Abstract
Acute and chronic exposure to arsenic and mercury is known to produce vasoconstriction. There is, however, no clarity concerning the pathways leading to this increased contraction. In this study we elicit and compare maximum contractility of rat aortas under resting conditions in the presence of arsenic and mercury, and delineate pathways mediating this effect. Phenylephrine (PE) induced hypercontraction of 37% and 32% were obtained when isolated aortic segments were exposed to 25 µM As(III) and 6 nM Hg(II), respectively. Isometric contraction measurements in presence of apocynin, verapamil and sodium nitroprusside indicates that the major causes of increased contraction are reactive oxygen species (ROS) and depletion of nitric oxide (NO). Calcium influx plays a minor role in arsenic and mercury caused hypercontraction. In unexposed aorta, eugenol causes relaxation by inhibiting ROS and elevating NO, linalool by blocking voltage dependent calcium channel (VDCC) and elevating NO, and carvone by blocking calcium influx through VDDC. Since the arsenic and mercury hypercontraction is mediated by increased ROS and depleted NO, we hypothesize that molecules which neutralize ROS or elevate NO will be better ameliorators. In line with this argument, we found eugenol to be the best ameliorator of arsenic and mercury hypercontraction followed by linalool and carvone.
Keywords: As(III), Hg(II), eugenol, carvone, linalool
Introduction
Arsenic and mercury are considered high-risk environmental pollutants impacting human health. Chronic exposure to these metals is associated with a wide range of illnesses including cancer, hyperkeratosis, diabetes, and cardiovascular diseases (1). Cardiovascular effects of arsenic and mercury toxicity include hypertension, atherosclerosis, peripheral vascular disorders and vasoconstriction (2, 3, 4). At low and high mercury concentrations in pre-contracted aorta, relaxation and hypercontraction, respectively, has been reported (5). Oxidative stress and endothelial dysfunction are consistently observed in arsenic and mercury exposed aortic smooth muscle, but emerging evidence suggests that these factors also have a causal role in calcium influx leading to vasoconstriction (6, 7). Reactive oxygen species (ROS) may directly alter vascular function or cause changes in vascular tone by altering nitric oxide (NO) bioavailability or signalling (8). ROS-producing entities involved in increased vascular oxidative stress, observed during hypertension, include NADPH oxidase, xanthine oxidase, the mitochondrial respiratory chain and uncoupled endothelial nitric oxide synthase (NOS) (9). Molecules which can quench ROS, inhibit calcium influx or increase availability of NO, will therefore be relaxant and can potentially ameliorate deleterious effects of As(III) and Hg(II) on smooth muscle.
Various essential oils are rich in eugenol, linalool and carvone, which are known to possess strong antioxidant, anti-inflammatory and anti-spasmodic properties. These active principles are reported to show relaxation effect on the cardiovascular system (10-12). The relaxant effects of eugenol and linalool on both the aorta and trachea has been shown in our previous work (13, 14). Additionally, the calcium-dependent and calcium-independent inhibitory effect of eugenol has also been reported on various smooth muscle tissues (10, 15). Linalool, a monoterpene, is a known to be a vasorelaxant in cardiovascular systems (16). Its mechanism of action is still not clear, but a recent report shows a calcium antagonist effect of linalool with NO playing a partial role in mediating this relaxant effect (11). Carvone has been shown to cause vasorelaxation in various systems, its effect on tracheal chains and ileum smooth muscle has been attributed to its calcium antagonist activity (17, 18).
The effect of exposure of the aorta to arsenic and mercury, its underlying mechanism and the possible ameliorative effects of eugenol, linalool and carvone on As(III) and Hg(II) exposed aortic segments is largely unknown. In this study we elicit and compare maximum contractility of aortic segments under resting conditions with that of arsenic and mercury exposed aortas and have attempted to delineate pathways mediating this effect by employing various inhibitors. The relaxant and ameliorative effect of eugenol, (+)-linalool and (–)-carvone are investigated.
Methods
Animals
Male Wistar rats, weighing 300–500 g, (from Project No. 883) as approved by the Institutional Animal Ethical Committee (IAEC, no. 004/2013), Jamia Millia Islamia University (New Delhi, India) were used in this study. They were kept under conditions of constant temperature (27 ± 2 °C) with a standard light/dark cycle (12/12 h), and free access to food and water. All animals were cared for the compliance with the Guide for the Care and Use of Laboratory Animals, published by the US National Institute of Health (National Institute of Health, US).
