Abstract
Various studies have shown that pregnancy is associated with gastrointestinal complaints that might result from disturbance of the normal contractile pattern of smooth muscle. Progesterone is an important steroid hormone, which plays a crucial role in female pregnancy. Progesterone affects muscle cells by genomic mechanisms, through nuclear receptors, and non-genomic mechanisms, through unidentified pathways. Non-genomic actions were defined as those occurring within 10 min of progesterone exposure. The aim of the present study was to investigate the non-genomic effect of progesterone on Rho kinase II activity in gastric smooth muscle. Single smooth muscle cells of the stomach obtained from Sprague Dawley rats were used. Dispersed gastric smooth muscle cells were treated with progesterone or acetylcholine (ACh) separately. Cells designated for progesterone treatment were incubated with 1 μM progesterone for 10 min. Rho kinase II expression and both basal and ACh-induced Rho kinase II activity were measured via specifically designed enzyme-linked immunosorbent assay (ELISA) and activity assay kits respectively in both control and progesterone-treated groups. Progesterone inhibited the ACh-induced, but not the basal, Rho kinase II activity in dispersed gastric smooth muscle cells without affecting its expression level. This study suggested that progesterone can rapidly affect the contractile activity of isolated gastric smooth muscle cells in rats via inhibition of the Rho kinase II pathway.
Keywords: mooth muscle, contraction, Rho kinase, progesterone
Introduction
Pregnancy is usually associated with various gastrointestinal (GI) complaints such as nausea, vomiting, reduced colonic activity resulting in varying degrees of constipation, and various gastric emptying disorders (1,2,3). There is an accumulating body of evidence to suggest that the myoelectric and motor activities of the GI smooth muscle are disturbed during pregnancy. In support of this suggestion, it has been found that pregnancy is associated with decreased gallbladder contractivity (4, 5), lowered esophageal sphincter pressure (6,7,8), reduced gastric emptying (9, 10), and reduced small intestinal (11) and colonic transit times (12). However, the exact molecular mechanisms for such pregnancy-associated GI disorders are still poorly understood.
Progesterone and estrogen are significantly elevated in the serum of pregnant females. These hormones play central roles in the maintenance of pregnancy and the initiation of parturition by modulating myometrial contractility and excitability. In addition, recent studies have shown that sex hormones target other body organs besides the myometrium, such as the GI tract, bladder, and blood vessels (13,14,15).
Functionally, progesterone affects mammalian cells by both genomic and non-genomic mechanisms. The genomic actions of progesterone are mediated via two sub-nuclear receptors, A and B, which act as transcription factors (16). Genomic actions of progesterone on the GI smooth muscle might explain some disturbances that complicate pregnancy and female functional disorders. For example, it has been shown that progesterone signaling regulates G protein expression level in female slow transit chronic constipation, which correlates with an over-expression of progesterone receptors (17).
The mechanisms responsible for the non-genomic effects of progesterone are not fully understood. Non-genomic effects of progesterone have been defined as those occurring within 10 min of exposure (18, 19). It has been proposed that progesterone interacts with plasmalemmal receptors and might lead to rapid activation of tyrosine kinases and phospholipases (20), mitogen-activated protein kinase (MAPK) (21), or inhibition of membrane transport systems (22). Furthermore, the types of changes induced by the non-genomic actions may be tissue specific, as diverse effects have been demonstrated in muscle, neural, endocrine, and reproductive cells (22,23,24).
Physiologically, smooth muscle is an important component of the GI tract and maintenance of its normal contractile behavior is essential for proper GI functions. Rho kinase II, a serine/threonine kinase, is an important downstream effector of the small G protein RhoA that has been found to be important in developing smooth muscle tone by maintaining the level of myosin light chain (MLC20) phosphorylation, —the essential step in smooth muscle contraction (25). The importance of the RhoA/Rho kinase pathway has been demonstrated in the pathogenesis of cardiovascular disorders and as a target in the development of new drugs such as fasudil, a Rho kinase inhibitor (25, 26). Exploring changes in Rho/Rho kinase pathway expression and activity in GI smooth muscle during pregnancy and the effect of this on muscle contractility could be an important step for a better understanding of the GI complaints that accompany pregnancy.
