Abstract
Pulmonary arterial hypertension (PAH) is defined as an intractable disease characterized by a progressive elevation of pulmonary vascular resistance (PVR) and pulmonary arterial pressure (PAP), leading to right heart failure and premature death. The five-year survival rate after diagnosis is approximately 57%. Although extensive research has identified some factors associated with the cause of PAH, the etiology and pathogenesis remain unclear. In addition to Ca2+ channel blockers (nifedipine, diltiazem), three categories of drug have been developed for the treatment of PAH based on the pathological mechanisms: prostacyclin and its analogues (epoprostenol, treprostinil, iloprost), endothelin receptor antagonists (bosentan, ambrisentan), and phosphodiesterase type 5 inhibitors (sildenafil, tadalafil). However, screening of novel types of drug acting on the signal pathway associated with the pathological mechanism underlying PAH is ongoing. We recently found that the extracellular Ca2+-sensing receptor (CaSR), which belongs to family C of the G protein-coupled receptor (GPCR) superfamily, is upregulated in pulmonary arterial smooth muscle cells (PASMCs) from patients with idiopathic PAH (IPAH). The upregulated CaSR is necessary for the enhanced Ca2+ signaling and the augmented cell proliferation in PASMCs from IPAH patients. Most importantly, blockage of CaSR with an antagonist, NPS2143, prevents the development of pulmonary hypertension and right ventricular hypertrophy in animal models of pulmonary hypertension. The use of calcilytics, antagonists of CaSR, may be a novel therapeutic approach for PAH patients.
Keywords: Ca2+-sensing receptor, pulmonary hypertension, pulmonary artery, smooth muscle, calcilytics
Introduction
Pulmonary arterial hypertension (PAH) is caused by functional and structural changes in the pulmonary vasculature that can lead to increased pulmonary vascular resistance (PVR) and pulmonary arterial pressure (PAP). The elevated PAP induces extensive changes in heart structure followed by right heart failure, and eventually death. PAH is clinically defined by PAP chronically increasing due to various causes and resting mean PAP being ≥25 mmHg. The total number of PAH patients is estimated to by approximately 100,000 globally. The five-year survival rate of this condition after diagnosis is ∼57%. The mean duration between symptom onset and diagnostic catheterization is 2.8 years. In the United States, the mean age of PAH patients was 36.4 years in the 1980s, but it was 53.0 years in 2007 due to improved diagnosis, treatment, and management (1, 2).
Clinical classification of pulmonary hypertension
Pulmonary hypertension falls into five diagnostic classifications in terms of its pathogenesis (3, 4) (Table 1). Group 1 is PAH that may occur in different clinical conditions depending on the associated disease. This subgroup includes patients with idiopathic PAH (IPAH) corresponding to sporadic disease in which there is neither family history of PAH nor an identified risk factor, as well as patients with heritable PAH (HPAH) with germline mutations in the bone morphogenetic protein receptor type 2 (BMPR2), activin receptor-like kinase type 1 (ALK1), and endoglin genes. PAH can also be induced by some drugs and chemicals. In addition, PAH associated with connective tissue disease (CTD), human immunodeficiency virus (HIV) infection, portal hypertension, and congenital heart disease (CHD) represents an important clinical subgroup. Group 2 is pulmonary hypertension with left heart disease including left-sided ventricular or valvular disease that may produce an increase in left arterial pressure, with passive backward transmission of the pressure leading to increased PAP. Group 3 is pulmonary hypertension due to lung diseases and/or hypoxia. The predominant cause in this group is alveolar hypoxia as a result of lung disease, impaired control of breathing, or chronic exposure to high altitude. Group 4 is chronic thromboembolic pulmonary hypertension (CTEPH). The incidence of CTEPH is unclear, but it occurs in ∼4% of patients after an acute pulmonary embolism. Group 5 consists of several forms of pulmonary hypertension for which the etiology is unclear and/or multifactorial.
Table 1. Clinical classification of pulmonary hypertension (Dana Point, 2008).
