Abstract
Chikungunya virus (CHIKV) has experienced 2 major expansion events in the last decade. The most recently emerged sublineage (ECSA-V) was shown to have increased efficiency in a historically secondary vector, Aedes albopictus, leading to speculation that this was a major factor in expansion. Subsequently, a number of experimental studies focused on the vector competence of CHIKV, as well as transmission modeling efforts. Mathematical models have used these data to inform their own investigations, but some have incorrectly parameterized the extrinsic incubation period (EIP) of the mosquitoes, using vector competence data. Vector competence and EIP are part of the same process but are not often correctly reported together. Thus, the way these metrics are used for model parameterization can be problematic. We offer suggestions for bridging this gap for the purpose of standardization of reporting and to promote appropriate use of experimental data in modeling efforts.
Keywords: Chikungunya, Aedes aegypti, vector competence, Aedes albopictus, extrinsic incubation period, basic reproductive number, vectorial capacity, mathematical modeling, arbovirus, data
Historically, the distribution of chikungunya virus (CHIKV), a mosquito-borne Alphavirus, has been limited to the tropical areas of the Eastern hemisphere including Southeast Asia and Africa, where it was first identified in the 1950s. CHIKV was primarily transmitted by the mosquito Aedes aegypti and transmission was most notable in Asia, particularly the southeast [1, 2]. The epidemic genotype of these often-sporadic outbreaks is known as the Asian or Southeast Asian (SEA) genotype [2]. Infection with CHIKV manifests as a severe flu-like illness with severe musculoskeletal pain and inflammation earning its name, which translates to “that which bends up.” Further, individuals infected with CHIKV can develop long-term, possibly prolonged arthritis [1, 3].
In 2005–2006, an outbreak occurred that expanded the known parameters associated with CHIKV transmission. Unlike previous outbreaks, there were hundreds of thousands of suspected cases on the island of La Reunion alone, including the first reported fatality [4, 5]. In addition, a historically secondary vector, Aedes albopictus, was implicated as the primary vector of that epidemic [6]. Further investigation of this La Reunion outbreak revealed that a mutation at position 226 of one of the envelope (E1) proteins resulted in an amino acid substitution (alanine to valine) [7]. This mutation (E226 V) was in part used to identify a new sublineage known as ECSA-V, owing to its phylogenetic grouping in the East/Central/South African (ECSA) genotype.
One of the primary determinants of the enhanced transmission phenotype of this ECSA-V sublineage was the increased efficiency of viral replication within A. albopictus mosquitoes [8]. E226 V was not likewise associated with increased fitness in vertebrate cell lines or mice [2, 9], further attributing the augmented epidemic potential of this sublineage to the difference in fitness in A. albopictus. However, the ecology of A. albopictus differs from that of A. aegypti because the former resides in less urbanized areas than the latter. In fact, several outbreaks of CHIKV infection in which ECSA-V and A. albopictus were implicated occurred in suburban areas or around agricultural installations, such as banana and rubber plantations [10–12], and likely played an important role in the expansion of CHIKV.
In late 2013, another extensive emergence event occurred in the Caribbean, where CHIKV quickly spread through the region where the human population was nearly completely susceptible. The epidemic was of the SEA genotype in the primary vector A. aegypti [13, 14], which surprised many, given the assumption that the ECSA-V sublineage was fitter and had been implicated in several recent outbreaks around the globe [10–12]. In addition, the implication of A. aegypti as the primary vector was generally unanticipated. This response to the finding of the Asian genotype in A. aegypti could be attributed to a general lack of recent experimentation surrounding these 2 components of CHIKV transmission.
Indeed, predictions from mathematical models also lent support to the assumption that the ECSA-V sublineage would continue to expand and emerge (and likely within A. albopictus populations) by inappropriately translating vector competence into an extrinsic incubation period (EIP) estimate that gave the ECSA-V–A. albopictus combination an advantage [15]. The correct parameterization of models is critical to ensure that predictions are reliable, particularly as these models increasingly inform public health policy decisions. Further, the basic reproductive number, R0, is one of the most widely used metrics to describe transmission. It defines the number of secondary cases that will occur, given a primary case; the vector-equivalent metric is vectorial capacity (V), itself an integral part of R0. R0 is directly calculable from model parameters [16], and thus incorrect parameterization of EIP can lead to incorrect estimates of R0 [15].
