Abstract
In this review, we highlight biological characteristics of Aedes aegypti and Aedes albopictus, 2 invasive mosquito species and primary vectors of chikungunya virus (CHIKV), that set the tone of these species' invasiveness, vector competence, and vectorial capacity (VC). The invasiveness of both species, as well as their public health threats as vectors, is enhanced by preference for human blood. Vector competence, characterized by the efficiency of an ingested arbovirus to replicate and become infectious in the mosquito, depends largely on vector and virus genetics, and most A. aegypti and A. albopictus populations thus far tested confer vector competence for CHIKV. VC, an entomological analog of the pathogen's basic reproductive rate (R0), is epidemiologically more important than vector competence but less frequently measured, owing to challenges in obtaining valid estimates of parameters such as vector survivorship and host feeding rates. Understanding the complexities of these factors will be pivotal in curbing CHIKV transmission.
Keywords: Aedes aegypti, Aedes albopictus, chikungunya, competitive displacement, invasiveness, satyrization, vectorial capacity, vector competence
Because most cases of chikungunya fever arise as a consequence of infectious bites by either of the mosquito species Aedes aegypti or Aedes albopictus, this review synthesizes aspects of the biology of these species that contribute to their public health threats. Both species are highly invasive, so we begin by considering features of A. aegypti and A. albopictus that have enabled their establishment in human-dominated environments across the globe. The second section of this contribution focuses on vectorial capacity (VC), namely the biological characteristics of females of these species that facilitate their infection by, replication of, and transmission of chikungunya viruses.
ATTRIBUTES OF INVASIVENESS
Invasion biology studies the introduction, establishment, and spread of nonnative species outside their native ranges. In this review, we distinguish invasive species from other nonnative species on the basis of their impacts. Particularly, invasive species, in contrast to other nonnative species, are of greater concern because of their realized or potential effects on native species and ecosystems and/or human activities and health [1].
A previous review distinguished between 9 invasive mosquitoes and 22 other, nonnative species that have become established outside their native ranges [1]. These 31 species combined were significantly more likely than mosquitoes that have not expanded their ranges to have drought-resistant eggs, presumably because this trait has enabled their transport and introduction into new regions; however, the proportions of invasive and nonnative species with this trait did not differ significantly (Table 1). Although larval development in container habitats did not differ significantly between invasive and nonnative mosquito species, significantly more invasive mosquito species than nonnative species were associated with human-dominated habitats (Table 1).
Table 1.
Associations of Biological Traits With Invasiveness Among Mosquito Species
| Biological Trait | Invasive Species |
Other Nonnative Species |
All Other Culicidae |
P Valuea | |||
|---|---|---|---|---|---|---|---|
| + | − | + | − | + | − | ||
| Desiccation-resistant eggs | 5 | 4 | 14 | 8 | 1012 | 2479 | .002 |
| Desiccation-resistant eggs | 5 | 4 | 14 | 8 | … | … | .693 |
| Container larval microhabitat | 7 | 2 | 10 | 12 | … | … | .134 |
| Human-dominated macrohabitat | 6 | 3 | 3 | 19 | … | … | .028 |
Data are modified from [1].
Abbreviations: +, presence of trait; −, absence of trait.
a By the Fisher exact test. The value in the first row is for the comparison of invasive species plus other nonnative species to all other Culicidae, whereas values in remaining rows are for comparisons of invasive species to other nonnative species.
Considering invasive animals and plants associated with human environments, Hufbauer et al [2] proposed that preadaptations in their native ranges predispose such species for future success in invaded environments. The anthropogenically induced adaptation to invade hypothesis of Hufbauer et al [2] has been applied to A. aegypti and A. albopictus to account for the invasive success of these vector species [3].
In its native range in Africa, A. aegypti is recognized as occurring in 2 forms, a feral morph sometimes recognized as subspecies formosus, and a domesticated form, subspecies aegypti [4]. Globally widespread by shipping in previous centuries [5], the majority of successful invasive establishments of A. aegypti, especially in tropical regions of Asia and the Americas, are genetically more closely related to the domestic morph [6], with accompanying adaptations such as preferences for human blood and occupancy of man-made containers in their immature stages [4].
