Abstract
The regulation of smooth muscle contraction and relaxation involves phosphorylation and dephosphorylation of regulatory proteins, particularly myosin. To elucidate the regulatory mechanisms, analyzing the phosphorylation signal transduction is crucial. Although a pharmacological approach with selective inhibitors is sensitive and a useful technique, it leads to speculation regarding a signaling pathway but does not provide direct evidence of changes at a molecular level. We developed a highly sensitive biochemical technique to analyze phosphorylation by adapting Phos-tag SDS-PAGE. With this technique, we successfully analyzed myosin light chain (LC20) phosphorylation in tiny renal afferent arterioles. In the rat afferent arterioles, endothelin-1 (ET-1) induced diphosphorylation of LC20 at Ser19 and Thr18 as well as monophosphorylation at Ser19 via ETB receptor activation. Considering that LC20 diphosphorylation can decrease the rate of dephosphorylation and thus relaxation, we concluded that LC20 diphosphorylation contributes, at least in part, to the prolonged contraction induced by ET-1 in the renal afferent arteriole.
Keywords: mooth muscle, renal arteriole, phosphorylation, Phos-tag, endothelin-1
Introduction
Smooth muscle is found everywhere in the body — the walls of blood vessels, lymphatic vessels and hollow organs, and the ciliary muscle and iris of the eye. Although they basically have the same contractile and regulatory apparatuses in common, they respond differently to various stimuli to achieve specific effects at individual times.
The regulation of smooth muscle contraction and relaxation involves phosphorylation of regulatory proteins, including myosin (1,2,3), which generates contractile force. To study the regulatory signal transduction pathways, a variety of physiological and pharmacological techniques have been employed. Although pharmacological observations with the use of selective inhibitors can lead to speculation about the signal transduction pathways, they will not provide conclusive evidence due to their general lack of specificity.
By combining biochemical and pharmacological techniques, changes at a molecular level will be measurable, and thus conclusive evidence will be attainable. In this review, I will give examples of phosphorylation analysis, especially in small smooth muscles, which had been difficult to study biochemically. And I will summarize our recent studies on a tiny blood vessel, the renal afferent arteriole.
Phosphorylation analysis in smooth muscles
It is widely accepted that the contraction of smooth muscle is primarily regulated by phosphorylation of myosin regulatory light chains (see reviews in Refs. (1,2,3)). Actin-activated myosin ATPase and motor activities increase upon phosphorylation of its 20-kDa regulatory light chain (LC20) at Ser19, resulting in contraction. These activities decrease when LC20 is dephosphorylated, resulting in relaxation.
The level of LC20 phosphorylation is determined by the balance between kinase and phosphatase activities. When smooth muscle receives contractile stimuli, intracellular Ca2+ concentration increases, and the Ca2+/calmodulin complex is formed (4). The Ca2+/calmodulin complex activates myosin light chain kinase (MLCK), resulting in accumulation of phosphorylated LC20 and contraction (5). Some contractile stimuli also activate another pathway whereby myosin phosphatase activity is decreased by phosphorylation of its myosin targeting subunit (MYPT1) (6) or by direct binding of phosphorylated 17-kDa PKC-potentiated inhibitory protein of PP1 (CPI-17) (7). This decrease in myosin phosphatase activity changes the balance between kinase and phosphatase activities, resulting in further accumulation of phosphorylated LC20 and thus stronger contraction. This mechanism is called "Ca2+-sensitization" (1, 2).
To elucidate the regulatory mechanisms of smooth muscle contraction, analyzing the phosphorylation signaling pathway is crucial. A variety of physiological and pharmacological techniques have been employed to this end. Pharmacological techniques exploiting selective inhibitors lead to speculation about the signaling pathways, but do not provide direct molecular evidence. For example, a Rho-associated protein kinase (ROCK) inhibitor, H1152, inhibited rabbit urethral smooth muscle contraction, suggesting that ROCK would phosphorylate MYPT1 and thus increase accumulation of phosphorylated LC20 (8). H1152, however, did not alter the level of LC20 phosphorylation in the rabbit urethrae. Things would become even more complicated if a less selective inhibitor was employed.
Biochemical approaches provide molecular evidence to test inconclusive speculations. By combining biochemical analysis with pharmacological techniques, we can measure changes in signal transduction pathways at a molecular level, and thus acquire conclusive evidence.
A variety of biochemical techniques have been applied to measure phosphorylation of myosin and other regulatory proteins in smooth muscle. For example, isoelectric focusing (9, 10), 2D-electrophoresis (11, 12), and urea/glycerol PAGE (13, 14, 15) have been used to measure myosin LC20 phosphorylation. In these electrophoreses, LC20 is separated into discrete bands or spots based on its phosphorylation state, and the ratio of the phosphorylated to unphosphorylated form(s) is quantified densitometrically. These conventional electrophoretic techniques, however, are relatively insensitive (> ∼300 pg LC20 are required) (16), and their application is, therefore, limited to relatively large smooth muscle samples.
Highly sensitive phosphorylation analysis by Phos-tag SDS PAGE and 3-step western blotting
Recently, we successfully improved the sensitivity of the LC20 phosphorylation analysis by combining Phos-tag SDS-PAGE with 3-step western blotting (17).