Solutions and drugs
Eugenol, (+)-linalool, (–)-carvone (Sigma, purity 98.0%) were first dissolved in a small volume of dimethyl sulfoxide (DMSO, Sigma Chemicals Co., St Louis, MO, USA) and the total volume made up with distilled water. Final vehicle concentration to which tissue was exposed was always less than 0.05%. At this concentration DMSO did not influence phenylephrine (PE)-induced contraction (19). PE, acetylcholine (ACh), sodium nitroprusside (SNP), apocynin, verapamil, NG-nitro-L-arginine methyl ester (L-NAME), arsenic (As(NO2)3) and mercury (HgCl2) were procured from Sigma Chemicals, St. Louis, USA. Sodium chloride, potassium chloride, magnesium sulphate, dextrose, calcium chloride, potassium dihydrogen phosphate and sodium bicarbonate obtained from Merck (India) were used for preparation of Krebs buffer with composition (in mM): 120 NaCl; 25 NaHCO3; 1.2 MgSO4; 1.2 KH2PO4; 4.72 KCl, 2.5; CaCl2 and 11 C6H12O6.
Measurement of aortic contractile activity
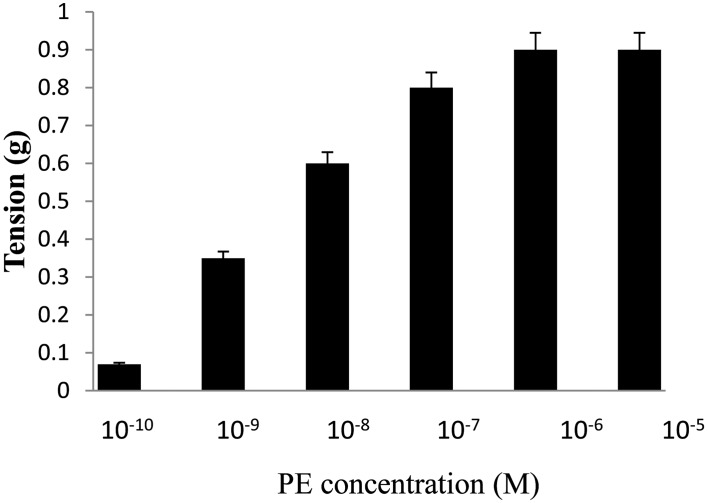
Rats were anesthetized with chloroform and sacrificed by cervical dislocation (20). Thoracic aortae were removed and immersed in Krebs medium at room temperature. After removing adhering fat and connective tissue, each aorta was cut transversally into cylindrical ring-like segments (4–5 mm) with care to avoid any damage to the endothelium. Rings were mounted in 15 mL organ baths containing Krebs medium continuously bubbled with 95% O2 and 5% CO2 at 37 °C. All experiments were performed after an equilibration period of 60 min with bathing medium renewed every 15 min which ruled out trauma or any other extraneous effect. Endothelium intact aortic rings were stretched with a passive tension of 2.0 g. Tension was recorded using an isometric force transducer (MLT0420, AD Instruments, Australia) connected to a PC-based Data acquisition system from AD Instruments (PL3508 PowerLab 8/35). Control contractions were induced at 1 µM PE as we get maximum response at this concentration (Fig. 1), this was in good agreement with other reports (21). Each aortic preparation was challenged at the beginning of the experiment with 1 µM of ACh, and if the vasorelaxant response to ACh was greater than 50% of PE-induced contraction, the aortic segment was considered to possess an intact endothelium.
Fig. 1.
Concentration response curve for PE (10−10–10−5 M) on ring segments of the rat aorta. The data represents the mean ± S.E.M. (n=5); n: number of tissues.
Four series of experiments were performed as follows.
1) Series 1
This series of experiments were carried out to assess the acute effects of 40 min incubation of aortic segments with 10 µM, 25 µM, 50 µM arsenic and 6 nM, 0.5 µM, 1 µM mercury on PE-induced contractions of the endothelium-intact aortic rings.
2) Series 2
In this series of experiments, investigation on the possible mechanisms involved in arsenic and mercury induced hypercontraction was performed by pre-incubating the aortic segments with apocynin (100 µM), verapamil (100 µM) or SNP (1 µM) for 40 min followed by exposure to either As(III) or Hg(II) for another 40 min. The relaxant effect of apocynin (100 µM), verapamil (1 µM) or SNP (1 µM) alone was determined by incubating non-contracted rings for 80 min before eliciting a contractile response.
