Abstract
Electrogastrography (EGG) is a non-invasive diagnostic motility for recording gastric myoelectrical activity. Gastric myoelectrical activity was first recorded in 1922. Advances in recording equipment enabled widespread use of cutaneous EGG after 1985. Later, introduction of multichannel EGG (M-EGG) enabled measurement of electrical activity transmission. At present, M-EGG findings are used as objective indicators of gastric motility disorders caused by various diseases. EGG measures two categories of gastric electrical activity: electrical response activity, or spike potentials; and electrical control activity, or slow waves. The appearance of abnormal rhythmic electrical activity is indicative of abnormalities in gastric motility. The normal frequency range of gastric electrical activity (normogastria) is around 3 cycles per min. Multiple EGG parameters assist in the assessment of gastric myoelectrical activity, and significant correlations between EGG and other gastric motility tests have been demonstrated in many studies. In Japan, however, EGG remains in the exploratory stage, and its clinical use is limited. There are large variations in procedures and systems used in previous studies, thus there is a need for standardization of EGG procedures and technical terminology. Here, we outline the current status of EGG and report the M-EGG procedures used in our department in addition to our M-EGG findings.
The abstract of this manuscript was presented during an educational seminar titled "Current status of gastrointestinal motility tests and keys for immediate implementation" at the 54th Annual Meeting of the Japan Society of Smooth Muscle Research
Keywords: electrogastrography, gastric myoelectrical activity, slow waves, %normal, %slow wave coupling
Introduction
Electrogastrography (EGG) is a diagnostic modality for recording gastric myoelectrical activity. This non-invasive technique records the myoelectrical activity using cutaneous electrodes placed on the skin of the abdomen over the stomach (1). The resulting electrogastrogram is a convenient and simple measurement of gastric electrical activity.
EGG provides objective indicators for gastric motility disorders caused by various diseases, such as diabetes (2,3,4,5,6), collagen disease (7,8,9,10,11,12), and functional dyspepsia (13,14,15,16,17,18). It has also been used to assess the efficacy of gastroprokinetic agents (19, 20) and the outcome of gastrointestinal surgery (21,22,23,24,25,26,27). EGG is useful not only for assessing disease conditions, but also for providing new insights into gastrointestinal motility. However, in Japan, the clinical use of EGG is still under investigation. Here, we outline the current status of EGG and report the procedures for multichannel EGG (M-EGG) use in our department in addition to our M-EGG findings.
History of EGG
The first study demonstrating gastric myoelectrical activity was reported by Alvarez in 1922 (28). EGG recording was initially performed on the mucosal or serosal membrane because gastric electrical potentials were small, and early EGG devices using cutaneous electrodes could not distinguish EGG signals from cardiac and respiratory artifacts. Since then, advances in recording equipment led to the first cutaneous EGG recording reported by Bellahsene in 1985 (29), which was a breakthrough in the clinical application of EGG. Multichannel EGG (M-EGG) was subsequently introduced and enabled measurement of transmission of electrical activity, as demonstrated by Chen et al. using cutaneous M-EGG (30).
Principles of EGG
The gastric electrical activity measured by EGG can be subdivided into two categories. The first is called electrical response activity (ERA), or spike potentials, which are spikes initiated from the gastric mucosa and are associated with gastric smooth muscle contractions. ERA is reported to reflect the peristaltic activity of the stomach (31). The second is called electrical control activity (ECA), or slow waves, which are regularly recurring electrical potentials originating in gastric smooth muscle cells, but that are not associated with gastric contractions (32). ECA is generated by pacemaker cells, namely, the interstitial cells of Cajal (33) in the greater curvature, and travels toward the pylorus. The frequency of ECA in the human stomach is roughly 3 cycles per min (cpm) (34, 35). Both ECA and ERA are identifiable in EGG recordings (36). Although EGG signals are not a direct representation of gastric motility, abnormalities in gastric electrical activity (ECA and ERA) detected by cutaneous EGG have been shown to correlate with abnormal gastric motility (37).
Indications and contraindications for EGG
The American Motility Society proposed the following indications for EGG: (1) unexplained nausea and vomiting; or (2) functional dyspepsia (37). Because of its non-invasive nature, EGG has no contraindications and is therefore used to measure gastric electrical activity in patients with a variety of diseases. However, meal loading prior to EGG is contraindicated in those who have difficulties with food intake. Consequently, only fasting EGG should be performed in patients with pyloric stenosis and dysphagia. In addition, patients must be in the supine position during EGG and have fasted for at least 6 h before EGG to eliminate the influence of residual gastric contents. Various medications (e.g., prokinetics, narcotic analgesics, anticholinergics, and anti-emetic agents) do not prohibit EGG recording, but as they could influence EGG results, their use must be taken into consideration when analyzing the electrical activity.