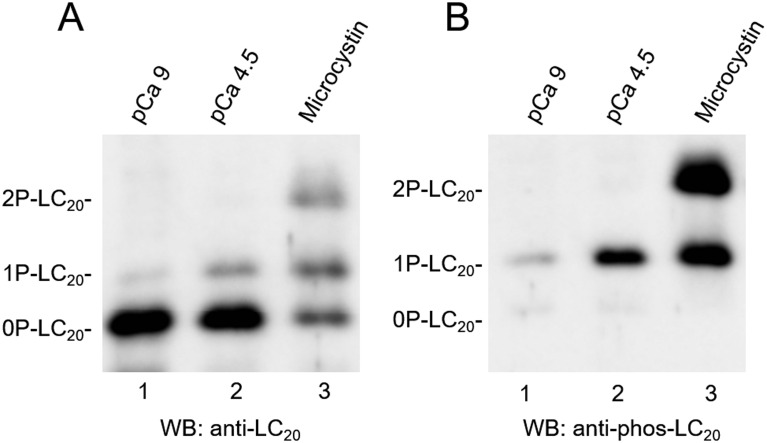
Phos-tag SDS-PAGE provides separation of phosphorylated proteins from their unphosphorylated forms based on the number and position of the phosphorylated sites (18, 19). Immobilized phosphate-affinity ligand (Phos-tag reagent) in a Laemmli SDS gel slows the migration of phosphorylated proteins due to binding to the ligand. Thus, the higher the stoichiometry of phosphorylation, the slower the migration rate through the gel (Fig. 1) (13, 17).
Fig. 1.
Phosphorylation-based LC20 separation by Phos-tag SDS-PAGE. Skinned rat tail artery strips were treated with pCa 9 (lane 1), pCa 4.5 (lane 2), or 1 μM microcystin (lane 3). (A) All forms of LC20s, regardless of the phosphorylation state, were detected by western blotting with pan anti-LC20 antibody. In a Phos-tag gel, phosphorylated LC20s (monophosphorylated, 1P-LC20; diphosphorylated, 2P-LC20) migrated more slowly than the unphosphorylated form (0P-LC20). (B) Phosphorylation-based LC20 separation was confirmed by using phospho-specific antibody against pSer19-LC20, which also recognizes diphosphorylated-(pThr18, pSer19)-LC20. This figure was reproduced from ref. (17).
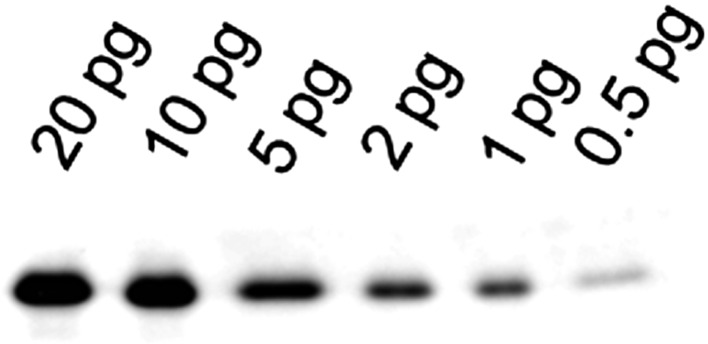
Three-step western blotting with the use of biotin-avidin binding significantly improved the sensitivity (17). By optimizing the conditions of western blotting, we are able to detect as little as 0.5 pg LC20 (Fig. 2). This is sufficiently sensitive to quantify LC20 in tiny smooth muscle tissues. For example, we were able to quantify LC20 phosphorylation in rat cerebral artery (20, 21) and renal arterioles (see below and Refs. (17, 22)).
Fig. 2.
Highly sensitive three-step western blotting. Purified LC20 (0.5–20 pg) was electrophoresed in a Laemmli SDS-gel and detected by 3-step western blotting with primary anti-LC20 antibody, secondary biotin-conjugated anti-IgG antibody and tertiary HRP-conjugated NeutrAvidin. As little as 0.5 pg of LC20 was detectable.
Renal microvasculature
The renal afferent and efferent arterioles regulate the inflow and outflow resistance of the glomerulus, thereby controlling the pressure within the intervening glomerular capillaries (PGC). PGC is a primary determinant of glomerular filtration rate (GFR) and must be maintained within precise limits for normal renal function and to protect against hypertensive injury.
Angiotensin II (Ang II) is a renal vasoconstrictor that contributes to renal vascular resistance under normal physiological conditions and thus plays an important role in modulating renal hemodynamics (23). Bolus administration of Ang II elicits a transient constriction of the afferent arteriole (22, 24).
Endothelin-1 (ET-1) is also a potent renal vasoconstrictor. Unlike Ang II, ET-1 does not contribute to renal vascular resistance under normal physiologic conditions, but rather is implicated in abnormal renal vasoconstriction in a wide variety of pathologic states (25,26,27). Bolus administration of ET-1 elicits long-lasting constriction of the afferent arteriole (22, 24).
Although both Ang II and ET-1 are potent renal vasoconstrictors, the nature of renal vascular tone induced by these two agents is qualitatively different (22). In order to address the molecular determinants underlying these differences, biochemical analysis is essential.
The renal afferent arteriole is too small to detect molecular changes with conventional biochemical techniques. The isolated afferent arteriole is approximately one-tenth the size of a human eyelash (10–20 μm in diameter) and consists of < 100 smooth muscle cells on average. It contains ∼50 pg (2.5 fmol) of LC20, well below the limit of detection of conventional assays (300 pg or 15 fmol) (16).
By utilizing the newly developed Phos-tag electrophoresis and highly sensitive 3-step western blotting as described above, we successfully measured LC20 phosphorylation in isolated afferent arterioles and were able to address the question whether Ang II and ET-1 activate distinct signaling pathways, resulting in different contractile responses (22).