3) Series 3
This series of experiments were aimed to examine the inhibitory effects of exposure of the aortic rings for 40 min to eugenol, linalool and carvone at 100 µM each, on the contractile responses to PE (1 µM) in unexposed or As(III)/Hg(II) exposed aortic preparations with intact endothelium.
4) Series 4
In this series of experiments, the relaxation pathways for each of eugenol, linalool and carvone were investigated. Experiments were performed on endothelium containing aortic preparations incubated for 40 min with apocynin (100 µM), verapamil (1 µM) or L-NAME (30 µM) followed by another 40 min incubation of tissues with 100 µM of either eugenol or linalool or carvone.
The duration of the incubation in various experiments and controls in respect of As/Hg, eugenol, linalool and carvone was always 40 min. Incubation time for molecules employed as inhibitors i.e. apocynin, verapamil, SNP and L-NAME was always 80 min (both in experiments and controls).
Arsenic and mercury incubation did not lead to any significant change in the applied passive tension of 2.0 g. Of all the other tested molecules viz: apocynin, verapmail, L-NAME, SNP, eugenol, linalool and carvone, only SNP incubation lead to a decrease in the resting tension. This was reset to the passive tension of 2.0 g before eliciting contraction by PE. SNP caused relaxation has also been reported by other investigators (7).
Statistical analysis
Each response was tested on 5 thoracic aortic rings taken from different rats. Data is expressed as the mean ± standard error of the mean (S.E.M.). The significance (P≤0.05) of the results was assessed by means of unpaired Students t-test, and one-way analysis of variance (ANOVA) followed by Duncan's multiple range test.
Results
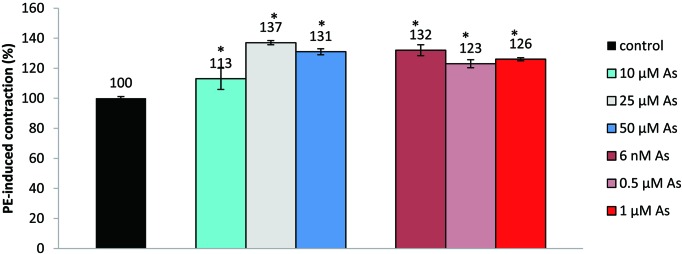
The normal concentration of arsenic in human plasma is 2.4 µg/L which can increase up to 38 µg/L in chronic arsenic exposure (22). Plasma arsenic concentration could vary from 2.2 µM to 44.0 µM in some acute exposures. Arsenic toxicity is found to be more severe in humans than in other species (23). The concentration of HgCl2 that is toxic for humans is about 1.5 µg/L of blood i.e. 7 nM (7). Unlike other studies where the effect of Hg(II) was seen on pre-contracted aortic segments, in this study we have compared the maximum contractile response of aortic segments that have been pre-incubated with As(III) and Hg(II) in the relevant concentration range (5). Optimum incubation time was found to be 40 min. Fig. 2 shows contractile response of aortic rings that have been incubated with various concentrations of As(III) and Hg(II) for 40 min. Increased PE-induced contractility in the case of As(III), compared to unexposed aortic segments, at 10 µM, 25 µM and 50 µM, was 13.0 ± 7.1%, 37.0 ± 1.5% and 31.0 ± 2.0%, respectively. As the optimum response was observed at 25 µM As(III), further experiments were performed at that concentration using As(NO2)3. Similar working concentrations have been employed by other investigators (24). The increase in contraction following incubation with Hg(II) as HgCl2 was 32.0 ± 3.8%, 23.0 ± 2.8% and 26.0 ± 1.1% at Hg(II) concentrations of 6 nM, 0.5 µM and 1 µM, respectively. As the optimal response was seen at 6.0 nM, further studies were performed at 6 nM to ensure healthy tissue and linearity in contractile response. Similar concentration ranges for Hg(II) have been shown to be effective by other workers (7). In the absence of agonist, arsenic 25 µM or mercury (6 nM) incubation did not lead to any significant tension change from the applied passive tension of 2.0 g.
Fig. 2.
Effect of various concentrations of arsenic and mercury on ring segments of the rat aorta. Values represent the mean ± S.E.M. (n=5); *P≤0.05; indicates a significant difference in PE-induced contraction with respect to control (ANOVA) followed by Duncan's multiple range test; n: number of tissues.
