Abstract
Ciliary muscle is a smooth muscle characterized by a rapid response to muscarinic receptor stimulation and sustained contraction. Although it is evident that these contractions are Ca2+-dependent, detailed molecular mechanisms are still unknown. In order to elucidate the role of Ser/Thr protein phosphatase 2A (PP2A) in ciliary muscle contraction, we examined the effects of okadaic acid and other PP2A inhibitors on contractions induced by carbachol (CCh) and ionomycin in bovine ciliary muscle strips (BCM). Okadaic acid inhibited ionomycin-induced contraction, while it did not cause significant changes in CCh-induced contraction. Fostriecin showed similar inhibitory effects on the contraction of BCM. On the other hand, rubratoxin A inhibited both ionomycin- and CCh-induced contractions. These results indicated that PP2A was involved at least in ionomycin-induced Ca2+-dependent contraction, and that BCM had a unique regulatory mechanism in CCh-induced contraction.
Keywords: mooth muscle, okadaic acid, bovine ciliary muscle, protein phosphatase
Introduction
Ciliary muscle is a smooth muscle with a parasympathetic innervation and characterized by a rapid response to muscarinic receptor stimulation and a subsequent sustained contraction (Fig. 1a) (1,2,3). Acetylcholine binds to Gq/11-coupled M3 receptors (3, 4) and induces rapid Ca2+ release from the sarcoplasmic reticulum, followed by a sustained Ca2+ influx through non-selective cation channels (NSCC) on the plasma membrane of ciliary myocytes (5,6,7).
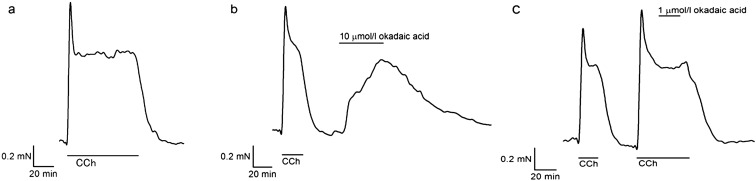
Fig. 1.
Effects of okadaic acid on bovine ciliary muscle strips. (a) A representative isometric tension recording showed that 2 µmol/l CCh induced a reversible contraction with a transient peak followed by a plateau phase in the BCM. After removal of CCh, it returned to the resting level. (b) Addition of 10 µmol/l okadaic acid to a relaxed BCM preparation produced a slow tension development. It slowly reverted to the resting level when okadaic acid was removed. (c) Addition of 1 µmol/l okadaic acid did not cause any change (98.1 ± 1.2%, n = 8, P = 0.16) in pre-contracted BCM with 2 µmol/l CCh.
One of the unique properties of ciliary muscle contraction is that high potassium depolarization with a muscarinic receptor inhibitor, atropine, does not cause contraction (1), suggesting the lack of voltage-dependent Ca2+ channels on ciliary muscle (8). Although it is evident that the Ca2+ entry through NSCC is necessary for sustained contraction (6), downstream regulatory mechanisms have not been elucidated.
Okadaic acid is a toxic polyether derivative of a C38 fatty acid, source of diarrhetic food poisoning, isolated from the black sponge, Halichondria okadai. It has been reported that okadaic acid caused Ca2+-independent contraction in various smooth muscle preparations (9,10,11,12,13,14). Following such reports, lower concentrations of okadaic acid were found to inhibit agonist- or depolarization-induced contractions in those preparations (15,16,17,18).
These concentration-dependent opposite effects of okadaic acid could be attributable to the difference in inhibition potency against Ser/Thr protein phosphatase type 1 (PP1) and type 2A (PP2A). While okadaic acid at lower concentration selectively inhibits PP2A (Ki = 34 pmol/l), it potently inhibits both PP1 (Ki = 147 nmol/l) and PP2A at higher concentration (19). Therefore, smooth muscle contraction with high okadaic acid could be due to the inhibition of myosin light chain phosphatase, classified as PP1, and accumulation of phosphorylated myosin. On the other hand, the inhibitory effect of okadaic acid at lower concentration on smooth muscle contraction might be attributable to PP2A inhibition. However, the true targets of okadaic acid and underlying mechanisms still remain an open question.
In this study, we examined the effects of okadaic acid on bovine ciliary muscle strips (BCM), and tried to elucidate a role of PP2A in the contraction of BCM. Okadaic acid at higher concentrations induced contraction in BCM as it does in other smooth muscle preparations (9,10,11,12,13,14, 20). Interestingly, it failed to inhibit carbachol (CCh)-induced contraction. Rubratoxin A, a more selective PP2A inhibitor, blocked CCh-induced contraction.
Methods
Ethical approval
All experimental procedures conformed to the "Guidelines for Proper Conduct of Animal Experiments" approved by the Science Council of Japan, and a protocol reviewed by the Animal Care and Use Committee of Asahikawa Medical University.
Tissue preparation
Fresh bovine eyes were obtained from a local slaughterhouse and placed in ice-cold physiological saline solution (PSS) after enucleation. The eyes were incised circumferentially about 5 mm posterior to the limbus. After the vitreous humour and lens were removed, the ciliary muscle was carefully dissected out from the scleral spur.
The smooth muscle of the guinea pig taenia caeci was used as a control. Male guinea pigs (3–10 weeks old) were anesthetized with sevoflurane and sacrificed by exsanguination. The taenia caeci was carefully removed and placed in ice-cold PSS until use.
Isometric force measurement
Both the ciliary muscle and taenia caeci were cut into strips about 0.5 mm in width and 2 mm in length. The ends of strips were tied with rayon monofilaments to fine needles connected to a force transducer (Minebea Co., Tokyo, Japan) and mounted in a 340 µl-chamber filled with PSS kept at 30 °C. After attachment, smooth muscle strips were equilibrated under a resting tension of 40 mg for about 30 min. PSS was changed every 10 min. In this study, while we used a non-perfused chamber to save expensive drugs, this did result in noisy tension traces.
