Abstract
Background:
Due to the high number of women affected by cervical cancer and the importance of an early diagnosis, combined with the frequent incidence of false-negative Papanicolaou (Pap) smear screening results for this disease, several studies have been conducted in recent years in order to find better tests. Liquid-based cytology (LBC) tests, including the liquid-based thin layer method, have demonstrated the highest potential for reducing false-negative cases and improved sample quality. This study aimed to compare the strength of the Pap smear test with fluid cytology and conventional tests in detecting cervical dysplasia.
Materials and Methods:
This descriptive-analytic study was conducted on 366 women who attended private laboratories for a Pap smear. The Pap smear sampling was conducted simultaneously using two methods: conventional Pap (CP) smear and LBC), from the cervix.
Results:
The mean age of the participants was 32 ± 8.8 years. Diagnostic results of endocervical cells, epithelial cells, vaginitis cells, and metaplastia were consistent with both conventional and liquid cytology smears, and the kappa coefficient was determined to be significant (P < 0.001). In total, 40.5% of diagnostic cases indicated bacterial inflammation 80.3% of the diagnoses in both methods were P1 and 3.9% of cases diagnosed were P2, the overall diagnostic consistency was 83.9% between the two sampling methods. The inflammation diagnosis was 40.5% and this was consistent in both methods of LBC and CP. There was one case of a false-negative diagnosis in the LBC method and 14 cases in the CP method.
Conclusion:
Results showed that the LBC may improve the sample's quality and reduce the number of unsatisfactory cases more than with the CP method.
Keywords: Cervical dysplasia, liquid-based cytology, Papanicolaou smears examination
INTRODUCTION
Cancer is the disease of the century and the leading cause of death around the world resulting in 13% of all causes of death in 2008. Cervical cancer is the second highest cause of cancer-related mortality in women,[1,2] and more than 80% of new cervical cancer cases occur in developing and under developed countries.[3] The progression of this fetal cancer is slow with the precancerous period of 10–20 years. The only sign of this cancer in the early stages is the loss of abnormal cells[4] and clinical signs of the disease appear only after cancer has reached advanced stages, with low survival rate.[5] Conversely, this cancer in precancerous conditions is completely curable.[5]
All of these issues have suggested the importance of cancer screening endeavors,[4,5] and screening with conventional Papanicolaou (CP) has reduced the mortality by 70%.[3,4] The sensitivity of this screening procedure is 5–60% and reaches 80% at best.[4,6] On the other hand, it is also associated with a significant number of false-negative cases (20–50%).[5,6,7] In 1996, liquid-based cytology (LBC) method was developed hoping to overcome the disadvantages of the previous method expecting to have good features such as high sensitivity, sample adequacy, and faster sample preparation,[8,9,10,11] according to the literature, the LBC method decreased the rate of inadequate smears,[12,13,14,15,16,17,18,19,20] however, studies conducted in Iran have not confirmed this conclusion.[12] Some papers have reported no differences between the sensitivity of these two methods,[13,14,15,16,17,18,19,20,21,22,23,24] while others and especially national studies have supported LBC as having a higher sensitivity.[25,26] There is also confusion with regard to the negative and positive prediction values of these methods is not clearly demonstrated.[20,23]
According to the World Health Organization's annual report, this cancer currently results in 581 deaths per year in Iran.[27,28,29] Given the importance of cervical cancer screening, the need to find the best screening method for detection of the disease, and discrepancies in results and sensitivity of CP and LBC environments in previous studies, this study has been designed to compare these two methods.
This study will help to identify the most optimal way for screening cervical cancer, and through adoption of appropriate policies, the mortality rate of this fetal cancer could be significantly reduced.
MATERIALS AND METHODS
In this descriptive-analytic study, women who visited obstetrics and gynecology laboratories for a Papanicolaou (Pap) smear test were enrolled. All of the women had attended private laboratories for their Pap smear test due to the lack of LBC methods available in the governmental facilities. Pap smear samples were collected by two expert gynecologists using both techniques with specialized; spatula, cotton swabs, and endocervical brush from the cervical region, simultaneously. Exclusion criteria were pregnancy, hysterectomy, women who had received medication for cervical intraepithelial cancer treatment over the last 5 years.