Drug therapy for PAH
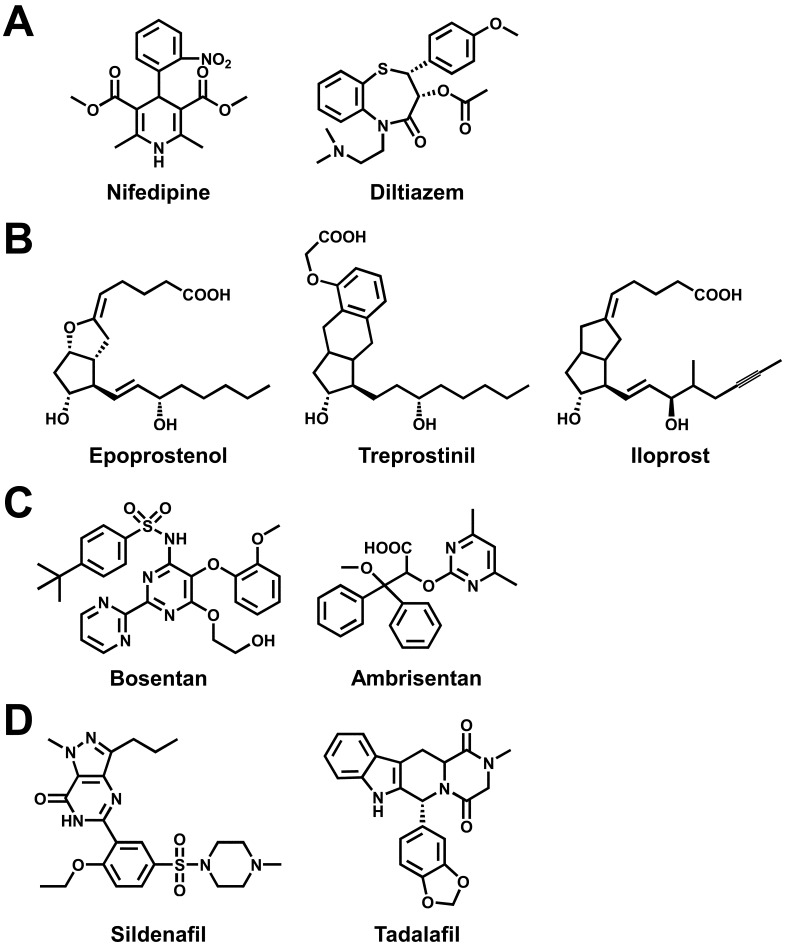
On the basis of our understanding of the pathological mechanisms of PAH, drug therapy for PAH has progressed in recent years via the development of several specific drugs that offer an effective alternative to voltage-dependent Ca2+ channel blockers such as nifedipine and diltiazem (4) (Fig. 1A). Epoprostenol (prostacyclin, also known as prostaglandin I2, PGI2; Fig. 1B), a potent vasodilator produced by vascular endothelium, was the first drug approved by the U.S. Food and Drug Administration (FDA) for the treatment of PAH. Epoprostenol improves exercise capacity, hemodynamics, and quality of life (QOL), as well as improving survival in PAH patients. Two prostacyclin analogues, treprostinil and iloprost, are also available for PAH treatment. In the pathophysiology of PAH, endothelin plays a key role for exerting vasoconstrictor and mitogenic effects by binding to endothelin receptors in pulmonary arterial smooth muscles. Bosentan (Fig. 1C), an endothelin receptor antagonist, improves exercise capacity, hemodynamics, and clinical deterioration. There is also ambrisentan, a selective endothelin receptor (ETA) antagonist for the treatment of PAH. In addition, two phosphodiesterase type 5 (PDE5) inhibitors, sildenafil and tadalafil (Fig. 1D), which increase cGMP and have been used to treat erectile dysfunction (ED), are also approved for PAH treatment. Specific pulmonary vasodilators have now been approved for treatment of PAH; moreover, numerous potential candidates either have been submitted for approval or are in development. Despite recent major therapeutic advances, no current treatments of PAH can cure this life-threatening disease.
Fig. 1.
Chemical structures of drugs for pulmonary arterial hypertension. In addition to Ca2+ channel blockers (A), prostacyclin and its analogues (B), endothelin receptor antagonists (C), and phosphodiesterase type 5 (PDE5) inhibitors (D) are now widely used for the treatment of pulmonary arterial hypertension (PAH).
