Abstract
Chikungunya fever, an acute and often chronic arthralgic disease caused by the mosquito-borne chikungunya virus (CHIKV), has reemerged since 2004 to cause millions of cases. Because CHIKV exhibits limited antigenic diversity and is not known to be capable of reinfection, a vaccine could serve to both prevent disease and diminish human amplification during epidemic circulation. Here, we review the many promising vaccine platforms and candidates developed for CHIKV since the 1970s, including several in late preclinical or clinical development. We discuss the advantages and limitations of each, as well as the commercial and regulatory challenges to bringing a vaccine to market.
Keywords: Chikungunya, alphavirus, vaccine, arthralgia
Chikungunya fever (CHIKF), an acute febrile disease accompanied by severe, debilitating arthralgia and arthritis, is caused by the reemerging mosquito-borne chikungunya virus (CHIKV) [1]. In contrast to infections due to dengue virus and to many other arboviruses, 75%–95% of CHIKV infections are symptomatic, with up to 60% of patients exhibiting joint pain years after onset [2]. CHIKF is distributed evenly across age groups, with 90%–95% of cases resulting in fever, myalgia, and polyarthralgia and about half resulting in rash. Fatal disease, while rare, has been observed in neonates and elderly individuals, as well as those with preexisting conditions such as diabetes or cardiovascular, respiratory, and neurologic disorders [2].
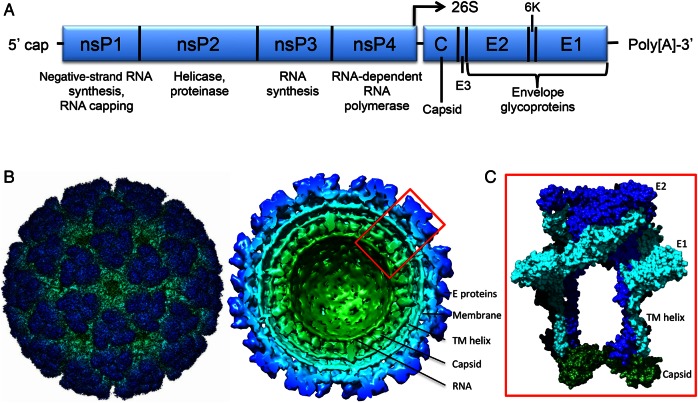
CHIKV, an alphavirus (Togaviridae), is characterized by 70-nm virions with 240 copies of the attachment and fusion heterodimeric proteins, E2 and E1, arranged as trimeric spikes surrounding an enveloped nucleocapsid that packages an approximately 12-kb positive-sense, single-stranded RNA genome (Figure 1) [3]. The virus is maintained in enzootic African cycles involving nonhuman primates and arboreal mosquitoes, with spillover into humans. An Asian enzootic cycle has also been suggested [4], but a nonurban vector has yet to be implicated, and spillback from human transmission cannot be excluded. Unlike other alphaviruses, sustained urban CHIKV transmission can occur between humans and anthropophilic mosquitoes, causing outbreaks with attack rates up to 90%. These epidemic cycles, along with the nearly global distribution of urban vectors, account for the estimated 1 million annual cases in >100 countries and territories. The ongoing outbreak in the Americas alone has resulted in >1.9 million suspected cases in >50 countries [5].
Figure 1.
Genomic and structural characteristics of chikungunya virus (CHIKV). A, Genome organization and functional roles of the nonstructural and structural polyprotein open reading frames, as well as the 26S subgenomic promoter. B and C, Cryo-electron microscopic reconstruction of CHIKV virus-like particles, and enlarged depiction of the trimeric spikes consisting of heterodimers of E1 and E2 envelope glycoproteins (C; adapted from [16] with permission). Abbreviations: E, envelope; TM, transmembrane.
Phylogenetic analyses reveal 4 main CHIKV lineages: West and East/Central/South African (ECSA) enzootic lineages and Asian and Indian Ocean Lineage (IOL) endemic/epidemic lineages [6]. During urban transmission, Aedes aegypti typically transmits CHIKV, except for certain IOL and ECSA strains with adaptive mutations that mediate efficient transmission by Aedes albopictus [7]. All CHIKV lineages essentially constitute a single serotype, and cross-protective herd immunity likely regulates the periodic nature of major epidemics. For example, in 2010, 19 years after a 1991 outbreak caused by an Asian lineage strain in Thailand, over one third of individuals previously infected had neutralizing antibodies (nAb) against the newly introduced IOL strain [8].
Owing to the lack of licensed vaccines and antiviral therapeutics, the primary response to CHIKF outbreaks is vector control. However, A. aegypti and A. albopictus populations continue to expand because of factors such as insecticide resistance and poor infrastructure, lack of education, and uncontrolled urban development. Thus, a vaccine still provides the best hope for limiting CHIKV infections and spread.
Several animal models of CHIKF have been described. Disease in the cynomolgus macaque (Macaca fascicularis) most accurately reflects human disease [9], and this model is commonly used to study pathogenesis and vaccine efficacy; the rhesus macaque (M. mulatta) has similar value [10]. Several murine models exist, but phenotypes vary and may only represent certain aspects of human disease. Immunocompetent mice (C57BL/6, CD1, or ICR) have varying susceptibility to disease, depending on age, and only simulate arthritic aspects of human CHIKF [11–13]; however, their intact immune system is useful for studying vaccine immunogenicity [10, 11, 14, 15]. Because CHIKV is sensitive to type I interferon (IFN), mouse strains lacking parts of the type-I IFN signaling pathway (eg, IFN-α/βR+/−, IFN-α/βR−/−, or A129 strains) develop disease after CHIKV infection, may mimic human cell/tissue tropism, and are used to assess vaccine efficacy to capitalize on lethal end points [17, 18]. However, immunogenicity of replication-competent vaccines in these models may be exaggerated owing to the lack of a type-I IFN response.
When evaluating CHIKF vaccines, both humoral and cellular immunity have been assessed. For protection from disease, human and animal studies suggest that nAb are critical. In a prospective longitudinal study of acute febrile illness in the Philippines, all symptomatic CHIKV infections occurred in individuals with initial CHIKV plaque reduction neutralization (PRNT) titers of <10, while there was an association between an initial PRNT titer of ≥10 and 100% protection from disease, supporting nAb as immune correlates of protection [19]. One study in Singapore reported a strong correlation between early production of neutralizing immunoglobulin G3 antibodies and protection from chronic joint pain, while a delayed antibody response correlated with progression to chronic symptoms [20]. Furthermore, convalescent human serum can protect IFN-α/βR−/−mice from fatal CHIKV infection. There is also evidence from passive transfer experiments for nAb as correlates of protection, while indicating that cellular responses are less important [21, 22].
