Abstract
Migrating motor complex (MMC) is well characterized by the appearance of gastrointestinal (GI) contractions in the interdigestive state. The physiological importance of gastric MMC is a mechanical and chemical cleansing of the empty stomach in preparation for the next meal. MMC cycle is mediated via the interaction between motilin and 5-hydroxytryptamine (5-HT) by the positive feedback mechanism in conscious dogs. Luminal administration of 5-HT initiates duodenal phase II and phase III with a concomitant increase of plasma motilin release. Duodenal 5-HT concentration is increased during gastric phase II and phase III. Intravenous infusion of motilin increases luminal 5-HT content and induces phase III. 5-HT4 antagonists significantly inhibit both of gastric and intestinal phase III, while 5-HT3 antagonists inhibit only gastric phase III. These suggest that gastric MMC is regulated via vagus, 5-HT3/4 receptors and motilin, while intestinal MMC is regulated via intrinsic primary afferent neurons (IPAN) and 5-HT4 receptors. We propose the possibility that maximally released motilin by a positive feedback depletes 5-HT granules in the duodenal EC cells, resulting in no more contractions. Stress is highly associated with the pathogenesis of functional dyspepsia (FD). Acoustic stress attenuates gastric phase III without affecting intestinal phase III in conscious dogs, via reduced vagal activity. Subset of FD patients shows reduced vagal activity and impaired gastric phase III. The impaired gastric MMC may aggravate dyspeptic symptoms following a food ingestion. Maintaining MMC cycle in the interdigestive state is an important factor to prevent the postprandial dyspeptic symptoms.
Keywords: autonomic nerves, enterochromaffin cells, motilin, serotonin
Introduction
Gastrointestinal (GI) motility in the fasted state is a cyclical phenomenon called the migrating motor complex (MMC). There are four phases of a normal MMC cycle in humans and dogs. Phase I is a quiescent period with virtually no contractions. Phase II consists of intermittent, irregular low-amplitude contractions. Phase III consists of short burst of regular high-amplitude contractions. Phase IV represents a short transition period back to the quiescence of phase I (1). Phase III contractions periodically occur every 90–120 min in humans and dogs.
Plasma motilin level is closely associated with the appearance of gastric phase III contractions. Plasma motilin levels vary in a cyclic fashion and its peaks regularly occur during the period of gastric phase III in dogs (2, 3) and humans (4, 5). Motilin administration causes gastric phase III contractions in dogs (2, 6) and humans (5). The mechanism of MMC still remains unclear and several serious questions have been raised.
In rats, motilin and motilin receptors have not been found and motilin administration fails to affect GI motility in rats (7). In 1999, ghrelin was discovered as the endogenous ligand for the growth hormone secretagogue receptor (GHS-R) from rat stomach (8). Because of a structural resemblance to motilin, ghrelin is known as a motilin-related peptide (9, 10). As it is rather difficult to distinguish 3 phases in rats and mice, these phases are called as phase I-like contractions and phase III-like contractions (11, 12). Ghrelin administration elicits phase III-like contractions of the stomach in rats (11, 13) and mice (12). Plasma ghrelin levels are highly associated with the occurrence of phase III-like contractions of the rat stomach (13). Spontaneous phase III-like contractions of the antrum are abolished by a GHS-R antagonist (11, 13). These suggest that the spontaneous phase III-like contractions of the antrum are mediated via ghrelin in rats (13).
As ghrelin fails to cause any phase III contractions of the dog stomach (14), action of ghrelin and motilin in mediating interdigestive gastric contractions are different among humans, dogs and rodents. It is widely accepted that motilin regulates gastric phase III contractions in dogs and humans, while ghrelin regulates gastric phase III-like contractions in rats and mice.
This review article focused on the mechanism of MMC associated with motilin in humans and dogs.
The mechanism of motilin release
The duodenum, which stores motilin, plays an important role to initiate gastric MMC in dogs and humans. Motilin-immunoreactive cells are most frequent in the duodenal and jejunal mucosa and are concentrated in the deeper portion of the crypt of the human and dog intestine. No motilin cells are found in the stomach, colon, and rectum (15).
After duodenectomy, no obvious phase III contractions are seen in the dog antrum. The plasma motilin concentration does not fluctuate and remains low after duodenectomy. On the other hand, the contractile response of the stomach to exogenous motilin after duodenectomy is similar to that of intact dogs (16). These indicate that released motilin from the duodenal mucosa and upper jejunum play an important role to mediate gastric phase III.
The presence of nutrient in the duodenum strongly suppresses the endogenous release of motilin. In an interdigestive state, luminal acidification and bile regulate motilin release from the duodenal mucosa (17). The existence of muscarinic receptors is demonstrated in motilin producing cells (18). Muscarinic receptors are responsible for motilin release from motilin-producing cells in perifusion system of the canine duodenum (19). Although the precise mechanism of motilin release still remains unclear, the release of motilin is likely to be controlled by non-vagal cholinergic innervation in normal conditions (3, 20). Truncal vagotomy does not influence the intermittent fluctuation or concentration of plasma motilin in the fasting state (21). Similarly, the spontaneous fluctuations in the plasma motilin concentration are not influenced by vago-sympathetic nerve blockade (3).
Exogenously-administered motilin stimulates the endogenous motilin release (22). The peak of plasma motilin level is observed during the late phase of gastric phase III or after finishing gastric phase III (23). Thus, Sarna et al. proposed that endogenous motilin does not initiate spontaneous phase III. Instead, phase III contractions release motilin. They suggested that a positive feedback mechanism might exist because phase IIIcontractions released motilin and that motilin, in turn, potentiates contractions (24).
Site of action of motilin
It has been controversial whether motilin acts through intrinsic neurons (25), extrinsic neurons (20), or smooth muscles (26). Motilin receptors are present in the myenteric plexus, mucosa and muscle cells of GI tract (27). These receptors may mediate its pharmacological and physiological actions. Motilin-induced contractions are not inhibited by tetrodotoxin of the rabbit antrum in vitro (26), suggesting the direct action on the smooth muscle cells. In contrast, others demonstrated that atropine and hexamethonium attenuate motilin-induced contractions of the isolated dog stomach (25). This indicates that intramural cholinergic pathway is involved in mediating motilin-induced contractions of the dog stomach in vitro.
Ex vivo isolated stomach does not show cyclic MMC pattern. Motilin-induced contractions are much less potent than that of in vivo studies (25). Gastric phase III, but not intestinal phase III, is abolished by blockade of the cervical vago-sympathetic nerve trunk in conscious dogs (3). As sympathetic receptor blockers do not affect the inhibitory effect of vagal blockade (28), gastric phase III seems to be regulated by vagus nerve. Spontaneous phase III contractions after vagotomy are also less potent than that of before vagotomy (29). These suggest that motilin-induced gastric phase III is vagal dependent in physiological conditions (20).