Measurement and analysis of gastric myoelectrical activity
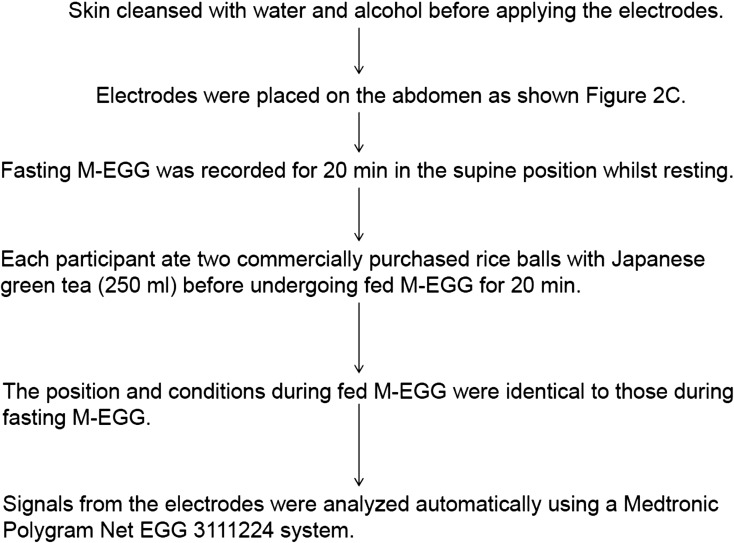
The procedure of M-EGG is shown as Fig. 1. The normal frequency range of gastric electrical activity (normogastria) is usually 2.25–3.75 cpm, while the range of abnormally low (bradygastria, slow pacemaker) or high (tachygastria, fast slow waves) frequencies is 1.00–2.25 cpm and 3.75–9.00 cpm, respectively. Frequencies outside the normal frequency are defined as arrhythmia. Typical gastric electrical recordings in a healthy volunteer are shown in Fig. 2A. Slow waves with a frequency of approximately 3 cpm was observed in the healthy volunteer, indicating normal gastric electrical activity. Because cutaneous electrodes are usually used during EGG, various types of artifacts exist in EGG signals. For example, the frequency ranges of electrical activity originating in the small intestine and respiratory muscles are 9–13 cpm and 12–24 cpm, respectively. The frequency of cardiac action potentials is around 60 cpm. Furthermore, artifacts occur as a consequence of body movement (38) but can be removed by digital filters. Today, many EGG recording systems include software that processes EGG signals. In our department, we used a M-EGG system PolyGraf® (Medtronic A/S, Skovlunde, Denmark) with a low pass filter of 15 cpm and a high pass filter of 1.8cpm, and we analyzed the acquired EGG signals using a Medtronic Polygram NET EGG 311244 system (Medtronic A/S). Specifically, raw signals acquired every 256 s are filtered and processed by Fast Fourier transformation to obtain a power spectrum, thereby automatically calculating the dominant frequency and dominant power. Reproducibility of early cutaneous EGG devices was poor, which made data analysis difficult. However, technological advances have resulted in compact and reliable EGG devices which produce results rapidly and automatically. For these reasons, cutaneous EGG is now used for bedside and outpatient examination.
Fig. 1.
Procedure for multichannel electrogastrography (M-EGG).
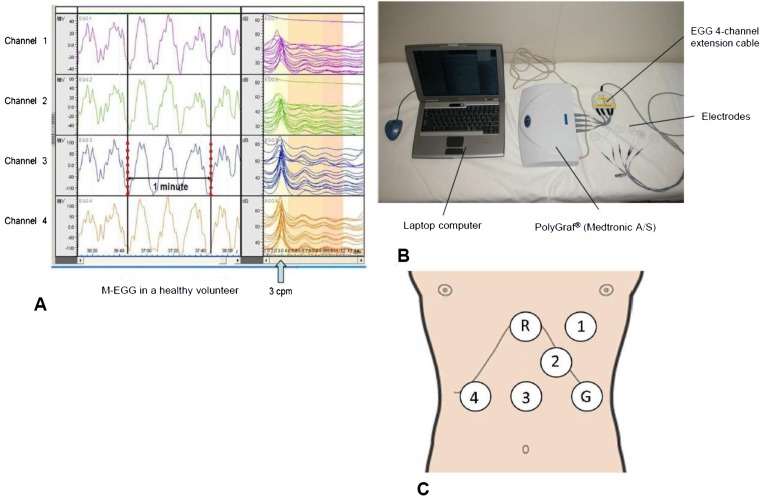
Fig. 2.
M-EGG recording. (A) The typical M-EGG waves recordings obtained from Channels 1–4 in a healthy volunteer. The slow wave is displayed to the left of the screen. Running spectrum analysis is displayed to the right of the screen. Running spectrum analysis shows many slow waves are 3 cycles per minute (cpm). (From Murakami et al. J Smooth Muscle Res. 2011; 15: J-37–J-44 (65). Reproduced by copyright permission of Japan Society of Smooth Muscle Research). (B) Equipment for M-EGG recording and analysis. (C) Cutaneous placement of multiple channels: Channel 3 was placed midway between the xiphoid process and the umbilicus; Channel 4 was placed 4 cm horizontal to Channel 3; Channels 2 and 1 were placed 45° to the upper left of Channel 3 at distances of 4 cm and 6 cm. The ground electrode was placed on the left costal margin horizontal to Channel 3. The reference electrode was placed on the xiphoid process. G: ground electrode; R: reference electrode.
Placement of cutaneous electrodes
In our department, we use six cutaneous electrodes designed for M-EGG (Medtronic A/S) (Fig. 2B). Use of multi-channel electrodes allows analysis of the relationship of slow waves between the electrodes as a measure of percentage of slow wave coupling (%SWC). %SWC is discussed below under EGG parameters. Electrode positions were as follows: the reference electrode on the xiphoid process; the Channel 3 electrode (Ch3) on the midpoint between the xiphoid process and the umbilicus; the Channel 4 electrode (Ch4) 4 cm to the right of Ch3; the Channel 2 (Ch2) electrode 4 cm to the upper-left of Ch3; the Channel 1 (Ch1) electrode 6 cm to the upper-left of Ch3; and the ground electrode to the left of Ch3 and on the left mid-clavicular line (Fig. 2C). Sufficient skin preparation by cleansing with alcoholic solution is necessary to reduce the resistance between the electrodes and the skin. M-EGG shows the skin resistance; an acceptable skin resistance is 11 kΩ or lower.