Herein we detail the nature of the gap between experimental vector competence reporting and its use in predictive models of CHIKV. We then offer suggestions for reporting experimental vector competence data for the purpose of standardization across publications, ease of comparisons, and promoting appropriate use of vector competence data in modeling efforts.
HOW IS VECTOR COMPETENCE REPORTED AND INTERPRETED?
Vector competence is the intrinsic ability of a vector to support viral replication so that the virus disseminates from the midgut to the salivary glands for transmission during subsequent vector blood meals. Or, from the virus' perspective, vector competence is the ability of the virus to disseminate through a vector for ultimate transmission. There have been many studies that report CHIKV vector competence and some that even compare genotype and strain differences [15]. The experimental studies report the proportion of mosquitoes that develop a disseminated infection and/or actively transmit virus. The difference in these proportions is, thus, the way in which differential competence for that particular pathogen is reported. Comparisons of vector competence are used to investigate differences in transmission potential across many other factors. For example, several strains of the ECSA genotype (both ECSA-A and ECSA-V sublineages) were shown to have different rates of dissemination at day 14 after exposure. In this study, there were significant differences among the 2 strains of the ECSA-V sublineages [8]. In addition, the relative dissemination efficiency among mosquito species subpopulations can also contribute to heterogeneity of the vector competence of CHIKV [17]. Finally, environmental conditions such as temperature and humidity are also known effectors of the vector competence of CHIKV [18]. Thus, vector competence is often cited as an important metric for understanding emergence or expansion potential (1) in places where mosquito populations exist, but virus has yet to be introduced; (2) where land use changes could alter the patterns of transmission due to altered vector ecology; or (3) in areas that are affected by weather anomalies or climate change.
WHAT IS THE EIP?
The EIP is the time it takes for a mosquito to become infectious once it has taken a viremic blood meal. It is the temporal component to vector competence. The EIP is affected by the same internal and external factors as vector competence (eg, temperature, humidity, and viral and vector populations), which is not surprising, as they are each interdependent parts of the same process.
The EIP is a variable rate, especially at the population level, where it describes the time it takes for virus to disseminate through a group of exposed mosquitoes. So when considering the comparison of vector species/populations or investigation into viral strain diversity, static vector competence estimates provide little insight into the EIP and the entire dynamic process of viral dissemination. Figure 1 shows what this process may look like among five hypothetical strains of CHIKV, with a period of exponential growth followed by a plateau at some maximum value interpreted as the ultimate dissemination rate (or maximum proportion of mosquitoes likely to become infectious). Measuring maximum dissemination at extended time points may not capture the totality of differences among transmission systems. As Figure 1 demonstrates, early and middle time points are important as the so-called first-one-out phenomenon might have more impact on cumulative transmission than the strain that ultimately disseminates in the greatest proportion of the population. In this hypothetical scenario involving 5 strains of virus, all 5 vector competence curves reach >99% dissemination by day 11 after exposure. However, the bottom strain has a significant lag in dissemination at early time points, which may provide the other 4 strains a competitive advantage: infection with strains 1–4 may result in several transmission events before strain 5 enters the exponential growth phase (around day 4 after exposure). This is especially important in the CHIKV system, which is driven by mosquitoes that take multiple blood meals in a single gonotrophic cycle [19]. Moreover, dissemination rates often fall short of 99%.
Figure 1.
Five hypothetical strains of chikungunya virus (CHIKV) with differing vector competence efficiencies. Data reflect the proportion of mosquitoes exposed that are likely infectious (vector competence) at varying days after exposure.
THE EIP IS OFTEN REPORTED AS A STATIC QUANTITY
Unfortunately, experimentalists often equate sampling time points with the EIP, and often these sampling time points are arbitrary. A previous review demonstrated that more than half of all studies reporting CHIKV vector competence did so at a single time point, which offers very little data regarding actual EIPs. In addition, day 14 after exposure accounted for 35.4% of all data points, while day 7 after exposure was a close second with 24.1% of data points [15].