Motilin-induced gastric phase III is antagonized by the systemic treatment with 5-hydroxytryptamine 3 (5-HT3) receptor antagonists in dogs (30, 31) and humans (32). Spontaneous gastric phase III is also antagonized by 5-HT3 antagonists (30, 31), indicating the mediation of endogenous releases of 5-HT.
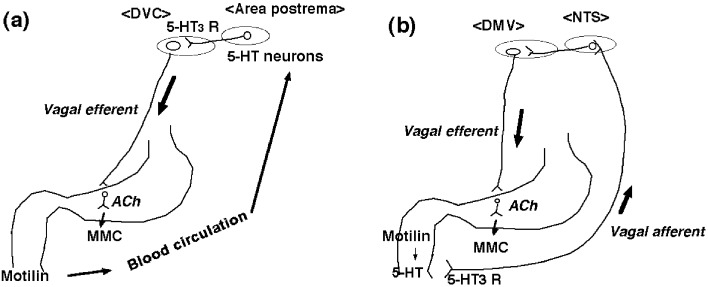
The area postrema is very rich in fenestrated capillaries, and supplied with numerous neurons including 5-HT neurons in the perivascular spaces around the capillaries and linked to the dorsal vagal complex (DVC) (33). Itoh (20) proposed the possibility that peripherally released motilin may stimulate motilin receptors of 5-HT neurons in the area postrema. Stimulation of 5-HT neurons by motilin activates vagal efferent through 5-HT3 receptors, resulting in gastric phase III (Fig. 1a). However, the existence of motilin receptors has not been demonstrated in the area postrema.
Fig. 1.
A mechanism of motilin-induced gastric MMC suggested by Dr. Itoh (20) (a). Released motilin from the duodenal mucosa reaches at the area postrema and activates 5-HT neurons at the dorsal vagal complex (DVC). Stimulation of 5-HT neurons activates vagal efferent through 5-HT3 receptors (a). A possible interaction of motilin and 5-HT mediating gastric MMC (b). Released motilin from the duodenal mucosa stimulates the release of 5-HT from the duodenal EC cells. Released 5-HT activates 5-HT3 receptors of the vagal afferent. The sensory information is carried to the brain stem [nucleus tractus solitarius (NTS) and dorsal motor nucleus of the vagus (DMV)] and activates vagal efferent. Finally, motilin initiates gastric phase III via vago-vagal reflex (b).
Gastric MMC and intestinal MMC
Although plasma motilin level is highly associated with the appearance of gastric phase III (1). phase III contractions in the small intestine sometimes occur without a concomitant increase in plasma motilin concentration (34). Motilin antiserum inhibits the occurrence of phase III contractions in the stomach, but not in the intestine (35). After duodenectomy, no obvious phase III contractions are seen in the gastric antrum, but intestinal phase III are seen in the upper jejunum (16). Chronic vagotomy reduces gastric phase III without affecting the intestinal phase III (29). These suggest that vagal innervation regulates gastric MMC, but not intestinal MMC.
Thus, gastric MMC and intestinal MMC are thought to be controlled by different mechanisms. 5-HT3 antagonists, which inhibit gastric phase III, have no effects on intestinal phase III (30, 31). When we carefully check MMC recordings in dogs, it is obvious that duodenal phase II/ III is frequently antecedent to gastric phase II/ III (1, 36,37,38,39). However, no reasonable explanation has been shown. What is the regulator of intestinal MMC?
Mystery of MMC in GI tract
It has been a mystery how MMC is regulated periodically every 90–120 min in GI tract. As exogenous motilin stimulates endogenous release of motilin (22), a positive feedback mechanism is likely to operate when the plasma motilin concentration increases during the interdigestive state. Accordingly, an inhibitory mechanism should be present to break the positive feedback system; otherwise, endogenous release of motilin will continue.
Luminal release of 5-HT
5-HT in GI tract is involved in regulating its motility. 5-HT stimulates phase II-like contractions when administered during phase I of the canine small intestine (40). In humans, 5-HT re-uptake inhibitor (paroxetine) shortens MMC cycle and increases the propagation velocity of intestinal phase III (41). This suggests that endogenous 5-HT plays an important role to regulate intestinal MMC in humans.
While 5-HT acts as a neurotransmitter of the enteric nervous system (42), the majority of 5-HT is stored in enterochromaffin (EC) cells of epithelial cells. EC cells have been considered to release 5-HT mainly into the blood vessels and/or intrinsic nerve terminal via a basolateral border (43). In contrast, others showed that 5-HT is also released into the intestinal lumen (44,45,46,47). Electrical stimulation of the vagus nerves or duodenal acidification evokes 5-HT release from EC cells into the intestinal lumen in concentrations as high as 2 µM (46, 48, 49).
In response to luminal pressure increase, 5-HT is released into the lumen, but not into the portal circulation of the rat colon (50). Luminal release of 5-HT is also studied by immunoelectron microscopic study of the rat duodenum (51). Aggregation of secretory granules of 5-HT is located in the apical as well as basolateral cytoplasm of EC cells in basal conditions. After the increase of intraluminal pressure, 5-HT particles are scattered over the apical cytoplasmic matrix and microvilli (51). 5-HT particles, which are primarily stored in the secretory granules of EC cells, are transported into the duodenal lumen through the apical cell membrane in response to intraluminal pressure increase (51). Luminally applied 5-HT can move by passive diffusion across the intestinal wall of the guinea pig ileum (52). 5-HT can cross the intestinal wall from the mucosa to the serosa (apical-to-basolateral direction) (53). Thus, 5-HT into the intestinal lumen could reach the synaptic circuitry resulting in stimulation of 5-HT receptors located on the lamina propria and/or enteric nervous system.
It is conceivable that luminally released 5-HT from EC cells stimulates 5-HT3 receptors located on the vagal sensory fibers. Through the brain stem [nucleus tractus solitarius (NTS) and dorsal motor nucleus of the vagus (DMV)], the sensory information is transferred and the vagal efferent stimulates the release of acetylcholine from the myenteric plexus, resulting in muscle contractions (54) (Fig. 1b). 5-HT also activates enteric afferent neurons to stimulate intestinal motor function (55,56,57).
In conscious dogs, luminally administered 5-HT initiates duodenal phase II followed by gastric phase II with the concomitant increase of plasma motilin levels (39). During duodenal phase II, luminal content of 5-HT of the duodenum is increased from 29 to 59 ng/mL. Luminal content of 5-HT of the duodenum is further increased to 250 ng/mL during gastric phase III. In contrast, the luminal concentration of 5-HT of the stomach does not change during phase I–III (39). These suggest that luminal concentration of 5-HT of the duodenum, but not the stomach, may play an important role to regulate MMC in GI tract.