At the time of the Pap smear sample collection, both CP and LBC methods were used to take samples from the cervix of each of the women simultaneously. In the CP method, the samples were taken from the exocervix and at the junction of the squamous and cylindrical tissues, using a special spatula; and sampling was performed using a cotton swab from the endocervical region. Samples were immediately transferred onto a glass slide and fixed with fixator solutions and transferred to the laboratory after they were completely fixed and dry. Then, the samples were stained by a qualified technician using Pap staining methods. A cervicovaginal cytology pathologist evaluated the processed slides.
LBC samples were coded and delivered to the pathologist anonymously and without prior knowledge of the CP results. The LBC was performed according to a liquid prep procedure, in which the back-end of the endocervical brush was placed into tight fitting standard vials containing standard specimen preservative solution (Ilia tec kimia sahand co.ltd) and sent to the laboratory. Next, approximately 2–4 ml of cleaning solution was poured in a 15–20 ml centrifuge tube labeled with the patient's name. After gentle mixing by an electrical shaker, the resulting solution was added to a centrifuge tube containing a cleaning solution. They were then coded and centrifuged in a vertical position for 10 min in 1000 ml of the solution at an ambient temperature. In the next step, the supernatant was removed, and a clean napkin was used to wipe the tube opening. Following dehydration, approximately 0.3–0.5 ml of cellular base solution was added to the sample tubes, mixed by a shaker for about 10 s, and 50 µL of the final yield was removed by laboratory sampler and placed on the slide surface in a circular manner. The prepared slides remained at ambient temperature until they were completely dry, then a qualified laboratory technician performed all the Pap standard staining. Next, an expert pathologist studied the stained slides. Finally, the prepared slides based on both techniques were classified according to Pap standard classification, and the rate of unsatisfactory results, inflammation, along with the presence of endocervical and metaplastic cells, were studied and compared between both groups.
Statistical analysis
Data were analyzed using SPSS (ver. 15) software package (SPSS Inc., Chicago, Illinois, USA). P < 0.05 was considered as significance level. The difference between the groups was determined by a Chi-square test. A kappa contingency coefficient was also used to determine consistency between the two methods.
RESULTS
A total of 366 subjects participated in the study. The mean age of the participants was 32 ± 8.8 years. 81.1% of study subjects were living in urban areas, (n = 297 and 69, respectively). Based on the results represented in Table 1, most of the consistency was observed in the diagnosis of atrophic cells. Overall, 28.7% of the test results were negative in both techniques, while 20.8% were positive [Table 2]. There was 49.5% consistency between the two methods, and the kappa contingency coefficient was 0.13, which was significant (P < 0.001).
Table 1.
Comparison of positive cases in both liquid and conventional Papanicolaou cytology
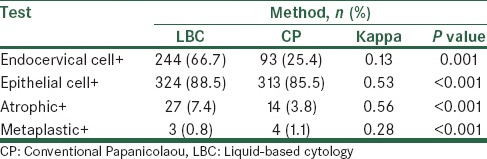
Table 2.
Comparison of diagnostic results of endocervical cells and epithelial cell in both liquid cytology and conventional Papanicolaou smears
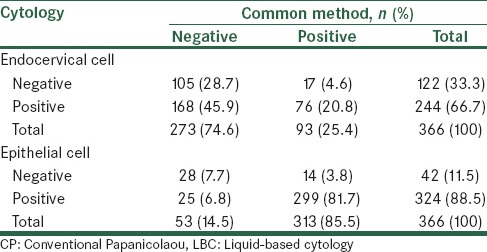
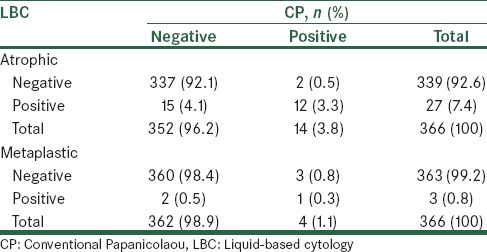
Table 2 shows a comparison of the diagnostic results of epithelial cells in LBC and CP, 7.7% of cases were negative and 81.7% cases were positive, using both methods. There was 89.4% consistency between the two methods, and a kappa contingency coefficient was 0.53 (P < 0.001). According to the results listed in Table 3, 92.1% of cases were negative and 3.3% cases were positive in both methods, in addition, there was 95.4% consistency between the two methods and a kappa contingency coefficient was 0.56 (P < 0.001). Table 3 shows that 98.4% of cases were negative and 0.3% cases were positive in both methods. There was 98.7% consistency between the two methods, and a kappa contingency coefficient was 0.28 (P < 0.001) in 91.3% of cases, while the sample condition was determined to be normal using both techniques.