Myosin LC20 phosphorylation in the renal microvasculature
We isolated afferent arterioles from agarose-supported rat kidney (17, 22). In brief, the left kidney of anesthetized rats was perfused in vivo with warmed Ca2+-free medium containing agarose. After the kidney was excised, it was chilled to solidify the agarose. The solidified agarose mimics the intraluminal pressure and thus allows the arterioles to maintain their physiological functions. Cortical slices were then treated with collagenase and dispase to separate microvessels from tubules. Individual arterioles were isolated and collected by using a dual-pipette micromanipulator. With this technique, we were able to measure molecular changes in afferent arterioles without contamination with tubules and other types of vessels.
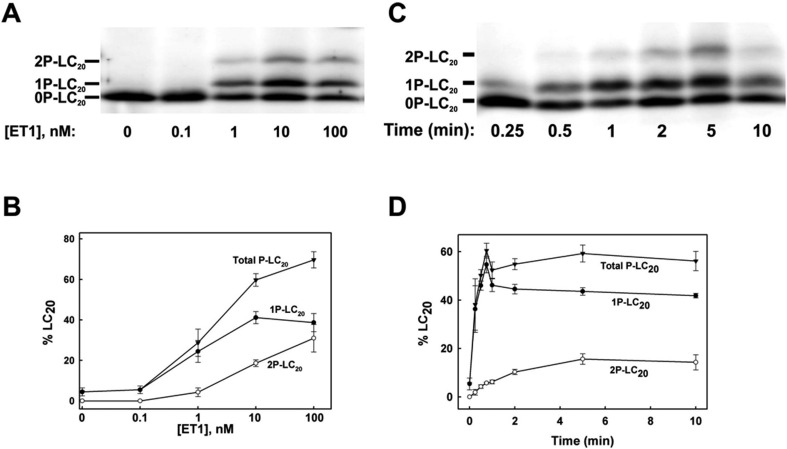
Ang II induced exclusively monophosphorylation of LC20 at Ser19. ET-1, on the other hand, induced not only monophosphorylation of LC20, but also diphosphorylation of LC20 (Fig. 3). The second phosphorylation site in ET-1-treated afferent arterioles was identified as Thr18 by western blotting with a diphosphorylation-specific antibody that recognizes LC20 only when phosphorylated at both Ser19 and Thr18 (22).
Fig. 3.
ET-1-induced LC20 phosphorylation in renal afferent arterioles of Wistar rats. (A) and (B) Isolated afferent arterioles were treated with the indicated concentrations of ET-1 for 5 min. Phosphorylated and unphosphorylated forms of LC20 were separated by Phos-tag SDS-PAGE and detected by a 3-step western blotting procedure with anti-LC20. A representative western blot is shown in (A) with cumulative quantitative data in (B). Data indicate the mean ± S.E.M. (n = 5 except for 10 nmol/L ET-1 where n = 7). (C) and (D) Time-courses of LC20 mono- and diphosphorylation in response to ET-1 (10 nmol/L). A representative western blot is shown in (C) with cumulative quantitative data in (D). Data indicate the mean ± S.E.M. (n = 4 except for 15 s, 45 s and 5 min where n = 3, 2 and 8, respectively). Percent phosphorylation was calculated from the following equations: % 1P-LC20 = [1P/(0P + 1P + 2P)] × 100%; % 2P-LC20 = [2P/(0P + 1P + 2P)] × 100%; % total P-LC20 = [(1P + 2P)/(0P + 1P + 2P)] × 100%. This work was originally published in Kidney International. Takeya K et al. Endothelin-1, but not angiotensin II, induces afferent arteriolar myosin diphosphorylation as a potential contributor to prolonged vasoconstriction. 2015; 87(2): 370–81. © International Society of Nephrology.
As seen in Fig. 3, ET-1 treatment increased LC20 diphosphorylation as well as monophosphorylation in a concentration- and time-dependent manner. The level of LC20 monophosphorylation reached a plateau of ∼40% at 10 nM ET-1. The second phosphorylation at Thr18 increased the total phosphorylation level to ∼60% at 10 nM ET-1 and to ∼70% at 100 nM ET-1. Monophosphorylation of LC20 increased rapidly following the application of 10 nM ET-1, reaching a steady-state level within 60 sec. Diphosphorylation of LC20, on the other hand, increased more slowly, reaching a maximum level within 5 min.
In contrast to ET-1, Ang II induced only monophosphorylation of LC20 even at a high concentration (100 nM), suggesting that only MLCK is involved in LC20 monophosphorylation in response to AngII (22).