Currently, there are >16 CHIKF vaccine candidates in preclinical and clinical development (Table 1), and each approach uses a different strategy with varied safety and immunogenicity trade-offs. Because these candidates are in different stages of development, animal models and immunogenicity assays that are used vary widely, making comparisons difficult. We summarize below vaccine development approaches and platforms.
Table 1.
Major Vaccines Developed and Under Development for Chikungunya Fever
| Name | Phase | Platform or Method | Doses | Immunogenicity Assay | Preclinical Immunogenicity (Model) | Preclinical Efficacy (Model) | Clinical Immunogenicity | Comments |
|---|---|---|---|---|---|---|---|---|
| Inactivated | ||||||||
| USAMRIID [24, 59] | 1 | Formalin inactivated, grown in green monkey kidney cells or chick embryonic cells | 1 or 2 doses | Log10 serum neutralization index, complement fixation, hemagglutination inhibition | NA | NA | Two doses 28 d apart; all 28 participants developed nAb | Doses determined by volume, not protein quantification; vaccine developed in chicken cells more immunogenic in mice than monkey cell–derived vaccine |
| DRDE-06 [26] | Preclinical | Formalin inactivated, virus grown in Vero cells, formulated with adjuvant | 3 × (10, 20, or 50 µg) | ELISA, PRNT90 | Unknown seroconversion rate; PRNT90 titer dose dependent with highest levels at 50 µg vaccine (Swiss albino mice) | Passive transfer of virus with convalescent mouse sera produced no disease in newborn mice (Swiss albino mice) | NA | Splenocytes from immunized mice produced high levels of IL-4, IL-5, IL-6, and GM-CSF after stimulation |
| Subunit | ||||||||
| CHIK-E1/E2 [27] | Preclinical | Recombinant E1/E2 produced in E. coli and formulated with adjuvant | 3 × 40 µg | ELISA, PRNT90 | Unknown seroconversion rate; PRNT90 = 64–512 (n = 6) 21 d after third dose; peak ELISA titers at 7 d after third dose, waning by day 21 (BALB/c mice) | Passive transfer of purified IgG from vaccinated mice into newborn mice, followed by 6 log10 PFU challenge, showed partial protection from death/viremia (BALB/c mice) | NA | Tested with three different adjuvant formulations, with alum and Freund's complete adjuvant performing the best |
| CHIK-E2 [29] | Preclinical | Recombinant E2 produced in E. coli and formulated with adjuvant | 2 × (10, 20, or 50 µg) | ELISA, CPE inhibition microneutralization | 100% seroconversion 14 d after second dose; nAb titers = 80–320 n = 6) 14 d after second dose; peak ELISA titers 14 d after second dose (BALB/c mice) | Partial protection from viremia/tissue viral load (genome copies) 14 and 140 d after second dose (BALB/c mice) | NA | rE2 derived from ECSA lineage; nAb titers 4-fold lower against Asian lineage |
| Virus-like particle | ||||||||
| VLP- NIH [3, 26] | 1 | VLPs produced from DNA transfected into human embryonic kidney VRC293 (HEK-293 derived) | 3 × (10, 20, or 40 µg) | ELISA and 50% neutralization using GFP reporter chimera | 100% seroconversion after 1 × 20 µg dose, boosted after 2 (n = 6; Rhesus macaque) | No detectable viremia after challenge (10 log10 PFU CHIKV LR2006-OPY-1 isolate, IV; Rhesus macaque) | 100% seroconversion in 10-µg and 40-µg group and 80% in 20-µg group after 1 dose; all titers increased with each booster | No severe adverse events reported |
| VLP-CHIKV-S27 [25, 31] | Preclinical | Baculovirus-vectored CHIKV VLPs formulated with adjuvant | 2 × 1 µg | PRNT95, modified protocol | 100% seroconversion after 2 doses (A129 mice) | 100% protection from lethal CHIKV infection (1000 TCID50 CHIKV S27 isolate) 6 wks after second dose (A129 mice) | NA | E1 or E2 protein-only controls elicited poor immunity and failed to protect all mice from lethal CHIKV challenge |
| Live-Attenuated and Live-Vectored | ||||||||
| 181/clone25 (TSI-GSD-218) [28] | 2 | Attenuation by serial, plaque-to-plaque MRC5 cell passages | (1 dose) 5 log10 PFU |
PRNT80 (preclinical), PRNT50 (clinical) | 100% seroconversion, PRNT80 = 20–2560 14 d after single immunization with 3.5–5.5 log10 PFU (Rhesus macaques) | 67%–100% protection against fatality after single immunization with 4.5–6.5 log10 PFU (18–21-day-old CD-1 outbred mice | 98.3% seroconversion, PRNT50 ≥1:20 (mean = approximately 600) 28 d after single immunization; 85% seropositive 1 y after single vaccination | 5 of 58 volunteers in phase 2 clinical trial developed mild, transient arthralgia; reversions in one of 2 attenuating mutations were detected in viremic vaccinees |
| Chimeric alphavirus [30] | Preclinical | recombinant alphavirus (EEEV, VEEV, or SINV) with CHIKV structural proteins | (1 dose) 4–6 log10PFU | PRNT80 | 100% seroconversion with PRNT80 = 20–320 by day 21 after vaccination (3-week-old Swiss Webster mice) | 100% protection from intranasal neurovirulent CHIKV (6.5 log10 PFU; C57BL/6 mice) | NA | Strong inducers of type I IFN; no viremia seen after vaccination |
| CHIKV/IRES [20, 33, 60] | Preclinical | Attenuation via inactivation of the CHIKV subgenomic promoter and introduction of an IRES to drive translation of the structural polyprotein ORF | (1 dose) 7 log10 PFU | ELISA, PRNT80 | 100% seroconversion, PRNT80 = 80–640 50 d after single immunization with 5.0 log10 PFU (cynomolgus macaques) | 100% protection from fever, hypothermia, and viremia after single immunization with 5.0 log10 PFU (cynomolgus macaques) | NA | Similar immunogenicity and efficacy in A129 mice |
