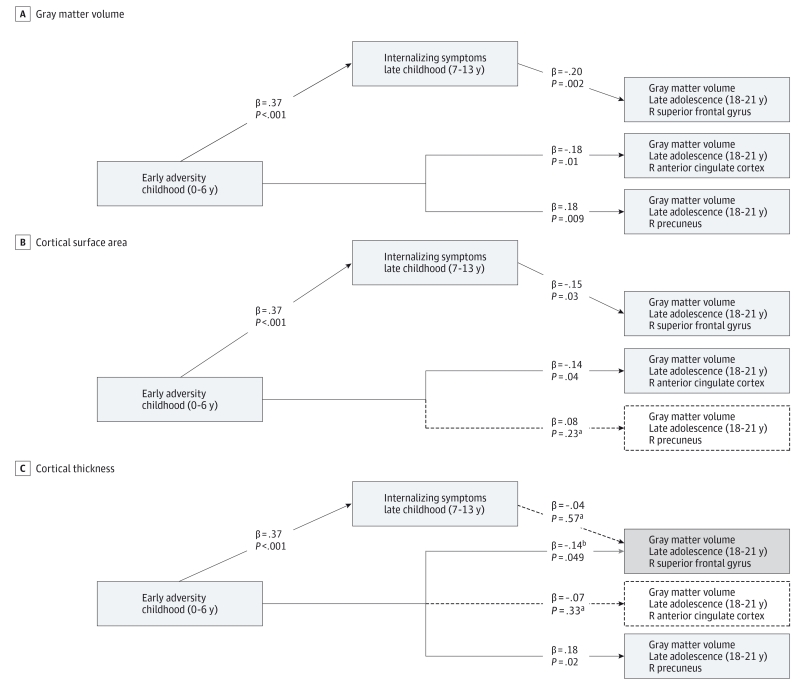
The path model (Figure 2A) showed that early adversity was associated directly with lower GM volume in the right ACC (β = −.18; P = .01) and with greater GM volume in the right precuneus (β = .18; P = .009). Childhood internalizing symptoms were associated with lower GM volume in the right superior frontal gyrus (β = −.20; P = .002). All analyses controlled for total brain volume, breastfeeding, prenatal adversity, and adolescent adversity.
Figure 2. Diagrams Illustrating the Multivariate Path Models of Direct Effects of Early Adversity and Childhood Internalizing Symptoms.
Standardized path coefficients for gray matter volume (A), surface area (B) and cortical thickness (C) analyses. Estimates of significant associations are presented in model A. Models B and C indicate whether findings from model A replicated for surface area (B) and thickness (C). Solid lines indicate paths that were replicated; dashed lines indicate paths that were not replicated (ie, it was not significant).a The gray line in model C indicates that this association was unique to model C (ie, it was not significant in model A).b The diagrams do not show control variables (total brain volume, duration of breastfeeding, and prenatal and adolescent adversity). All correlations between regions of interest were included in the model. R indicates right.
Indirect Effects
Because early adversity was associated with childhood internalizing symptoms, which were associated with structural variation in the superior frontal gyrus, we tested whether early adversity related to variation in GM volume in the superior frontal gyrus via higher levels of internalizing symptoms. Early adversity was associated indirectly with lower GM volume in the superior frontal gyrus via child internalizing symptoms (β = −.08; 95% CI, −0.14 to −0.01; P = .02) (Table 3).
Table 3. Indirect Effects of Early Adversity on Brain Structure via Internalizing Symptoms.
| Pathway | Age at Time of Measurement | Estimate | P Value | Bootstrapping, 95% CI |
||
|---|---|---|---|---|---|---|
| Early Childhood (0-6 y) | Childhood (7-13 y) | Late Adolescence (18-21 y) | ||||
| Indirect Effect on Cortical GM Volume | ||||||
| A | Early adversity | Internalizing symptoms | R superior frontal gyrus | −0.08 | .02 | −0.14 to −0.01 |
| Indirect Effect on Cortical Surface Area | ||||||
| B | Early adversity | Internalizing symptoms | R superior frontal gyrus | −0.06 | .08 | −0.12 to 0.01 |
Abbreviations: GM, gray matter; L, left; R, right.