The feral morph A. aegypti formosus preferentially occupies ecotonal, disturbed habitats on the Kenyan coast [7], which may have predisposed this species for subsequent domestic adaptations within Africa, as hypothesized by Tabachnick [8]. In its native Asian range, A. albopictus is also ecotonal, occurring preferentially on forest fringes, which is the habitat preference also observed in its invasive range [9] and has probably contributed to its invasive success worldwide [3].
FURTHER ADAPTATIONS FAVORING INVASIVENESS
Reproductive Competition and Character Displacement
The arrival and spread of A. albopictus in the southeastern United States, in the 1980s, were associated with rapid reductions and displacements of the resident container mosquito species, A. aegypti [10]. A similarly speedy competitive reduction of A. aegypti by A. albopictus in Bermuda was witnessed in the early 2000s by Kaplan et al [11]. Possible displacements of A. aegypti by A. albopictus have also been reported from central Africa [12] and Reunion and Mayotte islands in the Indian Ocean [13, 14].
Although the superiority of A. albopictus over A. aegypti in larval competition in resource-limited environments was formerly proposed as the most likely causal mechanism for observed displacements [5], recently a form of asymmetric reproductive interference, satyrization, has been proposed as a more likely cause of the rapid displacements and reductions of A. aegypti [15]. Interspecific matings between these species occur in nature wherever their ranges overlap but yield no offspring, and A. aegypti populations are affected more deleteriously by the errant matings than A. albopictus. This is because virgin female A. aegypti but not A. albopictus females are sterilized by accessory gland substances from heterospecific males [15]. Satyrization has been suggested to be an adaptation, or exaptation (a nonadaptive change), favoring the invasive success of A. albopictus [16].
In response to satyrization by A. albopictus, populations of A. aegypti in nature have evolved resistance to reproductive competition via character displacement, which favors coexistence of the two species by reducing the frequencies of cross-matings [16, 17]. Selection for satyrization resistance in A. aegypti is accompanied by fitness costs in laboratory populations, such as reduced fecundity and slower time to mating with members of their own species [16]. These costs probably account for the low levels of interspecific mating still observed in sympatric populations of these species in nature [16].
Anthropophily
In keeping with its preferred occupancy of urban environments, most invasive populations of domestic A. aegypti feed predominantly on human blood, which facilitates its vectoring of arboviruses in human-mosquito cycles in the absence of zoonotic reservoirs [18]. Similar to endophilic malaria vector species in Africa, domestic A. aegypti may often consume blood multiple times per gonotrophic cycle, thereby increasing its potential as an arbovirus vector [18].
In contrast, A. albopictus preferentially occurs in vegetated and rural habitats, especially where it is sympatric with A. aegypti [19]. However, when A. aegypti is absent, this species can be highly productive in urban habitats [20]. A. albopictus has been regarded traditionally as a mammal-feeding generalist [19], but at many locations in its native and invasive ranges, humans account for the preponderance of blood meals identified from engorged specimens collected in nature (Table 2). Host-choice experiments on female A. albopictus from Reunion Island, where this species transmitted chikungunya virus (CHIKV) during the 2006–2007 southwestern Indian Ocean epidemic, showed the local population to prefer humans above all alternative blood-meal hosts [30].
Table 2.
Anthropophily of Aedes albopictus, Based on Identifications of Blood-Meal Hosts of Wild-Caught Females of this Species
| Country | Habitat | Number of Bloodmeal Identifications | Human Host, % of Feeding Episodes | Reference |
|---|---|---|---|---|
| Japan | Suburban/urban | 114 | 68.5 | [21] |
| Thailand | Rural | 105 | 94.3 | [22] |
| China (Macau) | Rural | 48 | 63.9 | [23] |
| India | Suburban/urban | 534 | 99.5 | [24] |
| Singapore | Rural | 37 | 91.9 | [25] |
| Cameroona | Rural | 170 | 100.0 | [26] |
| Italya | Urban | 243 | 91.1 | [27] |
| Brazila | Urban | 177 | 68.2 | [28] |
| USA(NJ)a | Urban | 86 | 68.8 | [29] |
Nonrandom examples of feeding predominantly on human hosts.
a Invasive range of this species.