| Measles-CHIKV [36, 37] | 1 | Recombinant measles virus (Schwartz strain) expressing CHIKV VLPs | Preclinical: 103–105 PFU; clinical trials: 1.5 × 104 TCID50 (low), 7.5 × 104 TCID50 (medium), 3 × 105 TCID50 (high) | ELISA, PRNT50, PRNT90 | 100% seroconversion after 1 dose by PRNT50, titers increased with dose and boost (CD46-IFNAR) | 100% protection from lethal CHIKV infection (100 PFU intraperitoneally with CHIKV06–49 isolate) at vaccine doses >4 log10 PFU (CD46-IFNAR) | 44% (n = 4), 92% (n = 11), or 90% (n = 10) seroconversion from low, medium, and high doses, respectively, after 1 immunization; 100% seroconversion after 2 doses | Preexisting immunity to measles did not affect vaccination |
| VSV-CHIKV [38] | Preclinical | Recombinant VSV with or without G protein gene expressing CHIKV structural proteins | (1 dose) 6 log10 PFU | PRNT80 | 100% seroconversion; PRNT80 = 160 to >640 (ΔG) or PRNT80 = 80–320 (G) 30 d after 1 dose (C57BL/6 mice) | 100% protection from viremia after challenge and partial protection from footpad swelling (C57BL/6 mice) | NA | Strong cellular immunity seen (ELISPOT) |
| Replication-Defective | ||||||||
| CAdVax-CHIKV [48] | Preclinical | Recombinant adenovirus expressing structural polyprotein genes of CHIKV and produced in HEK293 packaging cell line | (1 dose) 8 log10 infectious units | ELISA, CPE inhibition microneutralization | 100% seroconversion; nAb titers = 2000 (mean) 39 d after single immunization (C57BL/6 mice) | 100% protection from viremia and footpad swelling 46 d after single immunization (C57BL/6 mice) | NA | No data for preexisting immunity to Ad5 |
| MVA-CHIKV [41] | Preclinical | Host-restricted poxvirus vector expressing complete structural polyprotein of CHIKV | (1 dose) 7 log10 PFU | ELISA, 50% neutralization using luciferase reporter replicon | 100% seroconversion; nAb titers = 100–1000 (n = 5) 42 d after single immunization (C57BL/6 mice) | 100% protection from viremia and footpad swelling 49 d after single immunization (C57BL/6 mice) | NA | 1- and 2-dose regimens were tested, with the single-dose proving sufficient for protection |
| Plasmid DNA | ||||||||
| pMCE321 [46] | Preclinical | DNA plasmid containing sequence of E3, E2, and E1 genes under a CMV promoter (intramuscular electroporation) | 5 × 1 mg | CPE inhibition microneutralization | 100% seroconversion 14 d after fifth dose; nAb titers = 80–1280 (n = 4), 14 days after 5th dose (Rhesus macaque) | 100% protection against >30% weight loss after 3 doses (25 µg) on day following third dose, with no protection from viremia (BALB/c mice, intranasal challenge) | NA | Plasmids expressing E2 and E1 alone were also tested and proved less immunogenic than E3, E2, and E1 combined |
| iDNA [47] | Preclinical | DNA plasmid containing full genome of CHIKV strain 181/25. Launches live-attenuated vaccine in vivo (intramuscular electroporation) | 1 × 10 µg | PRNT80 | 100% seroconversion 21 d after 1 dose; PRNT80 = 160–1280 (n = 10 BALB/c mice) | 100% protection from viremia following 6.8 log10 PFU intranasal challenge with neurovirulent CHIKV strain | NA | Still need to assess potential reversion of 2 point mutations in 181/25 backbone |
| DREP-Env [61] | Preclinical | DNA plasmid encoding CHIKV replicon (nsP1 to nsP4) and CHIKV envelope (E1 to E3) (intradermal electroporation) | 2 × 10 µg | ELISA, 50% neutralization using luciferase reporter replicon | 100% seroconversion; nAb titers approximately 100–5000 (n = 5) 6 wk after 2 doses (C57BL/6 mice) | 100% protection from viremia and footpad swelling 7 wk after 2 doses (C57BL/6 mice) | NA | Also tested combinations of DREP-Env with adjuvanted peptide, MVA-CHIKV, or both |
Abbreviations: Ad5, adenovirus type 5; CAdVax, complex adenovirus vaccine vector; CHIK, chikungunya; CMV, cytomegalovirus; CPE, cytopathic effect; DRDE, Defense Research and Development Establishment; E. coli, Escherichia coli; EEEV, eastern equine encephalitis virus; ELISA, enzyme-linked immunosorbent assay; ELISPOT, enzyme-linked immunospot; GFP, green fluorescent protein; GM-CS, granulocyte macrophage colony-stimulating factor; IFN, interferon; IgG, immunoglobulin G.; IL-4, interleukin 4; IL-5, interleukin 5; IL-6, interleukin 6; IRES, internal ribosomal entry site; MVA, modified vaccinia Ankara; nAb, neutralizing antibody; ORF, open reading frame; PFU, plaque-forming units; PRNT, plaque reduction neutralization test; SINV, Sindbis virus virus; TCID, tissue culture infectious dose; USAMRIID, US Army Medical Research Institute of Infectious Diseases; VEEV, Venezuelan equine encephalitis virus; VLP, virus-like particle; VSV, vesicular stomatitis virus.
INACTIVATED AND SUBUNIT VACCINES
Inactivated and protein subunits are traditionally considered the safest vaccine platforms. Inactivation is achieved through exposing cell culture–derived virus to formaldehyde or irradiation, followed by purification to remove the chemical. The main concerns with this approach are the costs of manufacturing at high biocontainment and ensuring that all infectious virus has been inactivated; failing to do so has resulted in catastrophic events with other alphavirus vaccines [23]. However, the developmental pathway for inactivated vaccines is straightforward, does not require genetic manipulation of the virus, and has yielded successful vaccines for several viral diseases. This approach was first used for CHIKV in the 1970s. Harrison and others used formalin to inactivate CHIKV strain 15 561 and showed that this vaccine was safe when administered to newborn mice and caused no pathological or histological changes when injected into the central nervous system (intrathalmic, intraspinal) of rhesus macaques [24, 25]. Furthermore, all human volunteers who received 2 doses (either 0.5 mL or 1.0 mL) seroconverted, as measured by PRNT, complement-fixation, and hemagglutination assays. No adverse events were noted. A limitation of this study was that the inoculum dose given to all vertebrate subjects was measured by volume following purification and not by total protein or initial virus content, making comparisons with other vaccines difficult.