Interaction of motilin and 5-HT
It is highly likely that motilin-induced gastric phase III is mediated via vagal cholinergic pathways and 5-HT3 receptors. Motilin receptors are expressed in the muscle and mucosa of the human GI tract (27), while no motilin receptors have been documented in the vagus nerve or nodose ganglion. In contrast, 5-HT3 receptors are located on the nerve terminal of vagal afferent of the duodenal mucosa (58). Thus, nerve endings may be the targets for the 5-HT released from EC cells (59).
Exogenously applied motilin stimulates 5-HT release into the lumen of the duodenum in conscious dogs (39, 60). In vitro study also showed that motilin stimulates 5-HT release into the lumen of the canine jejunum (61). These findings raise the possibility that motilin initiates phase III through the release of mucosal 5-HT from the duodenum. Released 5-HT activates 5-HT3 receptors of the vagal afferent. The sensory information is carried to the brain stem [nucleus tractus solitarius (NTS) and DMV] and activates vagal efferent. Finally, motilin initiates gastric phase III via vago-vagal reflex (39) (Fig. 1b).
Mechanism mediating gastric MMC and intestinal MMC
Endogenous 5-HT affects motor activity during phase II and III and appears to be a candidate regulator of the intrinsic mechanisms governing the initiation and propagation of the intestinal MMC (40). Previous study showed that intraluminal administration of 5-HT into the duodenum causes phasic contractions in dogs (39, 62). Intra-duodenal administration of 5-HT (10−7 M) initiates duodenal phase II in conscious dogs. 5-HT (10−7 M)-induced duodenal phase II contractions migrated to the jejunum. In contrast, 10 times higher concentration of 5-HT (10−6 M) initiates duodenal phase II followed by gastric phase II/ III and plasma motilin release (39). We cannot exclude the possibility that 5-HT4 receptors of intrinsic primary afferent neurons (IPAN) are 10 times more sensitive than 5-HT3 receptors of the vagal afferent in the canine duodenum.
Gastric MMC is inhibited by a 5-HT3 receptor antagonist (ondansetron), while intestinal MMC is not affected by ondansetron. On the other hand, intestinal MMC as well as gastric MMC is significantly attenuated by a 5-HT4 receptor antagonist (GR 125,487) (39). These indicate that intestinal MMC is regulated by 5-HT4 receptors, while gastric MMC is regulated by 5-HT3 receptors and 5-HT4 receptors.
Pancreatic and biliary secretion into the duodenum is observed in the period of duodenal phase I in conscious dogs (63, 64). A recent study showed that the duodenal pressure is increases just before the occurrence of duodenal phase II (39). The accumulation of gastric, pancreatic and biliary juices may gradually increase the luminal pressure of the duodenum during phase I, which can stimulate 5-HT release from EC cells. Released 5-HT initiates duodenal phase II via 5-HT4 receptors of IPAN. Thus, the initiation of duodenal phase II is not due to the increase of plasma motilin release (65).
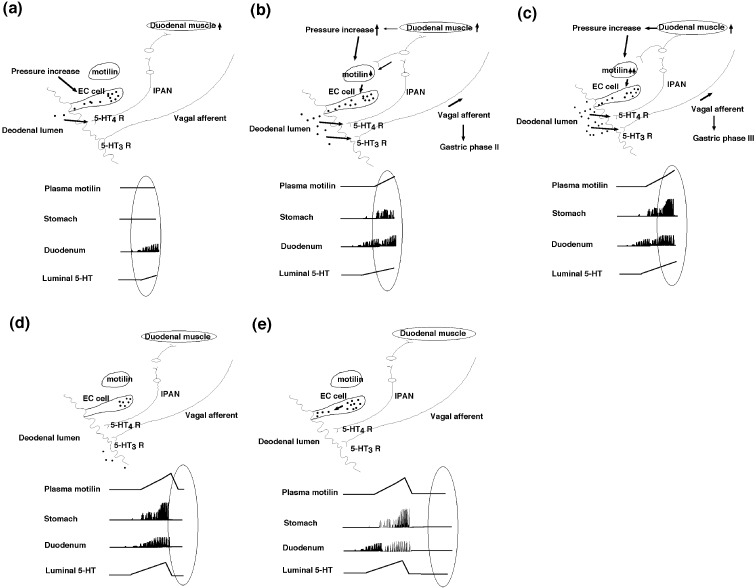
Duodenal phase II causes increase of duodenal pressure, which further stimulates the release of 5-HT. This positive circuit (pressure increase and 5-HT release) enhances the amplitude of duodenal phase II, leading to duodenal phase III. Finally, maximally increased duodenal pressure stimulates motilin release, resulting in gastric phase III (65). Briefly, initial stimulator of MMC is released 5-HT in response to intraluminal pressure increase during duodenal phase II. Maximally increased duodenal pressure stimulates motilin release from the duodenal mucosa. Released motilin further stimulates 5-HT release by a positive feedback mechanism. A large amount of 5-HT release stimulated by motilin acts on 5-HT3 receptors of the duodenal vagal afferent, in addition to 5-HT4 receptors of duodenal IPAN (65) (Fig. 2).
Fig. 2.
A possible mechanism of MMC during gastric phase I/duodenal phase II (a), gastric phase II/duodenal phase III (b), gastric/duodenal phase III (c), gastric/duodenal phase IV (d), and gastric/duodenal phase I (e). Plasma motilin levels, gastroduodenal contractions, and luminal 5-HT content in the duodenum in each phase are marked by oval-shaped circles. During phase I of GI tract, gastric, pancreatic and biliary juices are spontaneously secreted. Increased luminal pressure of the duodenum can stimulate 5-HT release from EC cells. Released 5-HT initiates duodenal phase II via 5-HT4 receptors of IPAN (a). Duodenal phase II causes increase of duodenal pressure, which further stimulates the release of 5-HT. This positive circuit (pressure increase and 5-HT release) gradually enhances the amplitude of duodenal phase II, leading to duodenal phase III. Finally, maximally increased duodenal pressure stimulates motilin release (b). Released motilin stimulates large amounts of 5-HT release which acts on 5-HT3 receptors of vagal afferent, in addition to 5-HT4 receptors of IPAN. Released motilin induces gastric phase II and III via vago-vagal reflex (c). Maximally released motilin by a positive feedback depletes 5-HT granules in the duodenal EC cells. Intraluminally released 5-HT are expelled distally during phase III of GI tract and no more present in the duodenal lumen, resulting in no more contractions (d). During phase I of GI tract, 5-HT granules are moving toward from the basolateral to apical cytoplasm, preparing for the next phase II of the duodenum (e). It may take time (for 40–60 min) to accumulate spontaneous gastric, pancreatic, and biliary secretion for increasing intaduodenal pressure, which leads to induce luminal release of 5-HT.