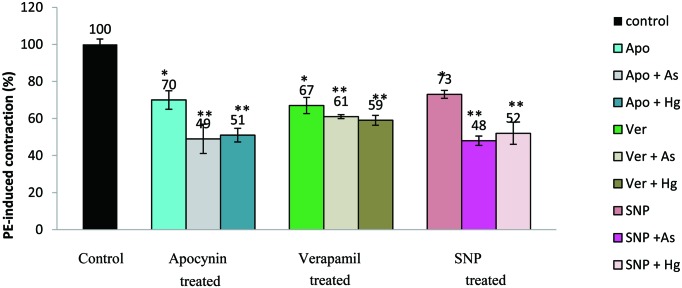
Fig. 3. shows the effects of apocynin, verapamil and SNP on PE-induced contractions for pollutant unexposed and As(III) or Hg(II) incubated aorta. Apocynin and verapamil themselves did not significantly affect the resting tension. SNP, however, caused relaxation of aortic segments from the applied passive tension of 2.0 g, which was reset to 2.0 g before eliciting contraction by PE. Apocynin, a naturally occurring acetophenone widely used as an antioxidant, caused 30.0 ± 5.0% relaxation in unexposed endothelium-intact aortic rings as compared to control rings. PE-induced contractile response was reduced to 33.0 ± 4.5% and 27.0 ± 2.2% in presence of verapamil (L-type calcium channel blocker) and SNP (NO donor), respectively. Pre-incubation of aorta with apocynin (100 µM) followed by pollutant exposure reduced the contractile response by 51.0 ± 7.9% and 49.0 ± 3.7% in the case of arsenic and mercury, respectively. With verapamil and SNP, this decrease in contraction was 39.0 ± 1.2% and 52.0 ± 2.8% for As(III) and 41.0 ± 2.6% and 48.0 ± 6.0% for Hg(II)-exposed tissue.
Fig. 3.
Effect of apocynin, verapamil and SNP on metal unexposed and metal exposed aortic rings. Values represent the mean ± S.E.M. (n=5); **P≤0.05; represents significant difference between effects of apocynin, verapamil, SNP on pollutant exposed and unexposed rings (unpaired Students t- test), *P≤0.05; indicates a significant difference in PE-induced contraction with respect to control (ANOVA) followed by Duncan's multiple range test; n: number of tissues.
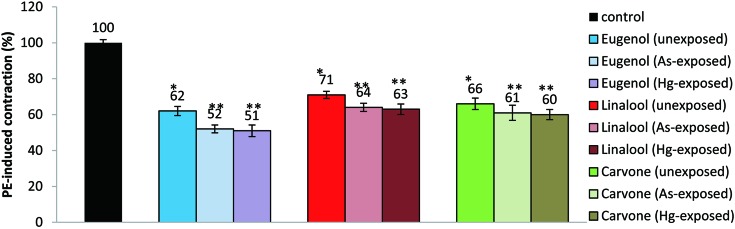
Fig. 4. shows the inhibitory effects of eugenol, linalool and carvone on the contractions induced by PE in rat aortic rings in unexposed and As(III) or Hg(II) exposed preparations. For pollutant unexposed rings optimal responses for eugenol, linalool and carvone was seen following 40 min incubation, so this duration was used for all experiments with these molecules. Eugenol, linalool and carvone alone did not cause any change in the applied passive tension in the absence of PE. Eugenol caused 38.0 ± 2.5%, 48.0 ± 2.2% and 49.0 ± 3.3% inhibition of contraction in metal unexposed, As(III) exposed and Hg(II) exposed aortic rings, respectively. Similar inhibition of contraction caused by linalool was 29.0 ± 2.0%, 36.0 ± 2.3% and 37.0 ± 2.9% for unexposed, As(III) exposed and Hg(II) exposed tissue. Carvone showed a relaxant effect of 34.0 ± 3.2% in unexposed; 39.0 ± 4.2% in arsenic exposed; and 40.0 ± 2.9% in mercury exposed aortic segments, respectively.
Fig. 4.
Effect of eugenol (100 µM), linalool (100 µM) and carvone (100 µM) on the contraction induced by PE in endothelium-intact thoracic aortic rings. Results are presented as the mean ± S.E.M. (n=5); **P≤0.05 compares a significant variation in contractions shown by these molecules in both unexposed and exposed ring segments of the rat aorta (unpaired Students t-test); *P≤0.05 shows significant difference of PE-induced contractions shown by eugenol, linalool and carvone versus control (ANOVA) followed by Duncan's multiple range test; n: number of tissues.