Pretest meals, patient position, and duration of EGG recording
Patients are usually placed in the supine position during EGG recording to eliminate motion artifacts. Meal loading is used to compare fasting and postprandial EGG measurements. However, no standard pre-test meals for EGG tests have been established. Studies in North America and Europe have often provided egg sandwiches and approximately 120 ml of orange juice (39, 40). Coffee (100 ml) has sometimes been added (40), and turkey sandwiches were provided in another study (12, 41). Meanwhile, in China, instant noodles with ham have been given (42). These choices indicate that an easily consumed meal is preferable. Considering that EGG is a modality for assessing autonomic function, it is likely that stress caused by difficulties in consuming a pre-test meal could influence EGG results. Thus, it is appropriate to choose an easily consumed meal before an EGG test. Total calories in the above meals were approximately 350–500 kcal. In Japan, on the other hand, enteral nutrients were used in one study (24).
On the basis of the pre-test meals used in different countries, we gave our subjects two commercially available rice balls and 250 ml of green tea because both are convenient and easy to consume. The nutritional value of one rice ball is 180 kcal, 4 g of protein, 0.5 g of fat, 40 g of carbohydrates, 1 g of salt, 25 mg of cholesterol, 1 g of fiber, and 0.6 mg of vitamin E. A 30- to 60-min duration of recording is usually used for both fasting and postprandial EGG (24, 30, 39, 40). However, some patients become uncooperative if the total examination time exceeds 60 min, and such long examinations are particularly inconvenient when performed as outpatient tests. For these reasons, we record both fasting and postprandial EGG signals for 20 min. This means that all procedures, including the explanation about EGG, consumption of a pre-test meal, and EGG recording, will be completed within 60 min. Although the duration of our EGG is 10 min less than the commonly used 30 min, we have not seen a notable impact of shorter recording times on EGG results.
EGG parameters
M-EGG parameters enable EGG to provide diverse information, but they also complicate the assessment of gastric electrical activity. The following four major types of parameters are used: (1) percentage of recording time in each of four frequency bands; (2) dominant frequency (DF); (3) dominant power; and (4) %SWC. The four frequency bands include normal (2.0–4.0 cpm), high (4.0–9.0 cpm), low (0.5–2.0 cpm), and extra high (>9.0 cpm) frequencies. The percentage of recording time is presented as %normal, %tachygastria, %bradygastria, and %arrhythmia, respectively. Just as abnormal waveforms indicate abnormalities in electrocardiography, abnormal rhythmic electrical activity in EGG indicates abnormalities in gastric motility. A parameter specific to M-EGG, %SWC, shows the percentage of time during which waves of a similar frequency are transmitted from one electrode to the other, and %SWC provides an assessment of transmission of gastric electrical activity. The slow waves in two channels are defined as coupled when the difference in their DF is ≤0.2 cpm (39). Wang et al. reported that %SWC represents the consistency of slow wave frequency (43). Lin and Chen reported that %SWC in functional dyspepsia patients was significantly lower than in healthy subjects (44).
Comparison of EGG with other gastric motility tests
Contrast radiography using barium has been used to examine gastric motility. Gastric myoelectrical activity measured by EGG was reported to be affected by contractions elicited by barium (45). The 13C-octanoate breath test correlated with the ratio of postprandial to fasting EGG power and half emptying time (T1/2) (46). Another report found that %normal values correlated with T1/2 and lag time (Tlag) (47). Furthermore, gastric emptying measured by abdominal ultrasound lay between the EGG power ratio and the motility index (48).
Relationship between gastric disorders and EGG
There are many reports that in patients with functional dyspepsia, %normal is low compared with that in healthy subjects (49,50,51,52,53). There are some reports that %tachygastria in functional dyspepsia patients was significantly higher after a test meal compared with that in healthy persons (49, 50, 52).
Kawagishi et al. reported that %normal in diabetes patients was significantly lower than in healthy subjects (54). Nohara et al. reported that diabetes patients with delayed gastric emptying in the 13C-octanoic acid breath test had lower postprandial %normal compared with healthy subjects (55). Two other studies reported that preprandial %bradygastria in diabetes patients was significantly higher than in healthy subjects (4, 56). Kamiya et al. used EGG in 24 patients with active gastric ulcer diseases and 10 healthy subjects as controls, and reported that the proportion of patients showing normogastria was significantly lower than in healthy controls (57). Patients with Helicobacter pylori infection and a duodenal ulcer or gastritis were reported to have lower %normogastria compared with healthy subjects (58, 59). Two studies reported that %normal in patients with H. pylori infection was increased after H. pylori eradication therapy (56, 60).
Imai et al. performed EGG after total or subtotal gastrectomy and found that 3 cpm power peaks were absent after total gastrectomy, confirming that EGG measures gastric electrical activity (24). However, two-thirds of patients who had undergone subtotal gastrectomy exhibited waveforms similar to those observed in healthy individuals, indicating that the region serving as the gastric pacemaker was not removed during gastrectomy in these patients. Schaap et al. reported that EGG signals contained a component at approximately 3 cpm in 22 of 33 partial gastrectomy patients (61). Homma et al. examined EGG recordings after subtotal gastrectomy, and reported that the postoperative to preoperative power ratio for the 3 cpm component was significantly reduced in the post prandial state (27). In systemic sclerosis patients, gastric dysrhythmias were correlated with certain gastrointestinal symptoms (7). A significant correlation was found between the symptom score and the percentage arrhythmia in patients in the fed state after bone marrow or stem cell transplantation (62). In patients with unresectable cancer, the severity of symptoms was significantly higher in patients with abnormal EGG results (63). We need to clarify the usefulness of these parameters and the relationship between EGG abnormalities and clinical symptoms in further studies.