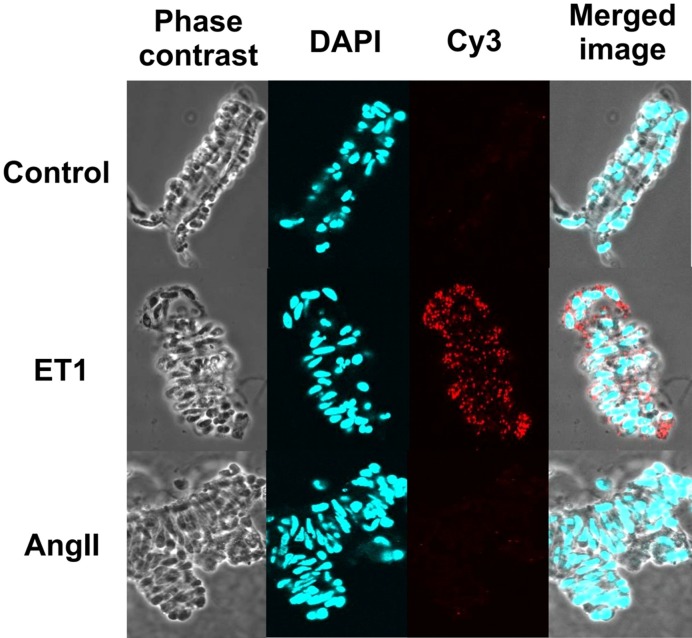
The diphosphorylation of LC20 at Ser19 and Thr18 in response to ET-1 treatment was confirmed by proximity ligation assay (Fig. 4), which provides higher sensitivity, specificity, and signal-to-noise ratio than regular immunostaining (28). When stained with pan anti-LC20 and anti-pT18, pS19-LC20 antibodies, strong fluorescent signals were observed in the smooth muscle cells in ET-1 treated afferent arteriole, but not in untreated control or AngII-treated vessels.
Fig. 4.
Proximity ligation assay for LC20 diphosphorylation. Untreated Wistar rat afferent arterioles (control) and afferent arterioles treated with ET-1 or Ang II (10 nmol/L for 5 min) were fixed, permeabilized and incubated with pan-LC20 antibody and anti-pT18, pS19-LC20. Bound antibodies in close proximity were detected by Cy3 staining. Panels show, from left to right, phase contrast images of the isolated arterioles, nuclear staining with DAPI, Cy3 fluorescence to illustrate LC20 diphosphorylation, and merged images. Results are representative of 18 (control), 4 (ET-1) and 4 (Ang II) independent experiments. This work was originally published in Kidney International. Takeya K et al. Endothelin-1, but not angiotensin II, induces afferent arteriolar myosin diphosphorylation as a potential contributor to prolonged vasoconstriction. 2015; 87(2): 370–81. © International Society of Nephrology.
Biochemical phosphorylation analysis also revealed that 1) inhibitors of MLCK and ROCK reduced ET-1-induced LC20 monophosphorylation as well as diphosphorylation, while they failed to abolish LC20 diphosphorylation, suggesting that other kinases (zipper-interacting protein kinase (ZIPK) (29) and/or integrin-linked kinase (ILK) (30)) are involved in the phosphorylation of LC20 at Thr18; 2) an ETB agonist (sarafotoxin 6c) induced LC20 diphosphorylation, suggesting that ETB, but not ETA receptors mediate the ET-1-induced LC20 diphosphorylation (22).
The observation that ET-1-induced constriction was relatively refractory to Ca2+ channel blockade (22) is also consistent with the involvement of Ca2+-independent kinases – ZIPK and/or ILK.
Role of LC20 diphosphorylation in the renal microvasculature
As it was reviewed previously (31, 32), diphosphorylation of LC20 was first demonstrated in vitro by high concentrations of MLCK (33). The additional phosphorylation at Thr18 increased actomyosin MgATPase activity, but not actin-filament velocity in the in vitro motility assay (33,34,35,36,37). Although LC20 diphosphorylation has been associated with pathophysiological conditions involving smooth muscle hypercontractility (38,39,40,41,42,43), the mechanism whereby LC20 diphosphorylation causes abnormal contraction remains controversial.
It has been proposed recently that the functional effect of LC20 diphosphorylation is to reduce the dephosphorylation rate and thus to slow smooth muscle relaxation (44). Based on this, we hypothesized that the prolonged constriction induced by ET-1 in the afferent arteriole was due to the diphosphorylation of LC20 via ETB-mediated signal transduction. To test this hypothesis, we administered a specific ETB agonist and antagonist to an in vitro perfused afferent arteriole (22).
Vasodilation following washout of the ETB agonist was slow and comparable to that following washout of ET-1, consistent with delayed vasorelaxation due to LC20 diphosphorylation (22). ETB receptor blockade significantly increased the rate of vasodilation following ET-1-induced vasoconstriction in the presence of the NO synthase inhibitor, L-NG-nitroarginine methyl ester (L-NAME), further implicating ETB receptors in the slow vasodilatory response following ET-1 washout (22).