After equilibration, strips were transiently stimulated with CCh (2 μmol/l) to induce contractile responses to confirm viability of the preparations. Muscle strips were then treated with CCh (2 μmol/l) or ionomycin (20 μmol/l) to obtain sustained contractions. After achieving a sustained contraction, various concentrations of protein phosphatase inhibitors, okadaic acid, fostriecin or rubratoxin A were administered. Thirty-minutes after the addition of each drug or when there were plateau responses, the tension was evaluated. Contraction changes were expressed as % of the response to CCh or ionomycin just prior to adding the inhibitors.
Solutions and Chemicals
PSS (mmol/l): NaCl 127, KCI 5.9, CaC12 2.4, MgC12 1.2, 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (Hepes) 20 and glucose 11.8, (pH = 7.4 at 30 °C). EGTA-PSS (mmol/l): NaCl 127, KCI 5.9, ethylene glycol-bis(2-aminoethylether)-N,N,N',N'-tetraacetic acid (EGTA) 0.1, MgC12 1.2, Hepes 20 and glucose 11.8, (pH = 7.4 at 30 °C).
Okadaic acid was kindly provided by the late Dr. Tsukitani (formerly Fujisawa Pharmaceutical Co., Tokyo, Japan). Ionomycin calcium salt was purchased from Wako Pure Chemical Industries. LTD. (Osaka, Japan). Carbachol, Y-27632 and Gö6983 were purchased from Sigma (St. Louis, MO, USA). Fostriecin and rubratoxin A were purchased from Bioaustralis Co. (Australia) and Microbial Chemistry Research Foundation (Tokyo, Japan), respectively.
Statistics
Data are presented as mean values ± SEM of n experiments. Statistical significance was assessed by paired or unpaired t-test for two groups. P < 0.05 was considered to be significant.
Results
Effects of okadaic acid on bovine ciliary muscle
We first examined the effects of okadaic acid on bovine ciliary muscle preparations (Fig. 1). Treatment of relaxed BCM with 10 μmol/l okadaic acid caused a slow increase in isometric tension (Fig. 1b). After removal of okadaic acid, it slowly relaxed back to the resting level. Interestingly, okadaic acid at a lower concentration (1 μmol/l), which was known to inhibit agonist- or depolarization-induced contraction in other smooth muscle tissues (15,16,17,18, 20), did not cause any changes (98.1 ± 1.2%, n = 8, P = 0.16) in BCM pre-contracted with 2 μmol/l CCh (Fig. 1c).
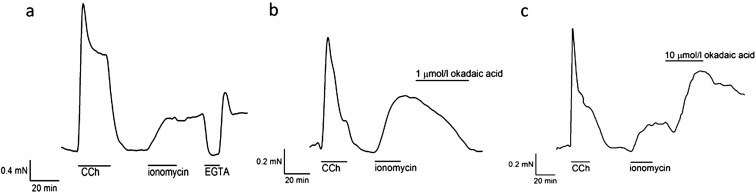
In order to avoid potential activation of complex regulatory pathways such as "Ca2+ sensitization (21, 22)" or "actin-reorganization mechanisms (23)" by CCh, we then examined the effects of okadaic acid on the Ca2+-induced contraction of the BCM. Since BCM have been shown not to have any voltage-dependent Ca2+ entry mechanism (1, 8), we employed the Ca2+ ionophore, ionomycin, to evoke Ca2+-induced contraction. Ionomycin (20 μmol/l) treatment for 20 min caused a slowly developed sustained contraction which lasted even after washout of ionomycin (Fig. 2a), suggesting that ionomycin remained intercalated in the plasma membrane allowing continuous entry of Ca2+. In contrast with CCh-induced contraction, 1 μmol/l okadaic acid attenuated ionomycin-induced contraction (31.0 ± 11.0%, n = 6, P < 0.01, Fig. 2b). Okadaic acid at 10 μmol/l initially caused a small decrease in tension and then induced strong tension development in the ionomycin-contracted BCM (227 ± 34%, n = 6, P = 0.013, Fig. 2c).
Fig. 2.
Effects of okadaic acid on ionomycin-induced contraction in bovine ciliary muscle strips. (a) Treatment with 20 µmol/l ionomycin for 20 min induced a long lasting contraction. The contraction continued even after wash out of the ionomycin. Removal of external Ca2+ with EGTA relaxed the strip, confirming the contraction was dependent on Ca2+ entry through the intercalated ionomycin. The tension developed again after re-addition of Ca2+ to the external solution. (b) One µmol/l okadaic acid attenuated ionomycin-induced contraction (31.0 ± 11.0%, n = 6, P < 0.01). (c) Ten µmol/l okadaic acid caused an initial small decrease in tension, followed by a strong tension development (227 ± 34%, n = 6, P = 0.013) which tended to reverse slowly when okadaic acid was removed.