Although numerous studies have examined the effect of progesterone on GI smooth muscle, its effect on gastric Rho kinase II has not been explored. Therefore, the present study was designed to investigate the non-genomic action of progesterone on the Rho kinase pathway in smooth muscle cells of the stomach. Because progesterone may affect various types of gastric cells, studying the role of progesterone on a single cell type in a multicellular preparation could be difficult. For this reason, this study was performed on single smooth muscle cells freshly isolated from the stomach of rat. Insights into the molecular basis of abnormal smooth muscle function will prove invaluable in the treatment of GI motility disorders associated with pregnancy.
Materials and Methods
Male Sprague Dawley (SD) rats were provided by the animal house of Jordan University of Science and Technology (JUST). Male rats were used in this study to avoid possible confounding influence from differing circulating progesterone levels in cycling female rats. All procedures were approved by the Animal Care and Use Committee (ACUC) at JUST. Male SD rats (6 weeks of age, 200–250 g) were sacrificed by an overdose of ether.
Assays were performed using a Rho kinase II assay kit (Cell Biolabs, INC., CA, USA), a Rho kinase II ELISA kit (Cusabio Biotech, Newark, DE, USA) and a Dc protein assay kit (Bio-Rad, Hercules, CA, USA). The Anti-calponin antibody (ab46794) was purchased from Abcam, Cambridge, MA, USA. The 500-μm Nitex mesh was purchased from Amazon. All remaining materials were purchased from Sigma, St Louis, MO, USA. Progesterone was dissolved and diluted in 99% ethanol while acetylcholine (ACh) was dissolved in distilled water.
Preparation of dispersed gastric smooth muscle cells
Smooth muscle cells were isolated from the circular muscle layer of the rat stomach by sequential enzymatic digestion, filtration, and centrifugation as described previously (27, 28). Briefly, strips of circular muscle from the stomach were dissected and incubated at 31 °C for 30 min in HEPES medium containing 120 mM NaCl, 4 mM KCl, 2.0 mM CaCl2, 2.6 mM KH2PO4, 0.6 mM MgCl2, 25 mM HEPES, 14 mM glucose, 2.1% Eagle's essential amino acid mixture, 0.1% collagenase, and 0.01% soybean trypsin inhibitor. The tissue was continuously gassed with 100% oxygen during the entire isolation procedure. After the partly digested strips were washed twice with 50 ml of enzyme-free medium, the muscle cells were allowed to disperse spontaneously for 30 min. The cells were harvested by filtration through 500-μm Nitex mesh and centrifuged twice at 350 g for 10 min to eliminate broken cells and organelles. The cells were counted in a hemocytometer and it is estimated that 95% of the cells excluded trypan blue. All the experiments were done within 2–3 h of cell dispersion.
Identification of smooth muscle cells
The smooth muscle identity of rat gastric muscle cells was verified by immunohistochemical staining of paraffin-embedded rat smooth muscle using ab46794 at 1/100 dilution with anti-calponin antibody.
Progesterone treatment
Aliquots (0.4 ml) of dispersed gastric smooth muscle cells were prepared. Aliquots were randomly distributed into either control or treatment groups. Aliquots which were designated for progesterone treatment were incubated with progesterone (1 µM) for 10 min in the presence or absence of ACh.
Measurement of Rho kinase II activity
Rho kinase II activity was analyzed by an enzyme immunoassay, using Cell Biolabs' 96-well Rho kinase II activity assay kit. Experiments were done according to the manufacture's protocol, using 10 µl of protein lysate. The total starting protein concentration for every sample was 1 mg/ml.
Measurement of Rho kinase II expression
Muscle cells were solubilized in Triton X-100-based lysis buffer plus protease and phosphatase inhibitors (100 µg/ml phenylmethanesulfonylfluoride (PMSF), 10 µg/ml aprotinin, 10 µg/ml leupeptin, 30 mM sodium fluoride and 3 mM sodium vanadate). After centrifugation of the lysates at 20,000 g for 10 min at 4 °C, the protein concentrations of the supernatant were determined with a Dc protein assay kit from Bio-Rad. Samples of equal amounts of proteins were quantitated by ELISA according to the manufacturers' instructions.
Analysis of data
Each experiment was performed on gastric smooth muscle cells that were harvested from six rats. Statistical analysis of all experiments was performed using Prism 5.0 software, GraphPad Software, San Diego, CA. For Rho kinase activity experiments, a one-way analysis of variance (ANOVA) was performed. Where the ANOVA was statistically significant, it was followed by Fisher's post-hoc analysis to determine the significance of differences between experimental groups. For the Rho kinase expression experiments, an unpaired student t-test was used to reveal significant differences between the compared groups. A P<0.05 was required for statistical significance in all the experiments. All data are shown as mean ± standard error of the mean (S.E.M).