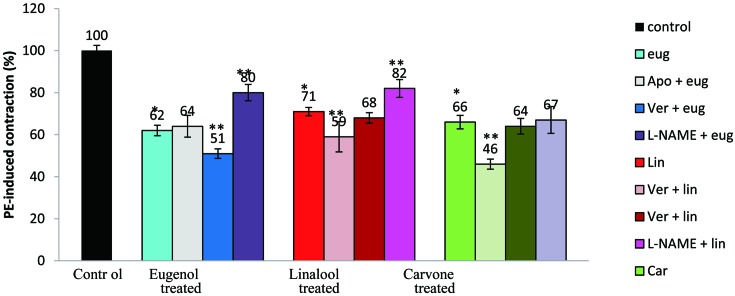
The effects of various inhibitors on the contraction elicited by aortic rings incubated with eugenol, linalool or carvone are illustrated in Fig. 5. The saturating effect producing concentration of apocynin (100 µM) caused relaxations of 36 ± 5.2%, 41.0 ± 7.2% and 54.0 ± 2.5% with eugenol, linalool and carvone, respectively. In the presence of verapamil (1 µM), eugenol, linalool and carvone showed 49.0 ± 2.3%, 32.0 ± 4.9% and 36.0 ± 1.5% inhibition of contraction, respectively. L-NAME (30 µM); a potent inhibitor of NO, caused a decrease in the contractile response of eugenol, linalool and carvone by 20.0 ± 3.99%, 18.0 ± 4.2% and 33.0 ± 6.4%, respectively. In an earlier study, we have shown eugenol to be a more effective relaxant in the presence of indomethacin, to be less effective in the presence of L-NAME, while there was no change in relaxation in the presence of apocynin (13). Co-incubation of eugenol with verapamil caused more relaxation than eugenol alone. Linalool and carvone were more effective relaxants in the presence of apocynin, whereas the presence of verapamil did not alter the aortic contractility in respect of either linalool or carvone. L-NAME decreased the effectiveness of linalool as a relaxant but made no difference to the relaxant capacity of carvone.
Fig. 5.
Effect of eugenol, linalool and carvone and in both the absence and presence of apocynin (100 µM), verapamil (1 µM) and L-NAME (30 µM) in pollutant unexposed ring segments of the rat aorta. Values represent the mean ± S.E.M. (n=5); **P≤0.05 represents significant contraction variation by these plant derivatives in the presence versus the absence of inhibitors (unpaired Students t- test), *P≤0.05 shows a significant difference in PE-induced contractions by eugenol, linalool and carvone versus control (ANOVA) followed by Duncan's multiple range test; n: number of tissues.
Discussion
In this study we have shown that in isolated aortic segments under resting conditions when exposed to arsenic and mercury, there is an increase in isometric tension measured via agonist stimulation in an organ bath. Incubation in the presence of 25 µM arsenic and 6 nM mercury did not have any effect on the applied passive tension (2.0 g) in the absence of agonist, but caused hypercontraction of 37% and 32% after PE-induction. Under similar in vitro conditions, arsenic and mercury also cause hypercontraction of tracheal smooth muscle (unpublished results). These results are in line with the existing literature suggesting aortic vasoconstriction with arsenic (4). Mercury has also been reported to enhance contraction responses in aortic segments (7). A biphasic effect of mercury has recently been reported, with vasorelaxation at lower concentrations and vasoconstriction at higher concentrations in pre-contracted aortic segments (5). We did not observe biphasic responses in this study, possibly because we measured the tension after 40 min of Hg(II) incubation of the resting aortic segments which may lead to generation of ROS.
The excitation-relaxation mechanism of smooth muscle is regulated by changes in the intracellular calcium concentration (25), vasodilation by endothelium released nitric oxide (26), and excessive production of ROS (27). In this study, each of these pathways were inhibited to gain insight into the hypercontraction mechanisms of As(III) and Hg(II). In unexposed rings, magnitude of the maximum inhibition of contraction (or relaxation) caused by apocynin, verapamil, and SNP was almost identical, indicating that all major pathways contribute almost equally to the measured contraction. The decreased contractile responses shown by the arsenic and mercury exposed aortic rings in the presence of apocynin and SNP was greater as compared to unexposed aortic segments. This difference was 21% and 25% for As(III), and 19% and 21% for Hg(II). The difference in relaxation given by verapamil for unexposed and As(III) or Hg(II) exposed aorta was only 6% and 8%, respectively. The significantly higher effectiveness of apocynin and SNP in the case of pollutant hypercontracted aortic segments indicates that ROS generation and NO depletion play major roles in causing hypercontraction. This seems to be in agreement with other studies which indicate that exposure to arsenic and mercury cause overproduction of ROS resulting in oxidative stress with subsequent damage to endothelium which leads to reduced NO bioavailability in the vascular system (7, 28). In the presence of verapamil, the small difference in contraction suggests that calcium influx plays a minor role in hypercontraction of smooth muscle tissues in response to acute exposure to either arsenic or mercury.