EGG results in our Department
In our Department, we used the PolyGraf (Medtronic A/S) M-EGG system in the three following investigations.
Investigation 1: Relationship of gastric emptying to %normal and %SWC
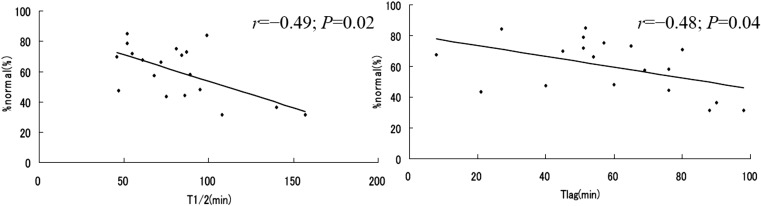
Nineteen subjects were simultaneously examined by M-EGG and subjected to the 13C gastric emptying test (64). Subjects comprised 13 healthy volunteers, two gastric cancer patients who were able to take food orally, three patients who had undergone lower esophageal sphincter- and vagus-preserving proximal partial gastrectomy (65), and one who had a gastric ulcer. EGG %normal in Ch1 correlated negatively with T1/2 (r=–0.49, P=0.02) and Tlag (r=–0.48, P=0.04), as determined by the 13C gastric emptying test (Fig. 3). EGG %SWC in any electrode pair correlated negatively with T1/2, Tlag, and the gastric emptying coefficient (66). Correlations between EGG parameters (%normal and %SWC) and gastric emptying parameters determined by the 13C gastric emptying test were statistically significant (66).
Fig. 3.
Correlation between %normal of the M-EGG and T/1/2 and Tlag of the 13C-acetate breath test. The %normal of Channel 1 was significantly correlated with T1/2 and Tlag. (From Murakami et al. J Smooth Muscle Res. 2011; 15: J-37–J-44 (65). Reproduced by copyright permission of Japan Society of Smooth Muscle Research).
Investigation 2: M-EGG of the remnant stomach in patients who had undergone distal gastrectomy with or without vagus preservation
M-EGG was performed in 20 patients who had undergone distal gastrectomy without vagus preservation (DG group), in 26 patients who had undergone distal gastrectomy with preservation of the fundic branches of the vagus nerve (VP-DG group) and in 12 healthy volunteers (67). M-EGG was performed 2 weeks after surgery. A 20-min fasting EGG recording was performed, followed by a 20-min postprandial recording. Two commercially purchased rice balls and green tea were provided after completion of the fasting recording. Longer periods of normal gastric function (normogastria, 2.0–4.0 cpm) were detected in Ch1 in the VP-DG group than in the DG group in the fasting and fed states (P<0.05). Thus, slow waves can be recorded non-invasively by M-EGG in the remnant stomach following gastrectomy. The VP-DG group showed better preservation of gastric myoelectric activity than the DG group (67).
Investigation 3: Relationship between electrical activity of the remnant stomach and postoperative complaints
M-EGG was performed in 15 patients in the DG group and 12 patients in the VP-DG group (67). The Japanese version of the Gastrointestinal Symptom Rating Scale (GSRS) was used for a postoperative complaint survey. No obvious correlations were noted between EGG parameters (%normal and %SWC) and GSRS scores (reflux, abdominal pain, and indigestion scores) in the DG group. In the VP-DG group, %SWC in the fed state correlated negatively with GSRS scores (reflux, r=–0.59, P=0.02; abdominal pain, r=–0.51, P=0.04; indigestion, r=–0.59, P=0.02; and total score, r=–0.75, P=0.02). Thus, preservation of the fundic branches of the vagus nerve resulted in the preservation of gastric activity of the remnant stomach, and EGG results (%SWC) correlated with GSRS scores (67).
Conclusions
M-EGG is a simple and non-invasive diagnostic modality for measuring gastric myoelectric activity and is useful for objective assessment of gastrointestinal motility. M-EGG is one of the few non-invasive procedures for examining gastrointestinal function and is thus expected to play a pivotal role in future developments. However, procedures and systems used in previous studies vary widely, indicating the need for standardization of M-EGG procedures and technical terminology.
Conflict of interest
The authors declare that they have no conflict of interest.
Acknowledgments
The authors thank Mr. Masakatsu Yamaguchi (Asahi Biomed Co., Ltd. Tokyo, Japan). for generous technical support.