Effects of other PP2A inhibitors on bovine ciliary muscle
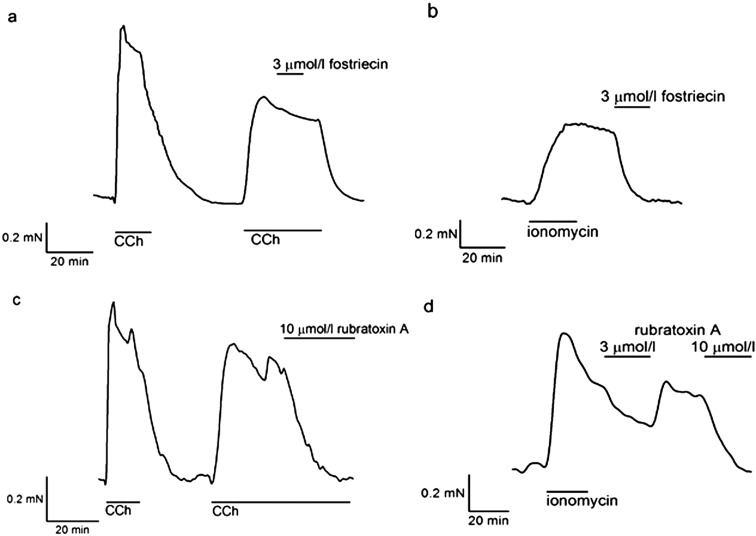
To confirm that those inhibitory effects of okadaic acid were due to specific inhibition of PP2A, we examined other selective PP2A inhibitors, fostriecin (IC50 = 3.2 nmol/l for PP2A and 131 μmol/l for PP1 (24)) and rubratoxin A (Ki = 28.7 nmol/l for PP2A (25)). Fostriecin at a lower concentration (3 μmol/l) completely inhibited ionomycin-induced contraction in BCM (2.0 ± 1.6%, n = 6, P < 0.01, Fig. 3b), while it failed to inhibit CCh-induced contraction (97.7 ± 3.4%, n = 6, P = 0.53, Fig. 3a). These inhibitory effects were consistent with those of okadaic acid at a lower concentration.
Fig. 3.
Effects of fostriecin and rubratoxin A on bovine ciliary muscle strips. Fostriecin and rubratoxin A were added to BCM strips pre-contracted by CCh or ionomycin. (a) Following CCh-induced contraction, 3 µmol/l fostriecin did not cause any change (97.7 ± 3.4%, n = 6, P = 0.53). (b) With ionomycin-induced contraction, 3 µmol/l fostriecin inhibited contraction completely (2.0 ± 1.6%, n = 6, P < 0.01). (c) Rubratoxin A inhibited CCh-induced contraction at 10 µmol/l (1.7 ± 2.2%, n = 6, P < 0.01). (d) It also inhibited ionomycin-induced contraction. Three µmol/l rubratoxin A decreased ionomycin-induced tension to 63.2 ± 6.8% (n = 6, P < 0.01), and 10 µmol/l relaxed it completely (1.5 ± 1.7%, n = 6, P < 0.01).
On the other hand, rubratoxin A showed partly different effects. Rubratoxin A, at 10 μmol/l, completely inhibited ionomycin-induced contraction like other PP2A inhibitors (1.5 ± 1.7%, n = 6, P < 0.01, Fig. 3d), while it also inhibited CCh-induced contraction (1.7 ± 2.2%, n = 6, P < 0.01, Fig. 3c). It did not cause any contractions even at a higher concentration (30 μmol/l).
Effects of PP2A inhibitors on guinea pig taenia caeci strips
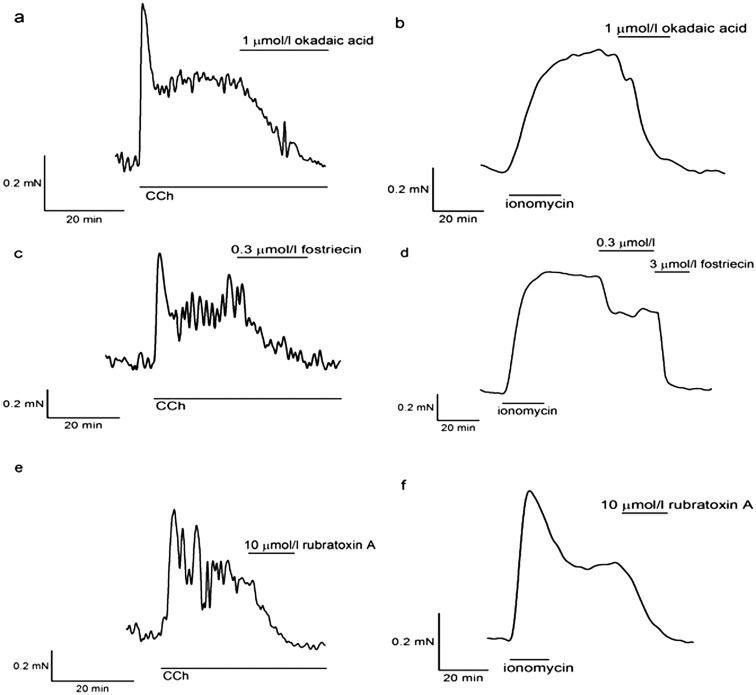
We also examined the effects of these PP2A inhibitors on another smooth muscle preparation from a different species to check their specificity. We chose the guinea pig taenia caeci strips as it has been well studied and okadaic acid has been shown to have the inhibitory effect on this smooth muscle (15). In the guinea pig taenia caeci strips, ionomycin-induced contraction was completely blocked by all three inhibitors at lower concentrations (Fig. 4b, d and f). In contrast to the BCM, they also inhibited CCh-induced contractions in strips of the guinea pig taenia caeci (Fig. 4a, c and e).
Fig. 4.
Effects of PP2A inhibitors on guinea pig taenia caeci strips. The spontaneous contractions of the guinea pig taenia caeci strips were enhanced by CCh. (a) One µmol/l okadaic acid blocked CCh-induced contraction, and caused relaxation completely. (b) One µmol/l okadaic acid also inhibited ionomycin-induced contraction (7.3 ± 2.0%, n = 8, P < 0.01). (c) Fostriecin of 0.3 µmol/l inhibited CCh-induced contraction. (d) In ionomycin-induced contraction, 0.3 µmol/l fostriecin decreased the tension by 67.8 ± 4.8% (n = 6, P < 0.01), and 3 µmol/l relaxed it completely. (e) Rubratoxin A of 10 µmol/l inhibited CCh-induced contraction. (f) In ionomycin-induced contraction, 10 µmol/l rubratoxin A relaxed it completely (0.83 ± 0.5%, n = 6, P < 0.01).