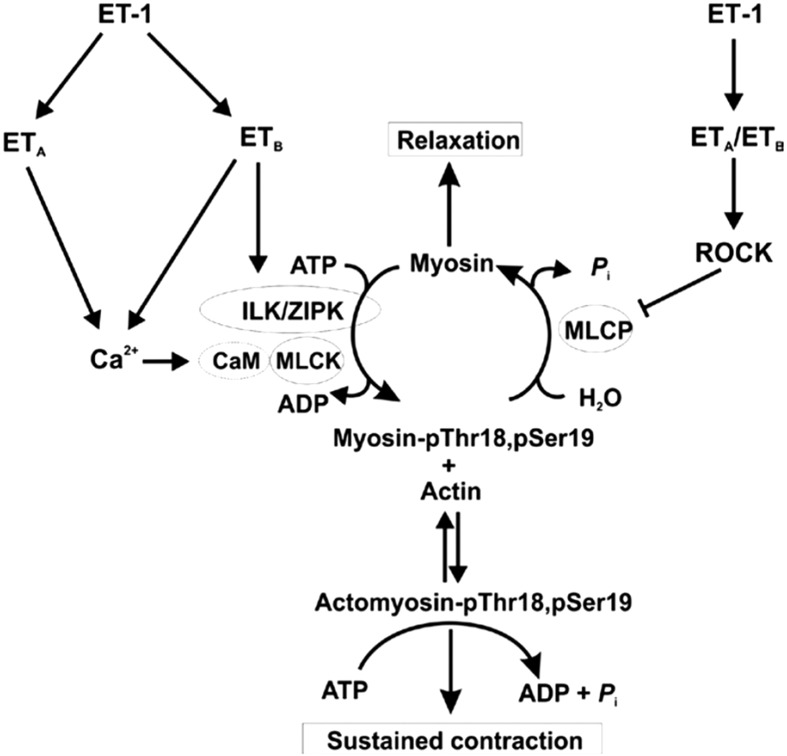
We concluded, therefore, that LC20 diphosphorylation via ETB receptor activation contributes, at least in part, to the prolonged contraction induced by ET-1 in the renal afferent arteriole (Fig. 5). Considering that ET-1 increases in ischemic kidney (45), ET-1-induced LC20 diphosphorylation and consequent prolonged contraction of afferent arterioles may contribute to ischemia/reperfusion-induced acute renal failure. Although endothelial ETB receptors may offer renal protection via NO synthesis (46), the activation of ETB receptors on the afferent arteriolar myocytes might contribute to abnormal vasoconstriction or vasospasm associated with recovery.
Fig. 5.
Proposed signaling pathways leading to ET-1-induced sustained vasoconstriction of the renal afferent arteriole. ET-1, acting via ETA receptors, triggers an increase in cytosolic free Ca2+ concentration ([Ca2+]i), largely through Ca2+ entry via voltage-gated Ca2+ channels (Cav), leading to binding of Ca2+ to calmodulin (CaM), activation of myosin light chain kinase (MLCK), phosphorylation of LC20 at Ser19 and cross-bridge cycling. Activation of ETA receptors also induces inhibition of myosin light chain phosphatase (MLCP) via activation of the Rho-associated kinase (ROCK) pathway, leading to increased LC20 phosphorylation at Ser19 due to the increase in MLCK: MLCP activity ratio. MLCP inhibition unmasks basal activity of integrin-linked kinase (ILK) and/or zipper-interacting protein kinase (ZIPK) that phosphorylate LC20 at both Thr18 and Ser19. Activation of ETB receptors in the vascular smooth muscle cells leads to activation of ILK and/or ZIPK (or possibly other kinase(s) capable of phosphorylating LC20 at Thr18 and Ser19). The rapid increase in Ser19 phosphorylation accounts for the initial phase of the contractile response to ET-1, while the slower diphosphorylation at Thr18 and Ser19, associated with reduced rates of LC20 dephosphorylation and relaxation (44), can account for the sustained contractile response to ET-1 and prolonged contraction that occurs following removal of the stimulus. This figure was originally published in Kidney International. Takeya K et al. Endothelin-1, but not angiotensin II, induces afferent arteriolar myosin diphosphorylation as a potential contributor to prolonged vasoconstriction. 2015; 87(2): 370–81. © International Society of Nephrology.
Conclusions
In this review, I have provided an example of highly sensitive phosphorylation analysis that is capable of measuring phosphorylation in tiny tissue samples. By combining this biochemical approach with physiological, pharmacological and immunocytochemical approaches, we will be able to evaluate physiological events at a molecular level. As I showed in this review, Phos-tag based phosphorylation analysis, combined with proximity ligation assay, is suitable for studying micro-samples with high sensitivity.
Conflict of Interest
The author declares that he has no conflict of interest.
Acknowledgments
The work described in this review was carried out in the laboratories of Drs. Michael P. Walsh and Rodger Loutzenhiser at the University of Calgary. I would like to thank the members of their laboratories for expert technical assistance. I am grateful to Dr. Walsh for proofreading the manuscript.
References
- 1.Somlyo AP, Somlyo AV. Ca2+ sensitivity of smooth muscle and nonmuscle myosin II: modulated by G proteins, kinases, and myosin phosphatase. Physiol Rev. 2003; 83(4): 1325–58 http://www.ncbi.nlm.nih.gov/pubmed/14506307. doi: 10.1152/physrev.00023.2003 [DOI] [PubMed] [Google Scholar]
- 2.Kim HR, Appel S, Vetterkind S, Gangopadhyay SS, Morgan KG. Smooth muscle signalling pathways in health and disease. J Cell Mol Med. 2008; 12(6A): 2165–80 http://www.ncbi.nlm.nih.gov/pubmed/19120701. doi: 10.1111/j.1582-4934.2008.00552.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ito M, Hartshorne DJ. Phosphorylation of myosin as a regulatory mechanism in smooth muscle. Prog Clin Biol Res. 1990; 327: 57–72 http://www.ncbi.nlm.nih.gov/pubmed/2181476. [PubMed] [Google Scholar]
- 4.Wilson DP, Sutherland C, Walsh MP. Ca2+ activation of smooth muscle contraction: evidence for the involvement of calmodulin that is bound to the triton insoluble fraction even in the absence of Ca2+. J Biol Chem. 2002; 277(3): 2186–92 http://www.ncbi.nlm.nih.gov/pubmed/11707462. doi: 10.1074/jbc.M110056200 [DOI] [PubMed] [Google Scholar]