Similar promising results were reported by Tiwari et al, using a formalin-inactivated ECSA lineage CHIKV strain [26]. Here, 3 protein-quantified doses (10, 25, or 50 µg) with alum adjuvant were given to mice. PRNT90 titers were greatest following the highest dose, and passive transfer of these immune sera mixed with virus was sufficient to protect naive newborn mice from lethal CHIKV infection.
In contrast to inactivation of wild-type CHIKV, which requires biosafety level 3 containment, recombinant proteins offer an alternative that does not require biocontainment. The favorable safety and manufacturing features of recombinant-protein subunit vaccines has prompted the development of a number of CHIKF vaccine candidates using this approach. Either a combination of adjuvanted E1 and E2 envelope proteins [27] or E2 and adjuvant alone produced in Escherichia coli [28, 29] require multiple doses, generate short-lived immunity, and provide only partial protection from viremia in BALB/c mice. Additional efficacy studies in other animal models are needed to more fully evaluate these candidates.
VIRUS-LIKE PARTICLE (VLP) APPROACHES
VLPs tend to be more immunogenic than inactivated or subunit vaccines yet remain equally safe. A variety of methods is used to produce self-assembling VLPs, all of which require expression of the complete CHIKV structural protein open reading frame (ORF). One method uses a virus such as an insect-specific baculovirus to generate large amounts of protein [30, 31]. Another requires cells to be transfected with nucleic acids encoding these genes, which secrete self-assembling VLPs into the cell culture supernatant [3]. With either approach, VLPs require purification.
Cells transfected with a DNA expression vector encoding the structural polypeptide yield VLPs in cell culture that are immunogenic and protect against CHIKV viremia upon challenge in rhesus macaques, prompting the advancement of this candidate into human clinical trials [3]. For the phase 1 trial, 25 participants were enrolled to receive 3 intramuscular injections of 10, 20, or 40 µg of total VLP protein at weeks 0, 4, and 20 [32]. Complete seroconversion was observed after 2 vaccine doses, with peak mean 50% nAb titers (using a chimeric Semliki Forest/CHIKV reporter virus) of 4525–8745 fourteen weeks after the third dose, waning to 717–1385 twenty-four weeks after the third dose, depending on the vaccine dose. The titers measured using this assay were not extrapolated to traditional PRNT titers used in prior studies, making comparisons impossible. Vaccine-attributable responses were dose dependent, with the most common reported side effects including tenderness at the injection site, malaise, nausea, headache, and myalgia. No serious adverse events were reported [33].
VLPs produced from the baculovirus expression system also show promise [30, 31]. All A129 mice vaccinated with 2 doses of VLPs containing 1 µg of total protein adjuvanted with Matrix M produce nAb and are fully protected against lethal CHIKV challenge. This level of antibody production and protection was not observed in A129 mice immunized with E1 or E2 alone. Some protection was observed in mice with low levels of nAb, suggesting a correlation between nAb levels and protection from death.
LIVE-ATTENUATED VACCINES
The first live-attenuated vaccine, known as 181/clone25 or TSI-GSD-218, progressed the furthest into clinical trials. This vaccine was created from the AF15561 CHIKV isolate from Thailand and passaged via 18 plaque passages in human lung cells (MRC-5) to generate an attenuated virus [10]. This resulted in a virus that produced smaller plaques as compared to the parental virus, was no longer neurovirulent in suckling mice, and induced nAb in adult rhesus macaques. In humans, this vaccine was also highly immunogenic but produced arthralgia in some vaccinees [34]. Although 10 nucleotide differences were observed between strain 181/clone25 and its parent AF15561, the attenuation was based upon only 2 nonsynonymous mutations in the E2 gene, and reversions at these positions occurred in human volunteers and mice [35]. This highlights the need to stabilize the attenuation mechanism of the 181/clone25 vaccine before additional development.
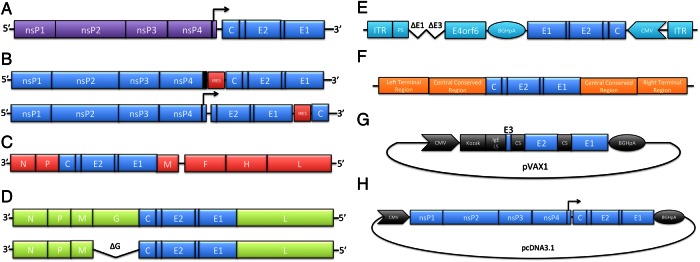
The first new live-attenuated vaccines to be published since strain 181/clone25 were chimeric alphaviruses genetically engineered based on a genomic complementary DNA (cDNA) clone of either Sindbis virus, a relatively benign virus, a naturally attenuated Brazilian strain of eastern equine encephalitis (now the species Madariaga virus), or the TC-83 strain of Venezuelan equine encephalitis virus [36]. The ORF for the structural polyprotein contained in the subgenomic RNA was substituted with that of the La Réunion strain of CHIKV (Figure 2A), and the rescued chimeric viruses replicated efficiently in Vero cells. When tested in immunocompetent mice, none produced any signs of disease or viremia, including after intracerebral inoculation of infant mice. Each induced complete seroconversion after a single dose of 3.8–5.8 log10 plaque-forming units (PFU), with mean 80% nAb titers of 40–256 [36]. When vaccinated mice were challenged with the neurovirulent Ross strain of CHIKV, all provided complete protection against fatal neurologic disease, viremia, and weight loss. Refined versions of the TC-83–based chimeric vaccines, encoding neither capsid nor nsP2 proteins capable of entering the nucleus to inhibit transcription and the antiviral response, are even more immunogenic and completely protect A129 mice from viremia and fatal disease after single doses of 4–5 log10 PFU [37].
Figure 2.