Therefore, released motilin induces gastric phase II/ III via vagal dependent mechanisms. This may be the reason why duodenal phase II contractions are antecedent to gastric phase II contractions. In contrast, motilin infusion did not elicit antecedent duodenal phase II contractions prior to the gastric phase II contractions (39). This suggests that motilin initiates gastric phase II contractions, but not duodenal phase II contractions.
A positive feedback mechanism is likely to operate when the plasma motilin concentration increases during the interdigestive state. Although an inhibitory mechanism should be present to break the positive feedback system, it still remains unknown how MMC is terminated. As the luminal release of 5-HT is the key factor to initiate MMC, the terminator should be involved in mediating the inhibition of 5-HT release. We propose the possibility that maximally released motilin by a positive feedback depletes 5-HT granules in the duodenal EC cells, resulting in no more contractions (Fig. 2d). Thus, released motilin, which initiates gastric phase II and III, also terminates gastric and intestinal phase III. It is our assumption that during phase I of GI tract, 5-HT granules are moving toward from the basolateral to apical cytoplasm, preparing for the next duodenal phase II (Fig. 2e).
Stress and MMC
Psychological stress plays a major role in functional GI disorders, such as irritable bowel syndrome (IBS) (66) and functional dyspepsia (FD) (67, 68). Experimental studies demonstrated that colonic motility is stimulated (69,70,71), while gastric emptying is delayed by stress (72, 73) in rodents. The inhibitory effects of stress on gastric emptying are mediated via reduced parasympathetic pathways (74, 75) or increased sympathetic pathways (73, 76) in rodents.
Acoustic stress forces to hear loud noise through earpieces in conscious dogs. Previous studies demonstrated that acoustic stress delays the occurrence of the next gastric MMC (77,78,79). Acoustic stress attenuates gastric phase III without affecting intestinal phase III. During acoustic stress, heart rate and sympathetic tone are increased, while parasympathetic tone is reduced. As gastric phase III, but not intestinal phase III, is regulated by vagal efferent, it is likely that the impaired gastric phase III induced by acoustic stress is mainly due to reduced vagal activity (80). Therefore, if we can improve reduced vagal activity associated with stress, impaired gastric phase III would be treatable. A recent study showed that somatosensory nerve stimulation restores impaired gastric phase III induced by acoustic stress in conscious dogs (80).
Clinical importance of MMC
The physiological importance of gastric MMC is a mechanical and chemical cleansing of the empty stomach in preparation for the next meal (20). When gastric phase III activity is impaired, the gastric content may stay for a longer period. Impaired gastric phase III activity may cause retention of the gastric contents and bacterial overgrowth, resulting in various symptoms. Bacterial overgrowth might be due to specific motility disorder, with namely a complete or almost complete absence of interdigestive MMC (81).
Absence of phase III activity has been found in dyspeptic Helicobacter pylori-positive patients more frequently than in those without the infection. After H. pylori eradication, the incidence of gastric phase III is not altered (82, 83). Therefore, the abnormal motility might be a predisposing condition for bacterial colonization of the gastric mucosa rather than its consequence.
FD is a symptom complex characterized by postprandial upper abdominal discomfort or pain, early satiety, nausea, vomiting, abdominal distension, bloating, and anorexia in the absence of organic disease. Approximately 50% of patients with FD have motor disorders, such as antral hypomotility, impaired accommodation reflex and gastric dysrhythmias. Studies using questionnaires showed that more than 75% of FD patients reported relationship between aggravation of symptoms and ingestion of meal (84).
In the clinical setting, abnormal motility patterns of gastric MMC have been demonstrated (85). The incidence of gastric phase III activity of the antrum is attenuated in FD patients (86,87,88). The impaired and/or irregular gastric MMC may aggravate dyspeptic symptoms following food ingestion. Dyspeptic symptoms in the postprandial state would be reduced once impaired gastric MMC in the interdigestive state is improved. Subsets of FD patients show the reduced activity of the vagus (89, 90). As vagus plays an important role to mediate gastric MMC, impaired activity of the vagus may contribute to the impaired gastric MMC in FD patients.
Conclusion
Luminal release of 5-HT from the duodenum initiates duodenal phase II followed by gastric phase III and intestinal phase III with a concomitant increase of plasma motilin release. 5-HT4 receptor antagonists inhibit both of gastric and intestinal phase III, while 5-HT3 receptor antagonists inhibit only gastric phase III. MMC cycle is mediated via the interaction between motilin and 5-HT by the positive feedback mechanism.
Acute stress attenuates gastric phase III without affecting intestinal phase III in conscious dogs. The impaired gastric phase III induced by stress is mediated via reduced vagal activity. Stress is highly associated with the pathogenesis of FD. In FD patients, incidence of gastric phase III is reduced. The impaired gastric MMC may aggravate dyspeptic symptoms following food ingestion. It is proposed that maintaining gastric MMC in the interdigestive state is an important factor to prevent the postprandial dyspeptic symptoms.
Conflict of interest
The author declares that he has no conflict of interest.