- 5.Kamm KE, Stull JT. Dedicated myosin light chain kinases with diverse cellular functions. J Biol Chem. 2001; 276(7): 4527–30 http://www.ncbi.nlm.nih.gov/pubmed/11096123. doi: 10.1074/jbc.R000028200 [DOI] [PubMed] [Google Scholar]
- 6.Hartshorne DJ, Ito M, Erdödi F. Role of protein phosphatase type 1 in contractile functions: myosin phosphatase. J Biol Chem. 2004; 279(36): 37211–4 http://www.ncbi.nlm.nih.gov/pubmed/15136561. doi: 10.1074/jbc.R400018200 [DOI] [PubMed] [Google Scholar]
- 7.Eto M. Regulation of cellular protein phosphatase-1 (PP1) by phosphorylation of the CPI-17 family, C-kinase-activated PP1 inhibitors. J Biol Chem. 2009; 284(51): 35273–7 http://www.ncbi.nlm.nih.gov/pubmed/19846560. doi: 10.1074/jbc.R109.059972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Walsh MP, Thornbury K, Cole WC, Sergeant G, Hollywood M, McHale N. Rho-associated kinase plays a role in rabbit urethral smooth muscle contraction, but not via enhanced myosin light chain phosphorylation. Am J Physiol Renal Physiol. 2011; 300(1): F73–85 http://www.ncbi.nlm.nih.gov/pubmed/20861082. doi: 10.1152/ajprenal.00011.2010 [DOI] [PubMed] [Google Scholar]
- 9.Kerrick WG, Hoar PE, Cassidy PS. Calcium-activated tension: the role of myosin light chain phosphorylation. Fed Proc. 1980; 39(5): 1558–63 http://www.ncbi.nlm.nih.gov/pubmed/7364052. [PubMed] [Google Scholar]
- 10.Silver PJ, Stull JT. Quantitation of myosin light chain phosphorylation in small tissue samples. J Biol Chem. 1982; 257(11): 6137–44 http://www.ncbi.nlm.nih.gov/pubmed/7076667. [PubMed] [Google Scholar]
- 11.Barron JT, Bárány M, Bárány K, Storti RV. Reversible phosphorylation and dephosphorylation of the 20,000-dalton light chain of myosin during the contraction-relaxation-contraction cycle of arterial smooth muscle. J Biol Chem. 1980; 255(13): 6238–44 http://www.ncbi.nlm.nih.gov/pubmed/6771267. [PubMed] [Google Scholar]
- 12.Kitazawa T, Gaylinn BD, Denney GH, Somlyo AP. G-protein-mediated Ca2+ sensitization of smooth muscle contraction through myosin light chain phosphorylation. J Biol Chem. 1991; 266(3): 1708–15 http://www.ncbi.nlm.nih.gov/pubmed/1671041. [PubMed] [Google Scholar]
- 13.Takeya K. [Phosphorylation analysis of contractile regulatory proteins in smooth muscle by using phos-tag SDS electrophoresis]. Seibutsu Butsuri Kagaku. 2012; 56 (1): s15–9. Available from: http://joi.jlc.jst.go.jp/JST.JSTAGE/sbk/56.s15?from=CrossRef.
- 14.Trybus KM. Filamentous smooth muscle myosin is regulated by phosphorylation. J Cell Biol. 1989; 109(6 Pt 1): 2887–94 http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2115938&tool=pmcentrez&rendertype=abstract. doi: 10.1083/jcb.109.6.2887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weber LP, Van Lierop JE, Walsh MP. Ca2+-independent phosphorylation of myosin in rat caudal artery and chicken gizzard myofilaments. J Physiol. 1999; 516(Pt 3): 805–24 http://www.ncbi.nlm.nih.gov/pubmed/10200427. doi: 10.1111/j.1469-7793.1999.0805u.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shiraishi M, Loutzenhiser RD, Walsh MP. A highly sensitive method for quantification of myosin light chain phosphorylation by capillary isoelectric focusing with laser-induced fluorescence detection. Electrophoresis. 2005; 26(3): 571–80 http://www.ncbi.nlm.nih.gov/pubmed/15690429. doi: 10.1002/elps.200410119 [DOI] [PubMed] [Google Scholar]
- 17.Takeya K, Loutzenhiser K, Shiraishi M, Loutzenhiser R, Walsh MP. A highly sensitive technique to measure myosin regulatory light chain phosphorylation: the first quantification in renal arterioles. Am J Physiol Renal Physiol. 2008; 294(6): F1487–92 http://www.ncbi.nlm.nih.gov/pubmed/18400874. doi: 10.1152/ajprenal.00060.2008 [DOI] [PubMed] [Google Scholar]
- 18.Kinoshita E, Kinoshita-Kikuta E, Takiyama K, Koike T. Phosphate-binding tag, a new tool to visualize phosphorylated proteins. Mol Cell Proteomics. 2006; 5(4): 749–57 http://www.ncbi.nlm.nih.gov/pubmed/16340016. doi: 10.1074/mcp.T500024-MCP200 [DOI] [PubMed] [Google Scholar]
- 19.Kinoshita E, Kinoshita-Kikuta E. Improved Phos-tag SDS-PAGE under neutral pH conditions for advanced protein phosphorylation profiling. Proteomics. 2011; 11(2): 319–23 http://www.ncbi.nlm.nih.gov/pubmed/21204258. doi: 10.1002/pmic.201000472 [DOI] [PubMed] [Google Scholar]