Genome organizations of live-attenuated, vectored, and DNA vaccines for chikungunya. A, Chimeric alphavirus with nonstructural polyprotein genes from attenuated strains of eastern or Venezuelan equine encephalitis or Sindbis viruses and structural polyprotein genes from chikungunya virus (CHIKV). B, CHIKV/IRES, version 1, with an inactivated subgenomic promoter and inserted IRES element, as well as CHIKV/IRES, version 2 (below), with a functional subgenomic promoter and translocated capsid with upstream IRES. C, Schwartz vaccine strain of measles virus expressing CHIKV structural polyprotein genes. D, Vesicular stomatitis virus with or without G glycoprotein gene expressing CHIKV structural polyprotein genes. E, Complex adenovirus with deletions in E1, E3, and parts of E4 with a cytomegalovirus (CMV) promoter driving expression of CHIKV structural polyprotein genes with bovine growth hormone polyadenylation signal (BGHpA) to improve gene expression. F, Modified vaccinia Ankara expressing CHIKV structural polyprotein genes. G, Plasmid DNA expressing E3, E2, and E1 separated by cleavage sequences (CS) with an immunoglobulin E leader sequence (LS) and BGHpA to improve gene expression. H, Plasmid DNA expressing the complete genome of CHIKV to launch live virus replication upon vaccination.
To improve the safety of the chimeric alphavirus vaccines for CHIKF, which retain residual ability to infect mosquito vectors, an internal ribosome entry site (IRES) shown previously to initiate translation inefficiently in insect cells [38] was used in a CHIKV cDNA based on the La Réunion strain [22]. The IRES was used in 2 different designs: (1) inactivation of the subgenomic promoter with 13 synonymous mutations and insertion of the IRES into the 5′ untranslated region of the subgenomic RNA or (2) retention of the promoter and insertion of the IRES downstream of the E1 gene, followed by the translocated capsid gene (Figure 2B) [22, 39]. In addition to the desired complete knockout of replication in mosquito cells, these modified CHIKV strains are also highly attenuated, based on reductions in structural protein expression. Unlike the 181/clone25 strain, the first IRES-based version produced no detectable viral load in 6-day-old outbred mice and only slight viremia with no weight loss, footpad swelling, or significant change in temperatures in 10-week-old A129 mice [22]. It induced complete seroconversion in A129 and immunocompetent C57BL/6 mice and protected completely against all measures of disease up to 247 days after a single dose [18]. Passive transfer of immune serum demonstrated that antibodies are sufficient for protection, and T cells play no detectible role [40]. In the cynomolgus macaque model, both genetic versions of the IRES-based vaccines were highly immunogenic when administered via the intradermal or subcutaneous routes and protected completely against fever and hypothermia, using telemetric monitoring, as well as viremia and all signs of disease when challenged 7 weeks [39] or up to 1 year after a single dose of the La Réunion [39] or a Caribbean challenge strain (C. Roy and S. C. Weaver, unpublished data).
LIVE VIRUS-VECTORED VACCINES
Another strategy in CHIKF vaccine development is the use of vaccine vectors such as measles virus (MV) and vesicular stomatitis viruses (VSV) that have been used to generate vaccine candidates for other diseases. In these vaccines, the structural CHIKV genes are inserted into the vector's genome to produce a virus that, in the case of MV, initiates expression of CHIKV structural proteins upon infection or, in the case of VSV, contains CHIKV structural proteins embedded in the virion (Figure 2C and 2D) [41].
MV-CHIKV was first tested in CD46-IFNAR mice (genetically modified to express the human CD46 protein and to lack the IFN-α/β receptor) with a prime-boost regimen and doses ranging from 103–105 50% tissue-culture infectious doses (TCID50) [42]. Mice seroconverted, with 90% nAb titers of 50–450 after 2 immunizations, and were protected against lethal CHIKV challenge. This vaccine underwent a phase 1 trial involving 42 participants receiving low (1.5 × 104), medium (7.5 × 104), or high (3 × 105) TCID50 doses [43]. Although a dose-dependent seroconversion was noted after 1 vaccination (44%, 92%, and 90% seroconversion from low, medium, and high doses, respectively), all participants seroconverted after the booster. While there was a slight reduction in the mean CHIKV nAb titers in individuals with preexisting measles immunity, the difference was not significant. Mild-to-moderate adverse events were described as headache, injection-site pain, and/or an influenza-like illness in all recipients who received the medium and high doses. No serious adverse events related to vaccination were noted.
Recombinant VSV with (VSV-CHIKV) or without (VSVΔG-CHIKV) the G protein gene also shows promise (Figure 2D) [44]. Compared with VSV-CHIKV, VSVΔG-CHIKV produced smaller plaques and displayed slower replication during the first 24 hours of infection. Regardless of this attenuated phenotype in vitro, VSVΔG-CHIKV appeared to be slightly more immunogenic in vivo. C57BL/6 mice vaccinated once with 106 PFU of vaccine all seroconverted by day 30, with higher 80% nAb titers seen in VSVΔG-CHIKV (160 to >640) than VSV-CHIKV (80–320). All mice were fully protected from disease upon challenge with 104 PFU of CHIKV in the footpad, as evidenced by a reduced/lack of footpad swelling and lack of viremia or weight gain.
REPLICATION-DEFECTIVE VECTORED VACCINES
Two replication-defective vaccine vectors, modified vaccinia Ankara (MVA) and complex adenovirus (CAdVax), have also been developed as CHIKF vaccines. MVA was developed by extensive passaging in primary chicken embryo fibroblasts, resulting in a loss of nearly 30 kb of the genome and restricting its replication to a few cell types, excluding human cells [45]. CAdVax, on the other hand, is a second-generation adenoviral vector with deletions in E1, E3, and E4 genes that are essential for replication but are provided in trans with a packaging cell line [46]. MVA and CAdVax were modified to express the complete structural ORF of CHIKV and grown in chicken embryo fibroblast or HEK-293 packaging cells, respectively (Figure 2E and 2F). Unlike the VSV-vectored vaccine, these modified viruses do not contain surface-expressed CHIKV antigens. Rather, CHIKV structural proteins are translated within the cells initially infected, without spreading the infection. A single vaccination with MVA-CHIKV (7 log10 PFU) or CAdVax (8 log10 infectious units) of C57BL/6 mice induced similar nAb titers that were sufficient to protect 100% of animals from viremia and footpad swelling <2 months after vaccination [47, 48]. While the safety profiles of these candidates were not empirically determined, previous applications of these vectors for other infectious diseases and their safety data were cited. The effect of preexisting vector immunity on immunogenicity was not directly assessed, perhaps building on previous applications of these vectors reporting a lack thereof [49, 50].