VECTORIAL CAPACITY
Viruses transmitted by A. aegypti and A. albopictus cause some of the most significant arthropod-borne viral diseases in the world, including the flaviviruses yellow fever virus (YFV), dengue virus (DENV), and Zika virus (ZIKV) and the alphavirus CHIKV. There are many reasons why these 2 mosquito species present such a great public health threat. Among these are environmental, behavioral, and genetic components. The threat to public health posed by A. aegypti in particular and A. albopictus less so is largely a consequence of their focused feeding on and close association with humans. Further facilitating viral spread is the ability of both the human host and mosquito vector to travel, the human actively and the mosquito more passively in immature stages, for example. Such a pattern is dangerous because it spreads viruses to new locations, hospitable to the vector, with naive human hosts. This section concentrates on selected components of virus-vector-vertebrate (human) interrelationships, focusing specifically on how interactions between vector, virus, and environment shape the patterns and intensity of transmission of one of the important viral pathogens mentioned above, CHIKV.
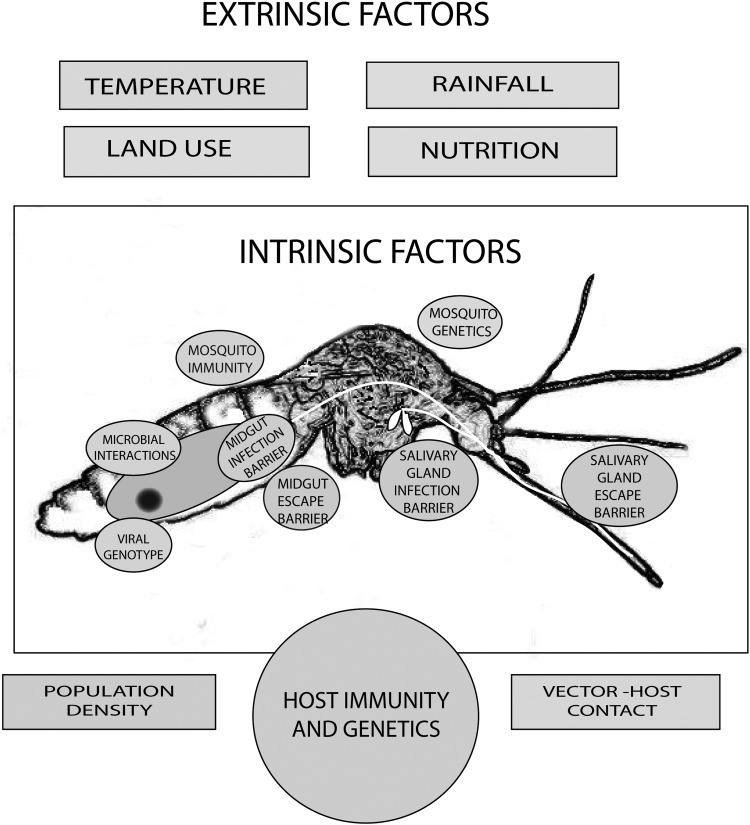
Mosquito-borne disease outbreaks are influenced by intrinsic (eg, vector and viral genetics, vector and host competence, and vector life-history traits) and extrinsic (eg, temperature, rainfall, and human land use) factors that affect virus activity and mosquito biology in complex and interconnected ways (Figure 1) [31]. Disease prevalence varies spatially and temporally, depending on VC, a concept that integrates intrinsic and extrinsic factors to address interactions of the virus with the arthropod and human host. VC leads to a clearer understanding of their complex interrelationships and how such relationships influence transmission of vector-borne pathogens. Determination of risk is measured through elucidation of the factors that compose VC. Other factors, such as human immune status and population density, also affect transmission dynamics, but they will not be discussed here. All of the contributing factors taken together have an impact on selective pressures shaping dynamic viral populations, host-virus outcomes, and, ultimately, epidemiological patterns.
Figure 1.
Factors affecting the vectorial capacity of a mosquito vector (modified from [31] with permission).