- 20.Johnson RP, El-Yazbi AF, Takeya K, Walsh EJ, Walsh MP, Cole WC. Ca2+ sensitization via phosphorylation of myosin phosphatase targeting subunit at threonine-855 by Rho kinase contributes to the arterial myogenic response. J Physiol. 2009; 587(Pt 11): 2537–53 http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2714019&tool=pmcentrez&rendertype=abstract. doi: 10.1113/jphysiol.2008.168252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.El-Yazbi AF, Johnson RP, Walsh EJ, Takeya K, Walsh MP, Cole WC. Pressure-dependent contribution of Rho kinase-mediated calcium sensitization in serotonin-evoked vasoconstriction of rat cerebral arteries. J Physiol. 2010; 588(Pt 10): 1747–62 http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2887992&tool=pmcentrez&rendertype=abstract. doi: 10.1113/jphysiol.2010.187146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Takeya K, Wang X, Kathol I, Loutzenhiser K, Loutzenhiser R, Walsh MP. Endothelin-1, but not angiotensin II, induces afferent arteriolar myosin diphosphorylation as a potential contributor to prolonged vasoconstriction. Kidney Int. 2015; 87(2): 370–81 http://www.nature.com/ki/journal/v87/n2/abs/ki2014284a.html?WT.ec_id=KI-201502. doi: 10.1038/ki.2014.284 [DOI] [PubMed] [Google Scholar]
- 23.Edwards RM, Aiyar N. Angiotensin II receptor subtypes in the kidney. J Am Soc Nephrol. 1993; 3(10): 1643–52 http://www.ncbi.nlm.nih.gov/pubmed/8318680. [DOI] [PubMed] [Google Scholar]
- 24.Loutzenhiser R, Epstein M, Hayashi K, Horton C. Direct visualization of effects of endothelin on the renal microvasculature. Am J Physiol. 1990; 258(1 Pt 2): F61–8 http://www.ncbi.nlm.nih.gov/pubmed/2405711. [DOI] [PubMed] [Google Scholar]
- 25.Gómez-Garre D, Largo R, Liu XH, Gutierrez S, López-Armada MJ, Palacios I, Egido J. An orally active ETA/ETB receptor antagonist ameliorates proteinuria and glomerular lesions in rats with proliferative nephritis. Kidney Int. 1996; 50(3): 962–72 http://www.ncbi.nlm.nih.gov/pubmed/8872972. doi: 10.1038/ki.1996.397 [DOI] [PubMed] [Google Scholar]
- 26.Orth SR, Esslinger JP, Amann K, Schwarz U, Raschack M, Ritz E. Nephroprotection of an ET(A)-receptor blocker (LU 135252) in salt-loaded uninephrectomized stroke-prone spontaneously hypertensive rats. Hypertension. 1998; 31(4): 995–1001 http://www.ncbi.nlm.nih.gov/pubmed/9535426. doi: 10.1161/01.HYP.31.4.995 [DOI] [PubMed] [Google Scholar]
- 27.Wilhelm SM, Stowe NT, Robinson AV, Schulak JA. The use of the endothelin receptor antagonist, tezosentan, before or after renal ischemia protects renal function. Transplantation. 2001; 71(2): 211–6 http://www.ncbi.nlm.nih.gov/pubmed/11213061. doi: 10.1097/00007890-200101270-00007 [DOI] [PubMed] [Google Scholar]
- 28.Söderberg O, Gullberg M, Jarvius M, Ridderstråle K, Leuchowius KJ, Jarvius J, Wester K, Hydbring P, Bahram F, Larsson LG, Landegren U. Direct observation of individual endogenous protein complexes in situ by proximity ligation. Nat Methods. 2006; 3(12): 995–1000 http://www.ncbi.nlm.nih.gov/pubmed/17072308. doi: 10.1038/nmeth947 [DOI] [PubMed] [Google Scholar]
- 29.Niiro N, Ikebe M. Zipper-interacting protein kinase induces Ca(2+)-free smooth muscle contraction via myosin light chain phosphorylation. J Biol Chem. 2001; 276(31): 29567–74 http://www.ncbi.nlm.nih.gov/pubmed/11384979. doi: 10.1074/jbc.M102753200 [DOI] [PubMed] [Google Scholar]
- 30.Deng JT, Van Lierop JE, Sutherland C, Walsh MP. Ca2+-independent smooth muscle contraction. a novel function for integrin-linked kinase. J Biol Chem. 2001; 276(19): 16365–73 http://www.ncbi.nlm.nih.gov/pubmed/11278951. doi: 10.1074/jbc.M011634200 [DOI] [PubMed] [Google Scholar]
- 31.Walsh MP. Vascular smooth muscle myosin light chain diphosphorylation: mechanism, function, and pathological implications. IUBMB Life. 2011; 63(11): 987–1000 http://www.ncbi.nlm.nih.gov/pubmed/21990256. doi: 10.1002/iub.527 [DOI] [PubMed] [Google Scholar]
- 32.Takeya K, Wang X, Sutherland C, Kathol I, Loutzenhiser K, Loutzenhiser RD, Walsh MP. Involvement of myosin regulatory light chain diphosphorylation in sustained vasoconstriction under pathophysiological conditions. J Smooth Muscle Res. 2014; 50: 18–28 http://www.ncbi.nlm.nih.gov/pubmed/24770446. doi: 10.1540/jsmr.50.18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ikebe M, Hartshorne DJ. Phosphorylation of smooth muscle myosin at two distinct sites by myosin light chain kinase. J Biol Chem. 1985; 260(18): 10027–31 http://www.ncbi.nlm.nih.gov/pubmed/3839510. [PubMed] [Google Scholar]