The favorable safety and immunogenicity profiles of replication-defective vaccines has also prompted the development of a host-restricted alphavirus, Eilat virus, as a chimeric alphavirus vaccine for CHIKV, with replication restricted to insect cells only; however, this vaccine candidate is still in the early stages of development [54].
DNA VACCINES
The use of plasmid DNA allows for rapid development of multiagent vaccines in response to new outbreaks. Several CHIKF vaccine candidates have been developed using this approach. In 1 study, 1 mg of plasmid DNA encoding CHIKV E3, E2, and E1 under the control of a cytomegalovirus promoter (Figure 2G) was administered 5 times with intramuscular electroporation in rhesus macaques. Fourteen days after the fifth dose, all 4 animals seroconverted with robust nAb titers (80–1280); however, efficacy testing was not described [52]. In a slightly different approach, the complete genome of live-attenuated CHIKV strain 181/25 was cloned into a plasmid downstream of a cytomegalovirus promoter, allowing for in vivo transcription of the viral genome and ultimately resulting in infectious virus production (Figure 2H). This DNA-launched live-attenuated vaccine was tested in BALB/c mice with a single 10-µg dose delivered by intramuscular electroporation. All 10 mice seroconverted 21 days after vaccination with a mean 80% nAb titer of 368 (range, 160–1280) and were completely protected from viremia following intranasal challenge with the neurovirulent Ross strain of CHIKV [53]. Finally, a variation of this approach, whereby the capsid gene was deleted to produce a CHIKV replicon expressing just the envelope proteins, was tested in C57BL/6 mice and, following 2 doses, induced 100% seroconversion with 50% nAb titers approximately 103. This study also tested various prime/boost strategies and found that a prime with the DNA replicon followed by a boost with MVA-CHIKV (see previous section) induced the highest nAb response, with mean 50% titers approximately 105, and protected mice from viremia and footpad swelling 7 weeks after the boost [54]. This highlights the potential for improving immunogenicity and efficacy by combining multiple vaccines. While the immunogenicity profile of this candidate, combined with other benefits of DNA vaccines such as production capability, storage, and genetic stability, are desirable, further safety and efficacy testing in other animal models is needed.
COMMERCIAL AND REGULATORY CHALLENGES
As discussed above, diverse CHIKF vaccine candidates appear highly promising for protection against CHIKV. Because CHIKV is antigenically conserved with extensive cross-reactions of antibodies, including nAb [55], and there is no evidence of reinfection [1], a single vaccine could probably provide worldwide protection. However, commercial and regulatory challenges for bringing a vaccine to market are concerning. Although CHIKV has gained international attention since it emerged in 2004 and especially since it spread to Europe in 2007 and to the Americas in 2013, history suggests that, after outbreaks subside with increasing herd immunity, CHIKV may return to obscurity because it is rarely diagnosed during interepidemic periods lasting decades [1], when CHIKV infection is typically misdiagnosed as dengue or other acute febrile diseases in the absence of affordable, point-of-care diagnostic assays [1]. Although some improvements in diagnostic tests have been reported [56–58], only approximately 3% of the 1.9 million suspected cases in the Americas have been confirmed with laboratory tests to detect CHIKV or specific antibodies [59]. If CHIKV returns to obscurity despite widespread endemic circulation, demand and the potential market for a vaccine are likely to decline and commercial investments needed on the order of hundreds of millions of US dollars are unlikely to be forthcoming with uncertain profits.
Another challenge to bringing a CHIKF vaccine to market is the typical requirement of a phase 3 efficacy clinical trial for licensure. During interepidemic periods, when CHIKV infections are rarely detected in the absence of surveillance [25], the selection of a site with adequate incidence to demonstrate efficacy would be highly challenging and the costs difficult to estimate. Innovative new approaches are needed, including (1) improved surveillance to monitor locations and levels of CHIKV circulation that could inform market analyses and clinical trial design, (2) the development of protocols for the rapid implementation of efficacy trials during an epidemic, (3) alternative routes to licensure involving either efficacy demonstration in models such as the macaque that accurately recapitulate human disease or implementing human challenge studies, and/or (4) innovative and sustained partnerships among governments and international agencies, regulatory authorities, and industry to generate and fund the surveillance and product development needed to bring a CHIKF vaccine to market. Furthermore, the implementation of an international nAb standard reagent could facilitate comparisons between seroepidemiologic and vaccine studies and aid in establishing a validated correlate of protection that could possibly provide an alternative pathway to licensure. These goals are attainable if momentum does not wane as CHIKF and other emerging diseases such as Ebola retreat from the public eye.
Notes
Financial support. This work was supported by the National Institutes of Health (grants R01 AI093491 and AI120942).