Enhanced Ca2+ signaling in pulmonary myocytes from IPAH patients
The pathogenic mechanisms involved in the pulmonary vascular abnormalities including sustained pulmonary vasoconstriction and vascular remodeling in IPAH patients remain unclear. Sustained vasoconstriction and vascular remodeling owing to proliferation of pulmonary arterial smooth muscle cells (PASMCs) are major pathogenic events that lead to early morbidity and mortality. Excessive PASMC proliferation is predominantly caused by increased production of vasoconstrictive and mitogenic agonists, and increased proliferation and/or decreased apoptosis of PASMCs. An increase in cytosolic Ca2+ concentration ([Ca2+]cyt) in PASMCs is a major trigger for pulmonary vasoconstriction and an important stimulus for PASMC migration and proliferation, which subsequently cause pulmonary vascular remodeling followed by an increase in PVR. Enhanced Ca2+ signals have been reported in PASMCs from IPAH patients: 1) increase in [Ca2+]cyt at rest, 2) enhanced store-operated Ca2+ entry (SOCE), and 3) upregulation of receptor-operated Ca2+ entry (ROCE) (5,6,7). The cellular and molecular mechanisms involved in the cause and development of IPAH are, however, not fully explained (8). Moreover, an unknown Ca2+ influx pathway differing from voltage-dependent Ca2+ channels and Ca2+-sensitive ion channels (9,10,11) has been suggested to be involved in the pathological mechanism underlying IPAH.
Upregulation of Ca2+-sensing receptor in IPAH
Recently, we found that extracellular application of Ca2+ induced a large increase in [Ca2+]cyt in PASMCs from IPAH patients, but not in PASMCs from normal subjects and CTEPH patients (12). The extracellular Ca2+-induced [Ca2+]cyt increase was concentration-dependent, with an EC50 of 1.22 mM (Fig. 2A). The application of spermine (an activator for the Ca2+-sensing receptor, CaSR) elicited an increase in [Ca2+]cyt in IPAH-PASMCs. In addition, NPS R568 (a CaSR agonist) enhanced, whereas NPS2143 (a CaSR antagonist) attenuated the extracellular Ca2+-induced [Ca2+]cyt rise in IPAH-PASMCs. The protein expression level of CaSR in PASMCs and lung tissues from IPAH patients was greater than that from normal subjects (Fig. 2B). Downregulation of CaSR in IPAH-PASMCs with siRNA inhibited the extracellular Ca2+-induced increase in [Ca2+]cyt and attenuated cell proliferation. On the other hand, overexpression of CaSR in normal PASMCs augmented the extracellular Ca2+-induced [Ca2+]cyt rise and enhanced cell proliferation. These lines of evidence indicate that functionally upregulated CaSR and subsequently augmented CaSR-mediated [Ca2+]cyt increase contribute to the enhanced Ca2+ signaling and excessive cell proliferation in PASMCs from IPAH patients. Enhanced function of CaSR through intracellular Ca2+ signaling is a newly identified pathogenic mechanism involved in the initiation and progression of pulmonary vascular remodeling in patients with IPAH.
Fig. 2.
Enhanced Ca2+-sensing receptor function in idiopathic pulmonary arterial hypertension. (A) Representative traces of [Ca2+]cyt changes in response to extracellular application of 0.1, 0.5, 1.1, 2.2, and 10 mM Ca2+ and the dose response curves in pulmonary arterial smooth muscle cells (PASMCs) from normal subjects and patients with idiopathic pulmonary arterial hypertension (IPAH) (n=57∼183 cells). The EC50 for extracellular Ca2+-induced [Ca2+]cyt increase in IPAH-PASMCs is 1.22 mM. (B) Western blot analysis on Ca2+-sensing receptor (CaSR) in membrane proteins isolated from PASMCs and lung tissues of normal subjects (Nor) and IPAH patients. The CaSR protein levels were normalized to the β-tubulin level. [modified from ref. 12].