Exploratory Follow-up Analyses
As can be seen in Figure 2B, early adversity was associated directly with a smaller surface area of the ACC and childhood internalizing symptoms were associated directly with a smaller surface area of the superior frontal gyrus, suggesting that volumetric effects in these regions appear to be driven by smaller surface areas. Early adversity was not associated with variation in the surface area of the precuneus, but instead it was associated with greater cortical thickness in this region (Figure 2C). Early adversity was also related to lower thickness of the superior frontal gyrus.
We examined whether early adversity was associated indirectly with variation in the surface area of the superior frontal gyrus. Based on the results of the evaluation, this indirect effect was not significant (Table 3).
Discussion
This study examined the extent to which adversity within the first 6 years of life relates to altered cortical brain structure in male youths. In this section, we will first discuss direct effects through which early adversity and internalizing symptoms related to variation in GM brain structure. We will then discuss indirect effects through which early adversity may affect brain structure via increased levels of internalizing symptoms.
Direct Effects
The present study found that adversity within the first 6 years of life was directly associated with lower GM volume in the ACC and with greater GM volume in the precuneus. These findings support those of previous studies that found lower GM volume in the ACC to be associated with adverse childhood events7 and harsh parenting.5 The finding that early adversity is associated with larger precuneus GM volume and thickness is somewhat surprising given that another study8 found lower thickness in the precuneus related to maltreatment. However, the present study examined the effect of adversity in addition to internalizing symptoms. Although the hypothesis is speculative, the positive association between adversity and precuneus volume could also relate to a “positive” adaption to adversity.13 Internalizing symptoms were associated with lower GM volume in the right superior frontal gyrus. Associations between depression and reduced cortical frontal lobe volumes have been consistently reported14,17,29 in studies and meta-analyses.
Indirect Effects
A novel aspect of the present study is the finding that early adversity was related indirectly to variation in GM volume in the superior frontal gyrus via higher levels of childhood internalizing symptoms. This finding adds to the literature suggesting that volumetric differences in depression may, to some extent, relate to early adverse experiences. Previous studies12,14,15 have shown that lower GM volume in both cortical and subcortical structures in patients with depression correlates with childhood adversity. Still, we know of just one study9 showing that childhood adversity (maltreatment before the age of 12 years) relates to altered brain structure via increased levels of psychiatric disorders during childhood. We extend this finding by demonstrating prospective associations in which early adversity can account for some of the structural variation typically associated with depression via increased levels of internalizing symptoms. We also examined variation in GM in cortical rather than subcortical regions.
Associations With Changes in Surface Area vs Cortical Thickness
To the best of our knowledge, the finding of direct effects whereby early adversity and internalizing symptoms are associated with smaller surface areas of the ACC and the superior frontal gyrus, respectively, are novel. Studies20,21 in humans and nonhuman primates have found that early brain development is characterized by an initial expansion of the cortical surface area. The expansion of the surface area may be particularlysusceptibletoearlyrisksinterferingwithearlybrain development. This finding, however, needs to be tested with longitudinal brain imaging data or in animal models.
Early adversity was also associated with greater thickness of the precuneus, and it appears that more work is necessary to understand factors that relate to structural variation in this region. Finally, early adversity predicted lower thickness of the superior frontal gyrus. The association between early adversity and the structural properties of the superior frontal gyrus (in the form of variation in cortical thickness) was not observed in the volumetric analyses. It does, however, fit well with the indirect effect of early adversity on GM volume via childhood internalizing symptoms.
Limitations
The results of the present study should be examined in light of several limitations. First, we tested the hypothesis that early adversity relates to altered brain structure via childhood internalizing symptoms. Alternative models (eg, structural variation may precede early adversity and depression or early adversity may predict childhood internalizing symptoms via the effect of adversity on the brain) should be examined. Second, the study was limited to male participants. Third, the study was limited to regions associated with depression identified in our meta-analysis. We cannot rule out the possibility that other cortical and/or subcortical regions could also be associated with internalizing symptoms or adversity. Fourth, cumulative risk indices are statistically sensitive and fit with theoretical and empirical models showing that multiple risks are more harmful than single risks.39 However, a limitation of cumulative risk models is that they give all risks equal weight and do not allow for the separation effects associated with specific risks.39 Fifth, mothers reported on early adversity and childhood internalizing symptoms, which may introduce issues related to shared method variance and potential reporter bias. Finally, this study focused on adversity, but other factors may also have affected the participants’ brain development.