VC is essentially an entomological restatement of the basic reproductive rate (R0) of a pathogen, defined as the number of secondary infections expected to occur from the introduction of a single infection in a naive population. An equation formalizing VC was described by Macdonald [32] and later modified by others. One of these, described by Black and Moore [33], provides a useful platform for rational examination of selective forces that may shape Aedes-transmitted arboviruses. This formula is VC = [ma2 (I*T)pn]/−ln(p), where m is the vector density in relation to the host, a is the probability that a vector feeds on a host in 1 day (ie, the host preference index multiplied by the feeding frequency), p is the probability that a vector survives 1 day, n is the duration of the extrinsic incubation period (EIP) in days, I (infection rate) * T (transmission rate) is equal to vector competence (b) or the proportion of vectors ingesting an infective meal that are later able to transmit the infection, and 1/−ln(p) is the duration of the vector's life in days after surviving the EIP. This equation demonstrates that the abundance (m) and vector competence (b) of mosquito populations would influence the reproductive rate of the arbovirus linearly and, thus, relatively weakly. In contrast, host feeding (a), vector longevity (p), and EIP (n) would influence R0 much more powerfully (eg, as a square or exponent). It seems to follow that virus infectivity for mosquitoes, which would be incorporated into VC as b, would be of relatively minor importance as compared to viral factors, such as the speed of dissemination from the midgut, that would impact the duration of the EIP, which would influence VC as n [31]. Thus, natural selection might favor a poorly infectious but rapidly disseminating virus over a highly infectious virus that disseminates slowly. Similar predictions might be made about viral influences on other mosquito-associated factors, such as host preference and survivorship.
Frequency of feeding on the targeted host (host feeding [a]) is an important component of VC. As aforementioned, domestic A. aegypti feed predominantly on human blood [4, 22] and take multiple blood meals during each gonotrophic cycle [18]. Once female mosquitoes are infectious, they may transmit virus each time they probe or take a blood meal. Domestic A. aegypti live in close proximity to humans, where they may feed on blood frequently.
A. albopictus also may preferentially feed on humans, as previously stated (Table 2), but some populations of this species may be more opportunistic in their host preferences, feeding on a variety of mammalian and avian species if available [19]. A. albopictus also may be found in more-diverse environmental settings than A. aegypti, such as suburban and rural environments. However, in south Florida before A. albopictus arrived, A. aegypti could be found in rural environments. In southern China, outside the range of A. aegypti, A. albopictus is urban dwelling [20]. Therefore, the niche width of these 2 species is not absolute.
VECTOR COMPETENCE
Even though vector competence is of relatively minor importance in the VC equation, where it is represented as a linear function, the mosquito must be physiologically competent for transmission to occur. That said, relatively incompetent vectors are capable of initiating and sustaining arbovirus outbreaks in the presence of high population density [34]. Such incompetence may actually select for higher viremia titers in the human host.
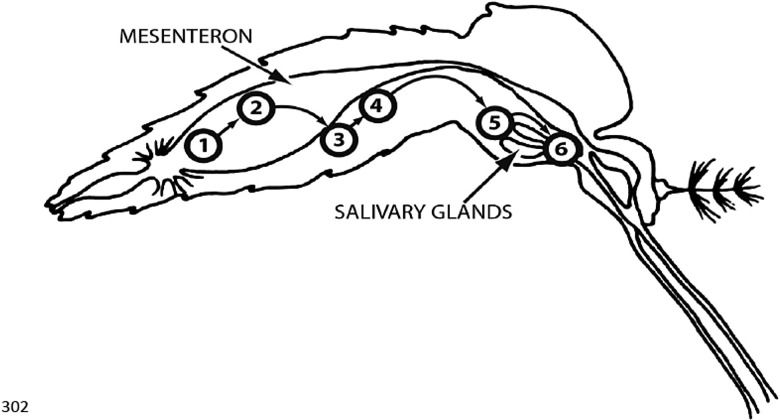
A competent mosquito is one in which virus (1) is ingested with the blood meal, (2) successfully infects and multiplies in mesenteronal epithelial cells, (3) disseminates to parenteral tissues where it replicates further, (4) infects salivary glands (or, alternatively, may infect salivary glands directly following release from the mesenteron), and (5) is released from the salivary gland epithelial cells into the salivary secretion and is transmitted during feeding (Figure 2) [35]. The susceptibility of a population is generally defined as the concentration of virus required to infect 50% of a mosquito population (ID50). Both virus and vector genetics influence vector competence. With DENV, variation occurs in both interspecific and intraspecific serotype-specific ID50 estimates. For example, the ID50 for DENV-1 and DENV-2 was lower than for DENV-3 and DENV-4 in one study in Vietnam [36], and field populations of A. aegypti demonstrated lower vector competence for the American genotype of DENV-2 than the Southeast Asian genotype [37]. It has been shown that people with asymptomatic and presymptomatic DENV infections had an approximately 100-fold lower ID50 and resulted in larger viral loads in infected mosquitoes, which was interpreted as increased transmission potential [38].