References
- 1.Itoh Z, Takeuchi S, Aizawa I, Mori K, Taminato T, Seino Y, Imura H, Yanaihara N. Changes in plasma motilin concentration and gastrointestinal contractile activity in conscious dogs. Am J Dig Dis. 1978; 23(10): 929–35. doi: 10.1007/BF01072469 [DOI] [PubMed] [Google Scholar]
- 2.Itoh Z, Honda R, Hiwatashi K, Takeuchi S, Aizawa I, Takayanagi R, Couch EF. Motilin-induced mechanical activity in the canine alimentary tract. Scand J Gastroenterol Suppl. 1976; 39: 93–110. [PubMed] [Google Scholar]
- 3.Hall KE, Greenberg GR, El-Sharkawy TY, Diamant NE. Vagal control of migrating motor complex-related peaks in canine plasma motilin, pancreatic polypeptide, and gastrin. Can J Physiol Pharmacol. 1983; 61(11): 1289–98. doi: 10.1139/y83-186 [DOI] [PubMed] [Google Scholar]
- 4.Vantrappen G, Janssens J, Peeters TL, Bloom SR, Christofides ND, Hellemans J. Motilin and the interdigestive migrating motor complex in man. Dig Dis Sci. 1979; 24(7): 497–500. doi: 10.1007/BF01489315 [DOI] [PubMed] [Google Scholar]
- 5.Janssens J, Vantrappen G, Peeters TL. The activity front of the migrating motor complex of the human stomach but not of the small intestine is motilin-dependent. Regul Pept. 1983; 6(4): 363–9. doi: 10.1016/0167-0115(83)90265-3 [DOI] [PubMed] [Google Scholar]
- 6.Wingate DL, Ruppin H, Green WE, Thompson HH, Domschke W, Wünsch E, Demling L, Ritchie HD. Motilin-induced electrical activity in the canine gastrointestinal tract. Scand J Gastroenterol Suppl. 1976; 39: 111–8. [PubMed] [Google Scholar]
- 7.Depoortere I, De Winter B, Thijs T, De Man J, Pelckmans P, Peeters T. Comparison of the gastroprokinetic effects of ghrelin, GHRP-6 and motilin in rats in vivo and in vitro. Eur J Pharmacol. 2005; 515(1-3): 160–8. doi: 10.1016/j.ejphar.2005.04.008 [DOI] [PubMed] [Google Scholar]
- 8.Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999; 402(6762): 656–60. doi: 10.1038/45230 [DOI] [PubMed] [Google Scholar]
- 9.Tomasetto C, Karam SM, Ribieras S, Masson R, Lefèbvre O, Staub A, Alexander G, Chenard MP, Rio MC. Identification and characterization of a novel gastric peptide hormone: the motilin-related peptide. Gastroenterology. 2000; 119(2): 395–405. doi: 10.1053/gast.2000.9371 [DOI] [PubMed] [Google Scholar]
- 10.Asakawa A, Inui A, Kaga T, Yuzuriha H, Nagata T, Ueno N, Makino S, Fujimiya M, Niijima A, Fujino MA, Kasuga M. Ghrelin is an appetite-stimulatory signal from stomach with structural resemblance to motilin. Gastroenterology. 2001; 120(2): 337–45. doi: 10.1053/gast.2001.22158 [DOI] [PubMed] [Google Scholar]
- 11.Fujino K, Inui A, Asakawa A, Kihara N, Fujimura M, Fujimiya M. Ghrelin induces fasted motor activity of the gastrointestinal tract in conscious fed rats. J Physiol. 2003; 550(Pt 1): 227–40. doi: 10.1113/jphysiol.2003.040600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zheng J, Ariga H, Taniguchi H, Ludwig K, Takahashi T. Ghrelin regulates gastric phase III-like contractions in freely moving conscious mice. Neurogastroenterol Motil. 2009; 21(1): 78–84. doi: 10.1111/j.1365-2982.2008.01179.x [DOI] [PubMed] [Google Scholar]
- 13.Ariga H, Tsukamoto K, Chen C, Mantyh C, Pappas TN, Takahashi T. Endogenous acyl ghrelin is involved in mediating spontaneous phase III-like contractions of the rat stomach. Neurogastroenterol Motil. 2007; 19(8): 675–80. doi: 10.1111/j.1365-2982.2007.00945.x [DOI] [PubMed] [Google Scholar]
- 14.Ohno T, Kamiyama Y, Aihara R, Nakabayashi T, Mochiki E, Asao T, Kuwano H. Ghrelin does not stimulate gastrointestinal motility and gastric emptying: an experimental study of conscious dogs. Neurogastroenterol Motil. 2006; 18(2): 129–35. doi: 10.1111/j.1365-2982.2005.00747.x [DOI] [PubMed] [Google Scholar]
- 15.Kobayashi S, Iwanaga T, Fujita T, Yanaihara N. Do enterochromaffin (EC) cells contain motilin? Arch Histol Jpn. 1980; 43(2): 85–98. doi: 10.1679/aohc1950.43.85 [DOI] [PubMed] [Google Scholar]
- 16.Suzuki H, Mochiki E, Haga N, Shimura T, Itoh Z, Kuwano H. Effect of duodenectomy on gastric motility and gastric hormones in dogs. Ann Surg. 2001; 233(3): 353–9. doi: 10.1097/00000658-200103000-00008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goll R, Nielsen SH, Holst JJ. Regulation of motilin release from isolated perfused pig duodenum. Digestion. 1996; 57(5): 341–8. doi: 10.1159/000201355 [DOI] [PubMed] [Google Scholar]
- 18.Poitras P, Dumont A, Cuber JC, Trudel L. Cholinergic regulation of motilin release from isolated canine intestinal cells. Peptides. 1993; 14(2): 207–13. doi: 10.1016/0196-9781(93)90031-B [DOI] [PubMed] [Google Scholar]
- 19.Yamada T, Mizumoto A, Satoh M, Haga N, Itoh Z. Muscarinic control of phase III contractions and motilin release in dogs. Peptides. 1997; 18(5): 673–80. doi: 10.1016/S0196-9781(97)00132-0 [DOI] [PubMed] [Google Scholar]
- 20.Itoh Z. Motilin and clinical application. Peptides. 1997; 18(4): 593–608. doi: 10.1016/S0196-9781(96)00333-6 [DOI] [PubMed] [Google Scholar]
- 21.Yoshiya K, Yamamura T, Ishikawa Y, Utsunomiya J, Mori K, Seino Y, Imura H, Yanaihara N. The failure of truncal vagotomy to affect motilin release in dogs. J Surg Res. 1985; 38(3): 263–6. doi: 10.1016/0022-4804(85)90036-8 [DOI] [PubMed] [Google Scholar]
- 22.Mochiki E, Satoh M, Tamura T, Haga N, Suzuki H, Mizumoto A, Sakai T, Itoh Z. Exogenous motilin stimulates endogenous release of motilin through cholinergic muscarinic pathways in the dog. Gastroenterology. 1996; 111(6): 1456–64. doi: 10.1016/S0016-5085(96)70006-9 [DOI] [PubMed] [Google Scholar]
- 23.Mochiki E, Inui A, Satoh M, Mizumoto A, Itoh Z. Motilin is a biosignal controlling cyclic release of pancreatic polypeptide via the vagus in fasted dogs. Am J Physiol. 1997; 272(2 Pt 1): G224–32. [DOI] [PubMed] [Google Scholar]