Concentration-dependent effects of PP2A inhibitors
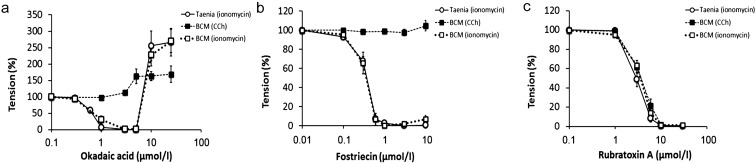
The concentration-dependent effects of okadaic acid, fostriecin and rubratoxin A on CCh- and ionomycin-induced contractions in BCM and ionomycin-induced contractions in strips of the guinea pig taenia caeci are summarized in Fig. 5. At lower concentrations (≤ 3 μmol/l), okadaic acid caused relaxation in ionomycin-contracted strips of both the BCM and taenia caeci, while it had no effect on CCh-induced contractions in BCM (Fig. 5a). Higher concentrations (≥ 10 μmol/l) of okadaic acid enhanced sustained tension in ionomycin-pre-contracted BCM and taenia caeci strips. In CCh-pre-contracted BCM, 5 μmol/l and higher concentrations of okadaic acid enhanced contraction.
Fig. 5.
Concentration-dependent effects of PP2A inhibitors. Effects of various concentrations of (a) okadaic acid (n = 6–10), (b) fostriecin (n = 6–8) and (c) rubratoxin A (n = 6) on the contractions induced by CCh (■), ionomycin (□) in BCM and ionomycin in guinea pig taenia caeci strips (○) were summarized. The effects of inhibitors on the CCh-induced contractions of the taenia caeci strips were omitted because they were difficult to quantitate due to their automaticity. Okadaic acid and fostriecin showed similar concentration-dependent inhibitory effects on ionomycin-induced contraction of both BCM and taenia caeci strips, while they failed to inhibit CCh-induced contractions of the BCM. Rubratoxin A inhibited CCh- and ionomycin-induced contractions both in both BCM and taenia caeci strips in a similar concentration-dependent manner. Only okadaic acid enhanced contractions at 10 µmol/l and higher.
Fostriecin (≥ 0.3 μmol/l) blocked ionomycin-induced contractions in strips of both the BCM and taenia caeci, while it failed to block CCh-induced contractions in BCM (Fig. 5b). Rubratoxin A inhibited both ionomycin- and CCh-induced contractions in both the BCM and taenia caeci in a similar concentration-dependent manner (Fig. 5c). In contrast with okadaic acid, neither fostriecin nor rubratoxin A enhanced contractions at higher concentrations.
Effects of a ROCK inhibitor
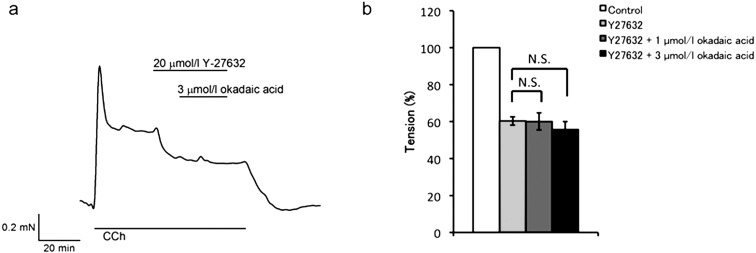
We hypothesized that the force-inhibiting effect of okadaic acid was masked by the Ca2+-sensitization mechanism (21, 22) in CCh-induced contractions of BCM. To test this hypothesis, we examined the effects of okadaic acid in the presence of Y-27632, a Rho-kinase inhibitor (Fig. 6). Addition of 20 μmol/l Y-27632 to CCh-contracted BCM decreased tension by about half (60.3 ± 2.3%, n = 6, P < 0.01 vs. control), suggesting the involvement of the Rho-kinase dependent Ca2+-sensitization mechanism in CCh-induced contractions of BCM. Surprisingly, however, subsequent addition of okadaic acid at 1 or 3 μmol/l showed no effect on the contraction (60.0 ± 4.6%, n = 3, P = 0.12, and 55.7 ± 4.3%, n = 3, P = 0.11, respectively, vs. Y-27632 alone, Fig. 6b).
Fig. 6.
Effects of the ROCK inhibitor on bovine ciliary muscle strips. (a) A representative tension trace showed that Y-27632 (20 µmol/l) attenuated CCh-induced contraction of the BCM. Addition of 3 µmol/l okadaic acid did not cause further change. It did not tend to reverse when Y-27632 and okadaic acid were removed. (b) Observed tension was normalized to that of pre-contraction with CCh (white column). Y-27632 decreased the tension by 60.3 ± 2.3% (pale gray column, n = 6, P < 0.01 vs. control). Addition of okadaic acid did not cause any change at either 1µmol/l (dark gray column, 60 ± 4.6%) or 3 µmol/l (black column, 55.7 ± 4.3%). Statistical assessments by paired t-test did not show significant differences in Y-27632 with 1 or 3 µmol/l okadaic acid (P = 0.12, n = 3 and P = 0.11, n = 3, respectively) compared with Y-27632 alone.