Figure 2.
Sequential steps required for a competent female mosquito to transmit an arbovirus after ingestion of an infective blood meal (modified from [35] with permission).
Transmission of East/Central/South African CHIKV by A. albopictus was facilitated after an amino acid change from alanine to valine at position 226 of CHIKV E1 glycoprotein (E1-A226 V), causing increased replication, midgut infection, dissemination, and transmission in this species, with no significant changes observed in A. aegypti's competence [39]. This adaptive mutation enhancing the vector competence of A. albopictus is thought to have occurred on at least 3 independent occasions in the Indian Ocean region, the Indian subcontinent, and Central Africa, supporting the hypothesis of convergent evolution [40]. Additional adaptive mutations in CHIKV have been identified in A. albopictus. These are E2 substitutions, with 1 mutation also involving an E3 substitution [41]. As with the E1 mutation, these changes enhance initial infection of the mosquito midgut and have little, if any, effect on infection of A. aegypti.
Additionally, vector competence is a quantitative trait that has been found to be highly variable in wild populations of A. aegypti [42]. When A. albopictus transmission potential for CHIKV was measured in 6 worldwide vector populations, with 2 virus strains and in 2 ambient temperatures (20° and 28°C), strong effects of the 3-way interaction of mosquito population, virus strain, and temperature were observed. This highlights the importance of studies that focus on genotype by genotype by environment interactions [43]. Differences in transmission efficiency of CHIKV by A. aegypti and A. albopictus were noted in populations of both species from the Americas, while dissemination rates were similar. This confirms that salivary glands may act as an anatomical barrier to virus transmission and may vary with mosquito and viral genetics, as well as viral dose [44]. Nonetheless, transmission efficiency reached rates as high as 83% and 97% in A. aegypti and A. albopictus populations, respectively [45].
Composition of the midgut bacterial community also has an impact on vector competence. One study showed 10–100-fold higher bacterial abundance in midguts of a DENV-resistant strain of A. aegypti as compared to susceptible and unselected strains [46]. Another study demonstrated that regulation of genes in the innate immune pathway (Toll pathway) was stimulated by natural gut microbiota [47]. These investigators further showed that mosquitoes reared without the presence of endogenous bacterial flora were less responsive immunologically to DENV, which was present in midguts at 2-fold higher titers as compared to wild-type mosquitoes.
Insect-specific viruses (ie, RNA viruses that replicate only in insects and not in vertebrate hosts) also may affect vector competence. They represent a broad range of families, including Flaviviridae and Togaviridae. It is suspected that competitive inhibition may diminish competence for some but not all secondarily infecting arboviruses [48].
Environmental or abiotic factors may have large effects on vector competence and VC. The climatic suitability for CHIKV and DENV outbreaks is dependent on bioclimatic factors that influence both vector and virus. Temperature is recognized to have a stronger influence on A. albopictus abundance than precipitation [49], but low rainfall levels may lead to an extinction of the A. albopictus population [50]. In a study that evaluated the impact of diurnal temperature range, A. aegypti lived longer and was more likely to become infected under moderate temperature fluctuations, typical of the high DENV transmission season, than under large temperature fluctuations, typical of the low DENV transmission season [51]. Temperature also may affect critical components of VC, such as mosquito developmental time and consequent population density, survivorship, blood feeding, fecundity, and the EIP. This points to the complexity of understanding the dynamics of transmission of arthropod-borne diseases.