Alternatively, the temporal component of vector competence is used to describe early dissemination/transmission, even when the proportions are very small. For example, in 2 of the most recent lineage expansions of arboviruses, vector competence changes have been suggested as a major contributing factor to the success of these new lineages. In 2002, a new genotype of West Nile virus emerged in North America (WN02), for which the EIP was shown to be 4 days shorter than the originally invading strain (NY99) [20]. This was determined as the earliest time to detection of transmitting Culex mosquitoes: 5 days after exposure for WN02 (2 of 200 tested mosquitoes) versus 9 days after exposure for NY99 (2 of 179 tested mosquitoes). Similarly, the emergence of the ECSA-V sublineage was also partially attributed to its increased efficiency in A. albopictus [7]. This was reported, however, as a day-by-day comparison of the 2 strains, with statistical significance observed only at day 7 after exposure. At 7 days after exposure, the LR2006 strain had approximately 66% dissemination, and the Asian strain had approximately 28% dissemination in A. albopictus (the percentages were determined by using PlotDigitizer). Interestingly, no statistically significant difference was found between the ECSA-V sublineage strain and the Asian genotype strain at the other days post exposure [7]. So again, a single day determined this efficiency difference. Interestingly, no difference was found between the ECSA-V sublineage strain and the Asian genotype strain [7].
These examples provide evidence that static estimates are problematic and subject to bias. However, it is important to note that these day-by-day comparisons—whether they involve the time to earliest detection or midpoint comparisons 7 days after exposure—are not without value, as discussed above.
HOW IS THE VECTOR COMPETENCE/EIP INCORPORATED INTO MODELS?
Direct measures of vector competence itself are rarely included in mathematical models. Instead, the EIP is often used to parameterize the average rate of dissemination. The implicit assumption of the majority of models is that the EIP is described by an exponential distribution, whose cumulative distribution function (CDF) is illustrated by 5 hypothetical curves, depicted in Figure 2. The distribution is defined by a single rate parameter, which represents the inverse of the mean EIP. The equation for the exponential CDF is given by , where λ is the rate parameter. But in the context of vector competence and EIP, if we define N as the day after exposure and v as vector competence, then .
Figure 2.
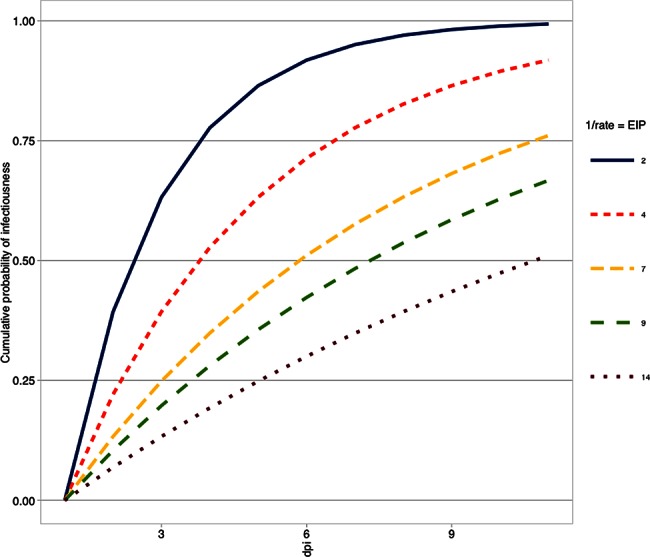
The cumulative probability of infectiousness on varying days after exposure is demonstrated by altering the value of the rate parameter (lines) on the basis of hypothetical average extrinsic incubation period (EIP) estimates.
Figure 2 shows the effect of different EIP averages on the shape of the exponential CDF. It should be noted that any distribution can be used to describe the process of EIP, and others have shown that perhaps other distributions are more appropriate in some cases [21, 22].