Results
Smooth Muscle Identity
The smooth muscle identity of rat gastric muscle cells was verified by immunohistostaining with anti-calponin antibody. The results showed that greater than 95% of cells stained positive for calponin (Fig. 1).
Fig. 1.
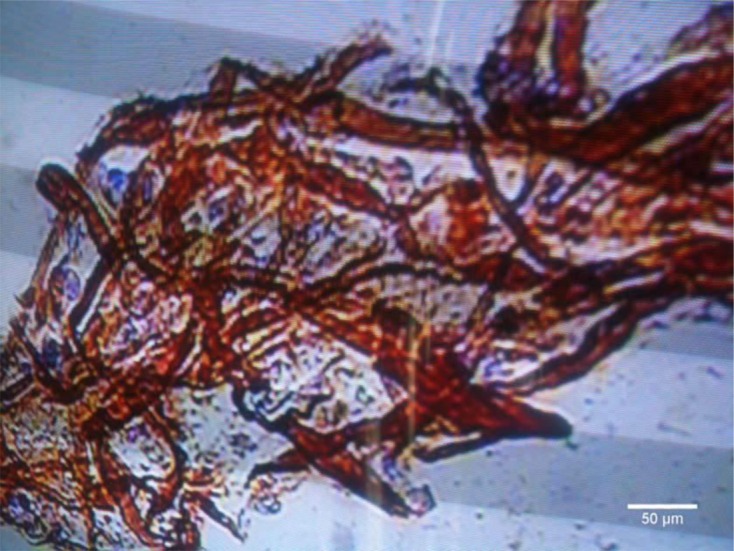
Immunohistochemical staining of paraffin-embedded rat smooth muscle using ab46794 at 1/100 dilution. Scale bar represents 50 μm.
Rho kinase II activity
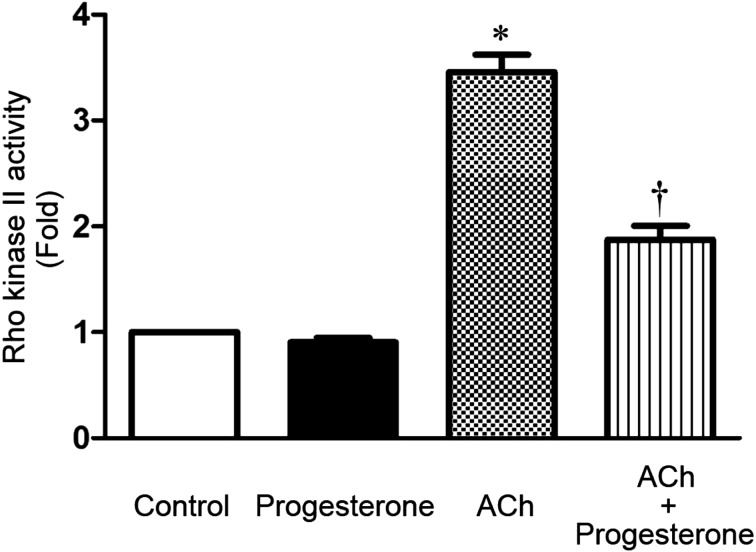
Treatment of freshly dispersed muscle cells with 0.1 μM ACh, a Gαq/13-coupled receptor agonist, for 10 min increased Rho kinase I—the smooth muscle predominant isoform (29)— activity above basal level (P<0.05, n=6). Importantly, incubation of gastric smooth muscle cells with 1 µM progesterone significantly inhibited ACh-stimulated Rho kinase II activity (P<0.05, n=6). Interestingly, progesterone had no effect on basal Rho kinase II activity (P>0.05, n=6) (Fig. 2).
Fig. 2.
Effect of progesterone incubation (1 μM for 10 min) on the basal and ACh-induced (ACh; 0.1 μM) activity of Rho kinase II in isolated rat gastric smooth muscle cells. Rho kinase II activity is expressed as multiples of the control levels (fold change). Progesterone didn't affect the basal Rho kinase activity (second column). ACh significantly augmented Rho kinase II activity (third column). Progesterone treatment significantly inhibited ACh-induced Rho kinase II activity (fourth column). Values shown are representative of at least four independent experiments performed in triplicate. (*, P<0.05 vs. control; †, P<0.05 vs. ACh, by two-way ANOVA followed by Bonferroni multiple comparison test).