Potential conflicts of interest. J. H. E. and S. C. W. hold patents and patents pending for the development of alphavirus vaccines. S. L. R. certifies no potential conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Weaver SC, Lecuit M. Chikungunya virus and the global spread of a mosquito-borne disease. N Engl J Med 2015; 372:1231–9. [DOI] [PubMed] [Google Scholar]
- 2.Simon F, Javelle E, Cabie A et al. French guidelines for the management of chikungunya (acute and persistent presentations). November 2014. Med Mal Infect 2015; 45:243–63. [DOI] [PubMed] [Google Scholar]
- 3.Akahata W, Yang ZY, Andersen H et al. A virus-like particle vaccine for epidemic chikungunya virus protects nonhuman primates against infection. Nat Med 2010; 16:334–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Apandi Y, Nazni WA, Noor Azleen ZA et al. The first isolation of chikungunya virus from nonhuman primates in Malaysia. J Gen Mol Virol 2009; 1:35–9. [Google Scholar]
- 5.Number of reported cases of chikungunya fever in the Americas, by country or territory 2015 (to week noted) cumulative cases epidemiological week / EW52. http://www.paho.org/hq/index.php?option=com_docman&task=doc_view&Itemid=270&gid=33091&lang=en. Accessed 19 July 2016. [Google Scholar]
- 6.Volk SM, Chen R, Tsetsarkin KA et al. Genome-scale phylogenetic analyses of chikungunya virus reveal independent emergences of recent epidemics and various evolutionary rates. J Virol 2010; 84:6497–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tsetsarkin KA, Chen R, Yun R et al. Multi-peaked adaptive landscape for chikungunya virus evolution predicts continued fitness optimization in Aedes albopictus mosquitoes. Nat Commun 2014; 5:4084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nitatpattana N, Kanjanopas K, Yoksan S et al. Long-term persistence of chikungunya virus neutralizing antibodies in human populations of North Eastern Thailand. Virol J 2014; 11:183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Labadie K, Larcher T, Joubert C et al. Chikungunya disease in nonhuman primates involves long-term viral persistence in macrophages. J Clin Invest 2010; 120:894–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Levitt NH, Ramsburg HH, Hasty SE, Repik PM, Cole FE, Lupton HW. Development of an attenuated strain of chikungunya virus for use in vaccine production. Vaccine 1986; 4:157–62. [DOI] [PubMed] [Google Scholar]
- 11.Gardner J, Anraku I, Le TT et al. Chikungunya virus arthritis in adult wild-type mice. J Virol 2010; 84:8021–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nakaya HI, Gardner J, Poo YS, Major L, Pulendran B, Suhrbier A. Gene profiling of chikungunya virus arthritis in a mouse model reveals significant overlap with rheumatoid arthritis. Arthritis Rheum 2012; 64:3553–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Poo YS, Rudd PA, Gardner J et al. Multiple immune factors are involved in controlling acute and chronic chikungunya virus infection. PLoS Negl Trop Dis 2014; 8:e3354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Couderc T, Khandoudi N, Grandadam M et al. Prophylaxis and therapy for chikungunya virus infection. J Infect Dis 2009; 200:516–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morrison TE, Oko L, Montgomery SA et al. A mouse model of chikungunya virus-induced musculoskeletal inflammatory disease: evidence of arthritis, tenosynovitis, myositis, and persistence. Am J Pathol 2011; 178:32–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sun S, Xiang Y, Akahata W et al. Structural analyses at pseudo atomic resolution of chikungunya virus and antibodies show mechanisms of neutralization. Elife 2013; 2:e00435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Couderc T, Chretien F, Schilte C et al. A mouse model for chikungunya: young age and inefficient type-I interferon signaling are risk factors for severe disease. PLoS Pathog 2008; 4:e29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Partidos CD, Weger J, Brewoo J et al. Probing the attenuation and protective efficacy of a candidate chikungunya virus vaccine in mice with compromised interferon (IFN) signaling. Vaccine 2011; 29:3067–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yoon IK, Alera MT, Lago CB et al. High rate of subclinical chikungunya virus infection and association of neutralizing antibody with protection in a prospective cohort in the Philippines. PLoS Negl Trop Dis 2015; 9:e0003764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kam YW, Lee WW, Simarmata D et al. Longitudinal analysis of the human antibody response to chikungunya virus infection: implications for serodiagnosis and vaccine development. J Virol 2012; 86:13005–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chu H, Das SC, Fuchs JF et al. Deciphering the protective role of adaptive immunity to CHIKV/IRES a novel candidate vaccine against chikungunya in the A129 mouse model. Vaccine 2013; 31:3353–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Plante K, Wang E, Partidos CD et al. Novel chikungunya vaccine candidate with an IRES-based attenuation and host range alteration mechanism. PLoS Pathog 2011; 7:e1002142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Franklin RP, Kinde H, Jay MT et al. Eastern equine encephalomyelitis virus infection in a horse from California. Emerg Infect Dis 2002; 8:283–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harrison VR, Eckels KH, Bartelloni PJ, Hampton C. Production and evaluation of a formalin-killed chikungunya vaccine. J Immunol 1971; 107:643–7. [PubMed] [Google Scholar]
- 25.Capeding MR, Chua MN, Hadinegoro SR et al. Dengue and other common causes of acute febrile illness in Asia: an active surveillance study in children. PLoS Negl Trop Dis 2013; 7:e2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tiwari M, Parida M, Santhosh SR, Khan M, Dash PK, Rao PV. Assessment of immunogenic potential of Vero adapted formalin inactivated vaccine derived from novel ECSA genotype of chikungunya virus. Vaccine 2009; 27:2513–22. [DOI] [PubMed] [Google Scholar]
- 27.Khan M, Dhanwani R, Rao PV, Parida M. Subunit vaccine formulations based on recombinant envelope proteins of chikungunya virus elicit balanced Th1/Th2 response and virus-neutralizing antibodies in mice. Virus Res 2012; 167:236–46. [DOI] [PubMed] [Google Scholar]
- 28.Kumar M, Sudeep AB, Arankalle VA. Evaluation of recombinant E2 protein-based and whole-virus inactivated candidate vaccines against chikungunya virus. Vaccine 2012; 30:6142–9. [DOI] [PubMed] [Google Scholar]
- 29.Weber C, Buchner SM, Schnierle BS. A small antigenic determinant of the chikungunya virus E2 protein is sufficient to induce neutralizing antibodies, which are partially protective in mice. PLoS Negl Trop Dis 2015; 9:e0003684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Metz SW, Martina BE, van den Doel P et al. Chikungunya virus-like particles are more immunogenic in a lethal AG129 mouse model compared to glycoprotein E1 or E2 subunits. Vaccine 2013; 31:6092–6. [DOI] [PubMed] [Google Scholar]