Table 3.
Comparison of diagnostic results of atrophic cells and metaplastic in liquid cytology and conventional Papanicolaou smears
There were 25 cases of Candida with LBC and 11 cases with a CP. Moreover, Trichomonas vaginalis was observed in 4 cases with LBC and in 5 cases with CP. Overall, 94.9% of cases produced the same diagnosis by both methods [Table 4]. Table 5 shows a comparison of inflammation results using LBC and CP.
Table 4.
Comparison of observed organisms in liquid cytology and conventional Papanicolaou smears
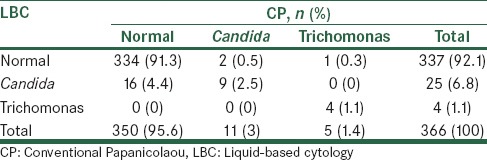
Table 5.
Comparison of inflammation results in liquid cytology and conventional Papanicolaou smears
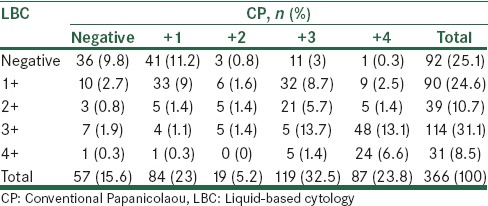
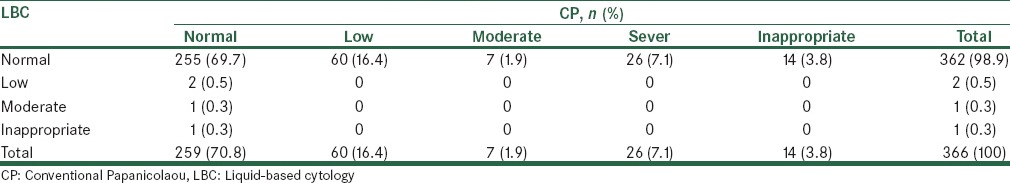
Based on Table 6, 80.3% of the diagnoses in both methods were P1 and 3.9% of cases diagnosed were P2, the overall diagnostic consistency was 83.9% between the two sampling methods. Based on the above data, using LBC 2 mild, 1 moderate, and 1 inappropriate case were diagnosed, while CP resulted in 60 cases of mild, 7 cases of moderate, 26 cases of severe, and 14 inappropriate cases were diagnosed, while only in the 69.7% of normal cases was agreement found between the two methods. Repeated Pap smears were carried out for inappropriate cases of sampling, but due to the lack of three consecutive positive cases of dysplasia, there were no indications for a biopsy [Table 7].
Table 6.
Comparison of neoplastic findings in both liquid and conventional Papanicolaou cytology
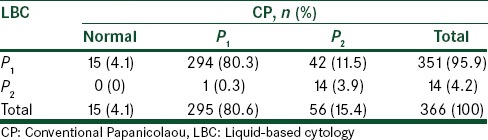
Table 7.