Likely more familiar to experimentalists are the generalized equations of R0 and V. R0 is often calculated as V × [c/r], where c represents vertebrate infectiousness, and r is the recovery rate (or the inverse of the average duration of infectiousness). V is often calculated as [ma2bpN]/−ln(p), where m is the density of mosquitoes, a is the mosquito biting rate, and p is the daily probability of mosquito survival. N is the day at which V is assessed, and b is the corresponding vector competence for that day. To calculate R0 and V, we held all mosquito parameters constant except for N, which we varied from 2 to 14 days after exposure. Because we wished to illustrate the value of parameterizing the true average EIP, we held all other parameters constant (values are published elsewhere [23–25]). Figure 3 illustrates how both V and R0 change when parameterized with different values of EIP.
Figure 3.
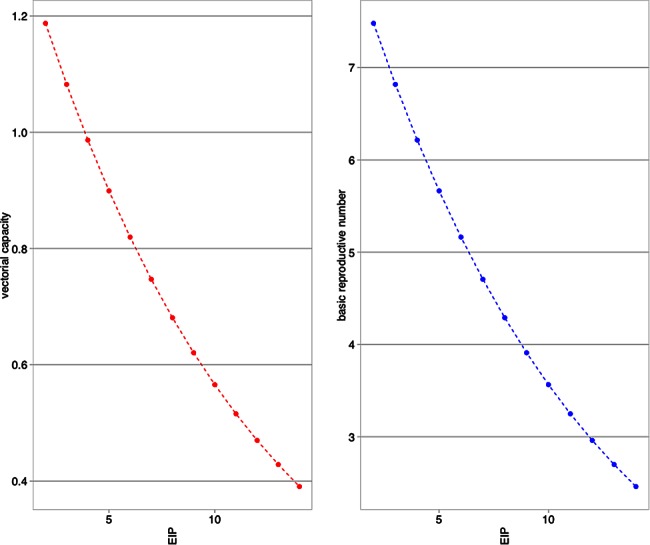
Changes in vectorial capacity (left) and the basic reproductive number (right) due to changes in the average extrinsic incubation period (EIP; all other parameters are held constant) illustrate the importance of appropriate use of vector competence data and EIP metrics.
BRIDGING THE GAP WITH THE EIP50
Use of vector competence data is a convenient way to summarize the vector-driven differences in arbovirus transmission systems. It is widely used, and we by no means suggest that experimentalists should abandon the use of vector competence. However, mathematical models depend on published data and often are used for predictive purposes and in public health policy decision-making. It is critical that the understanding of transmission events continues to be enhanced through the use of tools offered by mathematical modeling, but also that those developing these tools fully appreciate the processes underlying these events.
As vector competence is a biological process, it is incumbent upon those who intimately understand the nuances of vector competence to bridge this gap. We propose a wider use of distribution fitting to vector competence data, to calculate the extrinsic incubation time 50 (EIP50). The EIP50 was first used to describe differences in West Nile virus efficiency due to changes in temperature [26]. The interpretation of the EIP50 is similar to that of the successful standardized virus-quantification methods of tissue culture infective dose 50 (TCID50) and lethal dose 50 (LD50). Thus, the EIP50 is the time after exposure at which 50% of mosquitoes are likely infectious. Although this was originally published in 2006, only 2 other studies have used the EIP50 since [27, 28].
By promoting the use of EIP50, experimentalists gain several advantages independent of model parameterization. First, data would be easily interpreted by those not in the field. This would broaden the scope of understanding to audiences who are not familiar with vector competence but are familiar with similar metrics (eg, TCID50 and LD50). Second, it would allow for straightforward parameterization of modeling studies, thereby reducing the risk of inappropriate characterization of the EIP and potential bias in model predictions. Third, since the static estimates would be used to formulate a curve (given appropriate sample sizes and number of sampling times), comparisons across articles and studies with different sampling regimens would be facilitated.
CONCLUSIONS
The EIP50 represents the time (in days) that it takes for CHIKV strains to disseminate through a population of exposed mosquitoes such that 50% of those mosquitoes are infectious. The EIP50, as a standard way to report vector competence, still allows for direct comparisons of strain phenotypes or altered transmission conditions. The EIP50 is based on the functional fit (linear or otherwise) to multiple time points in the growth phase of the process of vector competence, indicating that single-time-point sampling is insufficient for characterization of virus efficiency within the vector. Further, as multiple time points are used to fit a continuous curve, sampling time points do not necessarily need to match across studies to still facilitate comparisons. This means the EIP50 reporting paradigm would enhance post hoc or metadata analyses across large collections of studies.