Structure of CaSR
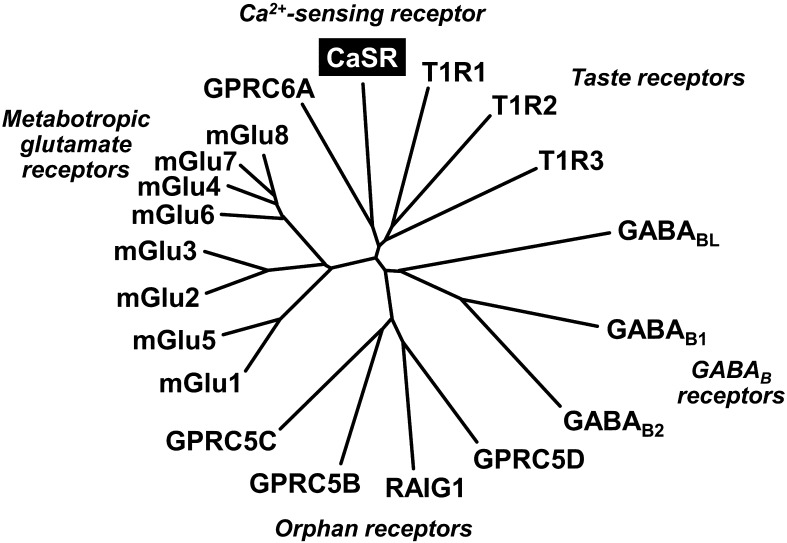
CaSR was originally cloned from bovine parathyroid glands, which secrete parathyroid hormone (PTH), an extracellular Ca2+-elevating hormone (13). CaSR (also known as GPRC2A) belongs to family C of the G protein-coupled receptor (GPCR) superfamily, which includes the metabotropic glutamate receptors (mGluRs), GABAB receptors, and taste receptors as well as orphan receptors (14, 15) (Fig. 3). Human CaSR consists of 1,078 amino acids (GenBank accession number: NM_000388); it forms a homodimeric configuration. Like the other members, CaSR constitutively presents as a large N-terminal extracellular domain containing 612 amino acid residues, a central core of 250 amino acids with seven transmembrane domains that are a hallmark of the GPCR superfamily, and an intracellular C-terminal tail of 216 amino acids (16, 17). CaSR is capable of coupling to several G-proteins including Gq that activates phospholipase C (PLC) and the inositol-1,4,5-trisphosphate (IP3) cascade, and Gi that inhibits adenylate cyclase (AC) and the cAMP pathway. CaSR also regulates multiple signaling pathways such as mitogen-activated protein kinase (MAPK) cascades: MAPK kinase (MEK), extracellular signal-regulated kinases (ERK1/2), and c-Jun N-terminal kinase (JNK) (17, 18).
Fig. 3.
Phylogenetic tree of family C of G-protein-coupled receptors. The Ca2+-sensing receptor (CaSR, also known as GPRC2A) belongs to family C of the G protein-coupled receptor (GPCR) superfamily, which includes the metabotropic glutamate receptors (mGluRs), GABAB receptors, and taste receptors, as well as orphan receptors. The structure of CaSR consists of a large N-terminal extracellular domain, a central core with seven transmembrane domains, and an intracellular C-terminal tail. [adapted from refs. 14 and 15].
Physiological roles of CaSR
Change in [Ca2+]cyt plays a pivotal role in physiological functions including neurotransmission, muscle contraction, transcriptional regulation, hormonal secretion, and brain functions. CaSR in the plasma membrane is a key regulator that senses changes in [Ca2+] in the extracellular space to maintain extracellular Ca2+ homeostasis (16,17,18). In parathyroid glands, sensing an elevation of [Ca2+] in the serum through CaSR triggers the reduction of PTH secretion and results in maintenance of the normal level of extracellular Ca2+. In addition, CaSR is distributed in the kidney, bone, gastrointestinal tract, brain, and heart, where it plays crucial roles in extracellular Ca2+ homeostasis. CaSR is also expressed in vascular smooth muscle and endothelium. Because activation of CaSR in vascular myocytes increases [Ca2+]cyt and subsequently induces vasoconstriction, CaSR is thought to be involved in the regulation of myogenic tone, peripheral vascular resistance, and arterial blood pressure. Loss-of-function mutations in the CaSR gene can lead to familial hypocalciuric hypercalcemia (FHH) and neonatal severe hyperparathyroidism (NSHPT). On the other hand, gain-of-function mutations cause autosomal dominant hypocalcemia (ADH) and Bartter syndrome type V (16, 18).