Comparison of hemorrhagic results in liquid cytology and conventional Papanicolaou smears
DISCUSSION
For the 1st time, the results of this study showed that the highest agreement of positive results was observed in the detection of epithelial and atrophic cells. The detection of endocervical and epithelial cells were 49.5% and 89.4% of cases, respectively; and the results of both methods were consistent. In addition, in the diagnosis of atrophic and metaplastic cells, there was consistency between the two methods in 95.4% and 98.7% of cases, respectively (P < 0.001). In a study conducted by Yousefi et al., the rate of CP samples without the endocervical cells was 33.4% and 76.9% in the LBC method. The smears without inflammation in both the CP and LBC methods were 26.7% and 59.9%, respectively. The lack of endocervical cells and inflammation were considered as a part of the unsatisfactory smears statistics.[7]
Several studies have compared CP and LBC, in which the majority reported a more significant benefit for LBC than with the CP. This is in terms of sample adequacy and appropriateness, detection of malignancies and premalignant lesions.[30,31] In a study by Kirschner et al., CP and LBC were compared for the screening of cervical cancer, and their results showed that the cervical samples containing atypical or suspicious malignant cells were 40% and 23.3%, respectively, and they were higher in LBC than in the CP method.[32]
A study by Limaye et al. indicated that the detection of intraepithelial squamous lesions in LBC compared with CP was 160% (1.3% vs. 3.4%) higher.[31] The results of this study showed that the condition of samples was normal in 91.3% of cases, and 25 cases of Candida were observed in the LBC method, compared with 11 cases in the CP. Furthermore, 4 cases of trichomonas were detected in LBC and 5 cases in CP. The overall consistency between the two methods in terms of the detected cases was 94.9%. The inflammation diagnosis was 40.5% and this was consistent in both methods of LBC and CP. In 80.3% of the diagnoses, it was P1 using both methods and 3.9% were P2; while in 83.9% of the cases the detection rate was identical. The P2 class in LBC was lower than with the CP. Yousefi et al. in a comparison study of the two cytology methods found that in Pap system the CP and LBC methods were identical in 78.6% of cases. Maximum similarity was observed in Class 1 with 93.4%, and the lowest similarity was found in Class 2, with 60% consistency in the detection parameter.[7]
Based on the results of the current study, hemorrhagic detection was mild in 2 cases, moderate in 1 case, and unsatisfactory in 1 case (method LBC), whereas 60 cases of mild, 7 cases of moderate, and 26 cases of severe were found (method CP), and CP detected 14 cases of unsatisfactory hemorrhagia. There is only 69.7% consistency between these two methods. There were 14 cases of cell loss and 26 cases of hemorrhagia that made the sampling inappropriate in CP method. LBC was evaluated to be more satisfactory than a CP. The presence of unsatisfactory slides is an issue that physicians and their patients interface in the widespread cellular pathology of the cervix. In fact, these cases are considered uninterruptable in the pathology study. An extensive review of the literature revealed that cervical cancer screening using the LBC method led to a reduction in the number of unsatisfactory cases.[33] This was demonstrated in Leman's study where these cases occurred in 9.7% of CP and 2% in the LBC method.[34] Whereas, a study conducted in Turkey reported lower numbers of unsatisfactory cases with LBC (0.1%) than with CP (1.7%).