The fact that plant-derived agents, eugenol, linalool and carvone, possess powerful anti-oxidant and calcium antagonist activity, highlights the importance of understanding their effects on As(III) and Hg(II) caused hypercontraction and the underlying mechanisms. Pre-incubation of aortic segments with eugenol, linalool or carvone could effectively reduce PE-induced contraction. Similar relaxant actions by these active compounds have been shown by others investigators in various smooth muscle tissues (11, 12, 15). Eugenol has been reported to inhibit PE-induced contraction by the same magnitude in both the absence and presence of apocynin, suggesting that it acts by inhibiting ROS (13). Increased inhibition of contraction seen when verapamil was co-incubated with eugenol points to different site of action of these two molecules. We have also observed similar antioxidant effects of eugenol in the tracheal system (14). These results are in line with those suggesting that eugenol exerts cardiopreventive effects through its antioxidant properties (29), while a calcium antagonist action of eugenol has also been observed, but at higher concentrations (10). The magnitude of relaxation shown by carvone or linalool in the presence of verapmail was unchanged, but these molecules when co-incubated with apocynin lead to significantly increased relaxation. This indicates that carvone and linalool cause relaxation via calcium channel blockage. These observations are consistent with reports suggesting that both linalool and carvone may act as calcium antagonists (11, 18). L-NAME, known to inactivate NOS (30), caused the relaxation elicited by eugenol and linalool to decrease, while that of carvone remained unchanged. This indicates that the vasorelaxation caused by eugenol and linalool is NO-dependent whereas the vasorelaxing actions of carvone are NO-independent. The results obtained in this study are supported by reports that both eugenol and linalool induce in vivo antispasmodic effects which are endothelium released nitric oxide dependent whereas carvone acts by NO-independent mechanisms in rat isolated stomach preparations (11, 31, 32).
To summarize, in rat aortas eugenol causes relaxation by inhibiting ROS and elevating NO, linalool inhibits contraction by blocking L-type voltage dependent calcium channels (VDCC) and elevating NO. Carvone causes relaxation by blocking calcium influx through VDDC. As(III) and Hg(II) caused hypercontraction is mediated by increased ROS and depletion of NO. Based on this we hypothesize that molecules which neutralize ROS or elevate NO will be ameliorators of arsenic and mercury caused vasoconstriction. In line with this we find eugenol, which neutralizes ROS and elevates NO, to be best ameliorator followed by linalool which only elevates NO in this system. Carvone with its major effect only of VDCC blocking comes out as poor ameliorator of As(III) and Hg(II) caused hypercontraction of aorta.
Conflict of interest
The authors declare no conflict of interest.
Acknowledgments
This work was supported by the funding from UGC (SAP), DRS-I (F.3–20/2011) to Prof. SFB and Prof. LAK.
References
- 1.ATSDR (Agency for Toxic Substances and Disease Registry) Priority list of hazardous substances. [Internet]. Department of Health and Human Services, Public Health Service, USA, Atlanta, 2011. Available from: http://www.atsdr.cdc.gov/spl/.