Rho kinase II expression
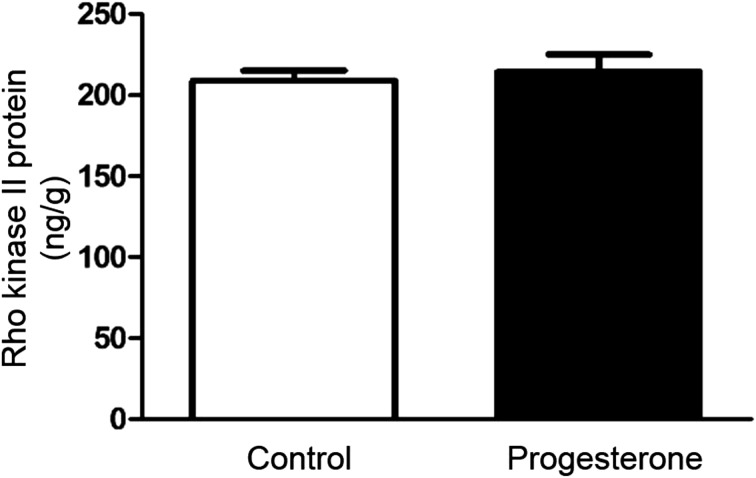
To determine whether progesterone had any effect on Rho kinase II expression profile we examined the expression level of Rho kinase II protein in control and progesterone-treated cells by ELISA. Despite the lower agonist-stimulated Rho kinase II activity in progesterone-treated cells compared to control, the expression of Rho kinase II protein was not different in both groups of cells (P>0.05, n=6) (Fig. 3). Consistent with the indifferent expression level of Rho kinase II protein, basal Rho kinase II activity was similar in both groups (P>0.05, n=6) (Fig. 2).
Fig. 3.
Effect of progesterone incubation (1 μM for 10 min) on the expression levels of Rho kinase II protein in rat isolated gastric smooth muscle cells. Rho kinase II protein expression level is expressed as ng/g of total protein. Rho kinase II protein expression was not affected by progesterone treatment. Values shown are representative of at least four independent experiments performed in triplicate. (P>0.05 by unpaired t test).
Discussion
It is well-known now that pregnancy is commonly associated with GI complaints such as nausea, vomiting, and improper gastric emptying of solids and liquids. Recent research has reported disturbances in the myoelectric, mechanical, and motor properties and activities of the GI smooth muscle during pregnancy (4,5,6,7,8,9,10,11,12). Still, the precise mechanism for such gut effects is poorly understood. In this study we found that progesterone treatment for 10 min rapidly decreased the ACh-induced activity level of Rho kinase II in rat gastric smooth muscle cells without affecting Rho kinase II expression. Based on these findings, we propose that this rapid progesterone inhibitory effect may contribute to the change in gastric motility during pregnancy.
This rapid hormonal effect on Rho kinase II represents mostly non-genomic action of progesterone, as the short incubation time of the hormone would not allow changes in protein expression levels to occur (18, 19). Our expression data negate an effect for short progesterone treatment on Rho kinase II protein levels. In support of these findings, basal Rho kinase II activity was not affected by progesterone incubation. Indeed, several previous reports have indicated that progesterone induces rapid, within 10 min, non-genomic effects in a variety of tissue types. For example, Bielefeldt et al. (22) found, using a human intestinal smooth muscle cell line, that progesterone reduced calcium currents consistent with blocking the L-type calcium channel. Both of these effects occurred very rapidly (within 1 min) and were not blocked by progesterone antagonists, which would impede genomic actions of progesterone and other progestins (19, 22). In addition, Xio et al. (30) reported that progesterone transiently inhibited calcium release from storage sites of colonic muscle cells and blocked the contraction to cholecystokinin (CCK-8) and neurokinin A (NKA). Moreover, a group of researchers showed in a well-designed experiment that cell-impermeant albumin-conjugated progesterone decreased thromboxane A2 receptor agonist-stimulated vascular smooth muscle calcium responses (31). This conjugated progesterone is believed not to rapidly cross the cell membrane and thus acts extracellulary. These findings might explain the rapid vasodilator action of progesterone in the primate coronary artery and isolated vascular smooth muscle. In spermatozoa, progesterone was also found to increase intracellular calcium levels by acting on a distinct non-genomic cell surface receptor. It is thought that this rapid effect of progesterone initiates the acrosomal reaction (19). These data strongly suggest the existence of an independent surface membrane progesterone receptor distinct from the classical nuclear progesterone receptor that is part of the transcription-activating superfamily. Furthermore, a progesterone binding membrane protein was isolated and cloned from porcine coronary artery muscle cells (32). Such a protein might be also expressed in the smooth muscle of other organs such as the stomach and could provide a possible explanation for the observed rapid non-genomic effects of progesterone on gastric Rho kinase II. Whether progesterone mediates its non-genomic action via affecting other membrane receptors such as G protein receptors is unknown so far.