Effects of a PKC inhibitor
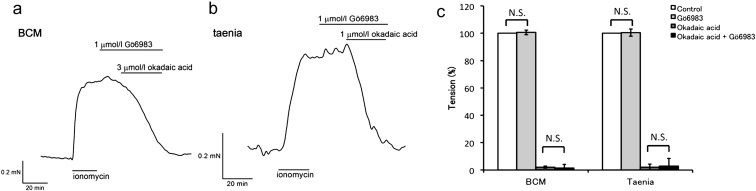
Protein kinase C alpha (PKCα) was reported to be involved in the mechanism by which okadaic acid causes inhibition of preparations of the canine basilar artery (26, 27). To test this hypothesis in both BCM and guinea pig taenia caeci strips, we examined the effects of Gö6983, a broad spectrum PKC inhibitor, on okadaic acid-induced relaxation. Figures. 7a and b showed representative tension traces of the BCM and strips of the guinea pig taenia caeci, respectively. Treatment with Gö6983 alone did not cause significant change in either tissue (101.0 ± 1.7%, n = 6, P = 0.71 for BCM and 101.0 ± 2.7%, n = 6, P = 0.86 for taenia caeci, vs. control), suggesting that PKC (specifically PKCα, β, γ, δ and ζ isoforms) might not have basal activity under these conditions. Gö6983 failed to attenuate inhibitory effect of okadaic acid both in BCM and guinea pig taenia caeci strips (1.5 ± 2.6%, n = 6, P = 0.81, and 2.8 ± 5.7%, n = 6, P = 0.91 respectively, vs. okadaic acid alone, Fig. 7c).
Fig. 7.
Effects of the PKC inhibitor. Gö6983 (1 µmol/l), a broad PKC inhibitor, had no effect on ionomycin-induced contraction in both BCM (a) and guinea pig taenia caeci strips (b). It did not attenuate the inhibitory effect of okadaic acid in either case. (c) The observed tension was normalized to that of pre-contraction with ionomycin (white column). Treatment with Gö6983 alone did not cause significant change in either of the preparations (pale gray column, 101.0 ± 1.7%, P = 0.71 for BCM and 101.0 ± 2.7%, P = 0.86 for taenia caeci, vs. control). Okadaic acid (3 µmol/l) induced complete relaxation in the absence (dark gray column, 2.2 ± 0.7% for BCM and 2.2 ± 2.1% for taenia caeci) or presence (black column, 1.5 ± 2.6% for BCM and 2.8 ± 5.7% for taenia caeci) of Gö6983. Each column represents the mean ± SEM of 6 experiments. Statistical assessments by Student's t-test did not show significant difference in the force inhibiting effect of okadaic acid between the absence and presence of Gö6983 (P = 0.81 for BCM and P = 0.91 for taenia caeci).
Discussion
In this study, we examined the effects of okadaic acid and other PP2A inhibitors on smooth muscle contraction in the BCM and guinea pig taenia caeci strips. As shown in Fig. 5, okadaic acid caused relaxation in ionomycin-contracted BCM and taenia caeci strips preparations at lower concentrations (≤ 3 μmol/l), while it had no effect on CCh-induced contraction in those of the BCM (Fig. 5a). Fostriecin showed similar inhibitory effects to okadaic acid, as it blocked ionomycin-induced, but not CCh-induced contractions in BCM (Fig. 5b). Rubratoxin A had more potent inhibitory effect, that is, both ionomycin- and CCh-induced contractions were blocked (Fig. 5c).
Considering that three PP2A inhibitors with different structures blocked Ca2+-induced contractions in the BCM and Ca2+- and CCh-induced contraction in strips of the taenia caeci, it seems reasonable to assume that the inhibitory effect of okadaic acid on smooth muscle contraction is due to PP2A inhibition, but not to its off-target effect. In other words, PP2A could play a role in force maintenance in these smooth muscle contractions.
The force developing effect at higher concentrations was observed only with okadaic acid. This could be explained by a different potency to PP1. Okadaic acid would inhibit PP1 with a Ki of 147 nmol/l (19), while fostriecin (24) and rubratoxin A (25) would not inhibit PP1 at the concentrations we tested in the present study. In the previous studies, potent PP1 inhibitors, such as calyculin A and tautomycin, induced Ca2+-independent contractions in various smooth muscle preparations (28, 29). Therefore, our tentative conclusion is that the force-developing effect of okadaic acid at higher concentrations could be due to the inhibition of PP1.
It is noteworthy that, in the BCM, okadaic acid enhanced CCh-induced contraction at a lower concentration than the ionomycin-induced one (Fig. 5a). This result suggests that PP1 activity could be attenuated in CCh-induced contraction by the Ca2+-sensitization mechanism, which is consistent with the results in Figure. 6, in which rho-kinase inhibition decreased CCh-induced contraction. The involvement of Rho-kinase dependent mechanisms has also been reported in CCh-induced contractions in rabbit and monkey ciliary muscles (30).
One of the most intriguing questions in the present study is why okadaic acid (and fostriecin) did not show any inhibitory effects on CCh-induced BCM contraction. One could argue that the force inhibiting effect of okadaic acid was masked by Ca2+ sensitization through a Rho-kinase dependent mechanism (21, 22). However, this seems unlikely because okadaic acid did not attenuate CCh-induced contraction in the BCM even when Rho-kinase dependent Ca2+-sensitization was blocked by Y-27632 (Fig. 6).