CONCLUSIONS
CHIKV is now widespread worldwide and likely will continue to pose a public health threat globally wherever the invasive mosquitoes A. albopictus and A. aegypti are present and a naive human population exists. The biology of these 2 vectors makes them extremely difficult to control, and there is currently no Food and Drug Administration–approved vaccine to prevent disease. Several innovative approaches to vector control are in trial, one being population replacement with Wolbachia-infected A. aegypti. These mosquitoes have shorter life spans and high resistance to DENV, CHIKV, and YFV infection, and the intracellular Wolbachia bacteria remains established in the population [52]. However, A. albopictus is unaffected by this technique. An alternative, population-suppression approach being tested is release of male mosquitoes carrying dominant lethal genes [53]. At this time, basic personal measures are the most effective for prevention of CHIKV (eg, removal of standing water around homes and individual protection against mosquito bites). Important questions remaining to assist in control include further elucidation of the biology of A. aegypti and A. albopictus, increased understanding of the ecology and evolution of CHIKV in its natural setting, and evaluation of the potential for CHIKV to become enzootic outside its native range in Africa.
Notes
Financial support. This work was supported by the National Institutes of Health (grants R01 AI044793 and R21 AI095780 to L. P. L.).
Potential conflict of interest. Both authors: No reported conflicts. Both authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Juliano SA, Lounibos LP. Ecology of invasive mosquitoes: effects on resident species and on human health. Ecol Lett 2005; 8:558–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hufbauer RA, Facon B, Ravigne V et al. . Anthropogenically induced adaptation to invade (AIAI): contemporary adaptation to human-altered habitats within the native range can promote invasions. Evol Appl 2012; 5:89–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Juliano SA, Lounibos LP. Invasions by mosquitoes: the roles of behaviour across the life cycle. In: Weis JS, Sol D, eds. Behaviour in biological invasions. Cambridge, United Kingdom: Cambridge University Press, 2016. [Google Scholar]
- 4.Powell JR, Tabachnick WJ. History of domestication and spread of Aedes aegypti--a review. Mem Inst Oswaldo Cruz 2013; 108(suppl 1):11–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lounibos LP. Invasions by insect vectors of human disease. Annu Rev Entomol 2002; 47:233–66. [DOI] [PubMed] [Google Scholar]
- 6.Brown JE, McBride CS, Johnson P et al. . Worldwide patterns of genetic differentiation imply multiple ‘domestications’ of Aedes aegypti, a major vector of human diseases. Proc Roy Soc B 2011; 278:2446–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lounibos LP. Habitat segregation among African treehole mosquitoes. Ecol Entomol 1981; 6:129–54. [Google Scholar]
- 8.Tabachnick WJ. Evolutionary genetics and arthropod-borne disease: the yellow fever mosquito. Am Entomol 1991; 37:14–26. [Google Scholar]
- 9.Lounibos LP, O'Meara GF, Escher RL et al. . Testing predictions of displacement of native Aedes by the invasive Asian tiger mosquito Aedes albopictus in Florida, USA. Biol Inv 2001; 3:151–66. [Google Scholar]
- 10.Lounibos LP. Competitive displacement and reduction. J Am Mosq Control Assoc 2007; 23(suppl 2):276–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaplan L, Kendell D, Robertson D, Livdahl T, Katchikian C. Aedes aegypti and Aedes albopictus in Bermuda: extinction, invasion, invasion and extinction. Biol Inv 2010; 9:3277–88. [Google Scholar]
- 12.Kamgang B, Ngoagouni C, Manirakiza A, Nakoune E, Paupy C, Kazanji M. Temporal patterns of abundance of Aedes aegypti and Aedes albopictus (Diptera: Culicidae) and mitochondrial DNA analysis of Ae. albopictus in the Central African Republic. PLoS Negl Trop Dis 2013; 7:e2590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bagny L, Delatte H, Quilici S, Fontenille D. Progressive decrease in Aedes aegypti distribution in Reunion Island since the 1900s. J Med Entomol 2009; 46:1541–5. [DOI] [PubMed] [Google Scholar]