[35] In a study by Zafari et al., the number of inadequate smears in the CP technique was 11 cases (9.2%) and in the thin layer technique there were 5 cases (4.2%), while inadequate cases due to lower cellularity in the CP constituted 10 cases (8.3%) and in the thin layer there were 2 cases (1.7%) (P = 0.008).[36] There were 0.1% and 1.7% inadequate smear cases in the LBC method and CP, respectively, in the Tuncer et al. study.[35] However, in the Kirschner et al. study, these cases were 2.3% and 0.3%, respectively;[32] which is consistent with the current study findings. The LBC method reduced the number of inadequate cases, and its prevalence was 9.1% in the CP method, which reduced to 1.6% using the LBC method.[37] The results of Yousefi et al. showed that inadequate cases were 1 case (0.3%) with CP and 14 cases (1%) with the LBC method.[7] Moreover, Hodgson et al. investigated the possibility of replacing CP with the LBC method and found that replacement of the CP method led to a significant increase in the detection of low-grade squamous intraepithelial lesion (LSIL) and high-grade squamous intraepithelial lesion (HSIL), but it also resulted in an approximately 50% increase in the number of inadequate samples,[38] which is not consistent with the current study's results. When the pathological test involved a spread slide study, the results indicated Class 1 of the cervix and endocervical response, the test could be considered normal, without any signs of abnormalities, and it is expected that no undesirable issues would happen before the next screening test in about 1–3 years. If abnormal cells are observed in the pathology response report, based on the degree of abnormality of cells, an appropriate intervention is necessary.[39] However, if the pathology report contains inadequate results that are of concern to the patient and their physician, it is recommended that a Pap smear is repeated immediately, and if three consecutive positive samples of dysplastic changes occur, then a colposcopy and biopsy is necessary. In the current study, a repeated Pap smear for inadequate slides was carried out, but due to the lack of three consecutive positive dysplastic changes there were no indications for a biopsy. One of the causes of inadequate samples was the presence of confounding factors such as blood cells, inflammatory cells, or an insufficient number of cells on the cell spread slides, or the slide contained a thick layer of cells. Improper transfer of cells after their removal, exudate, necrotic materials, and improper smear fixation are some of other factors.[40]
The next issue that should be considered is the time required to study and interpreting the smear slides by the cytotechnologists. In various studies, the average time for the CP method was 4.6 min and 3 min in the LBC technique.[41] Another problem that must be considered is the cytologist's behavior during the cell-wide review. In CP methods, the break time is more extensive than when the cell is prepared using the LBC method.[42,43] There are conflicting conclusions in previous meta-analysis about LBC, so in five different meta-analysis only two found higher LSIL and HSIL detection in the liquid-based technique than in cytology, which were conducted by Klinkhamer et al.[44] and Bernstein et al.[30] The sensitivity and thin prep characteristics were higher than cytology in a meta-analysis conducted by Abulafia,[43] while in studies by Davey et al.[40] and Arbyn et al.,[22] the diagnostic accuracy of both tests was identical. However, the overall conclusion of these studies is that even if the diagnostic accuracy of the two tests are equivalent, the advantages of the liquid base methods are: The remaining solution can be used for human papillomavirus testing, reading LBC slides is easier and faster, automatic reading of slides is possible, the number of inadequate smears is lower, and this method is more economical and cost effective. The LBC technique was better for LSIL and ASCUS detection, and detecting atypical glandular cells is improved with the use of the LBC test. The overall sensitivity of the cytology tests for the detection of glandular neoplasia is lower than in squamous cell neoplasia (50–70% vs. 30–87%).