- 24.Sarna S, Chey WY, Condon RE, Dodds WJ, Myers T, Chang TM. Cause-and-effect relationship between motilin and migrating myoelectric complexes. Am J Physiol. 1983; 245(2): G277–84. [DOI] [PubMed] [Google Scholar]
- 25.Mizumoto A, Sano I, Matsunaga Y, Yamamoto O, Itoh Z, Ohshima K. Mechanism of motilin-induced contractions in isolated perfused canine stomach. Gastroenterology. 1993; 105(2): 425–32. doi: 10.1016/0016-5085(93)90716-P [DOI] [PubMed] [Google Scholar]
- 26.Van Assche G, Depoortere I, Thijs T, Janssens JJ, Peeters TL. Concentration-dependent stimulation of cholinergic motor nerves or smooth muscle by [Nle13]motilin in the isolated rabbit gastric antrum. Eur J Pharmacol. 1997; 337(2-3): 267–74. doi: 10.1016/S0014-2999(97)01317-4 [DOI] [PubMed] [Google Scholar]
- 27.Takeshita E, Matsuura B, Dong M, Miller LJ, Matsui H, Onji M. Molecular characterization and distribution of motilin family receptors in the human gastrointestinal tract. J Gastroenterol. 2006; 41(3): 223–30. doi: 10.1007/s00535-005-1739-0 [DOI] [PubMed] [Google Scholar]
- 28.Chung SA, Valdez DT, Diamant NE. Adrenergic blockage does not restore the canine gastric migrating motor complex during vagal blockade. Gastroenterology. 1992; 103(5): 1491–7. doi: 10.1016/0016-5085(92)91169-5 [DOI] [PubMed] [Google Scholar]
- 29.Tanaka T, Kendrick ML, Zyromski NJ, Meile T, Sarr MG. Vagal innervation modulates motor pattern but not initiation of canine gastric migrating motor complex. Am J Physiol Gastrointest Liver Physiol. 2001; 281(1): G283–92. [DOI] [PubMed] [Google Scholar]
- 30.Yoshida N, Mizumoto A, Iwanaga Y, Itoh Z. Effects of 5-hydroxytryptamine 3 receptor antagonists on gastrointestinal motor activity in conscious dogs. J Pharmacol Exp Ther. 1991; 256(1): 272–8. [PubMed] [Google Scholar]
- 31.Haga N, Mizumoto A, Satoh M, Mochiki E, Mizusawa F, Ohshima K, Itoh Z. Role of endogenous 5-hydroxytryptamine in the regulation of gastric contractions by motilin in dogs. Am J Physiol. 1996; 270(1 Pt 1): G20–8. [DOI] [PubMed] [Google Scholar]
- 32.Wilmer A, Tack J, Coremans G, Janssens J, Peeters T, Vantrappen G. 5-hydroxytryptamine-3 receptors are involved in the initiation of gastric phase-3 motor activity in humans. Gastroenterology. 1993; 105(3): 773–80. doi: 10.1016/0016-5085(93)90895-J [DOI] [PubMed] [Google Scholar]
- 33.Leslie RA. Comparative aspects of the area postrema: fine-structural considerations help to determine its function. Cell Mol Neurobiol. 1986; 6(2): 95–120. doi: 10.1007/BF00711065 [DOI] [PubMed] [Google Scholar]
- 34.Poitras P, Steinbach JH, VanDeventer G, Code CF, Walsh JH. Motilin-independent ectopic fronts of the interdigestive myoelectric complex in dogs. Am J Physiol. 1980; 239(3): G215–20. [DOI] [PubMed] [Google Scholar]
- 35.Lee KY, Chang TM, Chey WY. Effect of rabbit antimotilin serum on myoelectric activity and plasma motilin concentration in fasting dog. Am J Physiol. 1983; 245(4): G547–53. [DOI] [PubMed] [Google Scholar]
- 36.Itoh Z, Aizawa I, Honda R, Takeuchi S, Mori K. Regular and irregular cycles of interdigestive contractions in the stomach. Am J Physiol. 1980; 238(2): G85–90. [DOI] [PubMed] [Google Scholar]
- 37.Itoh Z, Honda R, Aizawa I. Diurnal pH changes in duodenum of conscious dogs. Am J Physiol. 1980; 238(2): G91–6. [DOI] [PubMed] [Google Scholar]
- 38.Itoh Z, Takahashi I. Periodic contractions of the canine gallbladder during the interdigestive state. Am J Physiol. 1981; 240(2): G183–9. [DOI] [PubMed] [Google Scholar]
- 39.Nakajima H, Mochiki E, Zietlow A, Ludwig K, Takahashi T. Mechanism of interdigestive migrating motor complex in conscious dogs. J Gastroenterol. 2010; 45(5): 506–14. doi: 10.1007/s00535-009-0190-z [DOI] [PubMed] [Google Scholar]
- 40.Ormsbee HS, 3rd , Silber DA, Hardy FE, Jr . Serotonin regulation of the canine migrating motor complex. J Pharmacol Exp Ther. 1984; 231(2): 436–40. [PubMed] [Google Scholar]
- 41.Gorard DA, Libby GW, Farthing MJ. 5-Hydroxytryptamine and human small intestinal motility: effect of inhibiting 5-hydroxytryptamine reuptake. Gut. 1994; 35(4): 496–500. doi: 10.1136/gut.35.4.496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gershon MD, Drakontides AB, Ross LL. Serotonin: Synthesis and Release from the Myenteric Plexus of the Mouse Intestine. Science. 1965; 149(3680): 197–9. doi: 10.1126/science.149.3680.197 [DOI] [PubMed] [Google Scholar]
- 43.Gershon MD. Nerves, reflexes, and the enteric nervous system: pathogenesis of the irritable bowel syndrome. J Clin Gastroenterol. 2005; 39(5 Suppl 3): S184–93. doi: 10.1097/01.mcg.0000156403.37240.30 [DOI] [PubMed] [Google Scholar]
- 44.Ahlman H, DeMagistris L, Zinner M, Jaffe BM. Release of immunoreactive serotonin into the lumen of the feline gut in response to vagal nerve stimulation. Science. 1981; 213(4513): 1254–5. doi: 10.1126/science.6168020 [DOI] [PubMed] [Google Scholar]
- 45.Gronstad K, Dahlstrom A, Florence L, Zinner MJ, Ahlman J, Jaffe BM. Regulatory mechanisms in endoluminal release of serotonin and substance P from feline jejunum. Dig Dis Sci. 1987; 32(4): 393–400. doi: 10.1007/BF01296293 [DOI] [PubMed] [Google Scholar]
- 46.Kellum J, McCabe M, Schneier J, Donowitz M. Neural control of acid-induced serotonin release from rabbit duodenum. Am J Physiol. 1983; 245(6): G824–31. [DOI] [PubMed] [Google Scholar]
- 47.Ferrara A, Zinner MJ, Jaffe BM. Intraluminal release of serotonin, substance P, and gastrin in the canine small intestine. Dig Dis Sci. 1987; 32(3): 289–94. doi: 10.1007/BF01297056 [DOI] [PubMed] [Google Scholar]