- 31.Metz SW, Gardner J, Geertsema C et al. Effective chikungunya virus-like particle vaccine produced in insect cells. PLoS Negl Trop Dis 2013; 7:e2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chang LJ, Dowd KA, Mendoza FH et al. Safety and tolerability of chikungunya virus-like particle vaccine in healthy adults: a phase 1 dose-escalation trial. Lancet 2014; doi:10.1016/S0140-6736(14)61185-5. [DOI] [PubMed] [Google Scholar]
- 33.DeZure AD, Berkowitz NM, Graham BS, Ledgerwood JE. Whole-inactivated and virus-like particle vaccine strategies for chikungunya virus. J Infect Dis 2016; 214(suppl 5):S497–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Edelman R, Tacket CO, Wasserman SS, Bodison SA, Perry JG, Mangiafico JA. Phase II safety and immunogenicity study of live chikungunya virus vaccine TSI-GSD-218. Am J Trop Med Hyg 2000; 62:681–5. [DOI] [PubMed] [Google Scholar]
- 35.Gorchakov R, Wang E, Leal G et al. Attenuation of chikungunya virus vaccine strain 181/clone 25 is determined by two amino acid substitutions in the e2 envelope glycoprotein. J Virol 2012; 86:6084–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang E, Volkova E, Adams AP et al. Chimeric alphavirus vaccine candidates for chikungunya. Vaccine 2008; 26:5030–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang E, Kim DY, Weaver SC, Frolov I. Chimeric chikungunya viruses are nonpathogenic in highly sensitive mouse models but efficiently induce a protective immune response. J Virol 2011; 85:9249–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Finkelstein Y, Faktor O, Elroy-Stein O, Levi BZ. The use of bi-cistronic transfer vectors for the baculovirus expression system. J Biotechnol 1999; 75:33–44. [DOI] [PubMed] [Google Scholar]
- 39.Roy CJ, Adams AP, Wang E et al. Chikungunya vaccine candidate is highly attenuated and protects nonhuman primates against telemetrically monitored disease following a single dose. J Infect Dis 2014; 209:1891–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Partidos CD, Paykel J, Weger J et al. Cross-protective immunity against o'nyong-nyong virus afforded by a novel recombinant chikungunya vaccine. Vaccine 2012; 30:4638–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ramsauer K, Tangy F. Chikungunya virus vaccines: viral vector based approaches. J Infect Dis 2016; 214(suppl 5):S500–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brandler S, Ruffie C, Combredet C et al. A recombinant measles vaccine expressing chikungunya virus-like particles is strongly immunogenic and protects mice from lethal challenge with chikungunya virus. Vaccine 2013; 31:3718–25. [DOI] [PubMed] [Google Scholar]
- 43.Ramsauer K, Schwameis M, Firbas C et al. Immunogenicity, safety, and tolerability of a recombinant measles-virus-based chikungunya vaccine: a randomised, double-blind, placebo-controlled, active-comparator, first-in-man trial. Lancet Infect Dis 2015. [DOI] [PubMed] [Google Scholar]
- 44.Chattopadhyay A, Wang E, Seymour R, Weaver SC, Rose JK. A chimeric vesiculo/alphavirus is an effective alphavirus vaccine. J Virol 2013; 87:395–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gomez CE, Perdiguero B, Garcia-Arriaza J, Esteban M. Clinical applications of attenuated MVA poxvirus strain. Expert Rev Vaccines 2013; 12:1395–416. [DOI] [PubMed] [Google Scholar]
- 46.McConnell MJ, Imperiale MJ. Biology of adenovirus and its use as a vector for gene therapy. Hum Gene Ther 2004; 15:1022–33. [DOI] [PubMed] [Google Scholar]
- 47.Garcia-Arriaza J, Cepeda V, Hallengard D et al. A novel poxvirus-based vaccine, MVA-CHIKV, is highly immunogenic and protects mice against chikungunya infection. J Virol 2014; 88:3527–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang D, Suhrbier A, Penn-Nicholson A et al. A complex adenovirus vaccine against chikungunya virus provides complete protection against viraemia and arthritis. Vaccine 2011; 29:2803–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Barouch DH, Pau MG, Custers JH et al. Immunogenicity of recombinant adenovirus serotype 35 vaccine in the presence of pre-existing anti-Ad5 immunity. J Immunol 2004; 172:6290–7. [DOI] [PubMed] [Google Scholar]
- 50.Gudmundsdotter L, Nilsson C, Brave A et al. Recombinant modified vaccinia Ankara (MVA) effectively boosts DNA-primed HIV-specific immune responses in humans despite pre-existing vaccinia immunity. Vaccine 2009; 27:4468–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nasar F, Gorchakov RV, Tesh RB, Weaver SC. Eilat virus host range restriction is present at multiple levels of the virus life cycle. J Virol 2015; 89:1404–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mallilankaraman K, Shedlock DJ, Bao H et al. A DNA vaccine against chikungunya virus is protective in mice and induces neutralizing antibodies in mice and nonhuman primates. PLoS Negl Trop Dis 2011; 5:e928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tretyakova I, Hearn J, Wang E, Weaver S, Pushko P. DNA vaccine initiates replication of live attenuated chikungunya virus in vitro and elicits protective immune response in mice. J Infect Dis 2014; 209:1882–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hallengard D, Lum FM, Kummerer BM et al. Prime-boost immunization strategies against chikungunya virus. J Virol 2014; 88:13333–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Powers AM, Brault AC, Tesh RB, Weaver SC. Re-emergence of chikungunya and o'nyong-nyong viruses: evidence for distinct geographical lineages and distant evolutionary relationships. J Gen Virol 2000; 81:471–9. [DOI] [PubMed] [Google Scholar]
- 56.Tan JJ, Capozzoli M, Sato M et al. An integrated lab-on-chip for rapid identification and simultaneous differentiation of tropical pathogens. PLoS Negl Trop Dis 2014; 8:e3043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Goh LY, Kam YW, Metz SW et al. A sensitive epitope-blocking ELISA for the detection of chikungunya virus-specific antibodies in patients. J Virol Methods 2015; 222:55–61. [DOI] [PubMed] [Google Scholar]
- 58.Erasmus JH, Needham J, Raychaudhuri S et al. Utilization of an Eilat virus-based chimera for serological detection of chikungunya infection. PLoS Negl Trop Dis 2015; 9:e0004119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.White A, Berman S, Lowenthal JP. Comparative immunogenicities of chikungunya vaccines propagated in monkey kidney monolayers and chick embryo suspension cultures. Appl Microbiol 1972; 23:951–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Plante KS, Rossi SL, Bergren NA, Seymour RL, Weaver SC. Extended preclinical safety, efficacy and stability testing of a live-attenuated chikungunya vaccine candidate. PLoS Negl Trop Dis 2015; 9:e0004007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hallengard D, Kakoulidou M, Lulla A et al. Novel attenuated chikungunya vaccine candidates elicit protective immunity in C57BL/6 mice. Journal of virology 2014; 88:2858–66. [DOI] [PMC free article] [PubMed] [Google Scholar]