In strips of the guinea pig taenia caeci, okadaic acid completely inhibited CCh-induced contraction (Fig. 4). Furthermore, it has also been reported that okadaic acid inhibited agonist-induced contractions in various smooth muscle preparations, in which Ca2+-sensitization mechanisms were supposed to be activated (15,16,17,18). These results also deny the masking hypothesis by Ca2+-sensitization, and suggest the involvement of a unique okadaic acid-resistant regulatory mechanism in CCh-stimulated BCM contraction. Considering that inhibition of the Rho-kinase dependent Ca2+-sensitization mechanism, in which the balance between myosin kinase and phosphatase would be altered, failed to unmask the force-inhibiting effect of okadaic acid in CCh-contracted BCM, we assume another mechanism rather than myosin phosphorylation could be important for the resistance. Furthermore, since ionomycin-induced BCM contraction did not show okadaic acid resistance, Ca2+ influx/efflux would not be the target. More detailed study is required to elucidate the resistant mechanism for okadaic acid.
In the previous study, it has been reported that extensive skinning of smooth muscle by β-escin or Triton X-100 diminished force inhibiting effect of okadaic acid (31). These phenomena suggest the existence of a relaxation factor, which may be lost during the skinning. Okadaic acid would activate it by inhibiting PP2A to relax smooth muscle. Considering that okadaic acid failed to relax CCh-contracted BCM (Fig. 1 and 5), this relaxation factor would remain inactive under these conditions.
One of the candidates of this relaxation factor is PKCα. In previous studies, Obara et al. proposed that unmasking the basal PKCα activity was the cause of the inhibitory effect of okadaic acid (26, 27). In their studies, conventional PKCα inhibitors attenuated the inhibitory effect of okadaic acid in canine artery preparations. On the other hand, in the BCM, it has been reported that PKC inhibition by H7 or myristoilated PKC substrate had little effect on CCh-induced contraction (32), suggesting that PKCα would not have any activity in the CCh-induced BCM contraction. If so, it would be reasonable to assume that okadaic acid failed to attenuate the contraction because there was no basal PKCα activity in CCh-simulated BCM.
However, things seem to be more complicated, because the force-inhibiting effect of okadaic acid was also observed in the ionomycin-contracted both the BCM and strips of the guinea pig taenia caeci in the presence of a broad spectrum PKC inhibitor (Fig. 7). Considering that PKCα inhibition did not cause any changes in these muscles, PKCα does not seem to have any basal activities under these conditions, either. These results suggest that there could be a more fundamental cause of the inhibitory effect of okadaic acid rather than unmasking PKCα activity by PP2A inhibition.
In the present study, although okadaic acid and fostriecin failed to inhibit CCh-induced BCM contraction (Fig. 1, 3 and 5), rubratoxin A inhibited it completely (Fig. 3 and 5). The reason of this different effects is not clear yet, but it would be due to the different potency against other phosphatases, such as PP4 and PP1. Further study is needed to address this problem.
In summary, we found that 1) okadaic acid inhibits smooth muscle contraction through PP2A inhibition, and 2) CCh activates a unique contractile mechanism in the BCM which is resistant to the inhibitory effect of okadaic acid. As far as we know, there are only a few reports of contraction which is resistant to the inhibitory effect of okadaic acid (e.g. Ref. (33)). This exceptional case might elucidate the inhibitory mechanism by comparing CCh-contraction with that with ionomycin in the BCM.
Conflict of Interest
The authors declare that they have no conflict of interest.
Acknowledgments
We are very grateful to the late Dr. Tsukitani (formerly Fujisawa Pharmaceutical Co., Tokyo, Japan), who passed away on June 10, 2014, for kindly providing okadaic acid. We would like to dedicate this paper to his memory. We thank Dr. Walsh (University of Calgary, Canada) for reading the manuscript and giving insightful advice. This work was supported in part by a Grant-in-Aid for Young Scientist from Asahikawa Medical University.
References
- 1.Suzuki R. Neuronal influence on the mechanical activity of the ciliary muscle. Br J Pharmacol. 1983; 78(3): 591–7 http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2044737/. doi: 10.1111/j.1476-5381.1983.tb08819.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kamikawatoko S, Tokoro T, Ishida A, Masuda H, Hamasaki H, Sato J, Azuma H. Nitric oxide relaxes bovine ciliary muscle contracted by carbachol through elevation of cyclic GMP. Exp Eye Res. 1998; 66(1): 1–7 http://www.ncbi.nlm.nih.gov/pubmed/9533825. doi: 10.1006/exer.1997.0408 [DOI] [PubMed] [Google Scholar]