Conclusions
This study found that adversity within the first 6 years of life was prospectively associated with higher levels of child-hood internalizing symptoms and altered brain structure in late adolescence. The association between early adversity and adolescent brain structure worked both directly and indirectly via higher levels of internalizing symptoms. The finding of an indirect effect of early adversity via childhood internalizing symptoms supports previous suggestions9,14,15 that some of the structural variation observed in people suffering from depression may partially relate to the early risk environment in addition to the effect of depression itself.
The finding that childhood experiences can affect the brain highlights early childhood not only as a period of vulnerability but also as a period of opportunity. Interventions toward adversity might help to prevent children from developing internalizing symptoms and protect against abnormal brain development.
Supplementary Material
At a Glance.
The extent to which brain structure variation typically associated with depression may also relate to early experiences of stress was examined within a large (n = 494) longitudinal birth cohort.
The study found that early adverse experiences predicted lower gray matter volume in the anterior cingulate cortex and greater gray matter volume in the precuneus in adolescence.
Early adversity was indirectly associated with lower gray matter volume in the superior frontal gyrus via higher levels of internalizing symptoms.
These results indicate that early childhood adversity is associated with altered brain structure, and the effects of depression on the brain may partly relate to early adversity.
Acknowledgments
Funding/Support: The UK Medical Research Council, the Wellcome Trust (grant 092731), and the University of Bristol provide core support for the Avon Longitudinal Study of Parents and Children (ALSPAC). This study was supported by grants from the National Institutes of Health: R01MH085772 (Axon, Testosterone and Mental Health during Adolescence; Dr Paus) and R01HD068437 (Epigenetic Pathways to Conduct Problem Trajectories: Early Environmental Risks; Dr Barker).
Role of the Funder/Sponsor: The funding organizations had no influence on the conduct of the study design, data collection, management, analysis or interpretation of data. The ALSPAC team reviewed and approved the study for publication. The National Institutes of Health did not review the study and had no influence on the decision to submit the manuscript for publication.
Footnotes
Author Contributions: Drs Paus and Barker contributed equally to the study. Ms Jensen had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Jensen, Paus, Barker.
Acquisition, analysis, or interpretation of data: All authors.
Drafting of the manuscript: Jensen, Barker.
Critical revision of the manuscript for important intellectual content: All authors.
Statistical analysis: Jensen, Barker.
Obtained funding: Jensen, Paus, Barker. Administrative, technical, or material support: Jensen, Dickie, Schwartz, Evans.
Study supervision: Dumontheil, Paus.
Conflict of Interest Disclosures: None reported.
Disclaimer: The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Additional Contributions: We are grateful to the families who took part in this study and the ALSPAC team, including midwifes, interviewers, computer and laboratory technicians, clerical workers, research scientists, volunteers, managers, receptionists, and nurses.
Supplemental content at jamapediatrics.com
REFERENCES
- 1.Green JG, McLaughlin KA, Berglund PA, et al. Childhood adversities and adult psychiatric disorders in the National Comorbidity Survey Replication I: associations with first onset of DSM-IV disorders. Arch Gen Psychiatry. 2010;67(2):113–123. doi: 10.1001/archgenpsychiatry.2009.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kendler KS, Karkowski LM, Prescott CA. Causal relationship between stressful life events and the onset of major depression. Am J Psychiatry. 1999;156(6):837–841. doi: 10.1176/ajp.156.6.837. [DOI] [PubMed] [Google Scholar]