We do recognize, however, that some vector species or populations and/or viral strains can still contribute to transmission cycles yet never reach 50% dissemination. In this case, the mathematical determination of an appropriate EIPN can be computed in which the upper limit of N is determined by the less fit strain (least common time). That is, if strain A reaches its peak of 40% at day 10 after infection, then comparisons of EIP40 can be made. The interpretation of this would be dependent on the context of the hypothesis being tested and the resulting data. On the modeling side, this might require more-advanced fitting, such as a multiparameter distribution, but the EIPN would indicate as much, rather than having modelers assume that N is in fact the average. Another approach is to describe the distribution of the EIP as previously published [28]. Briefly, EIP determination was conducted similar to how an interquartile range is used to describe raw data distributions. EIP10 and EIP90 were calculated, as well as EIP50, providing additional lower (10%) and upper (90%) boundaries for an intuitive description of EIP processes.
Studies evaluating the vector competence of CHIKV report highly variable results, indicating that viral fitness is unstable among populations of both A. aegypti and A. albopictus [15]. A direct and readily interpreted comparison of strain phenotypes with a metric such as the EIP50 will inform risk assessments and transmission models by providing more appropriate parameterization of the transition of mosquitoes from the exposed to infectious class.
In summary, vector competence informed by single time points needs to be recognized as an incomplete measure of the viral/vector-centric efficiency of the transmission system. Second, the times at which vector competence measures are made is not a measure of the EIP; it is a sampling time. Third, more temporal sampling is needed to characterize the EIP. Finally, EIP50 estimates are critical for parameterization of models for more accurate predictions and enhanced precision of public health policy decisions informed by such.
By bridging gaps between experimental data reporting and the use of said data for theoretical and predictive modeling, scientists will continue to build collaborative relationships that not only take data further than summary statistics, but also result in enhanced means of translating data into public health policy decisions.
Notes
Financial support. This work was supported by the National Institute of General Medical Sciences, National Institutes of Health (grant U01 GM097661).
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Powers AM, Logue CH. Changing patterns of chikungunya virus: re-emergence of a zoonotic arbovirus. J Gen Virol 2007; 88(Pt 9):2363–77. [DOI] [PubMed] [Google Scholar]
- 2.Tsetsarkin KA, Chen R, Leal G et al. . Chikungunya virus emergence is constrained in Asia by lineage-specific adaptive landscapes. Proc Natl Acad Sci USA 2011; 108:7872–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Simon F, Javelle E, Gasque P. Chikungunya Virus Infections. N Engl J Med 2015; 373:93–4. [DOI] [PubMed] [Google Scholar]
- 4.Gerardin P, Guernier V, Perrau J et al. . Estimating Chikungunya prevalence in La Reunion Island outbreak by serosurveys: two methods for two critical times of the epidemic. BMC Infect Dis 2008; 8:99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Staikowsky F, Talarmin F, Grivard P et al. . Prospective study of Chikungunya virus acute infection in the Island of La Reunion during the 2005–2006 outbreak. PLoS One 2009; 4:e7603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bagny L, Delatte H, Quilici S, Fontenille D. Progressive decrease in Aedes aegypti distribution in Reunion Island since the 1900s. J Med Entomol 2009; 46:1541–5. [DOI] [PubMed] [Google Scholar]
- 7.Tsetsarkin KA, Vanlandingham DL, McGee CE, Higgs S. A single mutation in chikungunya virus affects vector specificity and epidemic potential. PLoS Pathog 2007; 3:e201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vazeille M, Moutailler S, Coudrier D et al. . Two Chikungunya isolates from the outbreak of La Reunion (Indian Ocean) exhibit different patterns of infection in the mosquito, Aedes albopictus. PLoS One 2007; 2:e1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bordi L, Meschi S, Selleri M et al. . Chikungunya virus isolates with/without A226 V mutation show different sensitivity to IFN-a, but similar replication kinetics in non human primate cells. New Microbiol 2011; 34:87–91. [PubMed] [Google Scholar]