- 2.Chen CJ, Chiou HY, Chiang MH, Lin LJ, Tai TY. Dose-response relationship between ischemic heart disease mortality and long-term arsenic exposure. Arterioscler Thromb Vasc Biol. 1996; 16(4): 504–10. doi: 10.1161/01.ATV.16.4.504 [DOI] [PubMed] [Google Scholar]
- 3.Houston MC. Role of mercury toxicity in hypertension, cardiovascular disease, and stroke. J Clin Hypertens (Greenwich). 2011; 13(8): 621–7. doi: 10.1111/j.1751-7176.2011.00489.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee MY, Lee YH, Lim KM, Chung SM, Bae ON, Kim H, Lee CR, Park JD, Chung JH. Inorganic arsenite potentiates vasoconstriction through calcium sensitization in vascular smooth muscle. Environ Health Perspect. 2005; 113(10): 1330–5. doi: 10.1289/ehp.8000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Omanwar S, Saidullah B, Ravi K, Fahim M. Vasorelaxant effects of mercury on rat thoracic aorta: the nitric oxide signaling mechanism. Hum Exp Toxicol. 2014; 33(9): 904–10. doi: 10.1177/0960327113512341 [DOI] [PubMed] [Google Scholar]
- 6.Li JX, Shen YQ, Cai BZ, Zhao J, Bai X, Lu YJ, Li XQ. Arsenic trioxide induces the apoptosis in vascular smooth muscle cells via increasing intracellular calcium and ROS formation. Mol Biol Rep. 2010; 37(3): 1569–76. doi: 10.1007/s11033-009-9561-z [DOI] [PubMed] [Google Scholar]
- 7.Lemos NB, Angeli JK, Faria T O, Ribeiro Junior RF, Vassallo DV, Padilha AS, Stefanon I. Low mercury concentration produces vasoconstriction, decreases nitric oxide bioavailability and increases oxidative stress in rat conductance artery. PLoS One. 2012; 7(11): e49005. doi: 10.1371/journal.pone.0049005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang HD, Pagano PJ, Du Y, Cayatte AJ, Quinn MT, Brecher P, Cohen RA. Superoxide anion from the adventitia of the rat thoracic aorta inactivates nitric oxide. Circ Res. 1998; 82(7): 810–8. doi: 10.1161/01.RES.82.7.810 [DOI] [PubMed] [Google Scholar]
- 9.Clempus RE, Griendling KK. Reactive oxygen species signaling in vascular smooth muscle cells. Cardiovasc Res. 2006; 71(2): 216–25. doi: 10.1016/j.cardiores.2006.02.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Damiani CE, Rossoni LV, Vassallo DV. Vasorelaxant effects of eugenol on rat thoracic aorta. Vascul Pharmacol. 2003; 40(1): 59–66. doi: 10.1016/S1537-1891(02)00311-7 [DOI] [PubMed] [Google Scholar]
- 11.Anjos PJ, Lima AO, Cunha PS, De Sousa DP, Onofre AS, Ribeiro TP, Medeiros IA, Antoniolli AR, Quintans-Júnior LJ, Santosa MR. Cardiovascular effects induced by linalool in normotensive and hypertensive rats. Z Naturforsch C. 2013; 68(5-6): 181–90. doi: 10.5560/ZNC.2013.68c0181 [DOI] [PubMed] [Google Scholar]
- 12.Consolini AE, Berardi A, Rosella MA, Volonte M. Antispasmodic effects of aloysia polystachya and a. gratissima tinctures and extracts are due to non-competitive inhibition of intestinal contractility induced by acethylcholine and calcium. Rev Bras Farmacogn. 2011; 21(5): 889–900. doi: 10.1590/S0102-695X2011005000137 [DOI] [Google Scholar]
- 13.Shabir H, Kundu S, Basir SF, Khan LA. Modulation of Pb(II) caused aortal constriction by eugenol and carvacrol. Biol Trace Elem Res. 2014; 161(1): 116–22. doi: 10.1007/s12011-014-0081-x [DOI] [PubMed] [Google Scholar]
- 14.Shabir H, Kundu S, Basir SF, Khan LA. Amelioration of lead and cadmium- induced rat tracheal hypercontraction by linalool and eugenol. Toxicol Environ Chem. 2014; 96(2): 307–17. doi: 10.1080/02772248.2014.931520 [DOI] [Google Scholar]
- 15.Leal-Cardoso JH, Lahlou S, Coelho-de-Souza AN, Criddle DN, Pinto Duarte GI, Santos MA, Magalhães PJ. Inhibitory actions of eugenol on rat isolated ileum. Can J Physiol Pharmacol. 2002; 80(9): 901–6. doi: 10.1139/y02-117 [DOI] [PubMed] [Google Scholar]
- 16.Menezes IA, Barreto CM, Antoniolli AR, Santos MR, de Sousa DP. Hypotensive activity of terpenes found in essential oils. Z Naturforsch C. 2010; 65(9-10): 562–6. doi: 10.1515/znc-2010-9-1005 [DOI] [PubMed] [Google Scholar]