- 14.Bagny L, Delatte H, Elissa N, Quilici S, Fontenille D. Aedes (Diptera: Culicidae) vectors of arboviruses in Mayotte (Indian Ocean): distribution area and larval habitats. J Med Entomol 2009; 46:198–207. [DOI] [PubMed] [Google Scholar]
- 15.Tripet F, Lounibos LP, Robbins D, Moran J, Nishimura N, Blosser EM. Competitive reduction by satyrization? Evidence for interspecific mating in nature and asymmetric reproductive competition between invasive mosquito vectors. Am J Trop Med Hyg 2011; 85:265–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bargielowski IE, Lounibos LP. Satyrization and satyrization-resistance in competitive displacements of invasive mosquito populations. Ins Sci 2016; 23:162–74. [DOI] [PubMed] [Google Scholar]
- 17.Bargielowski IE, Lounibos LP, Carrasquilla MC. Evolution of resistance to satyrization through reproductive character displacement in populations of invasive dengue vectors. Proc Natl Acad Sci U S A 2013; 110:2888–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ritchie SA. Dengue vector bionomics: why Aedes aegypti is such a good vector. In: Gubler DJ, Ooi EE, Vasudevan S, Farrar J, eds. Dengue and dengue haemorrhagic fever. Oxfordshire, United Kindom: CABI International, 2014:455–80. [Google Scholar]
- 19.Hawley WA. The biology of Aedes albopictus. J Am Mosq Cont Assoc 1988; 4(suppl 1):1–40. [PubMed] [Google Scholar]
- 20.Li Y, Kamara F, Zhou G et al. . Urbanization increases Aedes albopictus larval habitats and accelerates mosquito development and survivorship. PLoS Negl Trop Dis 2014; 8:e3301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sawabe K, Isawa H, Hoshino K et al. . Host-Feeding habits of Culex pipiens and Aedes albopictus (Diptera: Culicidae) collected at the urban and suburban residential areas of Japan. J Med Entomol 2010; 47:442–50. [DOI] [PubMed] [Google Scholar]
- 22.Ponlawat A, Harrington LC. Blood feeding patterns of Aedes aegypti and Aedes albopictus in Thailand. J Med Entomol 2005; 42:844–9. [DOI] [PubMed] [Google Scholar]
- 23.Almeida APG, Baptista SS, Sousa CA et al. . Bioecology and vectorial capacity of Aedes albopictus (Diptera: Culicidae) in Macao, China, in relation to dengue virus transmission. J Med Entomol 2005; 42:419–28. [DOI] [PubMed] [Google Scholar]
- 24.Tandon N, Ray S. Host feeding pattern of Aedes aegypti and Aedes albopictus in Kolkata India. Dengue Bull 2000; 24:117–20. [Google Scholar]
- 25.Colless DH. Notes on the culicine mosquitoes of Singapore. VII. Host preferences in relation to the transmission of disease. Ann Trop Med Parasit 1959; 53:259–67. [DOI] [PubMed] [Google Scholar]
- 26.Kamgang B, Nchoutpouen E, Simard F, Paupy C. Notes on the blood-feeding behavior of Aedes albopictus (Diptera: Culicidae) in Cameroon. Parasit Vectors 2012; 5:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Valerio L, Marini F, Bongiorno G et al. . Host-Feeding patterns of Aedes albopictus (Diptera: Culicidae) in urban and rural contexts within Rome Province, Italy. Vector Borne Zoonotic Dis 2010; 10:291–4. [DOI] [PubMed] [Google Scholar]
- 28.Gomes AC, Silva NN, Marques GRAM, Brito M. Host-feeding patterns of potential disease vectors in the Paraiba Valley Region, State of São Paulo, Brazil. J Vect Ecol 2003; 28:74–8. [PubMed] [Google Scholar]
- 29.Farajhi A, Egizi A, Fonseca DM et al. . Comparative host feeding patterns of the Asian tiger mosquito, Aedes albopictus, in urban and suburban northeastern USA and implications for disease transmission. PLoS Negl Trop Dis 2014; 8:e3037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Delatte H, Desvars A, Bouetard A et al. . Blood-feeding behavior of Aedes albopictus, a vector of Chikungunya on La Reunion. Vector Borne Zoonotic Dis 2010; 10:249–58. [DOI] [PubMed] [Google Scholar]
- 31.Kramer LD, Ciota AT. Dissecting vectorial capacity for mosquito-borne viruses. Curr Opin Virol 2015; 15:112–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Macdonald G. Epidemiologic models in studies of vector-borne diseases. Pub Hlth Rep 1961; 76:753–64. [PMC free article] [PubMed] [Google Scholar]
- 33.Black WC, Moore CG. Population biology as a tool for studying vector-borne diseases. In: Beaty BJ, Marquardt WC, eds. The biology of disease vectors. Niwot, CO: University Press of Colorado, 1996:393–416. [Google Scholar]