Modulators of CaSR
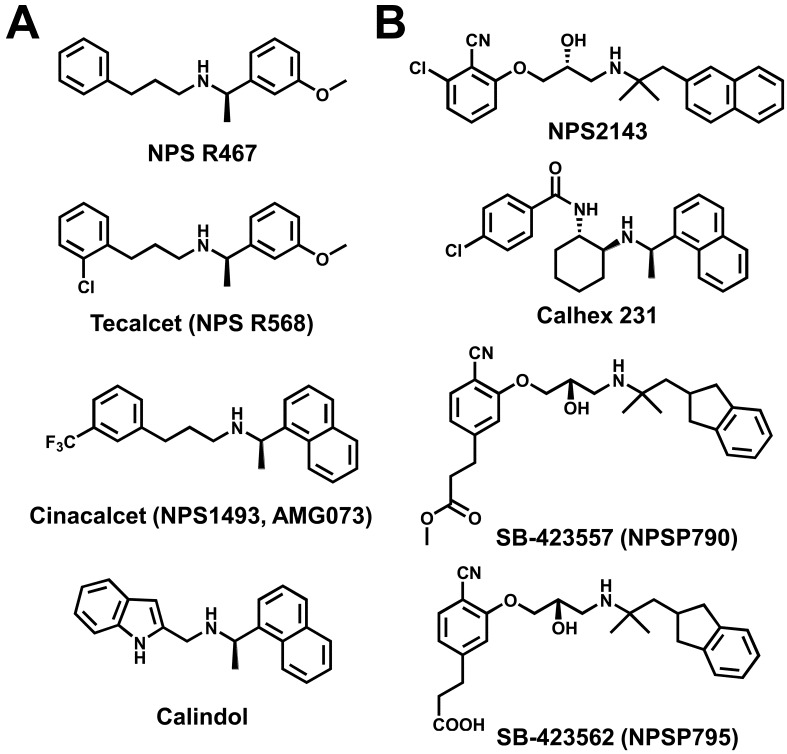
CaSR can be activated by several endo/exogenous ligands including polyvalent cations (Ca2+, Mg2+, Gd3+), polyamines (spermine, spermidine, putrescine), polypeptides (amyloid-β peptide, γ-glutamyl peptides), aminoglycoside antibiotics (neomycin, gentamicin, kanamycin), and amino acids (phenylalanine, tryptophan, glutamate). In addition, synthetic activators of CaSR, or calcimimetics (NPS R467, NPS R568, cinacalcet, calindol; Fig. 4A), and antagonists of CaSR, or calcilytics (NPS2143, Calhex 231, SB-423557, SB-423562; Fig. 4B), affect CaSR function (14, 15, 17, 18). Calcimimetics are positive allosteric modulators of CaSR, leading to the suppression of PTH secretion from the parathyroid glands in patients with overactivity of these glands. Tecalcet (also known as NPS R568) was developed for primary and secondary hyperthyroidism but dropped during clinical trials. Cinacalcet (also known as NPS1493 and AMG073) has been initially approved by the U.S. FDA for treating two conditions: secondary hyperparathyroidism in patients with chronic kidney disease (CKD) who are undergoing dialysis and hypercalcemia in patients with parathyroid cancer. It is the only calcimimetic currently approved for use in humans. On the other hand, calcilytics are negative allosteric modulators that indirectly stimulate PTH secretion through a decrease in CaSR activity. Calcilytics are potential drug candidates for the treatment of osteoporosis and other bone metabolism diseases. SB-423562 (also known as NPSP795) and its orally bioavailable precursor, SB-423557 (NPSP790), have recently undergone clinical validation as a treatment for osteoporosis or hypocalcemia due to the increased sensitivity of CaSR to extracellular Ca2+ (14, 15).
Fig. 4.