References
- 1.Yin J, Chen JD. Electrogastrography: methodology, validation and applications. J Neurogastroenterol Motil. 2013; 19(1): 5–17. doi: 10.5056/jnm.2013.19.1.5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pfister CJ, Hamilton JW, Nagel N, Bass P, Webster JG, Tompkins WJ. Use of spectral analysis in the detection of frequency differences in the electrogastrograms of normal and diabetic subjects. IEEE Trans Biomed Eng. 1988; 35(11): 935–41. doi: 10.1109/10.8673 [DOI] [PubMed] [Google Scholar]
- 3.Abell TL, Johnson WD, Kedar A, Runnels JM, Thompson J, Weeks ES, Minocha A, Griswold ME. A double-masked, randomized, placebo-controlled trial of temporary endoscopic mucosal gastric electrical stimulation for gastroparesis. Gastrointest Endosc. 2011; 74(3): 496–503. e3. [DOI] [PMC free article] [PubMed]
- 4.Posfay-Barbe KM, Lindley KJ, Schwitzgebel VM, Belli DC, Schäppi MG. Electrogastrography abnormalities appear early in children with diabetes type 1. Eur J Gastroenterol Hepatol. 2011; 23(10): 881–5. doi: 10.1097/MEG.0b013e32834967b6 [DOI] [PubMed] [Google Scholar]
- 5.Dirgenali F, Kara S, Okkesim S. Estimation of wavelet and short-time Fourier transform sonograms of normal and diabetic subjects’ electrogastrogram. Comput Biol Med. 2006; 36(12): 1289–302. doi: 10.1016/j.compbiomed.2005.07.005 [DOI] [PubMed] [Google Scholar]
- 6.Gad-el-Hak N, Bakr AM. Gastric myoelectrical activity in diabetics with and without diabetic autonomic neuropathy. Hepatogastroenterology. 2001; 48(38): 590–3. [PubMed] [Google Scholar]
- 7.McNearney TA, Sallam HS, Hunnicutt SE, Doshi D, Wollaston DE, Mayes MD, Chen JD. Gastric slow waves, gastrointestinal symptoms and peptides in systemic sclerosis patients. Neurogastroenterol Motil. 2009; 21(12): 1269–e120. doi: 10.1111/j.1365-2982.2009.01350.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wollaston DE, Xu X, Tokumaru O, Chen JD, McNearney TA. Patients with systemic sclerosis have unique and persistent alterations in gastric myoelectrical activity with acupressure to Neiguan point PC6. J Rheumatol. 2005; 32(3): 494–501. [PubMed] [Google Scholar]
- 9.Franck-Larsson K, Hedenström H, Dahl R, Rönnblom A. Delayed gastric emptying in patients with diffuse versus limited systemic sclerosis, unrelated to gastrointestinal symptoms and myoelectric gastric activity. Scand J Rheumatol. 2003; 32(6): 348–55. doi: 10.1080/03009740410005016 [DOI] [PubMed] [Google Scholar]
- 10.McNearney T, Lin X, Shrestha J, Lisse J, Chen JD. Characterization of gastric myoelectrical rhythms in patients with systemic sclerosis using multichannel surface electrogastrography. Dig Dis Sci. 2002; 47(4): 690–8. doi: 10.1023/A:1014759109982 [DOI] [PubMed] [Google Scholar]
- 11.Marycz T, Muehldorfer SM, Gruschwitz MS, Katalinic A, Herold C, Ell C, Hahn EG. Gastric involvement in progressive systemic sclerosis: electrogastrographic and sonographic findings. Eur J Gastroenterol Hepatol. 1999; 11(10): 1151–6. doi: 10.1097/00042737-199910000-00013 [DOI] [PubMed] [Google Scholar]
- 12.Pfaffenbach B, Adamek RJ, Hagemann D, Busch S, Hoffmann K, Altmeyer P, Schaffstein J, Wegener M. Effect of progressive systemic sclerosis on antral myoelectrical activity and gastric emptying. Z Gastroenterol. 1996; 34(9): 517–21. [PubMed] [Google Scholar]
- 13.Friesen CA, Lin Z, Singh M, Singh V, Schurman JV, Burchell N, Cocjin JT, McCallum RW. Antral inflammatory cells, gastric emptying, and electrogastrography in pediatric functional dyspepsia. Dig Dis Sci. 2008; 53(10): 2634–40. doi: 10.1007/s10620-008-0207-0 [DOI] [PubMed] [Google Scholar]
- 14.Leung MW, Wong BP, Chao NS, Chung KW, Kwok WK, Liu KK. Electrogastrography in the management of pediatric functional dyspepsia and motility disorder. J Pediatr Surg. 2006; 41(12): 2069–72. doi: 10.1016/j.jpedsurg.2006.08.008 [DOI] [PubMed] [Google Scholar]
- 15.Chen CL, Hu CT, Lin HH, Yi CH. Clinical utility of electrogastrography and the water load test in patients with upper gastrointestinal symptoms. J Smooth Muscle Res. 2006; 42(5): 149–57. doi: 10.1540/jsmr.42.149 [DOI] [PubMed] [Google Scholar]
- 16.Zhang H, Xu X, Wang Z, Li C, Ke M. Correlation between gastric myoelectrical activity recorded by multi-channel electrogastrography and gastric emptying in patients with functional dyspepsia. Scand J Gastroenterol. 2006; 41(7): 797–804. doi: 10.1080/00365520500469750 [DOI] [PubMed] [Google Scholar]