- 34.Miller BR, Monath TP, Tabachnick WJ, Ezike VI. Epidemic yellow fever caused by an incompetent mosquito vector. Trop Med Parasitol 1989; 40:396–9. [PubMed] [Google Scholar]
- 35.Hardy JL. Susceptibility and resistance of vector mosquitoes. In: Monath TP, ed. The arboviruses: epidemiology and ecology. Vol 1 Boca Raton, FL: CRC Press, 1988:87–126. [Google Scholar]
- 36.Nguyet MN, Duong TH, Trung VT et al. . Host and viral features of human dengue cases shape the population of infected and infectious Aedes aegypti mosquitoes. Proc Natl Acad Sci U S A 2013; 110:9072–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Armstrong PM, Rico-Hesse R. Differential susceptibility of Aedes aegypti to infection by the American and Southeast Asian genotypes of dengue type 2 virus. Vect-Bor Zoon Dis 2001; 1:159–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Duong V, Lambrechts L, Paul RE et al. . Asymptomatic humans transmit dengue virus to mosquitoes. Proc Natl Acad Sci U S A 2015; 112:14688–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tsetsarkin KA, Vanlandingham DL, McGee CE, Higgs S. A single mutation in chikungunya virus affects vector specificity and epidemic potential. PLoS Path 2007; 3:e201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tsetsarkin KA, Chen R, Sherman MB, Weaver SC. Chikungunya virus: evolution and genetic determinants of emergence. Curr Opin Virol 2011; 1:310–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Coffey LL, Failloux AB, Weaver SC. Chikungunya virus-vector interactions. Viruses 2014; 6:4628–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Knox TB, Kay BH, Hall RA, Ryan PA. Enhanced vector competence of Aedes aegypti (Diptera: Culicidae) from the Torres Strait compared with mainland Australia for dengue 2 and 4 viruses. J Med Entomol 2003; 40:950–6. [DOI] [PubMed] [Google Scholar]
- 43.Zouache K, Fontaine A, Vega-Rua A et al. . Three-way interactions between mosquito population, viral strain and temperature underlying chikungunya virus transmission potential. Proc Roy Soc B 2014; 281:20141078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ciota AT, Ehrbar DJ, Van Slyke GA et al. . Quantification of intrahost bottlenecks of West Nile virus in Culex pipiens mosquitoes using an artificial mutant swarm. Infect Genet Evol 2012; 12:557–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vega-Rua A, Zouache K, Girod R, Failloux AB, Lourenco-de-Oliveira R. High level of vector competence of Aedes aegypti and Aedes albopictus from ten American countries as a crucial factor in the spread of Chikungunya virus. J Virol 2014; 88:6294–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Charan SS, Pawar KD, Severson DW, Patole MS, Shouche YS. Comparative analysis of midgut bacterial communities of Aedes aegypti mosquito strains varying in vector competence to dengue virus. Parasitol Res 2013; 112:2627–37. [DOI] [PubMed] [Google Scholar]
- 47.Xi Z, Ramirez JL, Dimopoulos G. The Aedes aegypti toll pathway controls dengue virus infection. PLoS Pathog 2008; 4:e1000098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vasilakis N, Tesh RB. Insect-specific viruses and their potential impact on arbovirus transmission. Curr Opin Virol 2015; 15:69–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Alto BW, Juliano SA. Temperature effects on the dynamics of Aedes albopictus (Diptera: Culicidae) populations in the laboratory. J Med Entomol 2001; 38:548–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tran A, L'Ambert G, Lacour G et al. . A rainfall- and temperature-driven abundance model for Aedes albopictus populations. Int J EnvironRes Pub Hlth 2013; 10:1698–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lambrechts L, Paaijmans KP, Fansiri T et al. . Impact of daily temperature fluctuations on dengue virus transmission by Aedes aegypti. Proc Natl Acad Sci U S A 2011; 108:7460–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Van den Hurk AF, Hall-Mendelin S, Pyke AT et al. . Impact of Wolbachia on infection with chikungunya and yellow fever viruses in the mosquito vector Aedes aegypti. PLoS Negl Trop Dis 2012; 6:e1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Alphey L. Genetic control of mosquitoes. Annu Rev Entomol 2014; 59:205–24. [DOI] [PubMed] [Google Scholar]