- 3.Yasui F, Miyazu M, Yoshida A, Naruse K, Takai A. Examination of signalling pathways involved in muscarinic responses in bovine ciliary muscle using YM-254890, an inhibitor of the Gq/11 protein. Br J Pharmacol. 2008; 154(4): 890–900 http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2439854&tool=pmcentrez&rendertype=abstract. doi: 10.1038/bjp.2008.140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Masuda H, Goto M, Tamaoki S, Kamikawatoko S, Tokoro T, Azuma H. M3-type muscarinic receptors predominantly mediate neurogenic quick contraction of bovine ciliary muscle. Gen Pharmacol. 1998; 30(4): 579–84 http://www.ncbi.nlm.nih.gov/pubmed/9522179. doi: 10.1016/S0306-3623(97)00312-1 [DOI] [PubMed] [Google Scholar]
- 5.Takai Y, Awaya S, Takai A. Activation of non-selective cation conductance by carbachol in freshly isolated bovine ciliary muscle cells. Pflugers Arch. 1997; 433(6): 705–12 http://www.ncbi.nlm.nih.gov/pubmed/9049160. doi: 10.1007/s004240050335 [DOI] [PubMed] [Google Scholar]
- 6.Takai Y, Sugawara R, Ohinata H, Takai A. Two types of non-selective cation channel opened by muscarinic stimulation with carbachol in bovine ciliary muscle cells. J Physiol. 2004; 559(Pt 3): 899–922 http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=1665188&tool=pmcentrez&rendertype=abstract. doi: 10.1113/jphysiol.2004.065607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sugawara R, Takai Y, Miyazu M, Ohinata H, Yoshida A, Takai A. Agonist and antagonist sensitivity of non-selective cation channel currents evoked by muscarinic receptor stimulation in bovine ciliary muscle cells. Auton Autacoid Pharmacol. 2006; 26(3): 285–92 http://www.ncbi.nlm.nih.gov/pubmed/16879494. doi: 10.1111/j.1474-8673.2006.00347.x [DOI] [PubMed] [Google Scholar]
- 8.Kageyama M, Shirasawa E. L-type voltage-dependent Ca2+ and ATP-sensitive K+ channels are not involved in the regulation of bovine ciliary muscle contractility. J Ocul Pharmacol Ther. 1995; 11(4): 553–63 http://www.ncbi.nlm.nih.gov/pubmed/8574819. doi: 10.1089/jop.1995.11.553 [DOI] [PubMed] [Google Scholar]
- 9.Shibata S, Ishida Y, Kitano H, Ohizumi Y, Habon J, Tsukitani Y, Kikuchi H. Contractile effects of okadaic acid, a novel ionophore-like substance from black sponge, on isolated smooth muscles under the condition of Ca deficiency. J Pharmacol Exp Ther. 1982; 223(1): 135–43 http://www.ncbi.nlm.nih.gov/pubmed/6214623. [PubMed] [Google Scholar]
- 10.Ozaki H, Ishihara H, Kohama K, Nonomura Y, Shibata S, Karaki H. Calcium-independent phosphorylation of smooth muscle myosin light chain by okadaic acid isolated from black sponge (Halichondria okadai). J Pharmacol Exp Ther. 1987; 243(3): 1167–73 http://www.ncbi.nlm.nih.gov/pubmed/2826758. [PubMed] [Google Scholar]
- 11.Ozaki H, Kohama K, Nonomura Y, Shibata S, Karaki H. Direct activation by okadaic acid of the contractile elements in the smooth muscle of guinea-pig taenia coli. Naunyn Schmiedebergs Arch Pharmacol. 1987; 335(3): 356–8 http://www.ncbi.nlm.nih.gov/pubmed/3035385. doi: 10.1007/BF00172811 [DOI] [PubMed] [Google Scholar]
- 12.Takai A, Bialojan C, Troschka M, Rüegg JC. Smooth muscle myosin phosphatase inhibition and force enhancement by black sponge toxin. FEBS Lett. 1987; 217(1): 81–4 http://www.ncbi.nlm.nih.gov/pubmed/3036577. doi: 10.1016/0014-5793(87)81247-4 [DOI] [PubMed] [Google Scholar]
- 13.Bialojan C, Rüegg JC, Takai A. Effects of okadaic acid on isometric tension and myosin phosphorylation of chemically skinned guinea-pig taenia coli. J Physiol. 1988; 398: 81–95 http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=1191760&tool=pmcentrez&rendertype=abstract. doi: 10.1113/jphysiol.1988.sp017030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hirano K, Kanaide H, Nakamura M. Effects of okadaic acid on cytosolic calcium concentrations and on contractions of the porcine coronary artery. Br J Pharmacol. 1989; 98(4): 1261–6 http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=1854834&tool=pmcentrez&rendertype=abstract. doi: 10.1111/j.1476-5381.1989.tb12672.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Karaki H, Mitsui M, Nagase H, Ozaki H, Shibata S, Uemura D. Inhibitory effect of a toxin okadaic acid, isolated from the black sponge on smooth muscle and platelets. Br J Pharmacol. 1989; 98(2): 590–6 http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=1854712&tool=pmcentrez&rendertype=abstract. doi: 10.1111/j.1476-5381.1989.tb12633.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ashizawa N, Kobayashi F, Tanaka Y, Nakayama K. Relaxing action of okadaic acid, a black sponge toxin on the arterial smooth muscle. Biochem Biophys Res Commun. 1989; 162(3): 971–6 http://www.ncbi.nlm.nih.gov/pubmed/2764949. doi: 10.1016/0006-291X(89)90768-7 [DOI] [PubMed] [Google Scholar]
- 17.Shibata S, Satake N, Morikawa M, Kown SC, Karaki H, Kurahashi K, Sawada T, Kodama I. The inhibitory action of okadaic acid on mechanical responses in guinea-pig vas deferens. Eur J Pharmacol. 1991; 193(1): 1–7 http://www.ncbi.nlm.nih.gov/pubmed/1710986. doi: 10.1016/0014-2999(91)90192-S [DOI] [PubMed] [Google Scholar]
- 18.Wang XL, Akhtar RA, Abdel-Latif AA. Studies on the properties of myo-inositol-1,4,5-trisphosphate 5-phosphatase and myo-inositol monophosphatase in bovine iris sphincter smooth muscle: effects of okadaic acid and protein phosphorylation. Biochim Biophys Acta. 1994; 1222(1): 27–36 http://www.ncbi.nlm.nih.gov/pubmed/8186262. doi: 10.1016/0167-4889(94)90021-3 [DOI] [PubMed] [Google Scholar]