Chemical structures of modulators for Ca2+-sensing receptor. (A) The chemical structure of calcimimetics, or positive allosteric modulators, NPS R467, tecalcet (also known as NPS R568), cinacalcet (NPS1493, AMG073), and calindol. Tecalcet was developed for primary/secondary hyperthyroidism but dropped during clinical trials. Cinacalcet is used for uremic secondary hyperparathyroidism. (B) The chemical structure of calcilytics, or negative allosteric modulators, NPS2143, Calhex 231, SB-423562 (NPSP795), and SB-423557 (NPSP790). SB-423562 and SB-423557, an orally bioavailable precursor for SB-423562, are currently in clinical trials for osteoporosis.
Calcilytics block development of pulmonary hypertension
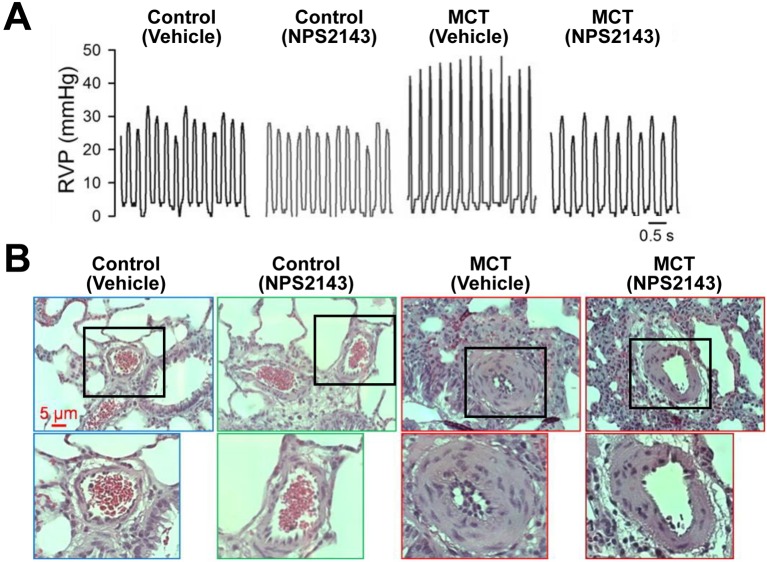
Similar to PASMCs from IPAH patients, upregulation of CaSR and enhanced CaSR-mediated increase in [Ca2+]cyt were observed in PASMCs isolated from two different animal models of pulmonary hypertension: monocrotaline (MCT)-induced pulmonary hypertension in rats and hypoxia-induced pulmonary hypertension (HPH) in mice (12, 19, 20). Finally, intraperitoneal injection of the calcilytic NPS2143 prevented the development of pulmonary hypertension and right ventricular hypertrophy in experimental animal models of pulmonary hypertension. Pulmonary hypertension characterized by increased right ventricular systolic pressure (RVSP; Fig. 5A) and vascular remodeling (Fig. 5B) in animal models, was improved by NPS2143. Pharmacological blockade of the upregulated CaSR with calcilytics may be a novel therapeutic approach for PAH patients who do not respond to the conventional drug therapy.
Fig. 5.
A calcilytic, NPS2143, rescues development of pulmonary hypertension. (A) Representative records of right ventricular pressure (RVP) in control and monocrotaline-induced pulmonary hypertensive (MCT) rats treated with vehicle or NPS2143. (B) Representative hematoxylin and eosin (H&E) images of small pulmonary arteries in control and MCT rats treated with vehicle or NPS2143. The magnified images of area surrounded by a black border in upper panels are shown in lower panels. [modified from ref. 12].
Conclusion
We recently found that CaSR is upregulated in PASMCs isolated from patients with IPAH and animals with experimental pulmonary hypertension (12, 19, 20). The upregulated CaSR is necessary for the enhanced extracellular Ca2+-induced increase in [Ca2+]cyt and the augmented cell proliferation in IPAH-PASMCs. Pharmacological blockade of CaSR with the calcilytic, NPS2143, inhibits the CaSR-mediated rise in [Ca2+]cyt and attenuates the development of experimental pulmonary hypertension in animal models. Functionally upregulated CaSR in PASMCs may play a novel pathogenic mechanism underlying sustained pulmonary vasoconstriction and excessive pulmonary vascular remodeling in IPAH patients. Currently, in addition to Ca2+ channel blockers (nifedipine, diltiazem), three therapeutic classes are widely used for the treatment of PAH: prostacyclins (epoprostenol, treprostinil, iloprost), endothelin receptor antagonists (bosentan, ambrisentan), and PDE inhibitors (sildenafil, tadalafil). Targeting CaSR in PASMCs, especially the calcilytic, may help develop a novel therapeutic approach for PAH.