- 17.van der Voort IR, Osmanoglou E, Seybold M, Heymann-Mönnikes I, Tebbe J, Wiedenmann B, Klapp BF, Mönnikes H. Electrogastrography as a diagnostic tool for delayed gastric emptying in functional dyspepsia and irritable bowel syndrome. Neurogastroenterol Motil. 2003; 15(5): 467–73. doi: 10.1046/j.1365-2982.2003.00433.x [DOI] [PubMed] [Google Scholar]
- 18.Holmvall P, Lindberg G. Electrogastrography before and after a high-caloric, liquid test meal in healthy volunteers and patients with severe functional dyspepsia. Scand J Gastroenterol. 2002; 37(10): 1144–8. doi: 10.1080/003655202760373344 [DOI] [PubMed] [Google Scholar]
- 19.Yagi M, Homma S, Kubota M, Iinuma Y, Kanada S, Kinoshita Y, Ohtaki M, Yamazaki S, Murata H. The herbal medicine Rikkunshi-to stimulates and coordinates the gastric myoelectric activity in post-operative dyspeptic children after gastrointestinal surgery. Pediatr Surg Int. 2004; 19(12): 760–5. doi: 10.1007/s00383-003-1053-y [DOI] [PubMed] [Google Scholar]
- 20.Chen JD, Ke MY, Lin XM, Wang Z, Zhang M. Cisapride provides symptomatic relief in functional dyspepsia associated with gastric myoelectrical abnormality. Aliment Pharmacol Ther. 2000; 14(8): 1041–7. doi: 10.1046/j.1365-2036.2000.00801.x [DOI] [PubMed] [Google Scholar]
- 21.Homma S, Kobayashi Y, Kosugi S, Ohashi M, Kanda T, Hatakeyama K. Local differences in electrogastrographic indices associated with total gastrectomy, total colectomy, distal gastrectomy and colonic replacement. J Smooth Muscle Res. 2010; 46(5): 235–48. doi: 10.1540/jsmr.46.235 [DOI] [PubMed] [Google Scholar]
- 22.Bures J, Kabelác K, Kopácová M, Vorísek V, Siroký M, Palicka V, Rejchrt S. Electrogastrography in patients with Roux-en-Y reconstruction after previous Billroth gastrectomy. Hepatogastroenterology. 2008; 55(85): 1492–6. [PubMed] [Google Scholar]
- 23.Hayashi T, Kinami S, Fushida S, Fujimura T, Miwa K, Inoue K. Evaluation of residual stomach motility after proximal gastrectomy for gastric cancer by electrogastrography. Dig Dis Sci. 2006; 51(2): 268–73. doi: 10.1007/s10620-006-3123-1 [DOI] [PubMed] [Google Scholar]
- 24.Imai K, Sakita M. Pre- and postoperative electrogastrography in patients with gastric cancer. Hepatogastroenterology. 2005; 52(62): 639–44. [PubMed] [Google Scholar]
- 25.Kauer WK, Stein HJ, Balint A, Siewert JR. Transcutaneous electrogastrography: a non-invasive method to evaluate post-operative gastric disorders? Hepatogastroenterology. 1999; 46(26): 1244–8. [PubMed] [Google Scholar]
- 26.Riezzo G, Pezzolla F, Giorgio I. Electrical activity recorded from abdominal surface before and after gastric surgery in man. Arch Physiol Biochem. 1996; 104(1): 50–6. doi: 10.1076/apab.104.1.50.12867 [DOI] [PubMed] [Google Scholar]
- 27.Homma S, Shimakage N, Yagi M, Hasegawa J, Sato K, Matsuo H, Tamiya Y, Tanaka O, Muto T, Hatakeyama K. Electrogastrography prior to and following total gastrectomy, subtotal gastrectomy, and gastric tube formation. Dig Dis Sci. 1995; 40(4): 893–900. doi: 10.1007/BF02064997 [DOI] [PubMed] [Google Scholar]
- 28.Alvarez WC. The electrogastrogram and what it shows. JAMA. 1922; 78(15): 1116–9. doi: 10.1001/jama.1922.02640680020008 [DOI] [Google Scholar]
- 29.Bellahsene BE, Hamilton JW, Webster JG, Bass P, Reichelderfer M. An improved method for recording and analyzing the electrical activity of the human stomach. IEEE Trans Biomed Eng. 1985; 32(11): 911–5. doi: 10.1109/TBME.1985.325623 [DOI] [PubMed] [Google Scholar]
- 30.Chen JD, Zou X, Lin X, Ouyang S, Liang J. Detection of gastric slow wave propagation from the cutaneous electrogastrogram. Am J Physiol. 1999; 277(2 Pt 1): G424–30. [DOI] [PubMed] [Google Scholar]
- 31.Szurszewski JH. Physiology of Gastrointestinal Tract Vol 2. LR Johnson (ed). New York: Raven Press; 1981. Electrical basis for gastrointestinal motility; p. 1435–66. [Google Scholar]
- 32.Abell TL, Malagelada JR. Electrogastrography. Current assessment and future perspectives. Dig Dis Sci. 1988; 33(8): 982–92. doi: 10.1007/BF01535995 [DOI] [PubMed] [Google Scholar]
- 33.Sanders KM, Koh SD, Ward SM. Interstitial cells of cajal as pacemakers in the gastrointestinal tract. Annu Rev Physiol. 2006; 68: 307–43. doi: 10.1146/annurev.physiol.68.040504.094718 [DOI] [PubMed] [Google Scholar]
- 34.Kwong NK, Brown BH, Whittaker GE, Duthie HL. Electrical activity of the gastric antrum in man. Br J Surg. 1970; 57(12): 913–6. doi: 10.1002/bjs.1800571211 [DOI] [PubMed] [Google Scholar]
- 35.Couturier D, Rozé C, Paolaggi J, Debray C. Electrical activity of the normal human stomach. A comparative study of recordings obtained from the serosal and mucosal sides. Am J Dig Dis. 1972; 17(11): 969–76. doi: 10.1007/BF02239136 [DOI] [PubMed] [Google Scholar]