Rho kinase II, the predominant Rho kinase expressed in smooth muscle, has been found to be important in developing smooth muscle tone by maintaining the level of MLC20 phosphorylation, the essential step in smooth muscle contraction (25). There is an accumulating body of evidence from previous reports to support a genomic inhibitory effect of progesterone on the contractility of smooth muscle in various organs such as the stomach (33), colon (12, 33), and gallbladder (34). This hormone effect might contribute to gastric dysmotility during pregnancy. Most importantly, progesterone was shown to induce rapid relaxation of KCl-induced contraction of rat aortic rings that was not mediated by gene transcription mechanisms. This relaxant effect of progesterone on aortic rings was partially dependent on endothelial NO production (35).
A non-genomic effect of progesterone on gastric smooth muscle contractility has not been investigated before. This study has shown for the first time that Rho kinase II is a target for rapid progesterone action in isolated smooth muscle cells of the stomach. Indeed, studying progesterone-mediated changes in the Rho kinase pathway activity and thus muscle contractility in the GI tract could be an important step for a better understanding of the GI complaints that accompany pregnancy. In conclusion, our results indicate that progesterone inhibited Rho kinase II activity in gastric smooth muscle cells occurs via non-genomic actions. Knowing the importance of the enzyme Rho kinase II in maintaining smooth muscle contraction and in support of the previous reports of progesterone action in the GI tract, we suggest that progesterone could affect the contractile activity of gastric smooth muscle cells in rats by inhibiting the Rho kinase II pathway. Future contraction studies using selective Rho kinase II inhibitors such as Y27632 and different concentrations of progesterone might further reinforce these findings.
Supportive foundations
This work was supported by Jordan University of Science & Technology, Irbid, Jordan (Grant Number 172/2012).
Conflict of interest
The authors declare that they have no conflict of interest.
Acknowledgments
This work was supported by Jordan University of Science & Technology, Irbid, Jordan (Grant Number 172/2012). The authors thank Dr. Omar Khabour for help and providing laboratory facilities.
References
- 1.Singer AJ, Brandt LJ. Pathophysiology of the gastrointestinal tract during pregnancy. Am J Gastroenterol. 1991; 86(12): 1695–712. [PubMed] [Google Scholar]
- 2.DiIorio C, van Lier D, Manteuffel B. Patterns of nausea during first trimester of pregnancy. Clin Nurs Res. 1992; 1(2): 127–40 discussion 141–3. doi: 10.1177/105477389200100202 [DOI] [PubMed] [Google Scholar]
- 3.Chandra K, Einarson A, Koren G. Taking ginger for nausea and vomiting during pregnancy. Can Fam Physician. 2002; 48: 1441–2. [PMC free article] [PubMed] [Google Scholar]
- 4.Everson GT, McKinley C, Lawson M, Johnson M, Kern F, Jr . Gallbladder function in the human female: effect of the ovulatory cycle, pregnancy, and contraceptive steroids. Gastroenterology. 1982; 82(4): 711–9. [PubMed] [Google Scholar]
- 5.Braverman DZ, Johnson ML, Kern F, Jr . Effects of pregnancy and contraceptive steroids on gallbladder function. N Engl J Med. 1980; 302(7): 362–4. doi: 10.1056/NEJM198002143020702 [DOI] [PubMed] [Google Scholar]
- 6.Brock-Utne JG, Dow TG, Dimopoulos GE, Welman S, Downing JW, Moshal MG. Gastric and lower oesophageal sphincter (LOS) pressures in early pregnancy. Br J Anaesth. 1981; 53(4): 381–4. doi: 10.1093/bja/53.4.381 [DOI] [PubMed] [Google Scholar]