- 19.Takai A, Sasaki K, Nagai H, Mieskes G, Isobe M, Isono K, Yasumoto T. Inhibition of specific binding of okadaic acid to protein phosphatase 2A by microcystin-LR, calyculin-A and tautomycin: method of analysis of interactions of tight-binding ligands with target protein. Biochem J. 1995; 306(Pt 3): 657–65 http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=1136572&tool=pmcentrez&rendertype=abstract. doi: 10.1042/bj3060657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Watanabe M, Nakano M. Force-inhibiting effect of okadaic acid on skinned rat uterus permeabilized with α-toxin. Pflugers Arch. 1995; 430(5): 754–6 http://www.ncbi.nlm.nih.gov/pubmed/7478929. doi: 10.1007/BF00386172 [DOI] [PubMed] [Google Scholar]
- 21.Uehata M, Ishizaki T, Satoh H, Ono T, Kawahara T, Morishita T, Tamakawa H, Yamagami K, Inui J, Maekawa M, Narumiya S. Calcium sensitization of smooth muscle mediated by a Rho-associated protein kinase in hypertension. Nature. 1997; 389(6654): 990–4 http://www.ncbi.nlm.nih.gov/pubmed/9353125. doi: 10.1038/40187 [DOI] [PubMed] [Google Scholar]
- 22.Somlyo AP, Somlyo AV. Ca2+ sensitivity of smooth muscle and nonmuscle myosin II: modulated by G proteins, kinases, and myosin phosphatase. Physiol Rev. 2003; 83(4): 1325–58 http://www.ncbi.nlm.nih.gov/pubmed/14506307. doi: 10.1152/physrev.00023.2003 [DOI] [PubMed] [Google Scholar]
- 23.Gunst SJ, Zhang W. Actin cytoskeletal dynamics in smooth muscle: a new paradigm for the regulation of smooth muscle contraction. Am J Physiol Cell Physiol. 2008; 295(3): C576–87 http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2544441&tool=pmcentrez&rendertype=abstract. doi: 10.1152/ajpcell.00253.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Walsh AH, Cheng A, Honkanen RE. Fostriecin, an antitumor antibiotic with inhibitory activity against serine/threonine protein phosphatases types 1 (PP1) and 2A (PP2A), is highly selective for PP2A. FEBS Lett. 1997; 416(3): 230–4 http://linkinghub.elsevier.com/retrieve/pii/S0014579397012106. doi: 10.1016/S0014-5793(97)01210-6 [DOI] [PubMed] [Google Scholar]
- 25.Wada S, Usami I, Umezawa Y, Inoue H, Ohba S, Someno T, Kawada M, Ikeda D. Rubratoxin A specifically and potently inhibits protein phosphatase 2A and suppresses cancer metastasis. Cancer Sci. 2010; 101(3): 743–50 http://www.ncbi.nlm.nih.gov/pubmed/20028386. doi: 10.1111/j.1349-7006.2009.01438.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Obara K, Ito Y, Shimada H, Nakayama K. The relaxant effect of okadaic acid on canine basilar artery involves activation of PKCalpha and phosphorylation of the myosin light chain at Thr-9. Eur J Pharmacol. 2008; 598(1-3): 87–93 http://www.ncbi.nlm.nih.gov/pubmed/18835557. doi: 10.1016/j.ejphar.2008.09.023 [DOI] [PubMed] [Google Scholar]
- 27.Obara K, Mitate A, Nozawa K, Watanabe M, Ito Y, Nakayama K. Interactive role of protein phosphatase 2A and protein kinase Calpha in the stretch-induced triphosphorylation of myosin light chain in canine cerebral artery. J Vasc Res. 2010; 47(2): 115–27 http://www.ncbi.nlm.nih.gov/pubmed/19729958. doi: 10.1159/000235966 [DOI] [PubMed] [Google Scholar]
- 28.Ishihara H, Ozaki H, Sato K, Hori M, Karaki H, Watabe S, Kato Y, Fusetani N, Hashimoto K, Uemura D. Calcium-independent activation of contractile apparatus in smooth muscle by calyculin-A. J Pharmacol Exp Ther. 1989; 250(1): 388–96 http://www.ncbi.nlm.nih.gov/pubmed/2545866. [PubMed] [Google Scholar]
- 29.Hori M, Magae J, Han YG, Hartshorne DJ, Karaki H. A novel protein phosphatase inhibitor, tautomycin. Effect on smooth muscle. FEBS Lett. 1991; 285(1): 145–8 http://www.ncbi.nlm.nih.gov/pubmed/1648511. doi: 10.1016/0014-5793(91)80745-O [DOI] [PubMed] [Google Scholar]
- 30.Fukiage C, Mizutani K, Kawamoto Y, Azuma M, Shearer TR. Involvement of phosphorylation of myosin phosphatase by ROCK in trabecular meshwork and ciliary muscle contraction. Biochem Biophys Res Commun. 2001; 288(2): 296–300 http://www.ncbi.nlm.nih.gov/pubmed/11606042. doi: 10.1006/bbrc.2001.5751 [DOI] [PubMed] [Google Scholar]
- 31.Watanabe M, Takano-Ohmuro H. Extensive skinning of cell membrane diminishes the force-inhibiting effect of okadaic acid on smooth muscles of Guinea pig hepatic portal vein. Jpn J Physiol. 2002; 52(2): 141–7 http://www.ncbi.nlm.nih.gov/pubmed/12139772. doi: 10.2170/jjphysiol.52.141 [DOI] [PubMed] [Google Scholar]
- 32.Thieme H, Nass JU, Nuskovski M, Bechrakis NE, Stumpff F, Strauss O, Wiederholt M. The effects of protein kinase C on trabecular meshwork and ciliary muscle contractility. Invest Ophthalmol Vis Sci. 1999; 40(13): 3254–61 http://www.ncbi.nlm.nih.gov/pubmed/10586950. [PubMed] [Google Scholar]
- 33.Harrison S, Spina D, Page CP. The effect of okadaic acid on non-adrenergic non-cholinergic contraction in guinea-pig isolated bronchus. Br J Pharmacol. 1997; 121(2): 181–6 http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=1564673&tool=pmcentrez&rendertype=abstract. doi: 10.1038/sj.bjp.0701114 [DOI] [PMC free article] [PubMed] [Google Scholar]