- 48.Larsson I. Studies on the extrinsic neural control of serotonin release from the small intestine. Acta Physiol Scand Suppl. 1981; 499: 1–43. [PubMed] [Google Scholar]
- 49.Zinner MJ, Jaffe BM, DeMagistris L, Dahlstrom A, Ahlman H. Effect of cervical and thoracic vagal stimulation on luminal serotonin release and regional blood flow in cats. Gastroenterology. 1982; 82(6): 1403–8. [PubMed] [Google Scholar]
- 50.Tsukamoto K, Ariga H, Mantyh C, Pappas TN, Yanagi H, Yamamura T, Takahashi T. Luminally released serotonin stimulates colonic motility and accelerates colonic transit in rats. Am J Physiol Regul Integr Comp Physiol. 2007; 293(1): R64–9. doi: 10.1152/ajpregu.00856.2006 [DOI] [PubMed] [Google Scholar]
- 51.Fujimiya M, Okumiya K, Kuwahara A. Immunoelectron microscopic study of the luminal release of serotonin from rat enterochromaffin cells induced by high intraluminal pressure. Histochem Cell Biol. 1997; 108(2): 105–13. doi: 10.1007/s004180050151 [DOI] [PubMed] [Google Scholar]
- 52.Cooke HJ, Montakhab M, Wade PR, Wood JD. Transmural fluxes of 5-hydroxytryptamine in guinea pig ileum. Am J Physiol. 1983; 244(4): G421–5. [DOI] [PubMed] [Google Scholar]
- 53.Martel F, Monteiro R, Lemos C, Vieira-Coelho MA. In vitro and in vivo effect of fluoxetine on the permeability of 3H-serotonin across rat intestine. Can J Physiol Pharmacol. 2004; 82(11): 940–50. doi: 10.1139/y04-083 [DOI] [PubMed] [Google Scholar]
- 54.Fukumoto S, Tatewaki M, Yamada T, Fujimiya M, Mantyh C, Voss M, Eubanks S, Harris M, Pappas TN, Takahashi T. Short-chain fatty acids stimulate colonic transit via intraluminal 5-HT release in rats. Am J Physiol Regul Integr Comp Physiol. 2003; 284(5): R1269–76. doi: 10.1152/ajpregu.00442.2002 [DOI] [PubMed] [Google Scholar]
- 55.Foxx-Orenstein AE, Kuemmerle JF, Grider JR. Distinct 5-HT receptors mediate the peristaltic reflex induced by mucosal stimuli in human and guinea pig intestine. Gastroenterology. 1996; 111(5): 1281–90. doi: 10.1053/gast.1996.v111.pm8898642 [DOI] [PubMed] [Google Scholar]
- 56.Bertrand PP, Kunze WA, Furness JB, Bornstein JC. The terminals of myenteric intrinsic primary afferent neurons of the guinea-pig ileum are excited by 5-hydroxytryptamine acting at 5-hydroxytryptamine-3 receptors. Neuroscience. 2000; 101(2): 459–69. doi: 10.1016/S0306-4522(00)00363-8 [DOI] [PubMed] [Google Scholar]
- 57.Hicks GA, Coldwell JR, Schindler M, Ward PA, Jenkins D, Lynn PA, Humphrey PP, Blackshaw LA. Excitation of rat colonic afferent fibres by 5-HT(3) receptors. J Physiol. 2002; 544(Pt 3): 861–9. doi: 10.1113/jphysiol.2002.025452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Glatzle J, Sternini C, Robin C, Zittel TT, Wong H, Reeve JR, Jr , Raybould HE. Expression of 5-HT3 receptors in the rat gastrointestinal tract. Gastroenterology. 2002; 123(1): 217–26. doi: 10.1053/gast.2002.34245 [DOI] [PubMed] [Google Scholar]
- 59.Berthoud HR, Kressel M, Raybould HE, Neuhuber WL. Vagal sensors in the rat duodenal mucosa: distribution and structure as revealed by in vivo DiI-tracing. Anat Embryol (Berl). 1995; 191(3): 203–12. doi: 10.1007/BF00187819 [DOI] [PubMed] [Google Scholar]
- 60.Tanaka T, Mizumoto A, Mochiki E, Haga N, Suzuki H, Itoh Z. Relationship between intraduodenal 5-hydroxytryptamine release and interdigestive contractions in dogs. J Smooth Muscle Res. 2004; 40(3): 75–84. doi: 10.1540/jsmr.40.75 [DOI] [PubMed] [Google Scholar]
- 61.Kellum JM, Maxwell RJ, Potter J, Kummerle JF. Motilin’s induction of phasic contractility in canine jejunum is mediated by the luminal release of serotonin. Surgery. 1986; 100(2): 445–53. [PubMed] [Google Scholar]
- 62.Tougas G, Allescher HD, Dent J, Daniel EE. Sensory nerves of the intestines: role in control of pyloric region of dogs. Adv Exp Med Biol. 1991; 298: 199–211. doi: 10.1007/978-1-4899-0744-8_18 [DOI] [PubMed] [Google Scholar]
- 63.Chen MH, Joffe SN, Magee DF, Murphy RF, Naruse S. Cyclic changes of plasma pancreatic polypeptide and pancreatic secretion in fasting dogs. J Physiol. 1983; 341: 453–61. doi: 10.1113/jphysiol.1983.sp014816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.DiMagno EP, Hendricks JC, Go VL, Dozois RR. Relationships among canine fasting pancreatic and biliary secretions, pancreatic duct pressure, and duodenal phase III motor activity--Boldyreff revisited. Dig Dis Sci. 1979; 24(9): 689–93. doi: 10.1007/BF01314466 [DOI] [PubMed] [Google Scholar]
- 65.Takahashi T. Mechanism of interdigestive migrating motor complex. J Neurogastroenterol Motil. 2012; 18(3): 246–57. doi: 10.5056/jnm.2012.18.3.246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mönnikes H, Tebbe JJ, Hildebrandt M, Arck P, Osmanoglou E, Rose M, Klapp B, Wiedenmann B, Heymann-Mönnikes I. Role of stress in functional gastrointestinal disorders. Evidence for stress-induced alterations in gastrointestinal motility and sensitivity. Dig Dis. 2001; 19(3): 201–11. doi: 10.1159/000050681 [DOI] [PubMed] [Google Scholar]
- 67.Feinle-Bisset C, Andrews JM. Treatment of Functional Dyspepsia. Curr Treat Options Gastroenterol. 2003; 6(4): 289–97. doi: 10.1007/s11938-003-0021-x [DOI] [PubMed] [Google Scholar]
- 68.Holtmann G, Kutscher SU, Haag S, Langkafel M, Heuft G, Neufang-Hueber J, Goebell H, Senf W, Talley NJ. Clinical presentation and personality factors are predictors of the response to treatment in patients with functional dyspepsia; a randomized, double-blind placebo-controlled crossover study. Dig Dis Sci. 2004; 49(4): 672–9. doi: 10.1023/B:DDAS.0000026317.00071.75 [DOI] [PubMed] [Google Scholar]