- 7.Jones MJ, Mitchell RW, Hindocha N, James RH. The lower oesophageal sphincter in the first trimester of pregnancy: comparison of supine with lithotomy positions. Br J Anaesth. 1988; 61(4): 475–6. doi: 10.1093/bja/61.4.475 [DOI] [PubMed] [Google Scholar]
- 8.Bainbridge ET, Nicholas SD, Newton JR, Temple JG. Gastro-oesophageal reflux in pregnancy. Altered function of the barrier to reflux in asymptomatic women during early pregnancy. Scand J Gastroenterol. 1984; 19(1): 85–9. [PubMed] [Google Scholar]
- 9.Parkman HP, Wang MB, Ryan JP. Decreased electromechanical activity of guinea pig circular muscle during pregnancy. Gastroenterology. 1993; 105(5): 1306–12. doi: 10.1016/0016-5085(93)90133-W [DOI] [PubMed] [Google Scholar]
- 10.Baron TH, Ramirez B, Richter JE. Gastrointestinal motility disorders during pregnancy. Ann Intern Med. 1993; 118(5): 366–75. doi: 10.7326/0003-4819-118-5-199303010-00008 [DOI] [PubMed] [Google Scholar]
- 11.Ryan JP. Effect of pregnancy on intestinal transit: comparison of results using radioactive and non-radioactive test meals. Life Sci. 1982; 31(23): 2635–40. doi: 10.1016/0024-3205(82)90739-1 [DOI] [PubMed] [Google Scholar]
- 12.Ryan JP, Bhojwani A. Colonic transit in rats: effect of ovariectomy, sex steroid hormones, and pregnancy. Am J Physiol. 1986; 251(1 Pt 1): G46–50. [DOI] [PubMed] [Google Scholar]
- 13.Barbagallo M, Dominguez LJ, Licata G, Shan J, Bing L, Karpinski E, Pang PK, Resnick LM. Vascular Effects of Progesterone : Role of Cellular Calcium Regulation. Hypertension. 2001; 37(1): 142–7. doi: 10.1161/01.HYP.37.1.142 [DOI] [PubMed] [Google Scholar]
- 14.Chen TS, Doong ML, Chang FY, Lee SD, Wang PS. Effects of sex steroid hormones on gastric emptying and gastrointestinal transit in rats. Am J Physiol. 1995; 268(1 Pt 1): G171–6. [DOI] [PubMed] [Google Scholar]
- 15.Coşkun T, Sevinç A, Tevetoğlu I, Alican I, Kurtel H, Yeğen BC. Delayed gastric emptying in conscious male rats following chronic estrogen and progesterone treatment. Res Exp Med (Berl). 1995; 195(1): 49–54. doi: 10.1007/BF02576773 [DOI] [PubMed] [Google Scholar]
- 16.Kastner P, Krust A, Turcotte B, Stropp U, Tora L, Gronemeyer H, Chambon P. Two distinct estrogen-regulated promoters generate transcripts encoding the two functionally different human progesterone receptor forms A and B. EMBO J. 1990; 9(5): 1603–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xiao ZL, Pricolo V, Biancani P, Behar J. Role of progesterone signaling in the regulation of G-protein levels in female chronic constipation. Gastroenterology. 2005; 128(3): 667–75. doi: 10.1053/j.gastro.2004.12.001 [DOI] [PubMed] [Google Scholar]
- 18.Sager G, Ørbo A, Jaeger R, Engström C. Non-genomic effects of progestins--inhibition of cell growth and increased intracellular levels of cyclic nucleotides. J Steroid Biochem Mol Biol. 2003; 84(1): 1–8. doi: 10.1016/S0960-0760(02)00269-8 [DOI] [PubMed] [Google Scholar]
- 19.Blackmore PF, Neulen J, Lattanzio F, Beebe SJ. Cell surface-binding sites for progesterone mediate calcium uptake in human sperm. J Biol Chem. 1991; 266(28): 18655–9. [PubMed] [Google Scholar]
- 20.Morrison T, Waggoner L, Whitworth-Langley L, Stith BJ. Nongenomic action of progesterone: activation of Xenopus oocyte phospholipase C through a plasma membrane-associated tyrosine kinase. Endocrinology. 2000; 141(6): 2145–52. [DOI] [PubMed] [Google Scholar]