Limitation
In one case, we suspected preneoplastic lesions, but after repeating the test 3 times, and due to the absence of three consecutive positive tests, biopsy was not conducted.
CONCLUSION
Results showed that the LBC method may improve the sample's quality and reduce the number of unsatisfactory cases more than the conventional CP method.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Behtash N, Mehrdad N. Cervical cancer: Screening and prevention. Asian Pac J Cancer Prev. 2006;7:683–6. [PubMed] [Google Scholar]
- 2.Oaknin A, Díaz de Corcuera I, Rodríguez-Freixinós V, Rivera F, del Campo JM. SEOM (Spanish Society of Clinical Oncology). SEOM guidelines for cervical cancer. Clin Transl Oncol. 2012;14:516–9. doi: 10.1007/s12094-012-0834-y. [DOI] [PubMed] [Google Scholar]
- 3.Singh E, Seth S, Rani V, Srivastava DK. Awareness of cervical cancer screening among nursing staff in a tertiary institution of rural India. J Gynecol Oncol. 2012;23:141–6. doi: 10.3802/jgo.2012.23.3.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Izadi Mood N, Dehdashti MR, Eftekhar Z, Ahmadi SA. The specimen adequacy and atypical squamous cell frequency: Conventional versus liquid-based cytology Pap smears. Tehran Univ Med J. 2009;66:900–6. [Google Scholar]
- 5.Anbiaei R, Yousefi Z, Anbiaei S, Sharifi N, Azmie R, Valaei N. [Experimental study for evaluation and comparison of conventional Pap smear and liquid-based smear in the daagnosis of cervical dysplasia] Pejouhandeh Dec 2006-Jan. 2007;11:325–30. [Persian] [Google Scholar]
- 6.Strander B, Andersson-Ellström A, Milsom I, Rådberg T, Ryd W. Liquid-based cytology versus conventional Papanicolaou smear in an organized screening program: A prospective randomized study. Cancer. 2007;111:285–91. doi: 10.1002/cncr.22953. [DOI] [PubMed] [Google Scholar]
- 7.Yousefi Z, Sharifi N, Ebrahimzadeh S, Anbyayy S. [Prevalence of inappropriate (unsatisfactory) cervical cytology cases in liquid based and conventional Pap smear methods] J Gorgan Univ Med Sci. 2007;9:12–6. [Persian] [Google Scholar]
- 8.Linder J, Zahniser D. The ThinPrep Pap test. A review of clinical studies. Acta Cytol. 1997;41:30–8. doi: 10.1159/000332302. [DOI] [PubMed] [Google Scholar]
- 9.Papillo JL, Zarka MA, St John TL. Evaluation of the ThinPrep Pap test in clinical practice. A seven-month, 16,314-case experience in Northern Vermont. Acta Cytol. 1998;42:203–8. doi: 10.1159/000331547. [DOI] [PubMed] [Google Scholar]
- 10.Bolick DR, Hellman DJ. Laboratory implementation and efficacy assessment of the ThinPrep cervical cancer screening system. Acta Cytol. 1998;42:209–13. doi: 10.1159/000331548. [DOI] [PubMed] [Google Scholar]
- 11.Payne N, Chilcott J, McGoogan E. Liquid-based cytology in cervical screening: A rapid and systematic review. Health Technol Assess. 2000;4:1–73. [PubMed] [Google Scholar]
- 12.Ronco G, Cuzick J, Pierotti P, Cariaggi MP, Dalla Palma P, Naldoni C, et al. Accuracy of liquid based versus conventional cytology: Overall results of new technologies for cervical cancer screening: Randomised controlled trial. BMJ. 2007 Jul 7;335(7609):28. doi: 10.1136/bmj.39196.740995.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Taylor S, Kuhn L, Dupree W, Denny L, De Souza M, Wright TC., Jr Direct comparison of liquid-based and conventional cytology in a South African screening trial. Int J Cancer. 2006;118:957–62. doi: 10.1002/ijc.21434. [DOI] [PubMed] [Google Scholar]