- 10.Kumar NP, Joseph R, Kamaraj T, Jambulingam P. A226 V mutation in virus during the 2007 chikungunya outbreak in Kerala, India. J Gen Virol 2008; 89(Pt 8):1945–8. [DOI] [PubMed] [Google Scholar]
- 11.Ng LC, Tan LK, Tan CH et al. . Entomologic and virologic investigation of Chikungunya, Singapore. Emerg Infect Dis 2009; 15:1243–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hapuarachchi HC, Bandara KB, Sumanadasa SD et al. . Re-emergence of Chikungunya virus in South-east Asia: virological evidence from Sri Lanka and Singapore. J Gen Virol 2010; 91(Pt 4):1067–76. [DOI] [PubMed] [Google Scholar]
- 13.Cauchemez S, Ledrans M, Poletto C et al. . Local and regional spread of chikungunya fever in the Americas. Euro Surveill 2014; 19:20854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leparc-Goffart I, Nougairede A, Cassadou S, Prat C, de Lamballerie X. Chikungunya in the Americas. Lancet 2014; 383:514. [DOI] [PubMed] [Google Scholar]
- 15.Christofferson RC, Chisenhall DM, Wearing HJ, Mores CN. Chikungunya viral fitness measures within the vector and subsequent transmission potential. PLoS One 2014; 9:e110538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heffernan JM, Smith RJ, Wahl LM. Perspectives on the basic reproductive ratio. J R Soc Interface 2005; 2:281–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vega-Rua A, Zouache K, Girod R, Failloux AB, Lourenco-de-Oliveira R. High level of vector competence of Aedes aegypti and Aedes albopictus from ten American countries as a crucial factor in the spread of Chikungunya virus. J Virol 2014; 88:6294–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zouache K, Fontaine A, Vega-Rua A et al. . Three-way interactions between mosquito population, viral strain, and temperature underlying chikungunya virus transmission potential. Proc Biol Sci 2014; 281:pii:20141078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Scott TW, Clark GG, Lorenz LH, Amerasinghe PH, Reiter P, Edman JD. Detection of multiple blood feeding in Aedes aegypti (Diptera: Culicidae) during a single gonotrophic cycle using a histologic technique. J Med Entomol 1993; 30:94–9. [DOI] [PubMed] [Google Scholar]
- 20.Moudy RM, Meola MA, Morin LL, Ebel GD, Kramer LD. A newly emergent genotype of West Nile virus is transmitted earlier and more efficiently by Culex mosquitoes. Am J Trop Med Hyg 2007; 77:365–70. [PubMed] [Google Scholar]
- 21.Chan M, Johansson MA. The incubation periods of Dengue viruses. PLoS One 2012; 7:e50972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tjaden NB, Thomas SM, Fischer D, Beierkuhnlein C. Extrinsic incubation period of dengue: knowledge, backlog, and applications of temperature dependence. PLoS Negl Trop Dis 2013; 7:e2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Adams B, Boots M. How important is vertical transmission in mosquitoes for the persistence of dengue? Insights from a mathematical model. Epidemics 2010; 2:1–10. [DOI] [PubMed] [Google Scholar]
- 24.Yakob L, Clements AC. A mathematical model of chikungunya dynamics and control: the major epidemic on Reunion Island. PLoS One 2013; 8:e57448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jansen CC, Williams CR, van den Hurk AF. The usual suspects: comparison of the relative roles of potential urban chikungunya virus vectors in Australia. PLoS One 2015; 10:e0134975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reisen WK, Fang Y, Martinez VM. Effects of temperature on the transmission of west nile virus by Culex tarsalis (Diptera: Culicidae). J Med Entomol 2006; 43:309–17. [DOI] [PubMed] [Google Scholar]
- 27.Paaijmans KP, Blanford S, Chan BH, Thomas MB. Warmer temperatures reduce the vectorial capacity of malaria mosquitoes. Biol Lett 2012; 8:465–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carrington LB, Armijos MV, Lambrechts L, Scott TW. Fluctuations at a low mean temperature accelerate dengue virus transmission by Aedes aegypti. PLoS Negl Trop Dis 2013; 7:e2190. [DOI] [PMC free article] [PubMed] [Google Scholar]