- 36.Smout AJ, van der Schee EJ, Grashuis JL. What is measured in electrogastrography? Dig Dis Sci. 1980; 25(3): 179–87. doi: 10.1007/BF01308136 [DOI] [PubMed] [Google Scholar]
- 37.Parkman HP, Hasler WL, Barnett JL, Eaker EY, American Motility Society Clinical GI Motility Testing Task Force. Electrogastrography: a document prepared by the gastric section of the American Motility Society Clinical GI Motility Testing Task Force. Neurogastroenterol Motil. 2003; 15(2): 89–102. doi: 10.1046/j.1365-2982.2003.00396.x [DOI] [PubMed] [Google Scholar]
- 38.Geldof H, van der Schee EJ, van Blankenstein M, Grashuis JL. Electrogastrographic study of gastric myoelectrical activity in patients with unexplained nausea and vomiting. Gut. 1986; 27(7): 799–808. doi: 10.1136/gut.27.7.799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Simonian HP, Panganamamula K, Parkman HP, Xu X, Chen JZ, Lindberg G, Xu H, Shao C, Ke MY, Lykke M, Hansen P, Barner B, Buhl H. Multichannel electrogastrography (EGG) in normal subjects: a multicenter study. Dig Dis Sci. 2004; 49(4): 594–601. doi: 10.1023/B:DDAS.0000026304.83214.50 [DOI] [PubMed] [Google Scholar]
- 40.Parkman HP, Harris AD, Miller MA, Fisher RS. Influence of age, gender, and menstrual cycle on the normal electrogastrogram. Am J Gastroenterol. 1996; 91(1): 127–33. [PubMed] [Google Scholar]
- 41.Chen JD, Lin ZY, Parolisi S, McCallum RW. Inhibitory effects of cholecystokinin on postprandial gastric myoelectrical activity. Dig Dis Sci. 1995; 40(12): 2614–22. doi: 10.1007/BF02220450 [DOI] [PubMed] [Google Scholar]
- 42.Zhao Y, Ke M, Wang Z, Wei J, Zhu L, Sun X, Zhang J. Pathophysiological and psychosocial study in patients with functional vomiting. J Neurogastroenterol Motil. 2010; 16(3): 274–80. doi: 10.5056/jnm.2010.16.3.274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang ZS, Elsenbruch S, Orr WC, Chen JD. Detection of gastric slow wave uncoupling from multi-channel electrogastrogram: validations and applications. Neurogastroenterol Motil. 2003; 15(5): 457–65. doi: 10.1046/j.1365-2982.2003.00430.x [DOI] [PubMed] [Google Scholar]
- 44.Lin X, Chen JZ. Abnormal gastric slow waves in patients with functional dyspepsia assessed by multichannel electrogastrography. Am J Physiol Gastrointest Liver Physiol. 2001; 280(6): G1370–5. [DOI] [PubMed] [Google Scholar]
- 45.Koch KL, Stewart WR, Stern RM. Effect of barium meals on gastric electromechanical activity in man. A fluoroscopic-electrogastrographic study. Dig Dis Sci. 1987; 32(11): 1217–22. doi: 10.1007/BF01296369 [DOI] [PubMed] [Google Scholar]
- 46.Gonlachanvit S, Chey WD, Goodman KJ, Parkman HP. Effect of meal size and test duration on gastric emptying and gastric myoelectrical activity as determined with simultaneous [13C]octanoate breath test and electrogastrography in normal subjects using a muffin meal. Dig Dis Sci. 2001; 46(12): 2643–50. doi: 10.1023/A:1012758925461 [DOI] [PubMed] [Google Scholar]
- 47.Kamiya T, Wada T, Ogasawara N, Kataoka H, Sasaki M. JOH T, Itho M. Impaired gastric motility in patients with functional dyspepsia. Jpn J Clin Physiol. 2007; 37: 251–6. [Google Scholar]
- 48.Shimada Y, Watanabe M, Shibahara N, Kita T, Itoh T, Terasawa K. Electrogastrographic power ratio in humans is not related to changes in antrum-skin distance but to antral motility. J Gastroenterol. 1998; 33(3): 310–7. doi: 10.1007/s005350050089 [DOI] [PubMed] [Google Scholar]
- 49.Riezzo G, Chiloiro M, Russo F, Clemente C, Di Matteo G, Guerra V, Di Leo A. Gastric electrical activity and gastrointestinal hormones in dyspeptic patients. Digestion. 2001; 63(1): 20–9. doi: 10.1159/000051868 [DOI] [PubMed] [Google Scholar]
- 50.Pfaffenbach B, Adamek RJ, Bartholomäus C, Wegener M. Gastric dysrhythmias and delayed gastric emptying in patients with functional dyspepsia. Dig Dis Sci. 1997; 42(10): 2094–9. doi: 10.1023/A:1018826719628 [DOI] [PubMed] [Google Scholar]
- 51.Chen TS, Lee YC, Chang FY, Wu HC, Lee SD. Psychosocial distress is associated with abnormal gastric myoelectrical activity in patients with functional dyspepsia. Scand J Gastroenterol. 2006; 41(7): 791–6. doi: 10.1080/00365520500495599 [DOI] [PubMed] [Google Scholar]
- 52.Leahy A, Besherdas K, Clayman C, Mason I, Epstein O. Abnormalities of the electrogastrogram in functional gastrointestinal disorders. Am J Gastroenterol. 1999; 94(4): 1023–8. doi: 10.1111/j.1572-0241.1999.01007.x [DOI] [PubMed] [Google Scholar]