- 21.Luconi M, Krausz C, Barni T, Vannelli GB, Forti G, Baldi E. Progesterone stimulates p42 extracellular signal-regulated kinase (p42erk) in human spermatozoa. Mol Hum Reprod. 1998; 4(3): 251–8. doi: 10.1093/molehr/4.3.251 [DOI] [PubMed] [Google Scholar]
- 22.Bielefeldt K, Waite L, Abboud FM, Conklin JL. Nongenomic effects of progesterone on human intestinal smooth muscle cells. Am J Physiol. 1996; 271(2 Pt 1): G370–6. [DOI] [PubMed] [Google Scholar]
- 23.Uchiyama T, Chess-Williams R. Muscarinic receptor subtypes of the bladder and gastrointestinal tract. J Smooth Muscle Res. 2004; 40(6): 237–47. doi: 10.1540/jsmr.40.237 [DOI] [PubMed] [Google Scholar]
- 24.Machelon V, Nomé F, Grosse B, Lieberherr M. Progesterone triggers rapid transmembrane calcium influx and/or calcium mobilization from endoplasmic reticulum, via a pertussis-insensitive G-protein in granulosa cells in relation to luteinization process. J Cell Biochem. 1996; 61(4): 619–28. doi: [DOI] [PubMed] [Google Scholar]
- 25.Murthy KS. Signaling for contraction and relaxation in smooth muscle of the gut. Annu Rev Physiol. 2006; 68: 345–74. doi: 10.1146/annurev.physiol.68.040504.094707 [DOI] [PubMed] [Google Scholar]
- 26.Hahmann C, Schroeter T. Rho-kinase inhibitors as therapeutics: from pan inhibition to isoform selectivity. Cell Mol Life Sci. 2010; 67(2): 171–7. doi: 10.1007/s00018-009-0189-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Murthy KS, Coy DH, Makhlouf GM. Somatostatin receptor-mediated signaling in smooth muscle. Activation of phospholipase C-beta3 by Gbetagamma and inhibition of adenylyl cyclase by Galphai1 and Galphao. J Biol Chem. 1996; 271(38): 23458–63. doi: 10.1074/jbc.271.38.23458 [DOI] [PubMed] [Google Scholar]
- 28.Murthy KS, Makhlouf GM. Functional characterization of phosphoinositide-specific phospholipase C-beta 1 and -beta 3 in intestinal smooth muscle. Am J Physiol. 1995; 269(4 Pt 1): C969–78. [DOI] [PubMed] [Google Scholar]
- 29.Murthy KS, Zhou H, Grider JR, Makhlouf GM. Sequential activation of heterotrimeric and monomeric G proteins mediates PLD activity in smooth muscle. Am J Physiol Gastrointest Liver Physiol. 2001; 280(3): G381–8. [DOI] [PubMed] [Google Scholar]
- 30.Xiao ZL, Cao W, Biancani P, Behar J. Nongenomic effects of progesterone on the contraction of muscle cells from the guinea pig colon. Am J Physiol Gastrointest Liver Physiol. 2006; 290(5): G1008–15. doi: 10.1152/ajpgi.00382.2005 [DOI] [PubMed] [Google Scholar]
- 31.Minshall RD, Pavcnik D, Browne DL, Hermsmeyer K. Nongenomic vasodilator action of progesterone on primate coronary arteries. J Appl Physiol 1985. 2002; 92(2): 701–8. doi: 10.1152/japplphysiol.00689.2001 [DOI] [PubMed] [Google Scholar]
- 32.Falkenstein E, Meyer C, Eisen C, Scriba PC, Wehling M. Full-length cDNA sequence of a progesterone membrane-binding protein from porcine vascular smooth muscle cells. Biochem Biophys Res Commun. 1996; 229(1): 86–9. doi: 10.1006/bbrc.1996.1761 [DOI] [PubMed] [Google Scholar]
- 33.Chen Q, Xiao ZL, Biancani P, Behar J. Downregulation of Galphaq-11 protein expression in guinea pig antral and colonic circular muscle during pregnancy. Am J Physiol. 1999; 276(4 Pt 1): G895–900. [DOI] [PubMed] [Google Scholar]
- 34.Everson GT. Gastrointestinal motility in pregnancy. Gastroenterol Clin North Am. 1992; 21(4): 751–76. [PubMed] [Google Scholar]
- 35.Zhang M, Wang GJ, Benishin CG, Pang PK. Rapid effect of progesterone on the contraction of rat aorta in-vitro. J Pharm Pharmacol. 2002; 54(11): 1529–34. doi: 10.1211/00223570263 [DOI] [PubMed] [Google Scholar]