Conflict of interest
I declare that I have no conflict of interest.
Acknowledgments
I would like to thank Dr. Hisao Yamamura (Nagoya City University, Nagoya, Japan) and Dr. Jason X.-J. Yuan and his lab members (University of Illinois at Chicago, Chicago, USA) for helpful advice. This work was supported in part by a Grant-in-Aid for Young Scientists (B) (25860068) from the Japan Society for the Promotion of Science.
References
- 1.Rich S, Dantzker DR, Ayres SM, Bergofsky EH, Brundage BH, Detre KM, Fishman AP, Goldring RM, Groves BM, Koerner SK, Levy PC, Reid LM, Vreim CE, Williams GW. Primary pulmonary hypertension. A national prospective study. Ann Intern Med. 1987; 107(2): 216–23. doi: 10.7326/0003-4819-107-2-216 [DOI] [PubMed] [Google Scholar]
- 2.Badesch DB, Raskob GE, Elliott CG, Krichman AM, Farber HW, Frost AE, Barst RJ, Benza RL, Liou TG, Turner M, Giles S, Feldkircher K, Miller DP, McGoon MD. Pulmonary arterial hypertension: baseline characteristics from the REVEAL Registry. Chest. 2010; 137(2): 376–87. doi: 10.1378/chest.09-1140 [DOI] [PubMed] [Google Scholar]
- 3.Simonneau G, Robbins IM, Beghetti M, Channick RN, Delcroix M, Denton CP, Elliott CG, Gaine SP, Gladwin MT, Jing ZC, Krowka MJ, Langleben D, Nakanishi N, Souza R. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol. 2009; 54 (1 Suppl): S43–54. doi: 10.1016/j.jacc.2009.04.012 [DOI] [PubMed] [Google Scholar]
- 4.Montani D, Günther S, Dorfmüller P, Perros F, Girerd B, Garcia G, Jaïs X, Savale L, Artaud-Macari E, Price LC, Humbert M, Simonneau G, Sitbon O. Pulmonary arterial hypertension. Orphanet J Rare Dis. 2013; 8(1): 97. doi: 10.1186/1750-1172-8-97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yu Y, Fantozzi I, Remillard CV, Landsberg JW, Kunichika N, Platoshyn O, Tigno DD, Thistlethwaite PA, Rubin LJ, Yuan JX. Enhanced expression of transient receptor potential channels in idiopathic pulmonary arterial hypertension. Proc Natl Acad Sci USA. 2004; 101(38): 13861–6. doi: 10.1073/pnas.0405908101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang S, Patel HH, Murray F, Remillard CV, Schach C, Thistlethwaite PA, Insel PA, Yuan JX. Pulmonary artery smooth muscle cells from normal subjects and IPAH patients show divergent cAMP-mediated effects on TRPC expression and capacitative Ca2+ entry. Am J Physiol Lung Cell Mol Physiol. 2007; 292(5): L1202–10. doi: 10.1152/ajplung.00214.2006 [DOI] [PubMed] [Google Scholar]
- 7.Song MY, Makino A, Yuan JX. STIM2 contributes to enhanced store-operated Ca2+ entry in pulmonary artery smooth muscle cells from patients with idiopathic pulmonary arterial hypertension. Pulm Circ. 2011; 1(1): 84–94. doi: 10.4103/2045-8932.78106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morrell NW, Adnot S, Archer SL, Dupuis J, Jones PL, MacLean MR, McMurtry IF, Stenmark KR, Thistlethwaite PA, Weissmann N, Yuan JX, Weir EK. Cellular and molecular basis of pulmonary arterial hypertension. J Am Coll Cardiol. 2009; 54 (1 Suppl): S20–31. doi: 10.1016/j.jacc.2009.04.