- 3.Kessler RC, McLaughlin KA, Green JG, et al. Childhood adversities and adult psychopathology in the WHO World Mental Health Surveys. Br J Psychiatry. 2010;197(5):378–385. doi: 10.1192/bjp.bp.110.080499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hanson JL, Chung MK, Avants BB, et al. Early stress is associated with alterations in the orbitofrontal cortex: a tensor-based morphometry investigation of brain structure and behavioral risk. J Neurosci. 2010;30(22):7466–7472. doi: 10.1523/JNEUROSCI.0859-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tomoda A, Sheu YS, Rabi K, et al. Exposure to parental verbal abuse is associated with increased gray matter volume in superior temporal gyrus. Neuroimage. 2011;54(suppl 1):S280–S286. doi: 10.1016/j.neuroimage.2010.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Teicher MH, Samson JA. Childhood maltreatment and psychopathology: a case for ecophenotypic variants as clinically and neurobiologically distinct subtypes. Am J Psychiatry. 2013;170(10):1114–1133. doi: 10.1176/appi.ajp.2013.12070957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cohen RA, Grieve S, Hoth KF, et al. Early life stress and morphometry of the adult anterior cingulate cortex and caudate nuclei. Biol Psychiatry. 2006;59(10):975–982. doi: 10.1016/j.biopsych.2005.12.016. [DOI] [PubMed] [Google Scholar]
- 8.Kelly PA, Viding E, Wallace GL, et al. Cortical thickness, surface area, and gyrification abnormalities in children exposed to maltreatment: neural markers of vulnerability? Biol Psychiatry. 2013;74(11):845–852. doi: 10.1016/j.biopsych.2013.06.020. [DOI] [PubMed] [Google Scholar]
- 9.Whittle S, Dennison M, Vijayakumar N, et al. Childhood maltreatment and psychopathology affect brain development during adolescence. J Am Acad Child Adolesc Psychiatry. 2013;52(9):940–952.e1. doi: 10.1016/j.jaac.2013.06.007. [DOI] [PubMed] [Google Scholar]
- 10.Evans GW, Kim P. Childhood poverty and young adults’ allostatic load: the mediating role of childhood cumulative risk exposure. Psychol Sci. 2012;23(9):979–983. doi: 10.1177/0956797612441218. [DOI] [PubMed] [Google Scholar]
- 11.Evans GW, Eckenrode J, Marcynyszyn LA. Chaos and the Macrosetting: The Role of Poverty and Socioeconomic Status. American Psychological Association; Washington, DC: 2010. pp. 225–238. [Google Scholar]
- 12.Treadway MT, Grant MM, Ding Z, Hollon SD, Gore JC, Shelton RC. Early adverse events, HPA activity and rostral anterior cingulate volume in MDD. PLoS One. 2009;4(3):e4887. doi: 10.1371/journal.pone.0004887. doi:10.1371/journal.pone.0004887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McEwen BS, Gianaros PJ. Central role of the brain in stress and adaptation: links to socioeconomic status, health, and disease. Ann N Y Acad Sci. 2010;1186(1):190–222. doi: 10.1111/j.1749-6632.2009.05331.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Frodl T, Reinhold E, Koutsouleris N, Reiser M, Meisenzahl EM. Interaction of childhood stress with hippocampus and prefrontal cortex volume reduction in major depression. J Psychiatr Res. 2010;44(13):799–807. doi: 10.1016/j.jpsychires.2010.01.006. [DOI] [PubMed] [Google Scholar]
- 15.Vythilingam M, Heim C, Newport J, et al. Childhood trauma associated with smaller hippocampal volume in women with major depression. Am J Psychiatry. 2002;159(12):2072–2080. doi: 10.1176/appi.ajp.159.12.2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bora E, Fornito A, Pantelis C, Yücel M. Gray matter abnormalities in major depressive disorder: a meta-analysis of voxel based morphometry studies. J Affect Disord. 2012;138(1-2):9–18. doi: 10.1016/j.jad.2011.03.049. [DOI] [PubMed] [Google Scholar]
- 17.Kempton MJ, Salvador Z, Munafò MR, et al. Structural neuroimaging studies in major depressive disorder: meta-analysis and comparison with bipolar disorder. Arch Gen Psychiatry. 2011;68(7):675–690. doi: 10.1001/archgenpsychiatry.2011.60. [DOI] [PubMed] [Google Scholar]
- 18.Lai CH. Gray matter volume in major depressive disorder: a meta-analysis of voxel-based morphometry studies. Psychiatry Res. 2013;211(1):37–46. doi: 10.1016/j.pscychresns.2012.06.006. [DOI] [PubMed] [Google Scholar]
- 19.Wierenga LM, Langen M, Oranje B, Durston S. Unique developmental trajectories of cortical thickness and surface area. Neuroimage. 2014;87:120–126. doi: 10.1016/j.neuroimage.2013.11.010. [DOI] [PubMed] [Google Scholar]