- 14.Akamatsu S, Kodama S, Himeji Y, Ikuta N, Shimagaki N. A comparison of liquid-based cytology with conventional cytology in cervical cancer screening. Acta Cytol. 2012;56:370–4. doi: 10.1159/000337641. [DOI] [PubMed] [Google Scholar]
- 15.Siebers AG, Klinkhamer PJ, Arbyn M, Raifu AO, Massuger LF, Bulten J. Cytologic detection of cervical abnormalities using liquid-based compared with conventional cytology: A randomized controlled trial. Obstet Gynecol. 2008;112:1327–34. doi: 10.1097/AOG.0b013e31818c2b20. [DOI] [PubMed] [Google Scholar]
- 16.Maccallini V, Angloni C, Caraceni D, Fortunato C, Venditti MA, Di Gabriele G, et al. Comparison of the conventional cervical smear and liquid-based cytology: Results of a controlled, prospective study in the Abruzzo region of Italy. Acta Cytol. 2008;52:568–74. doi: 10.1159/000325599. [DOI] [PubMed] [Google Scholar]
- 17.Sykes PH, Harker DY, Miller A, Whitehead M, Neal H, Wells JE, et al. A randomised comparison of SurePath liquid-based cytology and conventional smear cytology in a colposcopy clinic setting. BJOG. 2008;115:1375–81. doi: 10.1111/j.1471-0528.2008.01865.x. [DOI] [PubMed] [Google Scholar]
- 18.Treacy A, Reynolds J, Kay EW, Leader M, Grace A. Has the ThinPrep method of cervical screening maintained its improvement over conventional smears in terms of specimen adequacy? Diagn Cytopathol. 2009;37:239–40. doi: 10.1002/dc.20993. [DOI] [PubMed] [Google Scholar]
- 19.Patel C, Ullal A, Roberts M, Brady J, Birch P, Bulmer JN, et al. Endometrial carcinoma detected with SurePath liquid-based cervical cytology: Comparison with conventional cytology. Cytopathology. 2009;20:380–7. doi: 10.1111/j.1365-2303.2008.00621.x. [DOI] [PubMed] [Google Scholar]
- 20.Khaniki M, Nazari Z, Zendehdel K, Fakour F. Cervicovaginal cytopathology by liquid-prep TM a new liquid based method in comparison with conventional Pap smear. Iran J Pathol. 2009;4:59–64. [Google Scholar]
- 21.Roghaei MA, Afshar Moghaddam N, Pooladkhan Sh, Roghaie Sh. Adeguacy criteria and cytomorphological changes in liquid-prep TM versus conventional cervical cytology. Shiraz E Med J. 2010;11(4):173–82. [Google Scholar]
- 22.Arbyn M, Bergeron C, Klinkhamer P, Martin-Hirsch P, Siebers AG, Bulten J. Liquid compared with conventional cervical cytology: A systematic review and meta-analysis. Obstet Gynecol. 2008;111:167–77. doi: 10.1097/01.AOG.0000296488.85807.b3. [DOI] [PubMed] [Google Scholar]
- 23.Siebers AG, Klinkhamer PJ, Grefte JM, Massuger LF, Vedder JE, Beijers-Broos A, et al. Comparison of liquid-based cytology with conventional cytology for detection of cervical cancer precursors: A randomized controlled trial. JAMA. 2009;302:1757–64. doi: 10.1001/jama.2009.1569. [DOI] [PubMed] [Google Scholar]
- 24.Laiwejpithaya S, Rattanachaiyanont M, Benjapibal M, Khuakoonratt N, Boriboonhirunsarn D, Laiwejpithaya S, et al. Comparison between Siriraj liquid-based and conventional cytology for detection of abnormal cervicovaginal smears: A split-sample study. Asian Pac J Cancer Prev. 2008;9:575–80. [PubMed] [Google Scholar]
- 25.Zhu J, Norman I, Elfgren K, Gaberi V, Hagmar B, Hjerpe A, et al. A comparison of liquid-based cytology and Pap smear as a screening method for cervical cancer. Oncol Rep. 2007;18:157–60. [PubMed] [Google Scholar]
- 26.Chen C, Yang Z, Li Z, Li L. Accuracy of several cervical screening strategies for early detection of cervical cancer: A meta-analysis. Int J Gynecol Cancer. 2012;22:908–21. doi: 10.1097/IGC.0b013e318256e5e4. [DOI] [PubMed] [Google Scholar]
- 27.Castellsagué X, de Sanjosé S, Aguado T, Louie KS, Bruni L, Muñoz J, et al., editors. HPV and Cervical Cancer in the World. 2007 Report. WHO/ICO Information Centre on HPV and Cervical Cancer (HPV Information Centre) Available at: www.who.int/hpvcentre .