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yamamura A, Yamamura H, Zeifman A, Yuan JX. Activity of C2+ -activated Cl channels contributes to regulating receptor- and store-operated Ca2+ entry in human pulmonary artery smooth muscle cells. Pulm Circ. 2011; 1(2): 269–79. doi: 10.4103/2045-8932.83447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ko EA, Wan J, Yamamura A, Zimnicka AM, Yamamura H, Yoo HY, Tang H, Smith KA, Sundivakkam PC, Zeifman A, Ayon RJ, Makino A, Yuan JX. Functional characterization of voltage-dependent Ca2+ channels in mouse pulmonary arterial smooth muscle cells: divergent effect of ROS. Am J Physiol Cell Physiol. 2013; 304(11): C1042–52. doi: 10.1152/ajpcell.00304.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wan J, Yamamura A, Zimnicka AM, Voiriot G, Smith KA, Tang H, Ayon RJ, Choudhury MS, Ko EA, Wang J, Wang C, Makino A, Yuan JX. Chronic hypoxia selectively enhances L- and T-type voltage-dependent Ca2+ channel activity in pulmonary artery by upregulating Cav1.2 and Cav3.2. Am J Physiol Lung Cell Mol Physiol. 2013; 305(2): L154–64. doi: 10.1152/ajplung.00313.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yamamura A, Guo Q, Yamamura H, Zimnicka AM, Pohl NM, Smith KA, Fernandez RA, Zeifman A, Makino A, Dong H, Yuan JX. Enhanced Ca2+-sensing receptor function in idiopathic pulmonary arterial hypertension. Circ Res. 2012; 111(4): 469–81. doi: 10.1161/CIRCRESAHA.112.266361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brown EM, Gamba G, Riccardi D, Lombardi M, Butters R, Kifor O, Sun A, Hediger MA, Lytton J, Hebert SC. Cloning and characterization of an extracellular Ca2+-sensing receptor from bovine parathyroid. Nature. 1993; 366(6455): 575–80. doi: 10.1038/366575a0 [DOI] [PubMed] [Google Scholar]
- 14.Bräuner-Osborne H, Wellendorph P, Jensen AA. Structure, pharmacology and therapeutic prospects of family C G-protein coupled receptors. Curr Drug Targets. 2007; 8(1): 169–84. doi: 10.2174/138945007779315614 [DOI] [PubMed] [Google Scholar]
- 15.Urwyler S. Allosteric modulation of family C G-protein-coupled receptors: from molecular insights to therapeutic perspectives. Pharmacol Rev. 2011; 63(1): 59–126. doi: 10.1124/pr.109.002501 [DOI] [PubMed] [Google Scholar]
- 16.Brown EM. Clinical lessons from the calcium-sensing receptor. Nat Clin Pract Endocrinol Metab. 2007; 3(2): 122–33. doi: 10.1038/ncpendmet0388 [DOI] [PubMed] [Google Scholar]
- 17.Magno AL, Ward BK, Ratajczak T. The calcium-sensing receptor: a molecular perspective. Endocr Rev. 2011; 32(1): 3–30. doi: 10.1210/er.2009-0043 [DOI] [PubMed] [Google Scholar]
- 18.Hofer AM, Brown EM. Extracellular calcium sensing and signalling. Nat Rev Mol Cell Biol. 2003; 4(7): 530–8. doi: 10.1038/nrm1154 [DOI] [PubMed] [Google Scholar]
- 19.Yamamura A, Yamamura H, Guo Q, Zimnicka AM, Wan J, Ko EA, Smith KA, Pohl NM, Song S, Zeifman A, Makino A, Yuan JX. Dihydropyridine Ca2+ channel blockers increase cytosolic [Ca2+] by activating Ca2+-sensing receptors in pulmonary arterial smooth muscle cells. Circ Res. 2013; 112(4): 640–50. doi: 10.1161/CIRCRESAHA.113.300897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guo Q, Huang JA, Yamamura A, Yamamura H, Zimnicka AM, Fernandez R, Yuan JX. Inhibition of the Ca2+-sensing receptor rescues pulmonary hypertension in rats and mice. Hypertens Res. 2014; 37(2): 116–24. doi: 10.1038/hr.2013.129 [DOI] [PMC free article] [PubMed] [Google Scholar]