- 20.Rakic P. A small step for the cell, a giant leap for mankind: a hypothesis of neocortical expansion during evolution. Trends Neurosci. 1995;18(9):383–388. doi: 10.1016/0166-2236(95)93934-p. [DOI] [PubMed] [Google Scholar]
- 21.Li G, Nie J, Wang L, et al. Mapping region-specific longitudinal cortical surface expansion from birth to 2 years of age. Cereb Cortex. 2013;23(11):2724–2733. doi: 10.1093/cercor/bhs265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gilmore JH, Schmitt JE, Knickmeyer RC, et al. Genetic and environmental contributions to neonatal brain structure: a twin study. Hum Brain Mapp. 2010;31(8):1174–1182. doi: 10.1002/hbm.20926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Raznahan A, Shaw P, Lalonde F, et al. How does your cortex grow? J Neurosci. 2011;31(19):7174–7177. doi: 10.1523/JNEUROSCI.0054-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fraser A, Macdonald-Wallis C, Tilling K, et al. Cohort profile: the Avon Longitudinal Study of Parents and Children: ALSPAC mothers cohort. Int J Epidemiol. 2013;42(1):97–110. doi: 10.1093/ije/dys066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boyd A, Golding J, Macleod J, et al. Cohort profile: the “children of the 90s”: the index offspring of the Avon Longitudinal Study of Parents and Children. Int J Epidemiol. 2013;42(1):111–127. doi: 10.1093/ije/dys064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Khairullah A, Klein LC, Ingle SM, et al. Testosterone trajectories and reference ranges in a large longitudinal sample of male adolescents. PLoS One. 2014;9(9):e108838. doi: 10.1371/journal.pone.0108838. doi:10.1371/journal.pone.0108838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goodman A, Heiervang E, Collishaw S, Goodman R. The “DAWBA bands” as an ordered-categorical measure of child mental health: description and validation in British and Norwegian samples. Soc Psychiatry Psychiatr Epidemiol. 2011;46(6):521–532. doi: 10.1007/s00127-010-0219-x. [DOI] [PubMed] [Google Scholar]
- 28.Eickhoff SB, Laird AR, Grefkes C, Wang LE, Zilles K, Fox PT. Coordinate-based activation likelihood estimation meta-analysis of neuroimaging data: a random-effects approach based on empirical estimates of spatial uncertainty. Hum Brain Mapp. 2009;30(9):2907–2926. doi: 10.1002/hbm.20718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Drevets WC, Price JL, Furey ML. Brain structural and functional abnormalities in mood disorders: implications for neurocircuitry models of depression. Brain Struct Funct. 2008;213(1-2):93–118. doi: 10.1007/s00429-008-0189-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.May A. Experience-dependent structural plasticity in the adult human brain. Trends Cogn Sci. 2011;15(10):475–482. doi: 10.1016/j.tics.2011.08.002. [DOI] [PubMed] [Google Scholar]
- 31.Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci U S A. 2000;97(20):11050–11055. doi: 10.1073/pnas.200033797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Skrondal A, Rabe-Hesketh S. Generalized Latent Variable Modeling: Multilevel, Longitudinal, and Structural Equation Models. Champman & Hall/CRC Press; Boca Raton, FL: 2004. [Google Scholar]
- 33.Muthén LK, Muthén BO. Mplus: Statistical Analyses With Latent Variables: User’s Guide. Muthen & Muthen; Los Angeles, CA: 1998-2013. [Google Scholar]
- 34.Enders CK. Applied Missing Data Analysis. Guilford Press; New York, NY: 2010. [Google Scholar]
- 35.Kline RB. Principles and Practice of Structural Equation Modeling. 3rd ed. Guilford Press; New York, NY: 2010. [Google Scholar]
- 36.Bentler PM, Bonett DG. Significance tests and goodness of fit in the analysis of covariance structures. Psychol Bull. 1980;88(3):588–606. [Google Scholar]
- 37.Browne MW, Cudeck R, Bollen KA, Long JS. Alternative ways of assessing model fit. Sociol Med Res. 1992;21(2):230–258. [Google Scholar]
- 38.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J Roy Stat Soc B Met. 1995;57(1):289–300. [Google Scholar]
- 39.Evans GW, Li D, Whipple SS. Cumulative risk and child development. Psychol Bull. 2013;139(6):1342–1396. doi: 10.1037/a0031808. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.