- 69.Barone FC, Deegan JF, Price WJ, Fowler PJ, Fondacaro JD, Ormsbee HS, 3rd . Cold-restraint stress increases rat fecal pellet output and colonic transit. Am J Physiol. 1990; 258(3 Pt 1): G329–37. [DOI] [PubMed] [Google Scholar]
- 70.Gue M, Junien JL, Bueno L. Conditioned emotional response in rats enhances colonic motility through the central release of corticotropin-releasing factor. Gastroenterology. 1991; 100(4): 964–70. [DOI] [PubMed] [Google Scholar]
- 71.Taché Y, Martinez V, Wang L, Million M. CRF1 receptor signaling pathways are involved in stress-related alterations of colonic function and viscerosensitivity: implications for irritable bowel syndrome. Br J Pharmacol. 2004; 141(8): 1321–30. doi: 10.1038/sj.bjp.0705760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Martínez V, Wang L, Rivier J, Grigoriadis D, Taché Y. Central CRF, urocortins and stress increase colonic transit via CRF1 receptors while activation of CRF2 receptors delays gastric transit in mice. J Physiol. 2004; 556(Pt 1): 221–34. doi: 10.1113/jphysiol.2003.059659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nakade Y, Tsuchida D, Fukuda H, Iwa M, Pappas TN, Takahashi T. Restraint stress delays solid gastric emptying via a central CRF and peripheral sympathetic neuron in rats. Am J Physiol Regul Integr Comp Physiol. 2005; 288(2): R427–32. doi: 10.1152/ajpregu.00499.2004 [DOI] [PubMed] [Google Scholar]
- 74.Taché Y, Maeda-Hagiwara M, Turkelson CM. Central nervous system action of corticotropin-releasing factor to inhibit gastric emptying in rats. Am J Physiol. 1987; 253(2 Pt 1): G241–5. [DOI] [PubMed] [Google Scholar]
- 75.Broccardo M, Improta G. Pituitary-adrenal and vagus modulation of sauvagine- and CRF-induced inhibition of gastric emptying in rats. Eur J Pharmacol. 1990; 182(2): 357–62. doi: 10.1016/0014-2999(90)90294-G [DOI] [PubMed] [Google Scholar]
- 76.Lenz HJ, Burlage M, Raedler A, Greten H. Central nervous system effects of corticotropin-releasing factor on gastrointestinal transit in the rat. Gastroenterology. 1988; 94(3): 598–602. doi: 10.1016/0016-5085(88)90229-6 [DOI] [PubMed] [Google Scholar]
- 77.Gué M, Bueno L. Diazepam and muscimol blockade of the gastrointestinal motor disturbances induced by acoustic stress in dogs. Eur J Pharmacol. 1986; 131(1): 123–7. doi: 10.1016/0014-2999(86)90525-X [DOI] [PubMed] [Google Scholar]
- 78.Gue M, Fioramonti J, Frexinos J, Alvinerie M, Bueno L. Influence of acoustic stress by noise on gastrointestinal motility in dogs. Dig Dis Sci. 1987; 32(12): 1411–7. doi: 10.1007/BF01296668 [DOI] [PubMed] [Google Scholar]
- 79.Gue M, Honde C, Pascaud X, Junien JL, Alvinerie M, Bueno L. CNS blockade of acoustic stress-induced gastric motor inhibition by kappa-opiate agonists in dogs. Am J Physiol. 1988; 254(6 Pt 1): G802–7. [DOI] [PubMed] [Google Scholar]
- 80.Taniguchi H, Imai K, Ludwig K, Takahashi T. Effects of acupuncture on stress-induced gastrointestinal dysmotility in conscious dogs. Med Acupunct. 2012; 24(1): 43–9. doi: 10.1089/acu.2011.0832 [DOI] [Google Scholar]
- 81.Vantrappen G, Janssens J, Hellemans J, Ghoos Y. The interdigestive motor complex of normal subjects and patients with bacterial overgrowth of the small intestine. J Clin Invest. 1977; 59(6): 1158–66. doi: 10.1172/JCI108740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Testoni PA, Bagnolo F, Bologna P, Colombo E, Bonassi U, Lella F, Buizza M. Higher prevalence of Helicobacter pylori infection in dyspeptic patients who do not have gastric phase III of the migrating motor complex. Scand J Gastroenterol. 1996; 31(11): 1063–8. doi: 10.3109/00365529609036888 [DOI] [PubMed] [Google Scholar]
- 83.Testoni PA, Bagnolo F. In dyspeptic patients without gastric phase III of the migrating motor complex, Helicobacter pylori eradication produces no short-term changes in interdigestive motility pattern. Scand J Gastroenterol. 2000; 35(8): 808–13. doi: 10.1080/003655200750023165 [DOI] [PubMed] [Google Scholar]
- 84.Tack J, Bisschops R, Sarnelli G. Pathophysiology and treatment of functional dyspepsia. Gastroenterology. 2004; 127(4): 1239–55. doi: 10.1053/j.gastro.2004.05.030 [DOI] [PubMed] [Google Scholar]
- 85.Quigley EM, Donovan JP, Lane MJ, Gallagher TF. Antroduodenal manometry. Usefulness and limitations as an outpatient study. Dig Dis Sci. 1992; 37(1): 20–8. doi: 10.1007/BF01308337 [DOI] [PubMed] [Google Scholar]
- 86.Gu C, Ke M, Wang Z. Temporal and spatial relationship of pylorus to antroduodenal motility in functional dyspepsia. Chin Med J (Engl). 1998; 111(10): 906–9. [PubMed] [Google Scholar]
- 87.Kusano M, Sekiguchi T, Kawamura O, Kikuchi K, Miyazaki M, Tsunoda T, Horikoshi T, Mori M. Further classification of dysmotility-like dyspepsia by interdigestive gastroduodenal manometry and plasma motilin level. Am J Gastroenterol. 1997; 92(3): 481–4. [PubMed] [Google Scholar]
- 88.Kusano M, Sekiguchi T, Kawamura O, Kikuchi K, Nakamura K, Mori M. Disturbed initiation of gastric interdigestive migrating complexes despite high plasma motilin levels in patients with low gastric pH. Dig Dis Sci. 1998; 43(8): 1697–700. doi: 10.1023/A:1018815215724 [DOI] [PubMed] [Google Scholar]
- 89.Greydanus MP, Vassallo M, Camilleri M, Nelson DK, Hanson RB, Thomforde GM. Neurohormonal factors in functional dyspepsia: insights on pathophysiological mechanisms. Gastroenterology. 1991; 100(5 Pt 1): 1311–8. doi: 10.1016/0016-5085(91)70018-S [DOI] [PubMed] [Google Scholar]
- 90.Holtmann G, Goebell H, Jockenhoevel F, Talley NJ. Altered vagal and intestinal mechanosensory function in chronic unexplained dyspepsia. Gut. 1998; 42(4): 501–6. doi: 10.1136/gut.42.4.501 [DOI] [PMC free article] [PubMed] [Google Scholar]