- 34.Tanaka T, Sobue K, Owada MK, Hakura A. Linear relationship between diphosphorylation of 20 kDa light chain of gizzard myosin and the actin-activated myosin ATPase activity. Biochem Biophys Res Commun. 1985; 131(2): 987–93 http://linkinghub.elsevier.com/retrieve/pii/0006291X85913373. doi: 10.1016/0006-291X(85)91337-3 [DOI] [PubMed] [Google Scholar]
- 35.Ikebe M, Koretz J, Hartshorne DJ. Effects of phosphorylation of light chain residues threonine 18 and serine 19 on the properties and conformation of smooth muscle myosin. J Biol Chem. 1988; 263(13): 6432–7 http://www.ncbi.nlm.nih.gov/pubmed/2966156. [PubMed] [Google Scholar]
- 36.Bresnick AR, Wolff-Long VL, Baumann O, Pollard TD. Phosphorylation on threonine-18 of the regulatory light chain dissociates the ATPase and motor properties of smooth muscle myosin II. Biochemistry. 1995; 34(39): 12576–83 http://www.ncbi.nlm.nih.gov/pubmed/7548006. doi: 10.1021/bi00039a012 [DOI] [PubMed] [Google Scholar]
- 37.Umemoto S, Bengur AR, Sellers JR. Effect of multiple phosphorylations of smooth muscle and cytoplasmic myosins on movement in an in vitro motility assay. J Biol Chem. 1989; 264(3): 1431–6 http://www.ncbi.nlm.nih.gov/pubmed/2521481. [PubMed] [Google Scholar]
- 38.Obara K, Nishizawa S, Koide M, Nozawa K, Mitate A, Ishikawa T, Nakayama K. Interactive role of protein kinase C-δ with rho-kinase in the development of cerebral vasospasm in a canine two-hemorrhage model. J Vasc Res. 2005; 42(1): 67–76 http://www.ncbi.nlm.nih.gov/pubmed/15637442. doi: 10.1159/000083093 [DOI] [PubMed] [Google Scholar]
- 39.Katsumata N, Shimokawa H, Seto M, Kozai T, Yamawaki T, Kuwata K, Egashira K, Ikegaki I, Asano T, Sasaki Y, Takeshita A. Enhanced myosin light chain phosphorylations as a central mechanism for coronary artery spasm in a swine model with interleukin-1β. Circulation. 1997; 96(12): 4357–63 http://www.ncbi.nlm.nih.gov/pubmed/9416904. doi: 10.1161/01.CIR.96.12.4357 [DOI] [PubMed] [Google Scholar]
- 40.Shimokawa H, Seto M, Katsumata N, Amano M, Kozai T, Yamawaki T, Kuwata K, Kandabashi T, Egashira K, Ikegaki I, Asano T, Kaibuchi K, Takeshita A. Rho-kinase-mediated pathway induces enhanced myosin light chain phosphorylations in a swine model of coronary artery spasm. Cardiovasc Res. 1999; 43(4): 1029–39 http://www.ncbi.nlm.nih.gov/pubmed/10615430. doi: 10.1016/S0008-6363(99)00144-3 [DOI] [PubMed] [Google Scholar]
- 41.Harada T, Seto M, Sasaki Y, London S, Luo Z, Mayberg M. The time course of myosin light-chain phosphorylation in blood-induced vasospasm. Neurosurgery. 1995; 36(6): 1178–82 discussion 1182–3 http://www.ncbi.nlm.nih.gov/pubmed/7644000. doi: 10.1227/00006123-199506000-00018 [DOI] [PubMed] [Google Scholar]
- 42.Seto M, Yano K, Sasaki Y, Azuma H. Intimal hyperplasia enhances myosin phosphorylation in rabbit carotid artery. Exp Mol Pathol. 1993; 58(1): 1–13 http://www.ncbi.nlm.nih.gov/pubmed/8454033. doi: 10.1006/exmp.1993.1001 [DOI] [PubMed] [Google Scholar]
- 43.Cho YE, Ahn DS, Morgan KG, Lee YH. Enhanced contractility and myosin phosphorylation induced by Ca(2+)-independent MLCK activity in hypertensive rats. Cardiovasc Res. 2011; 91(1): 162–70 http://www.ncbi.nlm.nih.gov/pubmed/21378385. doi: 10.1093/cvr/cvr043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sutherland C, Walsh MP. Myosin regulatory light chain diphosphorylation slows relaxation of arterial smooth muscle. J Biol Chem. 2012; 287(29): 24064–76 http://www.ncbi.nlm.nih.gov/pubmed/22661704. doi: 10.1074/jbc.M112.371609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wilhelm SM, Simonson MS, Robinson AV, Stowe NT, Schulak JA. Endothelin up-regulation and localization following renal ischemia and reperfusion. Kidney Int. 1999; 55(3): 1011–8 http://www.ncbi.nlm.nih.gov/pubmed/10027938. doi: 10.1046/j.1523-1755.1999.0550031011.x [DOI] [PubMed] [Google Scholar]
- 46.Nishida M, Ieshima M, Konishi F, Yamashita J, Takaoka M, Matsumura Y. Role of endothelin B receptor in the pathogenesis of ischemic acute renal failure. J Cardiovasc Pharmacol. 2002; 40(4): 586–93 http://www.ncbi.nlm.nih.gov/pubmed/12352321. doi: 10.1097/00005344-200210000-00012 [DOI] [PubMed] [Google Scholar]