- 28.Cheung AN, Szeto EF, Leung BS, Khoo US, Ng AW. Liquid-based cytology and conventional cervical smears: A comparison study in an Asian screening population. Cancer. 2003;99:331–5. doi: 10.1002/cncr.11786. [DOI] [PubMed] [Google Scholar]
- 29.Castle PE, Bulten J, Confortini M, Klinkhamer P, Pellegrini A, Siebers AG, et al. Age-specific patterns of unsatisfactory results for conventional Pap smears and liquid-based cytology: Data from two randomised clinical trials. BJOG. 2010;117:1067–73. doi: 10.1111/j.1471-0528.2010.02650.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bernstein SJ, Sanchez-Ramos L, Ndubisi B. Liquid-based cervical cytologic smear study and conventional Papanicolaou smears: A metaanalysis of prospective studies comparing cytologic diagnosis and sample adequacy. Am J Obstet Gynecol. 2001;185:308–17. doi: 10.1067/mob.2001.116736. [DOI] [PubMed] [Google Scholar]
- 31.Limaye A, Connor AJ, Huang X, Luff R. Comparative analysis of conventional Papanicolaou tests and a fluid-based thin-layer method. Arch Pathol Lab Med. 2003;127:200–4. doi: 10.5858/2003-127-200-CAOCPT. [DOI] [PubMed] [Google Scholar]
- 32.Kirschner B, Simonsen K, Junge J. Comparison of conventional Papanicolaou smear and SurePath liquid-based cytology in the Copenhagen population screening programme for cervical cancer. Cytopathology. 2006;17:187–94. doi: 10.1111/j.1365-2303.2006.00384.x. [DOI] [PubMed] [Google Scholar]
- 33.French DP, Maissi E, Marteau TM. Psychological costs of inadequate cervical smear test results. Br J Cancer. 2004;91:1887–92. doi: 10.1038/sj.bjc.6602224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lerman C, Miller SM, Scarborough R, Hanjani P, Nolte S, Smith D. Adverse psychologic consequences of positive cytologic cervical screening. Am J Obstet Gynecol. 1991;165:658–62. doi: 10.1016/0002-9378(91)90304-a. [DOI] [PubMed] [Google Scholar]
- 35.Tuncer ZS, Basaran M, Sezgin Y, Firat P, Mocan Kuzey G. Clinical results of a split sample liquid-based cytology (ThinPrep) study of 4,322 patients in a Turkish institution. Eur J Gynaecol Oncol. 2005;26:646–8. [PubMed] [Google Scholar]
- 36.Zafari M, Behmanesh F, Tofighi M, Abasi E, Kialashaki A, Aghamohamadi A, et al. [A comparison of fluid-based thin layer Papanicolaou smear and conventional Pap smear] J Mazandaran Univ Med Sci. 2010;20:63–70. Persian. [Google Scholar]
- 37.Harkness CB, Theofrastous JP, Ibrahim SN, Galvin SL, Lawrence HC. Papanicolaou and thin-layer cervical cytology with colposcopic biopsy control. A comparison. J Reprod Med. 2003;48:681–6. [PubMed] [Google Scholar]
- 38.Hodgson W, Kaplan KJ, Rodriguez M, McHale MT, Rose GS, Elkas JC. The impact of converting to liquid-based cervical cytology in a military population. Gynecol Oncol. 2005;99:422–6. doi: 10.1016/j.ygyno.2005.06.046. [DOI] [PubMed] [Google Scholar]
- 39.Confortini M, Bulgaresi P, Cariaggi MP, Carozzi FM, Cecchini S, Cipparrone I, et al. Conventional Pap smear and liquid-based cervical cytology smear: Comparison from the same patient. Tumori. 2002;88:288–90. doi: 10.1177/030089160208800409. [DOI] [PubMed] [Google Scholar]
- 40.Davey E, Barratt A, Irwig L, Chan SF, Macaskill P, Mannes P, et al. Effect of study design and quality on unsatisfactory rates, cytology classifications, and accuracy in liquid-based versus conventional cervical cytology: A systematic review. Lancet. 2006;367:122–32. doi: 10.1016/S0140-6736(06)67961-0. [DOI] [PubMed] [Google Scholar]
- 41.Milanova E, Naumov J, Nikolovska E, Damcevski N. Correlation of conventional and liquid-based cytology and their meaning in management of precancerous cervical lesions. Akush Ginekol (Sofiia) 2005;44:60–2. [PubMed] [Google Scholar]
- 42.Marteau TM, Senior V, Sasieni P. Women's understanding of a “normal smear test result”: Experimental questionnaire based study. BMJ. 2001;322:526–8. doi: 10.1136/bmj.322.7285.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Abulafia O, Pezzullo JC, Sherer DM. Performance of ThinPrep liquid-based cervical cytology in comparison with conventionally prepared Papanicolaou smears: A quantitative survey. Gynecol Oncol. 2003;90:137–44. doi: 10.1016/s0090-8258(03)00176-8. [DOI] [PubMed] [Google Scholar]
- 44.Klinkhamer PJ, Meerding WJ, Rosier PF, Hanselaar AG. Liquid-based cervical cytology. Cancer. 2003;99:263–71. doi: 10.1002/cncr.11673. [DOI] [PubMed] [Google Scholar]