- 17.Boskabady MH, Kiani S, Azizi H. Relaxant effect of cuminum cyminum on guinea pig tracheal chains and its possible mechanism(s). Indian J Pharmacol. 2005; 37(2): 111–5. doi: 10.4103/0253-7613.15111 [DOI] [Google Scholar]
- 18.Souza FV, da Rocha MB, de Souza DP, Marçal RM. (-)-Carvone: antispasmodic effect and mode of action. Fitoterapia. 2013; 85: 20–4. doi: 10.1016/j.fitote.2012.10.012 [DOI] [PubMed] [Google Scholar]
- 19.Ansari HR, Nadeem A, Talukder MA, Sakhalkar S, Mustafa SJ. Evidence for the involvement of nitric oxide in A2B receptor-mediated vasorelaxation of mouse aorta. Am J Physiol Heart Circ Physiol. 2007; 292(1): H719–25. doi: 10.1152/ajpheart.00593.2006 [DOI] [PubMed] [Google Scholar]
- 20.Khalil-Manesh F, Gonick HC, Weiler EW, Prins B, Weber MA, Purdy RE. Lead-induced hypertension: possible role of endothelial factors. Am J Hypertens. 1993; 6(9): 723–9. [DOI] [PubMed] [Google Scholar]
- 21.Qian Q, Hunter LW, Du H, Ren Q, Han Y, Sieck GC. Pkd2+/- vascular smooth muscles develop exaggerated vasocontraction in response to phenylephrine stimulation. J Am Soc Nephrol. 2007; 18(2): 485–93. doi: 10.1681/ASN.2006050501 [DOI] [PubMed] [Google Scholar]
- 22.Heydorn K. Environmental variation of arsenic levels in human blood determines by neutron activation analysis. Clin Chim Acta. 1970; 28(2): 349–57. doi: 10.1016/0009-8981(70)90101-4 [DOI] [PubMed] [Google Scholar]
- 23.Chan PC, Huff J. Arsenic carcinogenesis in animals and in humans: mechanistic, experimental, and epi-demiological evidence. J Environ Sci Health Part C. 1997; 15(2): 83–122. doi: 10.1080/10590509709373492 [DOI] [Google Scholar]
- 24.Lee MY, Jung BI, Chung SM, Bae ON, Lee JY, Park JD, Yang JS, Lee H, Chung JH. Arsenic-induced dysfunction in relaxation of blood vessels. Environ Health Perspect. 2003; 111(4): 513–7. doi: 10.1289/ehp.5916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Berridge MJ. Smooth muscle cell calcium activation mechanisms. J Physiol. 2008; 586(21): 5047–61. doi: 10.1113/jphysiol.2008.160440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barthó L, Lefebvre RA. Nitric oxide-mediated contraction in enteric smooth muscle. Arch Int Pharmacodyn Ther. 1995; 329(1): 53–66. [PubMed] [Google Scholar]
- 27.Tsai MH, Jiang MJ. Reactive oxygen species are involved in regulating a1-adrenoceptor-activated vascular smooth muscle contraction. J Biomed Sci. 2010; 17(67): 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kumagai Y, Pi J. Molecular basis for arsenic-induced alteration in nitric oxide production and oxidative stress: implication of endothelial dysfunction. Toxicol Appl Pharmacol. 2004; 198(3): 450–7. doi: 10.1016/j.taap.2003.10.031 [DOI] [PubMed] [Google Scholar]
- 29.Choudhary R, Mishra KP, Subramanyam C. Prevention of isoproterenol-induced cardiac hypertrophy by eugenol, an antioxidant. Indian J Clin Biochem. 2006; 21(2): 107–13. doi: 10.1007/BF02912923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pfeiffer S, Leopold E, Schmidt K, Brunner F, Mayer B. Inhibition of nitric oxide synthesis by NG-nitro-L-arginine methyl ester (L-NAME): requirement for bioactivation to the free acid, NG-nitro-L-arginine. Br J Pharmacol. 1996; 118(6): 1433–40. doi: 10.1111/j.1476-5381.1996.tb15557.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pires AF, Madeira SV, Soares PM, Montenegro CM, Souza EP, Resende AC, Soares de Moura R, Assreuy AM, Criddle DN. The role of endothelium in the vasorelaxant effects of the essential oil of Ocimum gratissimum in aorta and mesenteric vascular bed of rats. Can J Physiol Pharmacol. 2012; 90(10): 1380–5. doi: 10.1139/y2012-095 [DOI] [PubMed] [Google Scholar]
- 32.Siqueira BPJ, Menezes CT, Silva JP, de Sousa DP, Batista JS. Antiulcer effect of epoxy-carvone. Rev Bras Farm. 2011; 22(1): 144–9. doi: 10.1590/S0102-695X2011005000172 [DOI] [Google Scholar]