- 53.Lin Z, Eaker EY, Sarosiek I, McCallum RW. Gastric myoelectrical activity and gastric emptying in patients with functional dyspepsia. Am J Gastroenterol. 1999; 94(9): 2384–9. doi: 10.1111/j.1572-0241.1999.01362.x [DOI] [PubMed] [Google Scholar]
- 54.Kawagishi T, Nishizawa Y, Emoto M, Maekawa K, Okuno Y, Taniwaki H, Inaba M, Ishimura E, Morii H. Gastric myoelectrical activity in patients with diabetes. Role of glucose control and autonomic nerve function. Diabetes Care. 1997; 20(5): 848–54. doi: 10.2337/diacare.20.5.848 [DOI] [PubMed] [Google Scholar]
- 55.Nohara S, Iwase M, Imoto H, Sasaki N, Nakamura U, Uchizono Y, Abe S, Doi Y, Iida M. Gastric emptying in patients with Type 2 diabetes mellitus and diabetes associated with mitochondrial DNA 3243 mutation using 13C-octanoic acid breath test. J Diabetes Complications. 2006; 20(5): 295–301. doi: 10.1016/j.jdiacomp.2005.07.007 [DOI] [PubMed] [Google Scholar]
- 56.Toporowska-Kowalska E, Wasowska-Królikowska K, Szadkowska A, Bodalski J. Electrogastrography in children and adolescents with type 1 diabetes: weak correlation with metabolic control parameters. Acta Paediatr. 2006; 95(11): 1439–45. doi: 10.1080/08035250600589025 [DOI] [PubMed] [Google Scholar]
- 57.Kamiya T, Kobayashi Y, Hirako M, Misu N, Nagao T, Hara M, Matsuhisa E, Ando T, Adachi H, Sakuma N, Kimura G. Gastric motility in patients with recurrent gastric ulcers. J Smooth Muscle Res. 2002; 38(1-2): 1–9. doi: 10.1540/jsmr.38.1 [DOI] [PubMed] [Google Scholar]
- 58.Budzyński A, Bobrzyński A, Lorens K, Konturek PC, Thor P, Konturek SJ. The influence of cholecystokinin on gastric myoelectrical activity in duodenal ulcer following Helicobacter pylori eradication--an electrogastrographic study. J Physiol Pharmacol. 2002; 53(2): 171–82. [PubMed] [Google Scholar]
- 59.Miyaji H, Azuma T, Ito S, Abe Y, Ono H, Suto H, Ito Y, Yamazaki Y, Kohli Y, Kuriyama M. The effect of helicobacter pylori eradication therapy on gastric antral myoelectrical activity and gastric emptying in patients with non-ulcer dyspepsia. Aliment Pharmacol Ther. 1999; 13(11): 1473–80. doi: 10.1046/j.1365-2036.1999.00634.x [DOI] [PubMed] [Google Scholar]
- 60.Lin Z, Chen JD, Parolisi S, Shifflett J, Peura DA, McCallum RW. Prevalence of gastric myoelectrical abnormalities in patients with nonulcer dyspepsia and H. pylori infection: resolution after H. pylori eradication. Dig Dis Sci. 2001; 46(4): 739–45. doi: 10.1023/A:1010783830093 [DOI] [PubMed] [Google Scholar]
- 61.Schaap HM, Smout AJ, Akkermans LM. Myoelectrical activity of the Billroth II gastric remnant. Gut. 1990; 31(9): 984–8. doi: 10.1136/gut.31.9.984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Xu X, Mandanas RA, Lin X, Chen JD. Impaired gastric slow wave rhythmicity in patients after bone marrow or stem cell transplant. Dig Dis Sci. 2002; 47(8): 1746–51. doi: 10.1023/A:1016484226110 [DOI] [PubMed] [Google Scholar]
- 63.Chasen M, Bhargava R. Gastrointestinal symptoms, electrogastrography, inflammatory markers, and PG-SGA in patients with advanced cancer. Support Care Cancer. 2012; 20(6): 1283–90. doi: 10.1007/s00520-011-1215-8 [DOI] [PubMed] [Google Scholar]
- 64.Murakami H, Matsumoto H, Kaida Y, Kubota H, Higashida M, Hirabayashi Y, Oka Y, Okumura H, Urakami A, Yamashita K, Hirai T. Examination on the gastric motility and the parameter (%normal, %SWC (Percentage slow wave coupling)) of multichannel electrogastrography. J Smooth Muscle Res. 2011; 15: J-37–44 doi: 10.1540/heikatsukinzashi.15.J37 [DOI] [Google Scholar]
- 65.Hirai T, Matsumoto H, Iki K, Hirabayashi Y, Kawabe Y, Ikeda M, Yamamura M, Hato S, Urakami A, Yamashita K, Tsunoda T, Haruma K. Lower esophageal sphincter- and vagus-preserving proximal partial gastrectomy for early cancer of the gastric cardia. Surg Today. 2006; 36(10): 874–8. doi: 10.1007/s00595-006-3265-y [DOI] [PubMed] [Google Scholar]
- 66.Ghoos YF, Maes BD, Geypens BJ, Mys G, Hiele MI, Rutgeerts PJ, Vantrappen G. Measurement of gastric emptying rate of solids by means of a carbon-labeled octanoic acid breath test. Gastroenterology. 1993; 104(6): 1640–7. doi: 10.1016/0016-5085(93)90640-X [DOI] [PubMed] [Google Scholar]
- 67.Murakami H, Matsumoto H, Kubota H, Higashida M, Nakamura M, Hirai T. Evaluation of electrical activity on multichannel electrogastrography after vagus nerve-preserving distal gastrectomy. J Smooth Muscle Res. 2013; 49: 1–14. doi: 10.1540/jsmr.49.1 [DOI] [PMC free article] [PubMed] [Google Scholar]